Abstract
Caspase-1 activation is a central event in innate immune responses to many pathogenic infections and tissue damage. The NLRP3 inflammasome, a multi-protein scaffolding complex that assembles in response to two distinct steps, priming and activation, is required for caspase-1 activation. However the detailed mechanisms of these steps remain poorly characterized. To investigate the process of LPS mediated NLRP3 inflammasome priming we used constitutively present proIL-18 as the caspase-1 specific substrate to allow study of the early events. We analyzed human monocyte caspase-1 activity in response to LPS priming followed by activation with ATP. Within minutes of endotoxin priming, the NLRP3 inflammasome is licensed for ATP induced release of processed IL-18, ASC, and active caspase-1, independent of new mRNA or protein synthesis. Moreover, extracellular-regulated kinase 1 (ERK1) phosphorylation is central to the priming process. ERK inhibition and siRNA mediated ERK1 knockdown profoundly impair priming. In addition, proteasome inhibition prevents ERK phosphorylation and blocks priming. Scavenging reactive oxygen species (ROS) with diphenylene-iodonium also blocks both priming and ERK phosphorylation. These findings suggest that ERK1-mediated post-translational modifications license the NLRP3 inflammasome to respond to the second signal ATP by inducing posttranslational events that are independent of new production of proIL-1β and NOD-like receptor components.
Keywords: human, inflammation, cytokines, monocytes/macrophages, lipopolysaccharide, cell activation, bacterial infections
Introduction
In response to the detection of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), a multi-protein complex assembles to activate caspase-1 from its zymogen form. This complex has been termed the inflammasome (1). Active caspase-1 cleaves the pro-inflammatory cytokines interleukin-1β (IL-1β) and interleukin-18 (IL-18) from their pro-forms into their secreted mature forms. In this context, the inflammasome plays a key role in the innate immune response to bacterial and viral infections as well as in responses to tissue damage. It has been implicated in a broad range of common inflammatory diseases such as sepsis (2–4), lung injury (3,5–7), diabetes mellitus (8,9), rheumatoid arthritis (10) as well as rarer inherited disorders such as familial Mediterranean fever (11) and assorted cryopyrin-associated auto-inflammatory diseases (12,13).
Inflammasomes can respond to specific PAMP/DAMPs and are categorized according their sensor proteins: NLRP1 (NOD-like receptor family, pyrin domain-containing-1), NLRP3 (NOD-like receptor family, pyrin domain-containing-3), NLRC4 (NOD-like receptor family CARD domain-containing protein 4), AIM2 (Absent in melanoma-2) and pyrin (14–18). The NLRP3 inflammasome is the most widely studied and responds to a broad range of PAMP/DAMPs. NLRP3 is part of the NOD-like receptor family which contain intracellular homologs of the Toll-like receptors (TLR). Similar to the TLRs, NLRP3 contains a leucine rich repeat region (LRR) in addition to a nucleotide binding domain (NBD) and a pyrin domain (PYD). It is the adaptor molecule, apoptosis-associated speck-forming complex containing CARD (ASC), which allows caspase-1 to bind to and dimerize in response to NLRP3 activation. How the NLRP3 inflammasome assembles in response to PAMP/DAMPs which have no structural homology is an area of active interest.
It is believed that the activation of the NLRP3 inflammasome in macrophages, dendritic and microglial cells requires two signals. The first signal is termed priming, and is commonly induced by a PAMP such as LPS. Current concepts suggest that priming depends upon the de novo synthesis of pro-IL-1β and the up-regulation of NLRP3 (19,20). However, these concepts were recently challenged by the demonstration that TLR-induced priming of the NLRP3 inflammasome does not require new protein synthesis or upregulation of NLRP3 (21,22). The second signal induces the assembly and activation of the inflammasome. Signal 2 can be induced by exogenous ATP activation of the P2X7 receptor, as well as by nigericin, silica, and pore-forming bacterial toxins, all of which induce efflux of potassium (23–26). Once cytosolic potassium levels fall, the primed inflammasome becomes functional, caspase-1 becomes catalytically active and mature IL-1β and IL-18 are released from the cell.
To understand the mechanism of TLR mediated priming in the two-step model of the NLRP3 inflammasome in human monocytes, we sought to study early signaling events that occur before protein translation. We show that pro-caspase-1, ASC and pro-IL-18 are constitutively expressed by resting fresh human monocytes. This knowledge allowed us to dissect the kinetics and signaling components of NLRP3 inflammasome priming. Our approach used the standard model of LPS priming followed by ATP. The use of short LPS priming times (5–30min in duration), as described for mouse macrophages before (21,22), eliminated the synthesis of new inflammasome components such as IL-1β and NLRP3 from contention as necessary components of LPS’s ability to ‘prime’ monocytes for the second signal, ATP. We show that in human monocytes using proIL-18 as the caspase-1 substrate, consistent with published findings in mouse macrophages (21,22), NLRP3 inflammasome priming is independent of new protein synthesis but is dependent on the generation of reactive oxygen species. We further show that NLRP3 inflammasome priming is dependent upon proteasome function and requires the activation of extracellular signal regulated kinase 1 (ERK 1).
Material and Methods
Cell culture and chemicals
Human PBMCs were isolated by Histopaque density gradients from fresh source leukocytes from the American Red Cross. Monocytes were isolated from PBMC by CD14 positive selection (Miltenyi Biotec). In brief, blood was layered on lymphocyte separation medium (Cellgro, USA) and spun at 600g for 20 min at room temperature with brakes off. The mononuclear layer was collected and washed three times with RPMI 1640. Monocytes were purified from PBMCs using positive selection with anti-CD14-coated magnetic beads following the manufacturer’s recommendations (Miltenyi Biotec). This method of purification yields greater than 98% pure monocytes based on flow cytometry analysis. Monocytes (1×106/ml) were grown in culture tubes in in RPMI 1640 (MediaTech, Inc) supplemented with 5% heat-inactivated FBS (Atlanta Biologicals) and 1% penicillin-streptomycin (Invitrogen Life Technologies). FBS lots were prescreened to confirm that they did not induce IL-18 release by ATP in the absence of LPS. Wild type, Casp1 and Nlrp3-deficient immortalized mouse bone marrow–derived macrophages (BMDMs) were generated as described earlier (19,27,28) and grown in RPMI 1640 supplemented with FBS and penicillin-streptomycin. Priming was provided by LPS (from Escherichia coli strain 0111:B4; Alexis Biochemicals, San Diego) and step 2 activation by ATP (5mM) from Sigma-Aldrich. MG132 (proteasome inhibitor), U0126 (ERK inhibitor), WP1130 (deubiquitinase inhibitor), G5 (ubiquitin isopeptidase inhibitor) and wortmannin (PI3K inhibitor) were from Calbiochem while SB203580 (P38 MAPK inhibitor) and A6355 (another ERK inhibitor) were from Sigma-Aldrich and SP600125 (JNK inhibitor) from Invivogen. Diphenylene iodonium chloride (DPI) was from Sigma-Aldrich. The IκBα inhibitor, BAY11-7082 was purchased from Invivogen while the AG126 was from Cayman Chemical.
Preparation of cell lysates and Western blotting
Cells were lysed in RIPA buffer (50 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM EDTA, 1mM NaF, 1% NP-40 and 0.25% Na-deoxycholate) supplemented with complete protease inhibitor cocktail (Sigma), 1 mM PMSF and 100 μM N-(methoxysuccinyl)-Ala-Ala-Pro-Val chloromethyl ketone - CMK). The protein concentrations were determined using Bio-Rad Dc protein Lowry assay (Bio-Rad). After SDS-PAGE gel electrophoresis, separated proteins were transferred to a PVDF (polyvinylidine fluoride) transfer membrane, probed with the antibody of interest, followed by HRP conjugated secondary antibody and developed by ECL (Amersham Biosciences) using autoradiography. Rabbit polyclonal antibodies against IL-1β, ASC and caspase-1 were developed in our laboratory. Anti-human IL-18 antibody was purchased from MBL, Ubiquitin antibody from Santa Cruz and actin from MP Biomedicals. Mouse anti-phospho-IκBα, rabbit anti-IκBα, rabbit anti-phospho-ERK1/2 and rabbit anti-ERK1/2 were purchased from Cell Signaling Tech. Released IL-1β was quantified using a sandwich ELISA format using our rabbit polyclonal as previously reported (29) but substituting monoclonal (MAB601) from R&D Systems for the capture antibody. In addition, IL-1β in the cell culture medium was detected by immunoblot of cell culture medium with rabbit polyclonal IL-1β antibody (lab generated). Released IL-18 was quantified by ELISA using MBL antibodies.
Cell death detection by quantification of lactate dehydrogenase (LDH) release in cell culture medium
LDH release into cell culture medium was used as an indicator of cell death using NAD+ reduction assay (Roche Applied Science). Monocytes were grown in culture tubes at the density 106/ml and pretreated with or without inhibitor for 30 minutes followed by LPS (1μg/ml) for 0.5 or 3 hours and finally activated with 5mM ATP for 30 minutes. Cell culture medium was collected, clarified by centrifugation and used for LDH assay. Total LDH content in cells (positive control) was measured in cells lysed with Triton X-100 (1% final concentration). Cell culture medium alone was used as a blank and OD values were subtracted from readings of samples and positive control. LDH concentration in the medium was detected at wavelength 490 nm. Cell death was calculated by the formula: , as described earlier (30).
Quantitative PCR
Total RNA from monocytes was extracted by TRIZOL method and converted into cDNA by ThermoScript RT system (both from Invitrogen Life Technologies). Quantitation of IL1B, IL8 and TNF genes expression was performed with SYBR Green I PCR Master Mix in the StepOne Real Time PCR System (both from Applied Biosystems) and expressed in relative copy numbers (RCN) as we described earlier (31).
Nucleofection
In knockdown experiments, small interfering RNA (siRNA) against ERK1 and scrambled siRNA were purchased from Sigma-Aldrich while Signalsilencer ® p42 MAPK (ERK2) siRNA II was purchased from Cell Signaling Company. siRNAs were delivered in monocytes by Amaxa nucleofector I (Lonza). 5′ –GACCGGAUGUUAACCUUUA-3′ and 5′-AAGCUGACCCUGAAGUUCA-3′ sequences were used to knock-down ERK1 and as scrambled control (32,33). For nucleofection, 5×106 monocytes were re-suspended in 100 μl of nucleofection solution containing 150 pmol siRNA for ERK1 and scrambled control, while siRNA against ERK2 was used according the manufacturer protocol. Nucleofection was performed with the Y-01 program. Immediately after nucleofection, monocytes were resuspended in RPMI medium supplemented with 10% FBS and left to recover overnight in polypropylene culture tubes to avoid adherence. The next morning, monocytes were counted with trypan blue showing that 90% of cells were viable. Then cells were treated with 1 μg/ml LPS for 30 minutes followed by 5 mM ATP for another 30 minutes. Released IL-18 in cell culture medium was measured using ELISA while cells were lysed and analyzed for proteins.
Statistical analysis
All experiments were performed a minimum of three independent times and expressed as mean values ± SEM. Comparison of groups for statistical difference were done using Student’s t test. P value ≤0.05 was considered significant.
Results
Monocyte priming for IL-1β versus IL-18
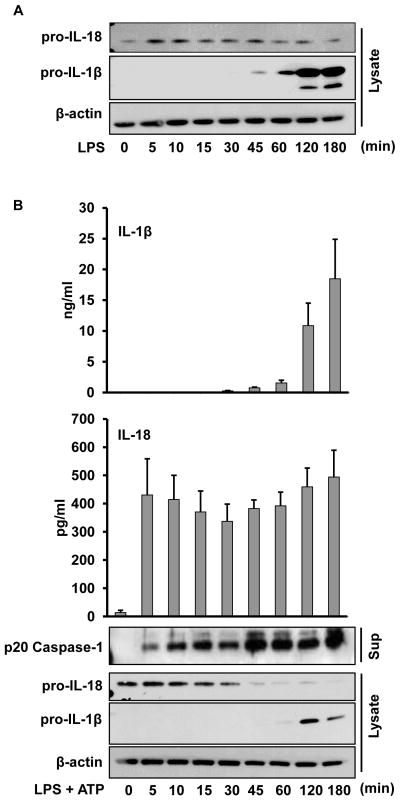
Priming with LPS and subsequent exogenous ATP-induced activation of the P2X7 receptor represents a classic model for NLRP3 inflammasome activation (23–25,34). However, the mechanistic details of the priming event remains poorly characterized. One proposed function of inflammasome priming is to generate the inflammasome substrate proIL-1β (19). Therefore, to better define priming we first analyzed the kinetics of the two known human monocyte caspase-1 substrates, IL-1β and IL-18. Human monocytes were purified by CD14 positive selection and treated with LPS (1 μg/ml) for intervals up to 180 min. Cells were lysed and analyzed for proIL-1β and proIL-18 by immunoblot. As demonstrated in Figure 1A, while purified human monocytes require almost an hour of priming to induce detectable levels of proIL-1β, the other caspase-1 substrate, proIL-18, is constitutively present. Thus, proIL-18 provides a tool to study earlier events in inflammasome priming.
Figure 1. IL-18 kinetics differs from IL-1β in response to LPS and ATP.
A. LPS priming induces expression of monocyte IL-1β but not IL-18. Fresh human monocytes were stimulated with LPS (1 μg/ml) for the specified time periods and then lysates analyzed for expression of proIL-18 and proIL-1β by immunoblots as compared to β-actin control. Shown is one representative blot of three. B. LPS stimulates rapid priming for ATP induced IL-18 and caspase-1 processing and release in monocytes. Monocytes were prepared and primed (step 1) as in Figure 1A for the indicated time points followed by 5 mM ATP for 30 min (step 2). Bar graphs represent released IL-1β and IL-18 from cell supernatants as determined by ELISA. Immunoblots show proIL-18, proIL-1β, and β-actin in the cell lysates and p20 caspase-1 released into supernatants, respectively. ELISA data represents the mean ± SEM for three separate monocyte donors and the blots are representative of repeated blots.
IL-18 processing and release is independent of new proteins synthesis but requires active caspase-1 and NLRP3 inflammasome
It has been presumed that the priming effect of LPS is dependent upon new NLRP3 protein expression along with generation of the proIL-1β substrate (19). However, recent studies clearly demonstrated that priming of the NLRP3 inflammasome in mouse macrophages does not require new protein synthesis or upregulation of NLRP3 (21,22). Having demonstrated that proIL-18 is constitutively present we elected to test whether new protein synthesis is required for priming in human monocytes. Monocytes were stimulated with LPS at brief intervals before addition of exogenous ATP. As shown in Figure 1B, whereas IL-1β processing and release was dependent upon new protein synthesis, this was not true for IL-18 since monocytes primed with LPS for 5 minutes followed by addition of ATP for 30 minutes without removal of LPS was sufficient to induce IL-18 release. Furthermore, the p20 functional form of caspase-1 was released into the supernatants concurrently with the IL-18 release, Figure 1B.
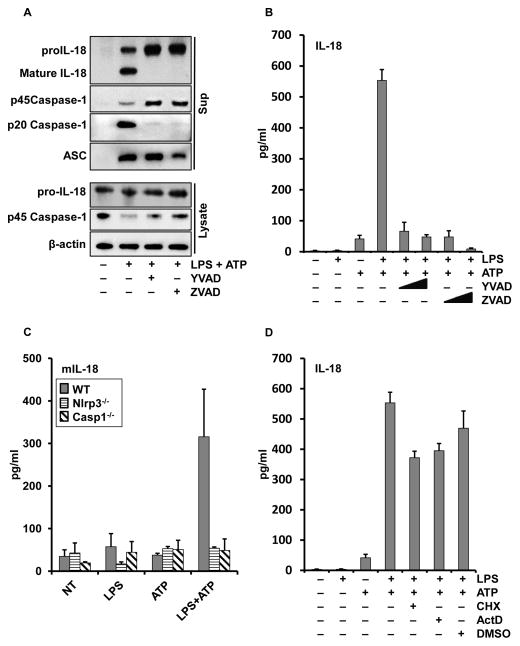
To confirm that the released IL-18 was a product of active caspase-1 (i.e. the inflammasome) and not simply the nonspecific release of proIL-18, we analyzed the supernatants by immunoblots. Importantly, the ELISA detection of IL-18 corresponded with the generation of 18 kDa processed form of IL-18, Figure 2A, B. This release of the mature form of IL-18 and caspase-1 was inhibited by pretreating the monocytes with caspase-1 inhibitors, YVAD and zVAD. Of note, although caspase-1 inhibition prevented the generation of mature IL-18 and p20 caspase-1, it did not prevent the release of proIL-18, procaspase-1 or ASC, Figure 2A. These results suggest that the inflammasome release pathway does not require functional caspase-1, as we have previously reported (35,36).
Figure 2. IL-18 processing and release is independent of new proteins synthesis but requires active caspase-1 and NLRP3 inflammasome.
Monocytes were pretreated with or without YVAD-cmk, ZVAD-fmk, cycloheximide or actinomycin D for 30 minutes prior to stimulation with LPS (1 μg/ml) for 30 min followed by 5 mM ATP for 30 min. (A) Immunoblots of IL-18, caspase-1 and ASC expressed in cell lysates (bottom group) and released in supernatants (top group) after pretreatment with YVAD or ZVAD (50uM). (B) Culture media were assayed by ELISA for the released IL-18 after LPS, ATP and YVAD or ZVAD at 10 or 50 μM, respectively. (C) Immortalized BMDM from WT, Nlrp3−/− and Casp1−/− were primed with LPS (1 μg/ml) for 30 minutes followed by 5 mM ATP for 30 min. Bar graphs represent released murine IL-18 from cell supernatants as determined by ELISA. (D) Monocytes were pretreated with cycloheximide (1 μg/ml) or actinomycin D (5 μg/ml) and IL-18 release after LPS/ATP stimulation was measured. ELISA data represents the mean ± SEM for three separate monocyte donors and the blots are representative of repeated blots.
The caspase-1 inhibition effect was further confirmed using BMDMs from mice with caspase-1 knockout (Figure 2C; Figure S1A), which showed that caspase-1 was essential for the LPS and ATP induced IL-18 processing.
Furthermore, extracellular potassium blocked IL-18 release from human monocytes (Figure S1B) which suggests that the human model represented the NLRP3 inflammasome. Rapid LPS priming of BMDMs from the NLRP3 knockout mice (Figure 2C, Figure S1A) failed to induce IL-18 processing suggesting that the early LPS priming targets the NLRP3 inflammasome.
Although unlikely, it is conceivable that within the 5 min of priming and 30 min of ATP, critical inflammasome regulatory proteins could be newly synthesized. To eliminate this possibility, monocytes were treated with cycloheximide, a translation inhibitor, or actinomycin D, a transcription inhibitor, 30 minute prior to the priming signal. These inhibitors completely blocked protein synthesis and mRNA transcription (Figure S2). Consistent with recent findings that priming of the NLRP3 inflammasome being a post-translational event (21,22), neither inhibition of protein synthesis or mRNA transcription inhibited the ability of LPS to prime for IL-18 processing and release (Figure 2D).
Priming regulated by proteasome
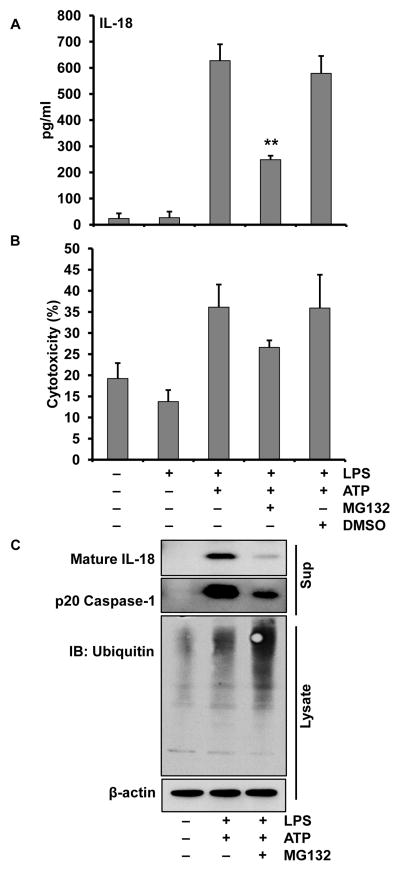
Having demonstrated that priming is indeed required, even for preformed substrates such as IL-18, and confirmed that this event is independent of new protein synthesis, we next wanted to determine whether post-translational protein modifications might be a component of priming. One possibility for post-translational modifications is the proteasome’s control of inflammasome-related protein levels. To test this we inhibited the proteasome using MG132 or bortezemib. Pretreating monocytes with MG132, 30 min prior to LPS priming, induced a significant deficit in ATP mediated IL-18 and caspase-1 processing and release while enhancing protein ubiquitination (Figure 3). In similar experiments, bortezemib also prevented priming of the IL-18 processing inflammasome (data not shown).
Figure 3. Proteasome inhibition suppresses mature IL-18 release by monocytes.
Monocytes were pretreated with or without MG132 (20μM) for 30 minutes, then primed with LPS (1 μg/ml) for 30 min followed by 30 min with or without 5 mM ATP. (A) Release of IL-18 in supernatants was significantly suppressed with MG132 **p < 0.01. (B) LDH release was not affected by MG132; (C) Immunoblots of cell supernatants for mature IL-18 and p20 caspase-1 (upper panels) and cell lysate for ubiquitinated proteins.
Proteasomal blockade also suppressed IL-1β processing and release. However, since MG132 prevented generation of proIL-1β, it was not possible to distinguish between the NFκB dependence of proIL-1β substrate generation and the effects of proteasomal inhibition on priming (Figure S3A). These results document the importance of using preformed proIL-18 as the optimum target substrate for studies of inflammasome priming events.
Since the half-life of intracellular constitutive IL-18 may be dependent upon NFκB activity, which itself is regulated by the proteasome, it is important to determine that proteasomal inhibition does not induce rapid loss of steady state proIL-18. We therefore examined the kinetics of proIL-18 in the presence of MG132 and protein synthesis inhibition (cycloheximide), Figure S3B,C. Neither inhibitor decreased intracellular proIL-18 levels at 1 h though proIL-18 levels began to decline by 2 h. Since we have shown that the priming and processing and release of IL-18 can occur as early as 35 minutes (5 min LPS priming and 30 min ATP) (Figure 1B), these data suggest that proteasomal regulation of proIL-18 inflammasome priming was not due loss of proIL-18 substrate. Of note, the rapidity of the priming event (5 min) coincides with the rapidity of the phosphorylation of the IKK substrate IκBα (Figure S3D) which, as expected, was preserved by MG132.
LPS induced ERK signaling events critical to priming
Having confirmed that inflammasome priming is independent of new protein synthesis, we turned to pathways important to priming upstream of NFκB transcriptional activation. Generation of IL-18 was significantly suppressed by the IKK inhibitor Bay 11 but even more powerfully by the ERK and tyrosine kinase inhibitor AG126 (Figure S4A). These data support the concept that priming involves a signaling step upstream of NFκB transcriptional activation in the LPS signaling pathway.
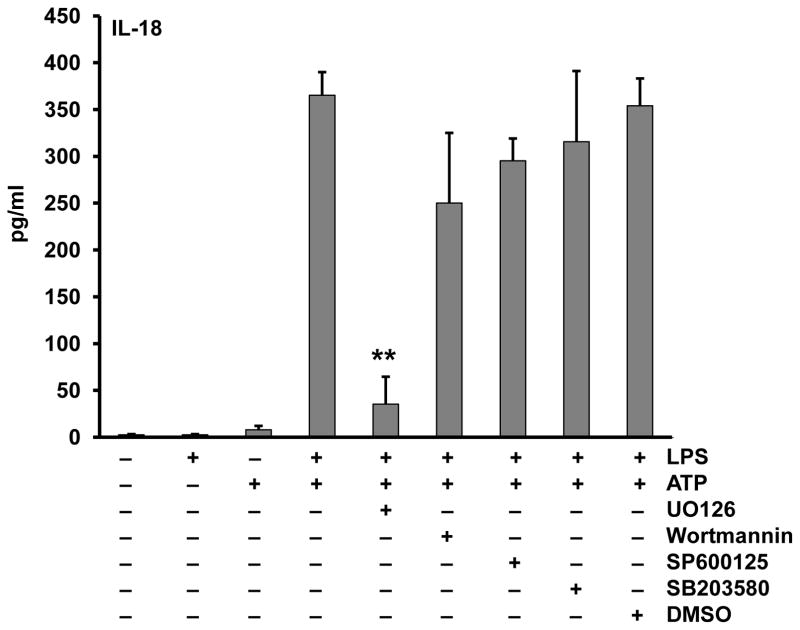
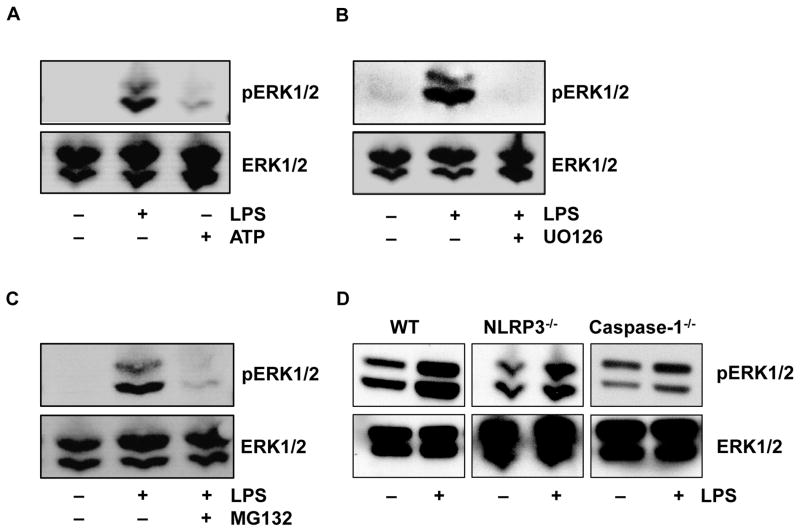
To further isolate the location of this priming event we compared the relative effects of inhibitors targeting MEK1/MEK2, PI3K, JNK and p38 kinase (Figure 4). Only MEK1/MEK2 inhibition demonstrated a profound suppression of priming and this finding is confirmed using another ERK inhibitor (A6355) (Figure S4B). To further analyze the specificity of this effect we found that ERK phosphorylation was induced by LPS priming but not by the second signal, ATP (Figure 5A), and ERK phosphorylation which is downstream of MEK1/MEK2 kinases was indeed inhibited by U0126 (Figure 5B). Lastly, regarding ERK phosphorylation, we considered whether the proteasomal inhibition also affected ERK signaling. As shown in Figure 5C, MG132 pretreatment prevented the LPS induced phosphorylation of ERK which confirms prior observations linking the proteasome to ERK (37).
Figure 4. Extracellular signal-regulated kinase (ERK) plays a crucial role in inflammasome priming.
Monocytes were pretreated with U0126 (ERK inhibitor) (10 μM), wortmannin (PI3K inhibitor) (10 μM), SB203580 (P38 MAPK inhibitor) (10 μM), or SP600125 (JNK inhibitor) (10 μM) for 30 min. Then cells were primed with LPS (1 μg/ml) for 30 min and activated with ATP (5 mM) for 30 min. Supernatants were assayed for the released IL-18. ** p = .0016.
Figure 5. ERK phosphorylation is induced by priming but not by ATP in absence of priming.
ERK phosphorylation was measured in monocyte lysates by immunoblotting with anti-phosphoERK specific antibody and total ERK antibody, respectively. (A) Comparison of ERK phosphorylation induced by LPS (1ug/ml) vs ATP 5mM, respectively. (B) UO126 (10μM) blocks phosphoERK induction by the LPS/ATP combination. (C) MG132 (20μM) blocks LPS induced ERK phosphorylation. (D) ERK phosphorylation in unstimulated versus LPS-primed WT, Nlrp3−/− and Casp1−/−BMDMs.
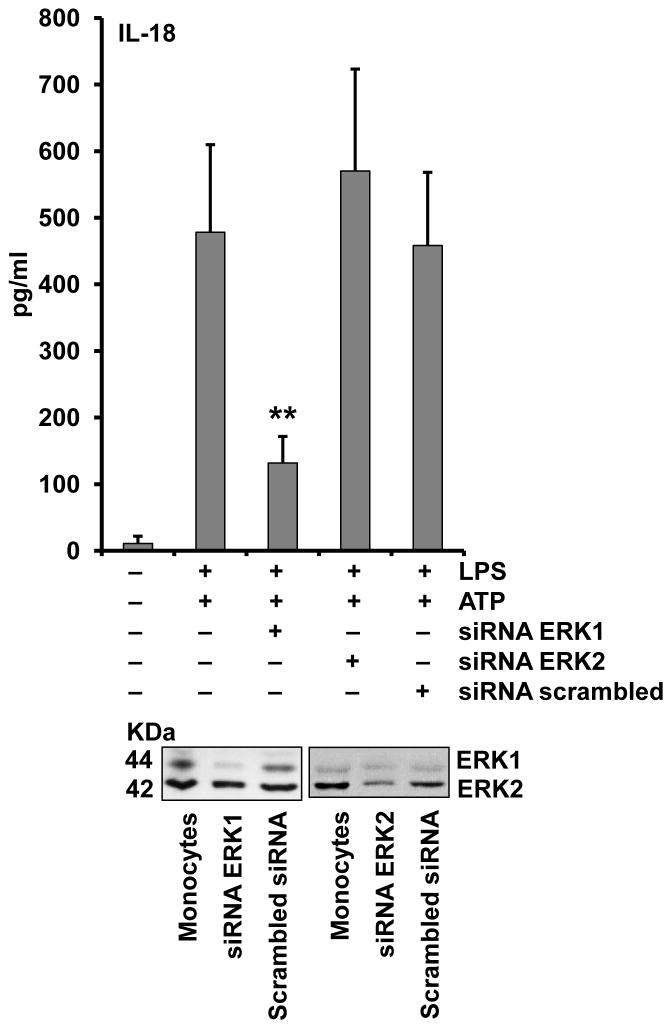
To further support the specificity of the ERK inhibitors UO126 and AG126, we down regulated ERK1 and ERK2 with siRNA. As shown in Figure 6, transient knockdown of ERK1 but not ERK2 was able to inhibit inflammasome priming. Together these data support the concept that ERK signaling is a central event in inflammasome priming. Additionally, we examined the role of ERK in priming of murine macrophages using immortalized BMDMs. Surprisingly, we found these cells have ERK MAPK activation at baseline, as shown in Figure 5D. Hence, these cells may bypass the need for ERK activation as part of the priming effect of LPS.
Figure 6. ERK1 knockdown suppresses IL-18 processing and release.
Monocytes were nucleofected with ERK1 and ERK2 specific siRNA or scrambled control and recovered overnight. Cells were then primed with LPS (1μg/ml) for 30 min and activated with ATP (5mM) for another 30 min. Supernatants were analyzed for IL-18 release and cell lysates were immunoblotted to confirm ERK protein down regulation using total ERK antibody. IL-18 ELISA represents the mean± SEM for three separate monocyte donors and the blots are representative of repeated blots. **p < 0.01 as compared to scrambled siRNA.
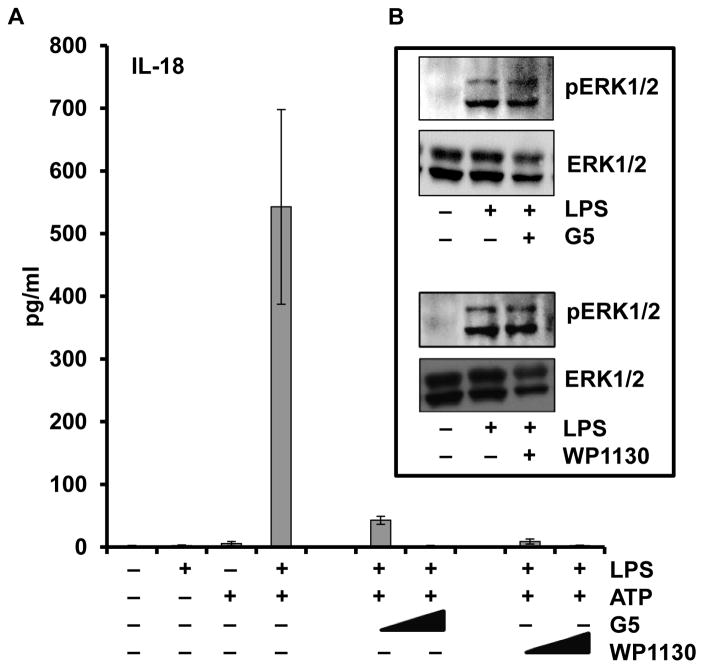
Previous reports suggested a role for deubiquitinases (DUB) in the post-translational regulation of the NLRP3 inflammasome (38,39), therefore we used the DUB inhibitors, WP1130 and G5, to evaluate the role of DUBs in the LPS rapid priming events. Interestingly, DUB inhibition diminished the amount of IL-18 released in a dose-dependent manner (Figure 7), confirming the published results in murine models. However, the DUB inhibitors did not affect ERK phosphorylation suggesting that these DUB enzymes may act downstream of, or parallel to, the ERK pathway.
Figure 7. DUBs inhibitors impair LPS priming of monocytes and IL-18 release.
Monocytes were pretreated with either G5 (0.1 and 0.5 μM) or WP1130 (1 and 5 μM) for 30 min prior to LPS (1 μg/ml) priming followed by the ATP (5mM) activation step. (A) Supernatants were analyzed for IL-18 release. (B) Cell lysates were analyzed by immunoblot for the effect of G5 (0.1μM) and WP1130 (1 μM) on the ERK phosphorylation.
ERK signaling and role of oxidants in priming
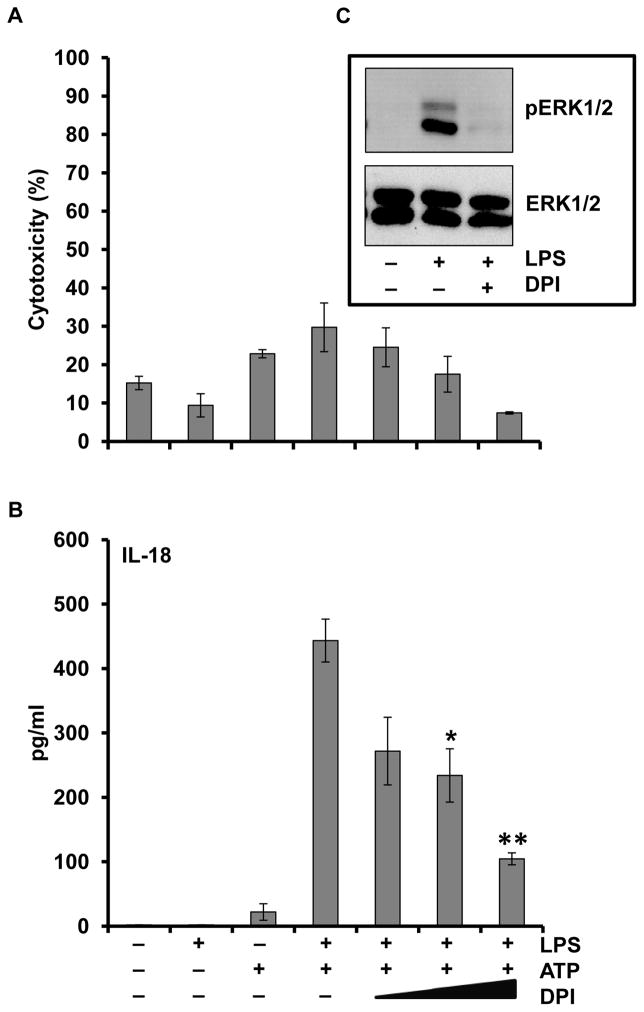
It has been shown that reactive oxygen species may activate the inflammasome but this has not been analyzed from the perspective of ERK mediated priming (21,22,40,41). We therefore adopted our LPS/ATP IL-18 model to this question. As shown in Figure 8A, B, the inhibitor of the NADPH oxidase diphenylene iodonium (DPI) was able to inhibit the ability of LPS/ATP to process proIL-18 in a dose-dependent manner. Of note, the prevention of IL-18 processing by DPI did inhibit ERK phosphorylation as shown in Figure 8C.
Figure 8. Inhibition of reactive oxygen species generation impairs LPS priming of monocytes and IL-18 release.
Monocytes were pretreated with DPI (1, 10, 20 and 50 μM) for 30 min prior to LPS (1 μg/ml) priming followed by the ATP (5mM) activation step. Supernatants were analyzed for (A) LDH and (B) IL-18 release, respectively. *p < 0.05 and **p < 0.01 as compared to LPS/ATP. (C) Cell lysates were immunoblotted for phosphoERK and total ERK.
These studies suggest a complex interplay between ERK and oxidants in which ERK function may be regulated in part by upstream oxidants which prevent phosphatases from suppressing ERK (42,43). Priming therefore represents a complex of regulated events involving kinases, phosphatases, oxidants and the proteasome that are linked to ERK signaling in a way that licenses the inflammasome constituents to respond to a second signal (results summarized in Figure 9).
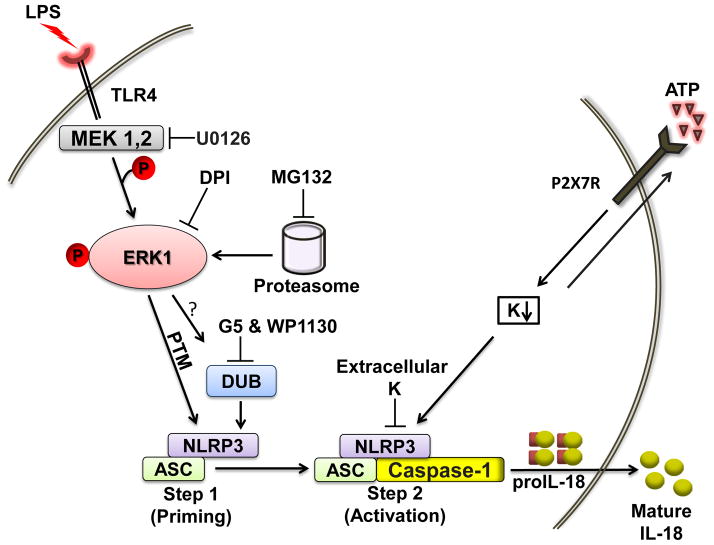
Figure 9. Model illustrating the signaling pathways involved in the ERK dependent rapid inflammasome priming.
Brief priming with LPS induces ERK1 phosphorylation that likely induces changes in either the localization or interaction of ASC with an NLR component of the inflammasome. This step 1 (priming event) can be blocked by UO126 and is controlled by reactive oxygen species and the proteasome. It seems that LPS-induced ERK phosphorylation leads to an ERK-dependent posttranslational modification of one or more of the key proteins involved in inflammasome function. DUBs may work downstream or parallel to the ERK pathway. Step 2 (provided by ATP in our experiments) lowers intracellular potassium levels. Step 2 is dependent upon this rapid signaling process since ATP without priming does not activate caspase-1.
Discussion
The inflammasome is involved in the pathogenesis of many inflammatory diseases and better understanding of how the inflammasome is regulated may identify new therapeutic agents that could be used to treat such diseases. In this context, it has been shown that inflammasome activation requires two distinct steps, i.e., step 1, priming, and step 2, activation. However, the mechanistic details of priming have been poorly characterized to date. Therefore, to better delineate what inflammasome priming is, we focused on those aspects of priming that do not require new protein synthesis.
First, we show that proIL-18 is constitutively expressed in fresh unstimulated monocytes. Based on this observation we utilized the LPS/ATP two-step model to identify some of the early regulatory events that occur before protein synthesis. Our approach depended upon a functional readout of inflammasome activation, the cleavage of caspase-1 and the maturation of proIL-18. Our results confirm that the widely accepted concept of priming as the induction of NLRP3 and the caspase-1 substrate proIL-1β is incomplete (19,20). Importantly in this regard, we show that the IL-18 processing inflammasome could be primed and activated after 5 minutes of LPS priming followed by 30 minutes of ATP. This finding is consistent with recent reports that showed activation of NLRP3 and cleavage of caspase-1 in bone marrow-derived macrophage (BMDMs) after priming of cells for short time with LPS followed by activation with ATP, or co-stimulation with TLR agonists plus ATP (22). We show that priming can be blocked by inhibitors of the NFκB signaling pathway but this effect was pre-transcriptional since kinetic analyses showed the inhibition occurred well before new protein synthesis. Furthermore, inhibitors of protein synthesis had no significant effect on priming of the NLRP3 inflammasome.
Attesting to the validity of the non-transcriptional model of inflammasome activation (21,22) we confirm by immunoblots that the released IL-18 is the mature processed form and we show that the processed IL-18 is accompanied by released p20 caspase-1 and ASC. This processing was dependent upon active caspase-1 since it was completely inhibited by caspase-1 specific tetrapeptide, YVAD-cmk. This result was further confirmed by using BMDMs from caspase-1 knockout mice. However, because these mice were Casp1/Casp11 double knockout, we cannot exclude the role of caspase 11 in this process.
Of note, however, inhibition of caspase-1 only inhibited processing but not the release of IL-18, suggesting that inflammasome release is not dependent upon active caspase-1, as we have previously observed (35,36). We interpret these results to mean that inflammasome priming by LPS causes a pre-transcriptional modification of the constituent proteins that control inflammasome readiness for step 2, ATP activation of the P2X7 receptor. Post-translational modifications of the key regulatory proteins by phosphorylation, ubiquitination and yet to be identified changes play crucial roles in the regulation of signal transduction in the cell. In this context, we previously showed that inhibition of tyrosine phosphatases leads to inflammasome activation and IL-1β secretion (32).
Also, recent reports show that LPS/ATP can induce deubiquitination of NLRP3, affecting inflammasome activation and caspase-1 cleavage (22,39). In addition, deubiquitinase (DUB) dependent regulation of signaling is required for assembly of the inflammasome and caspase-1 activation (38). These findings are in concert with our finding that MG132 preserves ubiquitinated protein levels and inhibits inflammasome priming and activation. Moreover, the inhibitors of DUB, G5 and WP1130, inhibited the rapid priming of the inflammasome suggesting that caspase-1-induced IL-18 release is DUB dependent.
We believe that our model reflects the NLRP3 inflammasome since we were able to block the rapid processing of proIL-18 by extracellular potassium which has previously been shown to be NLRP3 specific (26). In further support of NLRP3 is the abundance of data in murine models that support the LPS/ATP activation pathway as NLRP3 dependent (34,44). In addition, we used BMDMs for Nlrp3 knock-out mice, which showed inhibition of IL-18 release, confirming that NLRP3 is responsible for the rapid priming event in these macrophages
The post-translational nature of the priming event supported the idea that priming involves a kinase mediated event. Addressing the events critical to the LPS mediated priming we turned to classic signaling pathways. It is well established that lipopolysaccharide activates the MAPKs family including ERK, p38 MAPK and JNK as well as PI3K, another important mediator downstream of LPS/TLR4 signaling. To our knowledge the role of these pathways in this post-translational licensing of inflammasome function has not previously been attempted (27). We used specific inhibitors for each of the above kinase pathways. We found that the ERK inhibitor (U0126) could significantly block inflammasome priming and activation. The pharmacological inhibition was further confirmed with the effective siRNA knock-down of ERK1 which is a downstream target of MEK1/2. It seems that ERK1 is the key player in the early events associated with priming as suggested by the knock-down experiment but further work will be needed to confirm this finding. In contrast, the p38 MAPK inhibitor (SB203580), JNK inhibitor (SP600125) and the PI3K inhibitor (wortmannin) did not affect early priming and activation indicating that these conventional pathways activated by TLR signaling are not mandatory for the LPS-dependent inflammasome activation. Also, RIP2 signaling is not obligatory for inflammasome activation as some recent reports specified RIP2 as another target of SB203580 (45).
It should be noted that after our manuscript was submitted, a report demonstrated that Syk and Jnk are linked to inflammasome activity (46). This work used a 4h LPS priming step and nigericin model largely in a murine macrophage system. Unlike our current priming model, this work provided the inhibitors after the priming and failed to show an ERK effect. Interestingly, we found that ERK is spontaneously phosphorylated in these immortalized, proliferating murine BMDMs. We speculate that this background ERK activation provides “spontaneous” priming that is normally provided by LPS in our nondividing human monocytes, the focus of our model. As we have previously shown, providing ERK inhibition after priming is not able to block LPS/ATP mediated human monocyte inflammasome activation (36) whereas it does do so when added before priming. Thus methodological, cell type and species differences likely explain the variances with our current work regarding NLRP3 inflammasome regulation.
To discern whether the ERK activation was addressing the priming phase, we showed that ERK phosphorylation is dependent on LPS priming, as ATP alone did not induce ERK phosphorylation but LPS induced ERK phosphorylation was blocked by U0126. Of note, ERK has been found to phosphorylate more than 150 cytoplasmic and nuclear targets (47,48). Based upon the rapid kinetics, our findings suggest that ERK phosphorylation of transcription factor targets is not obligatory for the early priming events. It is likely, therefore, that ERK has other targets that play a key role in priming of the NLRP3 inflammasome. For example, it is conceivable that DUBs may be activated downstream of ERK. This post-translational modification could contribute to a DUB-dependent deubiquitination of NLRP3 leading to inflammasome assembly and caspase-1 cleavage and activation. Further work is needed to identify the target/s which could provide improved ways to manipulate inflammatory diseases.
One model for inflammasome priming and activation focuses on reactive oxygen species, ROS. Recent reports suggest that ROS interaction with the TXNIP-thioredoxin complex liberates TXNIP which then causes inflammasome assembly (49). We evaluated the role of ROS in the early events occurring after LPS priming. Inhibition of ROS production by DPI decreased IL-18 release, indicating that ROS plays a major role in early priming events as shown recently in mouse BMDMs (21,22,40,41). DPI was able to block ERK phosphorylation suggesting that ROS upstream of ERK can be regulatory. This is consistent with previous reports that ROS can initiate ERK activation by inhibition of ERK-directed phosphatases like DUSP1 and DUSP6 by the oxidation of their catalytic cysteine residues (50–52). Also, ROS can inhibit ERK tyrosine phosphatases, PP1 and PP2A, by inhibiting their cysteine catalytic sites (53). These ERK-directed phosphatases determine the outcome of RAS/Raf/ERK signaling (54,55). Finally, it also has been shown that some phosphatases are up-regulated in proteasome-inhibited cells including ERK specific phosphatases (37,56). Taken together, these prior reports substantiate our recent report that phosphatase inhibition itself can activate the inflammasome in PMA activated THP-1 cells (32) and provides additional support for the complex role that the proteasome plays in regulating caspase-1 activation (38). It seems that post-translational modifications are central to regulating inflammasome priming and activation.
In summary, our data reveal that priming (or licensing of the inflammasome) is an early event, independent of transcription and translation and centered upon the ERK signaling pathway. Further characterization of the regulatory mechanisms involved in the early inflammasome priming and activation should enhance our understanding and provide new opportunities to treat inflammasome-dependent disorders.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Susheela Tridandapani and Jon Butchar for their generous help.
Footnotes
This work was supported by NIH grants HL089440 and HL076278 to MDW and AR055398 to EA.
Reference List
- 1.Martinon F, Burns K, Tschopp J. The inflammasome. A molecular platform triggering activation of inflammatory caspases and processing of proIL-1b. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Fahy RJ, Exline MC, Gavrilin MA, Bhatt NY, Besecker BY, Sarkar A, Hollyfield JL, Duncan MD, Nagaraja HN, Knatz NL, Hall M, Wewers MD. Inflammasome mRNA expression in human monocytes during early septic shock. Am J Respir Crit Care Med. 2008;177:983–988. doi: 10.1164/rccm.200703-418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis EJ, van de Veerdonk FL, Mouktaroudi M, Raftogiannis M, Antonopoulou A, Joosten LA, Pickkers P, Savva A, Georgitsi M, van der Meer JW, Netea MG. Inhibition of caspase-1 activation in gram-negative sepsis and experimental endotoxemia. Crit Care. 2011;15:R27. doi: 10.1186/cc9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 6.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.dos Santos G, Kutuzov MA, Ridge KM. The inflammasome in lung diseases. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2012;303:L627–L633. doi: 10.1152/ajplung.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levandowski CB, Mailloux CM, Ferrara TM, Gowan K, Ben S, Jin Y, McFann KK, Holland PJ, Fain PR, Dinarello CA, Spritz RA. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc Natl Acad Sci U S A. 2013;110:2952–2956. doi: 10.1073/pnas.1222808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L, Katz SI, Kastner DL. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1b activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aksentijevich I, Putnam D, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, Hoffman HM, Kastner DL. The clinical continuum of cryopyrinopathies: Novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273–1285. doi: 10.1002/art.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman HM, Brydges SD. Genetic and molecular basis of inflammasome-mediated disease. J Biol Chem. 2011;286:10889–10896. doi: 10.1074/jbc.R110.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavrilin MA, Abdelaziz DHA, Mostafa M, Abdulrahman BA, Grandhi J, Akhter A, Abu Khweek A, Aubert DF, Valvano MA, Wewers MD, Amer AO. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol. 2012;188:3469–3477. doi: 10.4049/jimmunol.1102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2005;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 16.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 17.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–2. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu JW, Farias A, Hwang I, Fernandes-Alnemri T, Alnemri ES. Ribotoxic stress through p38 mitogen-activated protein kinase activates in vitro the human pyrin inflammasome. J Biol Chem. 2013 doi: 10.1074/jbc.M112.448795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243:174–190. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191:3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate Nlrp3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 24.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1b release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 25.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux Is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. The anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009 doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wewers MD, Pope HA, Miller DK. Processing proIL-1beta decreases detection by a proIL-1beta specific ELISA but increases detection by a conventional ELISA. J Immunol Methods. 1993;165:269–278. doi: 10.1016/0022-1759(93)90353-9. [DOI] [PubMed] [Google Scholar]
- 30.Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, Hall MW, Wewers MD. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982–7989. doi: 10.4049/jimmunol.0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavrilin MA, I, Bouakl J, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghonime MG, Shamaa OR, Eldomany RA, Gavrilin MA, Wewers MD. Tyrosine phosphatase inhibition induces an ASC-dependent pyroptosis. Biochem Biophys Res Commun. 2012;425:384–389. doi: 10.1016/j.bbrc.2012.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.vonThun A, Birtwistle M, Kalna G, Grindlay J, Strachan D, Kolch W, von KA, Norman JC. ERK2 drives tumour cell migration in three-dimensional microenvironments by suppressing expression of Rab17 and liprin-beta2. J Cell Sci. 2012;125:1465–1477. doi: 10.1242/jcs.092916. [DOI] [PubMed] [Google Scholar]
- 34.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 35.Wewers MD, Dare HA, Winnard AV, Parker JM, Miller DK. IL-1beta-converting enzyme (ICE) is present and functional in human alveolar macrophages: macrophage IL-1beta release limitation is ICE independent. J Immunol. 1997;159:5964–5972. [PubMed] [Google Scholar]
- 36.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1b and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 37.Cirit M, Grant KG, Haugh JM. Systemic perturbation of the ERK signaling pathway by the proteasome inhibitor, MG132. PLoS ONE. 2012;7:e50975. doi: 10.1371/journal.pone.0050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch SL, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases regulate the activity of caspase-1 and IL-1beta secretion via assembly of the inflammasome. J Biol Chem. 2012;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity. 2012 doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taxman DJ, Holley-Guthrie EA, Huang MTH, Moore CB, Bergstralh DT, Allen IC, Lei Y, Gris D, Ting JPY. The NLR adaptor ASC/pycard regulates DUSP10, MAP kinase (MAPK) and chemokine induction independent of the inflammasome. J Biol Chem. 2011;286:19605–19616. doi: 10.1074/jbc.M111.221077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacGillivray M, Herrera-Abreu MT, Chow CW, Shek C, Wang Q, Vachon E, Feng GS, Siminovitch KA, McCulloch CA, Downey GP. The protein tyrosine phosphatase SHP-2 regulates interleukin-1-induced ERK activation in fibroblasts. J Biol Chem. 2003;278:27190–27198. doi: 10.1074/jbc.M213083200. [DOI] [PubMed] [Google Scholar]
- 44.Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol. 2007;83:507–511. doi: 10.1189/jlb.0607362. [DOI] [PubMed] [Google Scholar]
- 45.Argast GM, Fausto N, Campbell JS. Inhibition of RIP2/RIck/CARDIAK activity by pyridinyl imidazole inhibitors of p38 MAPK. Mol Cell Biochem. 2005;268:129–140. doi: 10.1007/s11010-005-3701-0. [DOI] [PubMed] [Google Scholar]
- 46.Jankovic D, Ganesan J, Bscheider M, Stickel N, Weber FC, Guarda G, Follo M, Pfeifer D, Tardivel A, Ludigs K, Bouazzaoui A, Kerl K, Fischer JC, Haas T, Schmitt-Graff A, Manoharan A, Muller L, Finke J, Martin SF, Gorka O, Peschel C, Ruland J, Idzko M, Duyster J, Holler E, French LE, Poeck H, Contassot E, Zeiser R. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210:1899–1910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 49.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 50.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 51.Levinthal DJ, Defranco DB. Transient phosphatidylinositol 3-kinase inhibition protects immature primary cortical neurons from oxidative toxicity via suppression of extracellular signal-regulated kinase activation. J Biol Chem. 2004;279:11206–11213. doi: 10.1074/jbc.M314261200. [DOI] [PubMed] [Google Scholar]
- 52.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 53.Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem. 2003;278:37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- 54.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 55.Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Wei Q, Xia Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem. 2006;281:21652–21659. doi: 10.1074/jbc.M602105200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.