Abstract
Acrolein, an α,β-unsaturated aldehyde and a reactive product of lipid peroxidation, has been suggested as a key factor in neural post-traumatic secondary injury in SCI, mainly based on in vitro and ex vivo evidence. Here we demonstrate an increase of acrolein up to 300%; the elevation lasted at least two weeks in a rat SCI model. More importantly, hydralazine, a known acrolein scavenger can provide neuroprotection when applied systemically. Besides effectively reducing acrolein, hydralazine treatment also resulted in significant amelioration of tissue damage, motor deficits, and neuropathic pain. This effect was further supported by demonstrating the ability of hydralazine to reach spinal cord tissue at a therapeutic level following intraperitoneal application. This suggests that hydralazine is an effective neuroprotective agent not only in vitro, but in a live animal model of SCI as well. Finally, the role of acrolein in SCI was further validated by the fact that acrolein injection into the spinal cord caused significant SCI-like tissue damage and motor deficits. Taken together, available evidence strongly suggests a critical causal role of acrolein in the pathogenesis of spinal cord trauma. Since acrolein has been linked to a variety of illness and conditions, we believe that acrolein-scavenging measures have the potential to be expanded significantly ensuring a broad impact on human health.
Keywords: Aldehyde, oxidative stress, lipid peroxidation, acrolein scavenger
Introduction
A significant portion of damage sustained in spinal cord injuries stems from a delayed secondary biochemical reaction that amplifies the effects of physical insults and spreads additional damage throughout the cord. Oxidative stress, a hallmark of secondary injury, plays a critical role in mediating functional loss in SCI (Hall 1989). However, free radical scavengers have been largely unsuccessful at mitigating secondary injury in the spinal cord. We have recently demonstrated that acrolein, an α,β-unsaturated aldehyde and a reactive product of lipid peroxidation, is likely a key factor in neural post-traumatic secondary injury (Shi and Luo 2006; Hamann et al. 2008b; Hamann et al. 2008a; Shi et al. 2011a). This is based on its high toxicity to nervous tissue (Picklo and Montine 2001; Shi et al. 2002; Luo and Shi 2004; Luo et al. 2005a; Liu-Snyder et al. 2006b; Shi et al. 2011b), elevated concentrations in SCI (Luo et al. 2005b), and extended presence within biological systems (Esterbauer et al. 1991; Ghilarducci and Tjeerdema 1995; Luo et al. 2005b). Acrolein is capable of directly damaging proteins, DNA, and lipids, and stimulating the production of free radicals (Kehrer and Biswal 2000; Stevens and Maier 2008; Shi et al. 2011a). Furthermore, the neurotoxicity of acrolein has been shown to be mediated through poisoning mitochondria, compromising the integrity of neuronal membranes, and degrading myelin (Picklo and Montine 2001; Luo et al. 2005a; Luo and Shi 2005; Hamann et al. 2008a; Shi et al. 2011a; Shi et al. 2011b). Therefore, as both a product of and catalyst for lipid peroxidation (Esterbauer et al. 1991), acrolein appears to induce a vicious cycle of oxidative stress that dramatically amplifies its effects and is likely responsible for continuously propagating degeneration in SCI.
Based on existing evidence, we also hypothesize that suppressing acrolein will reduce neuronal damage and enhance functional recovery in SCI. To test such hypothesis, and to further support the active role of acrolein in SCI, we first demonstrated that hydralazine, an effective acrolein scavenger, can mitigate acrolein-mediated cell death in PC12 cells (Liu-Snyder et al. 2006a) as well as in isolated spinal cord segment (Hamann et al. 2008a). In addition, hydralazine also prevented acrolein-induced toxicity in cultured hepatocytes (Burcham et al. 2000; Burcham et al. 2004), and hepatoxicity and MS in mice (Kaminskas et al. 2004; Leung et al. 2011). Despite ample evidence of neuroprotection in vitro and the likelihood of a similar effect in vivo, the effect of acrolein scavenging therapy in live animal SCI has not been evaluated. Without this necessary information, the potential value of acrolein as a therapeutic target in the treatment of SCI remains uncertain.
The primary goal of this investigation is therefore to ascertain the neuroprotective role of acrolein scavengers in an animal model of SCI, a critical step to further ascertain the role of acrolein in the pathology of SCI in vivo. Such studies were also designed to test the effectiveness of the acrolein scavenger, hydralazine, an FDA-approved hypertensive medication in suppressing acrolein in a live animal model of SCI to reduce secondary injury and promote functional recovery.
Material and methods
Animal
Male Sprague-Dawley rats weighing 200–250 grams at the time of surgery were used. Rats were obtained from Harlan Laboratory (Indianapolis, IN, USA) housed and handled in compliance with the Purdue University Animal Care and Use Committee guidelines and ARRIVE guidelines. The institutional protocol number for this study is 1111000276. The animals were kept at least one week before surgery for acclimation.
Rat spinal cord contusion injury model
Rats were anesthetized with a ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture by an intraperitoneal (IP) injection. The spinous process and vertebrae lamina were removed to expose a dorsal surface of spinal cord at the T-10 spinal level. Two different severities of contusion SCI models were induced by New York University (NYU) impactor where a 10-gram rod was dropped from either 25 mm or 37.5 mm onto the intact dura mater to generate moderate and severely contused SCI models respectively. A sham operation was performed using only a laminectomy of the T-10 vertebra without a spinal cord contusion. After surgery, the animals were allowed to recover on a heating pad. Post-surgical care of SCI rats was performed including manual bladder expression daily until the return of reflexive bladder control was observed and 3.0 cc of saline administrated via subcutaneous injections to prevent dehydration. It is worth mentioning that ketamine, as a noncompetitive NMDA receptor antagonist, can be neuroprotective (Newcomer et al. 1999; Shibuta et al. 2006). However, in the current study both control and the treatment groups received the same regimen for general anesthesia. This avoids immediate concerns on ketamine administration.
Hydralazine Treatment
The hydralazine hydrochloride (Sigma, St. Louis, MO, USA) solution was dissolved in phosphate buffered saline. Hydralazine solution (5 mg/kg) was administered through a daily IP injection for 2 weeks, starting immediately within 5 minutes after SCI. To measure the acrolein-lysine adducts level by immunoblotting, hydralazine hydrochloride solution was administrated by IP injection immediately following and 24 hours after SCI. Rats were euthanized 2 hours after injection of the hydralazine hydrochloride solution. A dosage of 5 mg/kg was chosen to reduce acrolein levels in rats because its effectiveness and minimal influence on blood pressure has been proven through IP injection (Zheng et al. 2013). The injection volume was 2 ml/kg. Therefore, a rat of 250 g would receive a daily dosage of 1.25 mg dissolved in 0.5 ml of solution.
Quantification of Hydralazine in Central Nervous System
Paper spray mass spectrometry was set up following standard procedures before the analysis (Wang et al. 2011). To quantify the concentration of hydralazine in the tissues, brain and spinal cord from untreated rats were spiked artificially with different concentrations of hydralazine (from 15.625 ng/ml to 2000 ng/ml) and a fixed concentration of nicotine as an internal standard (250 ng/ml). A calibration curve was constructed with measurements from these samples. Quantitative analysis was performed using the intensity ratio of characteristic fragment at m/z 89 for hydralazine and m/z 132 for nicotine in single reaction monitoring (SRM) mode.
In the experimental group, rats were given an IP of either saline (control) or saline with 5 mg/kg (b.w.) hydralazine and sacrificed 2 hours after injection. The tissues of the rat were punched and transferred onto the paper for paper spray mass spectrometry analysis and quantification of hydralazine. The concentrations of hydralazine in the tissues were estimated by calibration curve fits.
Behavioral test for locomotor function
The recovery of locomotor function was assessed by using the Basso, Beattie and Bresnahan (BBB) Locomotor Rating Scale (Basso et al. 1996). The score is based on the locomotor ability of SCI rodent models. Briefly, the BBB scale is a 22-point scale which ranges from 0, no observable movement of hindlimb, to 21, normal movement of hindlimb. Rats were observed in an open field for 5 minutes after they had gently adapted to the field. Left and right hindlimb were assessed separately at day one and then weekly after SCI for 4 weeks. The score was obtained by taking an average value of both hindlimb results.
Pain assessment
The foot withdrawal threshold to mechanical stimuli was used as an indicator of mechanical hypersensitivity below injury level. The SCI rats were placed in a transparent plastic box on top of a metal mesh floor and left alone for at least 10 minutes to acclimate. For mechanical stimulation, a series of calibrated von Frey filaments (range: 0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0 and 15.0 grams, stoelting, Wood Dale, IL, USA) were applied perpendicular to the plantar surface of the hindlimb with sufficient bending force for 3–5 seconds and then removed. Stimuli were presented at 1 minute intervals. A brisk hindlimb withdrawal with or without licking and biting was considered as a positive response. In the event of paw withdrawal, a lower stimulus was presented; in the absence of a response, the filament of the next greater stimulus was applied. The mechanical thresholds were calculated by using the up-down method (Chaplan et al. 1994).
Isolation of spinal cord
The animals were anesthetized with an IP injection of a mixture of ketamine (80mg/kg) and xylazine (10mg/kg). When deeply anesthetized, they were perfused with oxygenated Kreb’s solution (all in mM): 124 NaCl, 2 KCl, 1.24 KH2PO4, 26 NaHCO3, 10 ascorbic acid, 1.3 MgSO4, 1.2 CaCl2, and 10 glucose. To remove the spinal cord, the whole vertebral column was rapidly removed and a dorsal laminectomy was performed along the vertebral column. The spinal cord was removed and cut into 1 cm sections for both histological analysis and acrolein concentration determination.
Artificial acrolein standard preparation
Solutions of 0.5 ml of 2 mg/ml rat albumin (CalBiochem) and 0.5 ml of 100 mM acrolein (Sigma) solution were carefully prepared and mixed together. The mixed solution was then incubated for 4 hours at 37 °C. If not used the same day, the mixed solution was then spread into ten different vials that could be stored at − 80 °C for up to 1 month. On the day of the experiment, serial dilutions with 0.1% BSA were then performed for up to 10000 folds for calibration standard curve construction.
Immunoblottings
The extracted spinal cord segments were incubated with 1% Triton solution and the corresponding amount of Protease Inhibitor Cocktails (Sigma-Aldrich, Product #: P8340) and then homogenized with a glass homogenizer (Kontes Glass Co.). The solution was then incubated on ice for at least 1 hour before centrifuged at 13500 g for at least 30 minutes at 4 °C. If the experiment was not performed on the same day, the sample was then stored at −80 °C and could be kept for up to two weeks. One additional round of centrifugation at 13500 g was performed after removal from −80 °C.
Prior to analysis, a BCA protein assay was performed to ensure equal loading for all samples. 200 µg of samples and artificially prepared acrolein standards were transfer at the same time to a nitrocellulose membrane using a Bio-Dot SF Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA), The membrane was then blocked for 1 h in blocking buffer (0.2% casein and 0.1% Tween 20 in PBS) before being transferred to 1:1000 primary mouse anti-acrolein antibody (ABCAM), in blocking buffer with 2% goat serum and 0.025% sodium azide, for 18 h at 4 °C. After the primary antibody incubation, the membrane was washed three times,10 minutes each, in blocking buffer before transferred to 1: 10 000 secondary alkaline phosphatase conjugated goat anti-mouse IgG antibody (VECTASTAIN ABC-AmP Kit) for 1 h at room temperature. After the secondary antibody incubation, the membrane was again washed for three times, 10 minutes each, in blocking buffer followed by 0.1% Tween 20 in Tris-buffered saline before exposed to Bio-Rad Immuno-Star Substrate and visualized by chemiluminescence. Density of bands were evaluated using Image J (NIH). A calibration curve was constructed with the artificial acrolein standard to correlate the band densities to tissue acrolein concentration in the samples.
Histology of rat spinal cord injury
Animals were deeply anesthetized and then perfused endocardially, initially with lactated ringers to flush out the blood and then with 10% formalin. The spinal cord was removed and post fixed for 24 hr in 10 % formalin, and embedded in paraffin. Horizontal sections at a thickness of 10 µm were cut and stained with Luxol fast blue and Cresyl violet. Images of sections midway through the lesion were captured at 4X. Images were analyzed in Image J such that the intact tissue and the cyst was circumscribed for each sample and the area measured by someone blind to the treatment. The percent of cyst to spinal cord area in the image was compared between treated and untreated groups with a student’s t-test using SAS statistical software. Measurements of two adjacent sections were averaged for each sample.
Acrolein microinjection into spinal cord and histological analysis
Following proper anesthesia, the rat spinal cord at the tenth thoracic (T10) vertebra was exposed by dorsal laminectomy. To facilitate micropipette insertion, the dural sheath was lanced with a needle point. The pipettes were pulled, beveled and loaded with either saline (vehicle) or acrolein in saline using a three-way valve and a syringe under negative pressure. Injections were performed using the PMI-100 pressure micro-injector (Dagan Corp., Minneapolis, MN). A volume of 1.6 µL of either sterile saline (left side-internal control) or acrolein (right side, 1.6 µmol) were injected to the spinal cord 0.6 mm lateral to the midline and 1.2 mm ventral to the cord surface at T10. Following injection, the surgical site was flushed with sterile saline. The muscle and dermal layers were sutured sequentially with interrupted sutures. The entire operation of microinjection was conducted under dim light to minimize light exposure to acrolein.
Histological analysis of the rat spinal cord following acrolein injection was performed 2 months following injury. Tissue was fixed, post-fixed and paraffin embedded. Hematoxylin and eosin stains were used to identify pathological changes resulting from in vivo acrolein injection into spinal cord based on conventional procedures. Briefly, the longitudinal paraffin sections were cut at a thickness of 15 microns using an AO820 microtome (American Optical, Buffalo, NY). Sections were deparaffinized and rehydrated, stained with hematoxylin and eosin, dehydrated, and coverslipped. Imaging was performed on an Olympus microscope at 4X and image-tile-stitching was performed in Image-J.
Statistical Analysis
Student’s t-test was used to compare data between two groups in various experiential conditions. ANOVA and Tukey tests were used in analysis when comparisons were made among more than two variables. The statistical significance level was set at p < 0.05. The averages were expressed in mean ± SEM.
To achieve reasonable statistical power analyses for experiments performed in this study, type II errors were controlled at 0.2 level (Power ≥ 0.8) for all the statistical tests. Treatment effects and variances for the studies were estimated from pilot studies and our previous publications (Hamann et al. 2008a; Zheng et al. 2013). Equal variance (ANOVA Model) was assumed and validated for each study. Given the above parameters, appropriate sample size for each study was estimated from SAS (Power Procedure).
Results
The elevation of acrolein following SCI
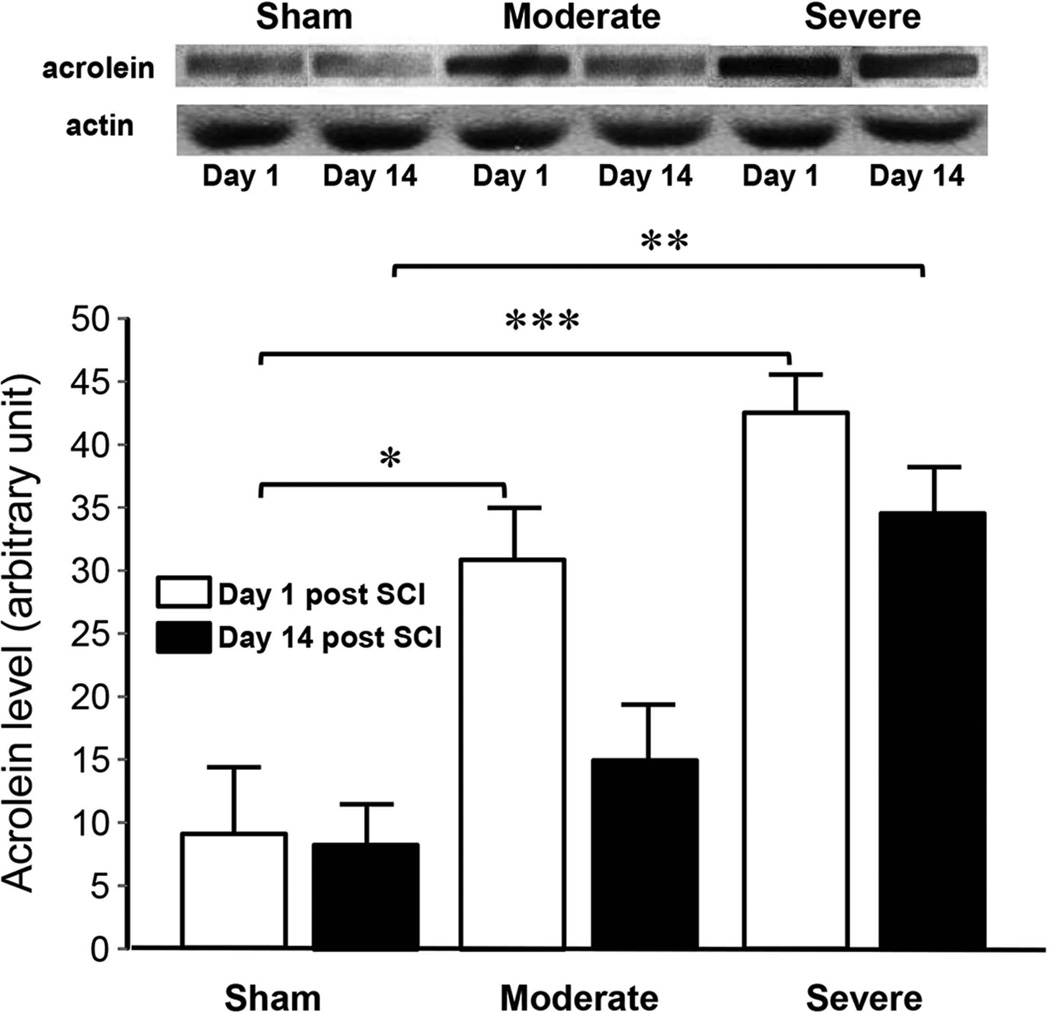
Using dot immunoblotting, we have found that the level of acrolein present in spinal cord tissue increased significantly at both days 1 and 14 post-SCI (Fig. 1). Specifically, at 1 day post SCI, the acrolein level of sham, moderately, and severely injured rats are: 9.1±5.3, 31.1± 4.0, and 42.7±3.1 respectively. A significant difference was detected between severe and sham (p < 0.005) as well moderate and sham (p < 0.05). At 14 days post-SCI, the acrolein level of sham, moderately injured, and severely injured rats were: 8.5±3.6, 15.3±4.0, and 34.7±3.6 respectively with a significance detected between severe and sham (p<0.01).
Figure 1. Elevation of acrolein in spinal cord tissue following various degrees of injury severity and time post contusion in rat.
Bar graph indicates that the overall acrolein-lysine conjugate level correlated well with the level of SCI severity, i.e. sham, moderately injured, and severely injured, at 1 day and 2 weeks post-injury. It also reveals significant elevation of acrolein one day following rat SCI in both moderate and severe injury groups based on dot immunoblotting quantification of acrolein-lysine adduct. In addition, elevation of acrolein persisted for at least two weeks in the severely injured group in comparison to the sham group. Photographic images (top) show representative blots for each experimental condition. Bar graph (bottom) displays that the acrolein-lysine levels in moderately injured rats 1 day post-SCI were significantly higher than the sham control (31.1± 4.0 au vs 9.1±5.3 au, p < 0.05, ANOVA) while no significance was found with a similar comparison in 14 day post-injury between sham and moderately injured (15.3±4.04 au vs 8.5±3.6 au, p > 0.05, ANOVA). However, in the severely injured group, acrolein levels were elevated significantly both at 1 day as well as 2 weeks post-SCI compared to the sham (42.7±3.1 au vs 9.1±5.3 au and 34.7±3.6 au vs 8.5±3.6, respectively. p < 0.005 for 1day and p < 0.01 for 14 day comparison). The samples in each condition were divided into two identical replicates. The first replicate was used to determine acrolein levels based on dot immunoblotting while the second one was used for actin quantification as an internal loading control. The actin level was quantified using anti-actin antibody measured from the Western blot. The intensity of the bands was quantified using Image J (NIH). N=4 for each group. * p < 0.05, ** p < 0.01, *** p < 0.005.
The level of hydralazine in CNS following IP injection
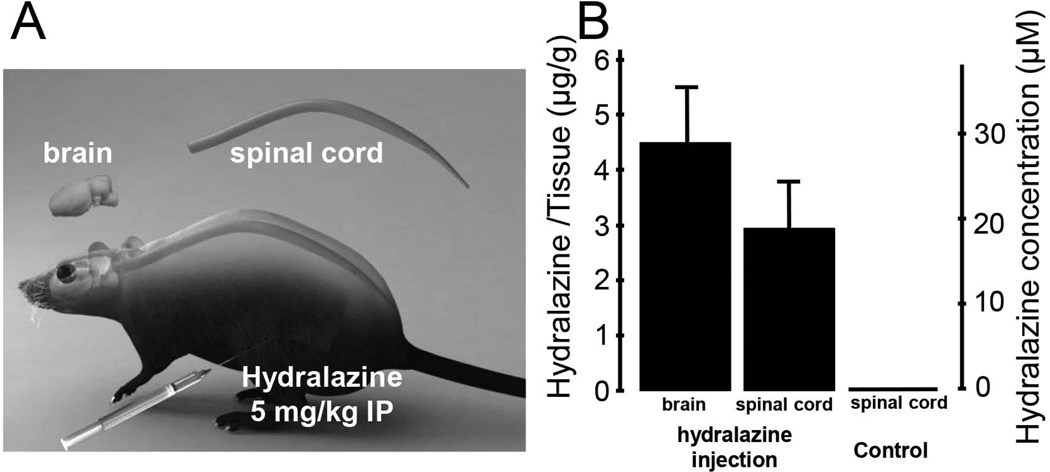
Prior to evaluating the effectiveness of hydralazine in lowering acrolein levels and offering neuoprotection in SCI, we first examined the level of hydralazine in spinal cord and brain tissue in the hours following IP injection. Hydralazine is known to have a short half-life of a few hours (Reece 1981); hence the animal was sacrificed approximately 2 hours after initial injection and brain and spinal cord were harvested for acrolein determination using the recently developed method of paper spray mass spectrometry (Wang et al. 2011). It appeared that a significant amount of hydralazine was present in the CNS within just two hours following IP injection. Specifically, at 2 hrs post IP injection, the concentrations of hydralazine in spinal cord and brain tissue were 2.9 ± 0.9 and 4.4 ± 1.1 µg/g, while none was detected in control (no hydralazine) animals (n=4 in all three groups). Since CNS tissue possesses a specific gravity value of approximately 1 (Hamann et al. 2008a), the hydralazine concentration in the CNS is calculated to be approximately 20 µM in spinal cord and 30 µM in brain (Fig. 2).
Figure 2.
Determination of hydralazine levels in CNS tissue following IP injection. A) Artist drawing of the acrolein injection method and the organs used for hydralazine quantification. The dosage of hydralazine was 5 mg/kg through intraperitoneal (IP) injection and the control group received the same amount of saline. B) Bar graph indicates the level of acrolein 2 hrs following both hydralazine (brain and spinal cord) and saline (spinal cord) injection. Specifically, brain and spinal cord issues were harvested and punched approximately 2 hours after hydralazine treatment (IP injection). The tissue was then transferred onto the paper for analysis using paper spray mass spectrometry to determine the level of hydralazine. The level of hydralazine was expressed both at µg/g as well as µM. Specifically, the levels of hydralazine in spinal cord and brain were 2.9±0.9 and 4.4±1.1 µg/g respectively. Using a previously determined gravity of spinal cord tissue at 1 (g/ml) (Hamann et al. 2008a), we estimated that hydralazine reached a level of close to 20 µM in spinal cord and 30 µM in brain tissue. No hydralazine was detected in the spinal cord in the control group where only saline was injected. N=4 in all three groups.
Hydralazine effectively decreases acrolein level in rat spinal cord following injury
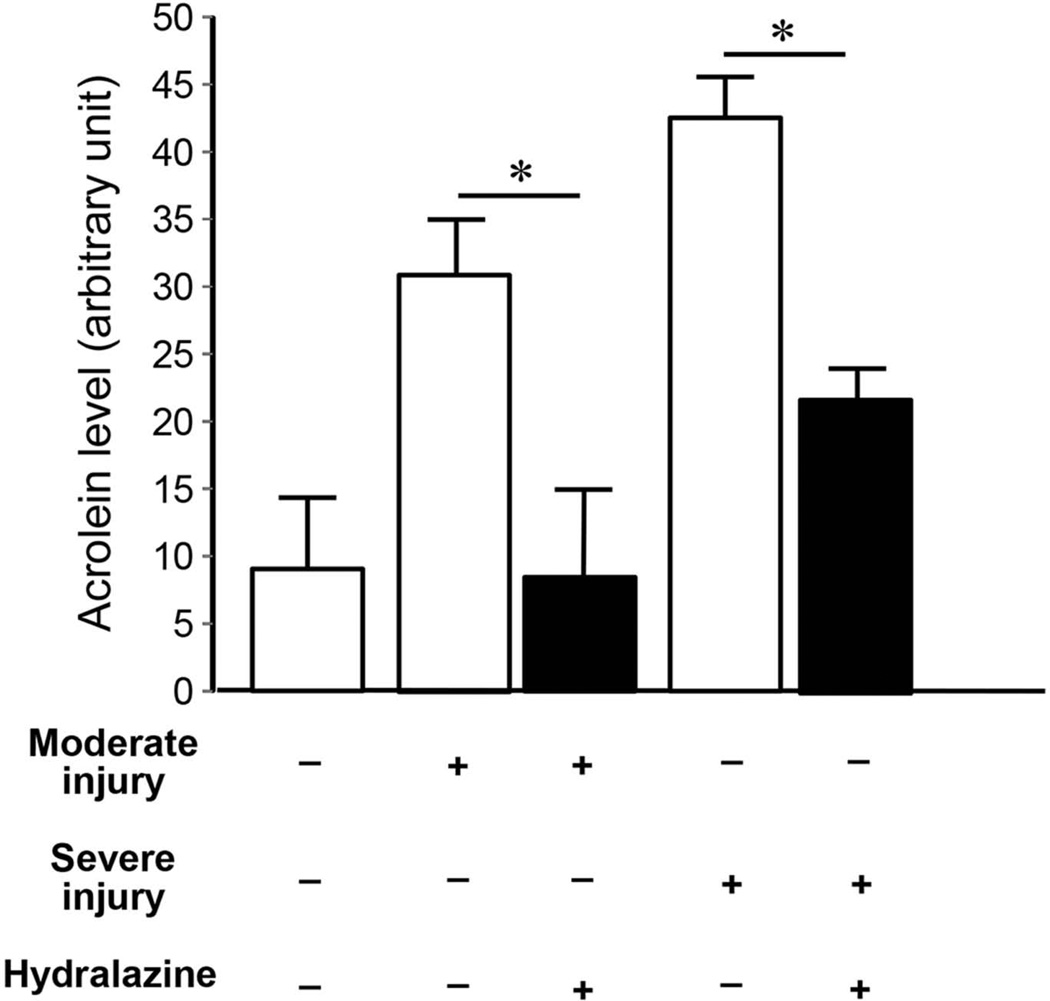
After confirming the availability of hydralazine in the CNS following IP injection, we tested the hypothesis that hydralazine actually reduces acrolein in the CNS in vivo. Acrolein was measured 24 hrs following SCI; hydralazine was applied twice during this period, immediately after injury and again 2 hours before the animal was sacrificed. As indicated in Figure 3, hydralazine treatment significantly reduced acrolein concentration from 31.1±4.0 au to 8.7± 6.4 au in moderate injury and from 42.7±3.1 au to 21.3±2.6 au in severe injury (p < 0.05 in both cases, Fig. 3).
Figure 3. Hydralazine effectively reduced acrolein level in rat SCI.
Hydralazine at a dosage of 5 mg/kg, was administered immediately after contusion injury and again 1 day post-injury in both moderately and severely injured rats. Approximately two hours following the second hydralazine application, spinal cord tissue was harvested for acrolein determination using dot immunoblotting. As indicated, such treatment of hydralazine significantly reduced the acrolein levels in both moderate and severe injury groups. The data used to generate the bar graph of sham, moderately injured and severely injured groups were the same as those used in Figure 1, included here for comparison purposes. Specifically, in the moderately injured and hydralazine treated group, the acrolein-lysine adducts level is 8.7±6.4 au which was significantly reduced from injured only group 31.1±4.0 au (p < 0.05, ANOVA). Similarly, hydralazine treatment also significantly reduced the level of acrolein-lysine adducts from 42.7±3.1 au to 21.3±2.6 au in the severely injured group (p < 0.05, ANOVA). Data and image analysis were similar to those performed for Fig. 1. N=4 in each condition. * p < 0.05
Hydralazine significantly reduces tissue damage after SCI
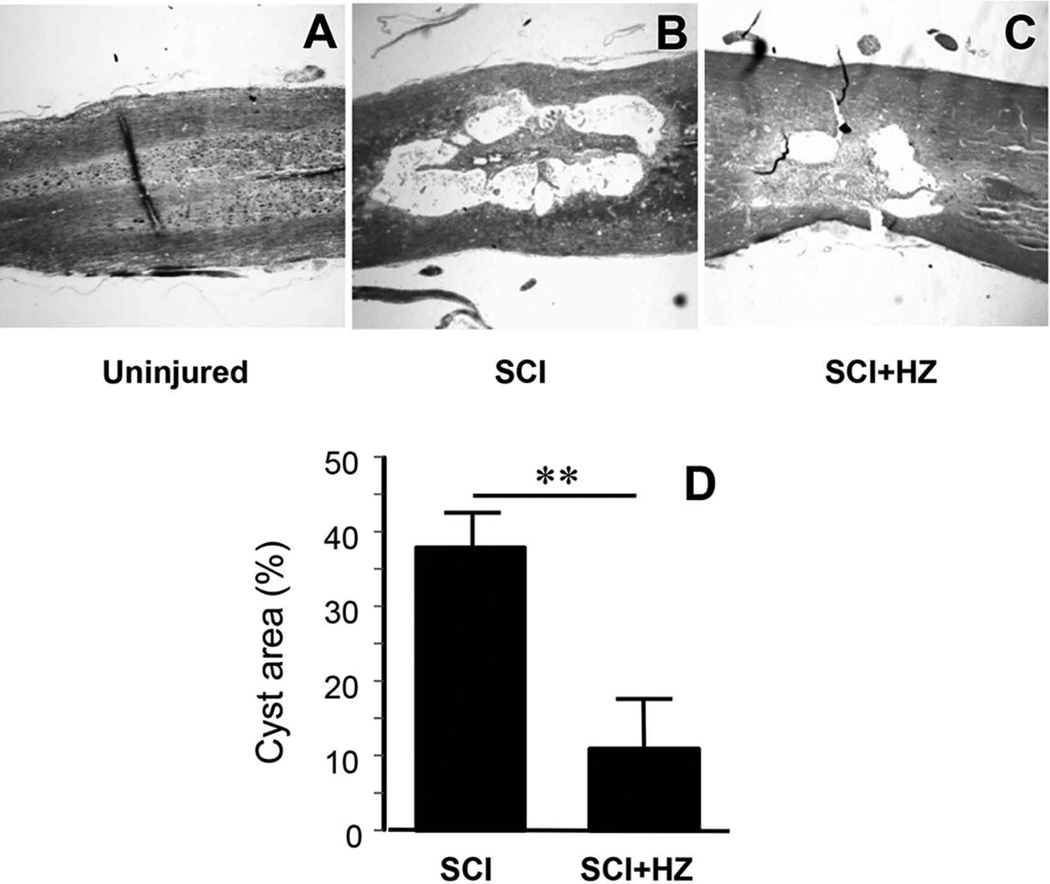
The extent of damage in rat spinal cord following SCI in groups treated with and without hydralazine were examined and quantified by measuring the percent area of the cyst relative to the rest of the tissue in the section. Specifically, the average of two adjacent longitudinal sections at the center of the cord was used. While no cysts were detected in uninjured cords, significant cysts were identified in rats that suffered SCI. Specifically, the cyst covered 38.0% of the cord for the SCI group. Hydralazine treatment significantly reduced this value to 11.4% (Fig. 4. p < 0.01. N=4 in both groups).
Figure 4. Hydralazine treatment significantly reduced the cyst covered area after SCI.
A–C) Conventional Luxol fast blue staining of horizontal sections of rat spinal cord reveals the size of the cyst at the injury site in uninjured (A), injury only (saline treatment) (SCI) (B), and injury treated with hydralazine (SCI+HZ) (C) groups 4 weeks following injury. Notice the lack of any noticeable cysts in the uninjured cord, the development of large cysts in the SCI group, and its significant reduction in hydralazine treated group. D) Quantitative comparison reveals that the size of the cyst in SCI-HZ group (11.4±5.4%) was significantly less that in SCI group (38.0±4.3%, p < 0.01, student’s t-test). N=4 in both groups. ** p < 0.01
Hydralazine improves motor behavioral recovery in rat SCI
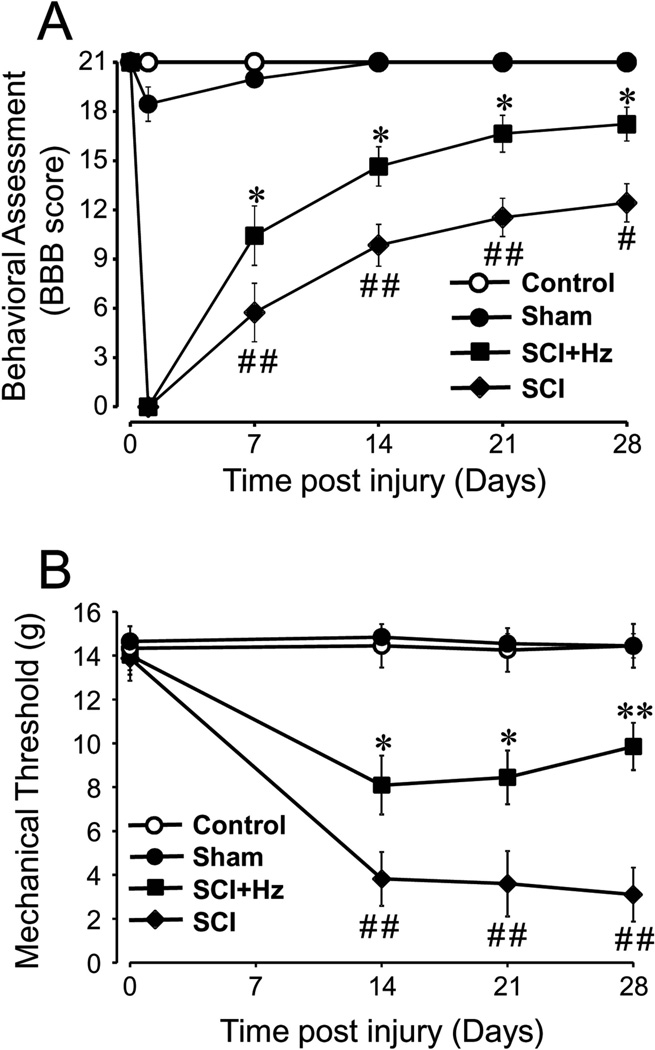
Behavioral motor function of SCI rats was evaluated using the BBB locomotor rating scale immediately before, one day after and then weekly for 4 weeks post SCI (Fig. 5A). Before SCI, the BBB scores of all groups, control, sham, SCI, and SCI treated with hydralazine (SCI + Hz), were 21. After SCI, all animals scored 0 one day after injury and then gradually recovered. The locomotor score of the hydralazine treatment group (N=13) displayed a significantly enhanced motor recovery compared to the SCI group (N=13) starting 1 week after SCI. Significant differences were also seen at 2, 3 and 4 weeks after SCI. At the conclusion of the experiment (4 weeks post SCI), the BBB score of the hydralazine treated group reached 16.1 ± 1.0 which is significantly higher than that of the SCI group, 12.4 ± 1.1 (p < 0.05). The BBB score of the sham group (N=6) was 18.5±1.1 one day after SCI, but normal gait was observed starting 1 week post-surgery. No motor abnormalities were detected in the control group during the entire 4 week experiment (N=10) (Fig. 5A).
Figure 5. Systemic application of hydralazine improved locomotor function recovery and alleviated acute neuropathic pain after spinal cord injury.
A) Locomotor function based on BBB score was assessed in control (uninjured), sham (sham injury), SCI (injured, saline treated), and SCI + Hz (injured treated with hydralazine). Notice that there are little or negligible locomotor deficits in control and sham groups. However, significant reduction of BBB score was observed in SCI group. Following spinal cord contusion in the SCI + Hz group, hydralazine (5 mg/kg) was applied daily through IP injection for 2 weeks immediately following injury. Such treatment significantly restored the BBB score at 1, 2, 3, and 4 weeks post-SCI when compared to the SCI group. Specifically, the final BBB score of SCI + Hz group at 4 weeks post-injury was 16.1 ± 1.0, which is significantly higher than that in SCI group, 12.4 ± 1.2. Similar differences were also observed in 1, 2, and 3 weeks following injury as indicated. B) The time courses of mechanical allodynia (the paw withdrawal threshold to von Frey filaments) in control, sham, SCI, and SCI + Hz are displayed here. Notice the lack of any appreciable allodynia in both control and sham groups, and severe mechanical allodynia development in the SCI group. It appears that hydralazine treatment significantly alleviated the mechanical allodynia by altering the paw withdrawal thresholds to von Frey filaments.
Values are mean ± SEM. # p < 0.05, ## p < 0.01 for comparison between sham and SCI. * p < 0.05 for comparison between SCI + Hz and SCI. ANOVA and Tukey test. N=6–13 in each group.
Hydralazine alleviated acute neuropathic pain following SCI in rat
Besides motor behavior, we were also interested in any benefits of acrolein scavenging on sensory abnormalities. In particular, we were interested to know if hydralazine-mediated acrolein reduction could mitigate neuropathic pain, a known symptom post-SCI in both animal and human patients. The behavioral test for mechanical allodynia, a type of neuropathic pain, was performed immediately before and 2, 3, 4 weeks following SCI. As indicated in Fig. 5B, mechanical allodynia was well established at two weeks post-SCI when the hindlimbs of the SCI rats could support their weight (Fig. 5A). Specifically, before SCI, the paw withdrawal thresholds, a measure of mechanical allodynia, in control, sham, SCI, and SCI treated with hydralazine groups were 14.0 ± 0.8 g, 14.7 ± 0.3 g, 14.3 ± 0.7 g and 13.9 ±1.0 g, respectively. As expected, no change of paw withdrawal thresholds was observed in control and sham groups. However, there was a marked decrease in paw withdrawal threshold in SCI rats, indicative of mechanical allodynia, examined 2, 3, and 4 weeks post-SCI. Furthermore, mechanical allodynia was significantly mitigated in the SCI + Hz group at 2, 3, and 4 weeks post SCI. Specifically, at 2 weeks post-SCI, the withdrawal threshold in SCI group was 3.7 ± 0.3 g which was significantly lower than sham or control (p < 0.01). Hydralazine treatment increased this value to 8.1 ± 1.2 g, making it significantly less sensitive than that in SCI rats (p < 0.05). Similar significant reductions of mechanical allodynia by hydralazine were also seen in 3 and 4 weeks post-SCI (p < 0.05 at 3 weeks p < 0.01 at 4 weeks. Fig. 5B). This indicates that hydralazine-mediated acrolein reduction was associated with decreased mechanical allodynia post-SCI.
Acrolein in vivo injection into spinal cord resulted in motor deficits and tissue damage
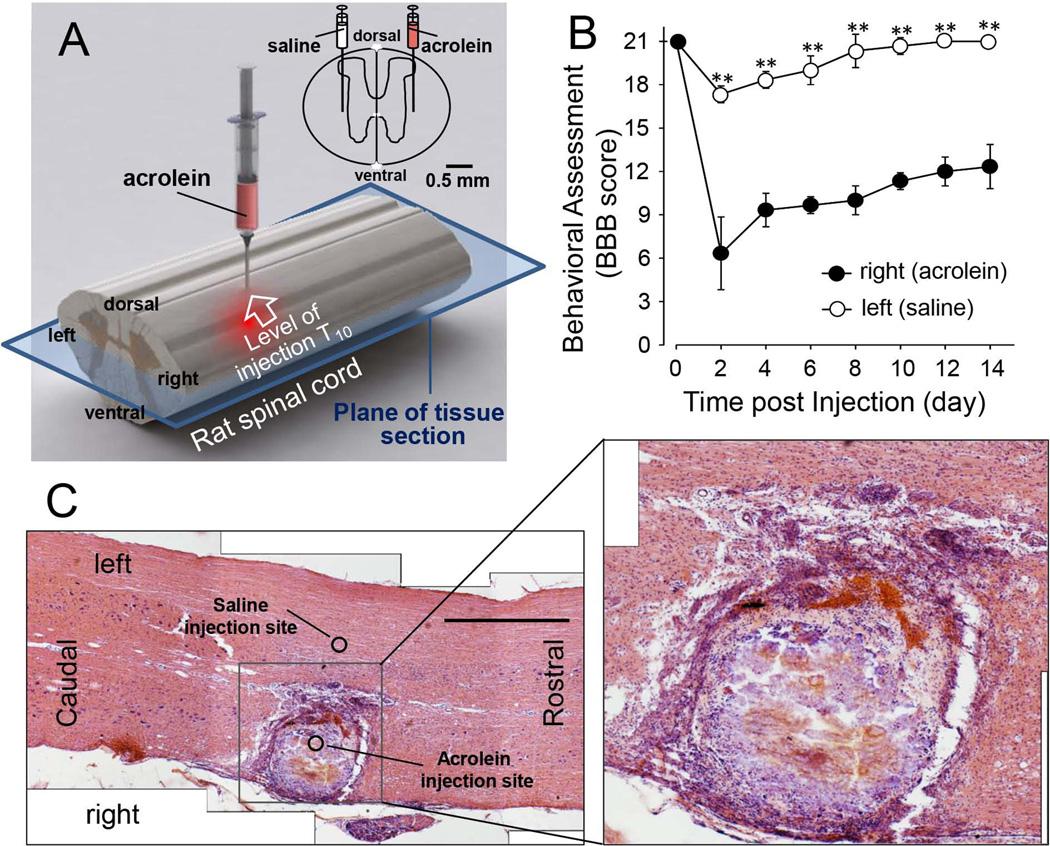
In order to examine the ability of acrolein alone to elicit neurological deficits in the absence of mechanical trauma, we injected a small amount of acrolein directly into the spinal cord of uninjured, healthy rats (Fig. 6A). As shown in Figure 6B, following injection of acrolein to the right side and equal volume of saline to the left side, we found that such treatment produced significant motor deficits on the side of acrolein injection, while administration of saline to the contralateral side resulted in the absence of persistent motor deficits. The acrolein injection also caused noticeable damage compared to the adjacent saline injection site (Fig. 6C). The eosinophilic lesion area is surrounded by an apparent glial scar region in which there are increased numbers of small-nucleus cells. Inside the scarred outline of the lesion, there doesn’t appear to be a substantial cellular presence except some large clusters of red blood cells in the medial aspect of the lesion. The lesion appears primarily composed of cellular debris with occasional intact cellular presence (Fig. 6C inset). This acrolein dependent lesion is primarily unilateral, with little noticeable damage appearing contralateral to the acrolein injection site where the same amount of saline was injected.
Figure 6. Motor and structural deficits resulted from acrolein injection to the spinal cord.
A) Diagram of injection locations and the plane of tissue section for the images displayed in (C). B) Laterally divided BBB scores for acrolein and saline injections over the course of 2 weeks. Notice the significant reduction of the BBB score on the side of acrolein injection compared to the contralateral or saline injected side. The values are mean ± SEM. ** p < 0.01 for comparison between right side (acrolein-injected) and left side (saline injected). Student’s t-test. N=6 in each group. C) H and E staining of a longitudinal spinal cord section (axiolateral plane as indicated in panel A) including the injection site. Note the difference between the right and left side of the cord, acrolein and saline injected respectively. No morphological abnormality is seen on the left. The right side presents an eosinophilic lesion area, surrounded by an apparent glial scar with elevated numbers of small-nucleus cells. The inset presents the enlarged central lesion area which reveals the detailed cellular destruction resulting from acrolein. The image appears mostly devoid of intact cells but rather cellular debris instead. Large clusters of red blood cells are visible in the medial aspect of the lesion. Scale bar in C: 1 mm.
Discussion
In the current study, we have found that acrolein is significantly elevated in rat spinal cord contusion injury and can be effectively reduced through systemic application of hydralazine, a proven acrolein scavenger. Besides neutralizing acrolein, hydralazine treatment also resulted in significant reduction of tissue damage, motor deficits, and neuropathic pain. In addition, acrolein injection into spinal cord caused significant SCI-like tissue damage and motor deficits. This data is consistent with repeated reports that hydralazine alleviates acrolein-mediated neuronal damage in in vitro experimentations (Liu-Snyder et al. 2006a; Hamann et al. 2008b; Hamann et al. 2008a; Hamann and Shi 2009). Taken together, available evidence strongly suggests a critical causal role of acrolein in the pathogenesis of spinal cord trauma.
Although acrolein was reported to be increased in spinal cord compression injury in guinea pig (Luo et al. 2005b), this is the first time elevated acrolein has been found in rat contusive SCI. We have also shown that acrolein was continuously elevated for at least two weeks in rats post-SCI (Fig. 1), a significant extension from the one week elevation reported in guinea pig. In addition, based on dot immunoblotting, the maximal increase of acrolein in rat SCI is 300% while the highest increase of acrolein in guinea pig was 60% measured by ELISA (Luo et al. 2005b). This discrepancy could be due to the difference in the animal species, the injury model, and the technique of acrolein estimation. However, in both cases, the maximal level of increase was found at 24 hours post injury. This suggests that acrolein elevation may peak at one day post physical trauma.
Although there are multiple reports that hydralazine mitigates neuronal damage in tissue culture and isolated nerve tissues (Liu-Snyder et al. 2006a; Hamann et al. 2008b; Hamann et al. 2008a; Hamann and Shi 2009), this is the first demonstration that hydralazine has the ability to exert an acrolein-scavenging effect and offer neuroprotection in vivo. Unlike in vitro and ex vivo conditions where the concentration of hydralazine could be well controlled (Liu-Snyder et al. 2006a; Hamann et al. 2008b; Hamann et al. 2008a), the tissue concentration of applied drug cannot be assumed in vivo due to the complexity of whole animal. Using a newly developed technique of quantifying hydralazine levels with paper spray mass spectrometry (Wang et al. 2011), we have shown that, following just 1 bolus systemic injection of 5 mg/kg, the concentration of hydralazine can reach an average of 20 µM in the spinal cord and 30 µM in the brain, 2 hrs post-injection. Since the minimal level of hydralazine that can significantly mitigate acrolein-mediated cellular damage is estimated to be no more than 25 µM (Liu-Snyder et al. 2006a), the level achieved in vivo in the current study is at the range capable of scavenging acrolein. This is consistent with the findings that hydralazine application leads to the reduction of acrolein in the spinal cord by 50–70% (Fig. 3). This finding also validates and further supports the notion that hydralazine is an effective neuroprotective treatment not only in vitro, but in a live animal model of SCI as well. Our data also echoes earlier findings that hydralazine can effectively reduce acrolein-mediated hepatic tissue damage in mice (Kaminskas et al. 2004). Since the half-life of hydralazine is approximately 1 hour (Reece 1981) and we only applied hydralazine once a day, it is possible that more frequent administration of hydralazine daily may boost its strength in suppressing acrolein in vivo.
In the current study, we have found that hydralazine treatment can significantly reduce cyst formation in the spinal cord, a severe form of tissue damage and dissolution seen, also known as syringomyelia in spinal cord trauma (Nurick et al. 1970; Mudge et al. 1984; Schurch et al. 1996; Rooney et al. 2009). It is likely that a cyst, observed from samples processed 4 weeks post-injury (Fig. 4), will eventually be filled with scar tissue, such as those seen in acrolein injected samples displayed in Figure 6 that were processed 8 weeks post-injury, as it has been shown previously in a rat spinal cord transection injury (Rooney et al. 2009). Therefore, it is reasonable to speculate that hydralazine-induced cyst reduction will lead to the reduction of scar formation, a major obstacle of axonal regeneration (Reier et al. 1983; Fitch et al. 1999).
It is clear in the current study that acrolein reduction through hydralazine was not only associated with improved motor behavior (Fig. 5A), but also accompanied by significant reduction of neuropathic pain (Fig. 5B), a recognized symptom associated with SCI (Hulsebosch et al. 2009). As a known pro-inflammation compound, acrolein has been shown to stimulate the production of multiple key inflammatory mediators (Facchinetti et al. 2007; Moretto et al. 2009). It is also well-established that inflammation is one of the major characteristics of SCI that contributes to the establishment and maintenance of neuropathic pain (Schnell et al. 1999; Jones et al. 2005; White et al. 2005; Trivedi et al. 2006; White et al. 2007; Donnelly and Popovich 2008; Hulsebosch 2008). Therefore, acrolein-meditated inflammation likely contributes to neuropathic pain, while the hydralazine-induced analgesic effect is possibly due to reduction of inflammation through acrolein scavenging. Taken together, acrolein scavenging strategy may be an important measure, among other effects, to reduce pain and improve the quality of life for SCI victims.
It has long been shown that phospholipid damage and breakdown occurs following traumatic spinal cord injury. For example, Horrockks and his colleagues reported an enhanced lipid breakdown associated with spinal cord injury in cats (Demediuk et al. 1985). While some did not find this phenomenon in rats (Murphy et al. 1994), others noted the comparable increase of lipid breakdown in rat spinal cord injury (Demediuk et al. 1989). Increases in oxidative stress, a known secondary injury mechanism in SCI, can lead to breakdown of lipids (for example, through lipid peroxidation) which in turn produces acrolein (Shi et al. 2011a). Therefore, acrolein could, at least conceptually, serve as an indicator of overall enhanced breakdown of lipid in SCI. As a related point of discussion, it has been observed that the levels of the lipids cholesterol and ethanolamine plasmalogen are significantly decreased following spinal cord injury due to enhanced breakdown of lipid (Demediuk et al. 1985; Demediuk et al. 1989). Since lowering acrolein using scavengers has been associated with reduced level of ROS and ROS can result in lipid breakdown through lipid peroxidation (Esterbauer et al. 1991; Hamann et al. 2008a; Hamann and Shi 2009; Shi et al. 2011a), it is likely that hydralazine could deter lipid breakdown and preserve cholesterol and ethanolamine plasmalogen. This hypothesis remained to be tested.
The current animal studies using hydralazine has provided a valuable proof-of-principle demonstration that acrolein-scavenging is an effective means of curtailing oxidative stress in SCI. However, despite the effectiveness of neuroprotection offered by hydralazine in the current animal study, the primary purpose of this investigation is not to suggest hydralazine as a first line therapeutic for SCI victims, but rather to demonstrate the utility of acrolein scavengers as a new treatment modality. In fact, it is important to point out some limitations for use of hydralazine as an acrolein scavenger. Specifically, hydralazine is a vasodilator (Khan 1953), which could lead to hypotension that is undesirable following SCI. In this regard, some other known acrolein scavengers, such as antidepressant phenelzine which does not significantly influence blood pressure, may also be appreciated as valuable options with the careful consideration of advantages and disadvantages (Cole and Weiner 1960; Wood et al. 2006).
By ascertaining the effectiveness of acrolein scavenging strategy, we have not only further implicated the pathological role of acrolein in SCI, but also demonstrated the potential of acrolein scavengers as a new treatment modality for SCI. Since the traditional treatment for SCI using free radical scavengers, such as methylprednisolone, has provided mostly marginal neuroprotection through its basic and clinical usage, acrolein scavenging may represent a novel, or at least an alternative strategy, to more effectively curtail oxidative stress and enhance neuronal recovery (Saunders et al. 1987; Anderson et al. 1994; Behrmann et al. 1994; Bartholdi and Schwab 1995; George et al. 1995; Chen et al. 1996; Pereira et al. 2009). It is expected that, once established, acrolein targeting therapeutics would not only benefit patients with SCI, but could also be used to treat other disorders where acrolein and oxidative stress are allegedly involved, such as multiple sclerosis (Smith et al. 1999; Leung et al. 2011; Shi et al. 2011b), Alzheimer’s disease (Lovell et al. 2001; Montine et al. 2002), and even cancer (Feng et al. 2006). Furthermore, acrolein has also been linked to the effects of aging (Montine et al. 2002), smoking (Feng et al. 2006), and exposure to pollution (Hesterberg et al. 2008). Beyond amplifying oxidative stress, acrolein synergistically exacerbates other parallel pathologies such as ischemia (Peasley and Shi 2003), inflammation (Facchinetti et al. 2007; Park and Taniguchi 2008; Moretto et al. 2009), and excitotoxicity (Lovell et al. 2000; Barger et al. 2007). Therefore, we believe that acrolein scavenging measures have the potential to be dramatically expanded and broadly impact human health.
Acknowledgments
This work was supported by the Indiana State Department of Health (Grant # 204200 to RS), National Institutes of Health (Grant # NS073636 to RS), and Indiana CTSI Collaboration in Biomedical Translational Research (CBR/CTR) Pilot Program Grant (Grant # RR025761 to FAW and RS). The authors would like to thank Melissa Tully and Jessica Page for critical reading of the manuscript and Michel Schweinsberg for illustration drawing.
Abbreviations foot note
- SCI
spinal cord injury
- MS
multiple sclerosis
- IP
intraperitoneal
- SRM
single reaction monitoring
Footnotes
There is not a conflict of interest for any of the authors.
Reference
- Anderson DK, Dugan LL, Means ED, Horrocks LA. Methylprednisolone and membrane properties of primary cultures of mouse spinal cord. Brain Res. 1994;637:119–125. doi: 10.1016/0006-8993(94)91224-6. [DOI] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdi D, Schwab ME. Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–186. doi: 10.1016/0006-8993(94)01410-j. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: Neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. 1994;126:61–75. doi: 10.1006/exnr.1994.1042. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Fontaine FR, Kaminskas LM, Petersen DR, Pyke SM. Protein adduct-trapping by hydrazinophthalazine drugs: mechanisms of cytoprotection against acrolein-mediated toxicity. Mol Pharmacol. 2004;65:655–664. doi: 10.1124/mol.65.3.655. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen A, Xu XM, Kleitman N, Bunge MB. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Experimental Neurology. 1996;138:261–276. doi: 10.1006/exnr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Cole RA, Weiner MF. Clinical and theoretical observations on phenelzine (nardil), an antidepressant agent. Am J Psychiatry. 1960;117:361–362. doi: 10.1176/ajp.117.4.361. [DOI] [PubMed] [Google Scholar]
- Demediuk P, Saunders RD, Anderson DK, Means ED, Horrocks LA. Membrane lipid changes in laminectomized and traumatized cat spinal cord. Proc Natl Acad Sci U S A. 1985;82:7071–7075. doi: 10.1073/pnas.82.20.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demediuk P, Daly MP, Faden AI. Changes in free fatty acids, phospholipids, and cholesterol following impact injury to the rat spinal cord. J Neurosci Res. 1989;23:95–106. doi: 10.1002/jnr.490230113. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A, Gigli PM, Catinella S, Civelli M, Patacchini R. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37:617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. Journal of Neuroscience. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ER, Scholten DJ, Buechler CM, Jordan-Tibbs J, Mattice C, Albrecht RM. Failure of methylprednisolone to improve the outcome of spinal cord injuries. Am Surg. 1995;61:659–663. discussion 663–654. [PubMed] [Google Scholar]
- Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Hall ED. Free radicals and CNS injury. Critical Care Clinics. 1989;5:793–805. [PubMed] [Google Scholar]
- Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem. 2008a;104:708–718. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008b;107:712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Lapin CA, Bunn WB. A comparison of emissions from vehicles fueled with diesel or compressed natural gas. Environ Sci Technol. 2008;42:6437–6445. doi: 10.1021/es071718i. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, McDaniel EE, Popovich PG. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des. 2005;11:1223–1236. doi: 10.2174/1381612053507468. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J Pharmacol Exp Ther. 2004;310:1003–1010. doi: 10.1124/jpet.104.067330. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicological Sciences. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Khan MA. Effect of hydralazine in hypertension. Br Med J. 1953;1:27–29. doi: 10.1136/bmj.1.4800.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience. 2011;173:150–155. doi: 10.1016/j.neuroscience.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J Neurosci Res. 2006a;84:219–227. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- Liu-Snyder P, McNally H, Shi R, Borgens RB. Acrolein-mediated mechanisms of neuronal death. J Neurosci Res. 2006b;84:209–218. doi: 10.1002/jnr.20863. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radic Biol Med. 2000;29:714–720. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiology of Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int. 2004;44:475–486. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46:243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int. 2005a;47:449–457. doi: 10.1016/j.neuint.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005b;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- Moretto N, Facchinetti F, Southworth T, Civelli M, Singh D, Patacchini R. alpha,beta-Unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. Am J Physiol Lung Cell Mol Physiol. 2009;296:L839–L848. doi: 10.1152/ajplung.90570.2008. [DOI] [PubMed] [Google Scholar]
- Mudge K, Van Dolson L, Lake AS. Progressive cystic degeneration of the spinal cord following spinal cord injury. Spine (Phila Pa 1976) 1984;9:253–255. doi: 10.1097/00007632-198404000-00005. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Nurick S, Russell JA, Deck MD. Cystic degeneration of the spinal cord following spinal cord injury. Brain. 1970;93:211–222. doi: 10.1093/brain/93.1.211. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Behrmann D, Bates CM, Horrocks LA. Lipid alterations following impact spinal cord injury in the rat. Mol Chem Neuropathol. 1994;23:13–26. doi: 10.1007/BF02858504. [DOI] [PubMed] [Google Scholar]
- Park YS, Taniguchi N. Acrolein induces inflammatory response underlying endothelial dysfunction: a risk factor for atherosclerosis. Ann N Y Acad Sci. 2008;1126:185–189. doi: 10.1196/annals.1433.034. [DOI] [PubMed] [Google Scholar]
- Peasley MA, Shi R. Ischemic insult exacerbates acrolein-induced conduction loss and axonal membrane disruption in guinea pig spinal cord white matter. J Neurol Sci. 2003;216:23–32. doi: 10.1016/s0022-510x(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhaes LG, Fornaro M, Di Scipio F, Geuna S, Mauricio AC, Varejao AS. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71–81. doi: 10.1016/j.expneurol.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Picklo MJ, Montine TJ. Acrolein inhibits respiration in isolated brain mitochondria. Biochim Biophys Acta. 2001;1535:145–152. doi: 10.1016/s0925-4439(00)00093-4. [DOI] [PubMed] [Google Scholar]
- Reece PA. Hydralazine and related compounds: chemistry, metabolism, and mode of action. Med Res Rev. 1981;1:73–96. doi: 10.1002/med.2610010105. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Stensaas LJ, Guth L. The astrocytic scar as an impediment to regeneration in the central nervous system. In: Kao CC, Bunge RP, Reier PJ, editors. Spinal Cord Reconstruction. Vol. 0. New York: Raven Press; 1983. pp. 163–195. [Google Scholar]
- Rooney GE, Endo T, Ameenuddin S, Chen B, Vaishya S, Gross L, Schiefer TK, Currier BL, Spinner RJ, Yaszemski MJ, Windebank AJ. Importance of the vasculature in cyst formation after spinal cord injury. J Neurosurg Spine. 2009;11:432–437. doi: 10.3171/2009.4.SPINE08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RD, Dugan LL, Demediuk P, Means ED, Horrocks LA, Anderson DK. Effects of methylprednisolone and the combination of alpha tocopherol and selinium on arachidonic acid metabolism and lipid peroxidation in traumatized spinal cord tissue. J Neurochem. 1987;49:24–31. doi: 10.1111/j.1471-4159.1987.tb03388.x. [DOI] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. Journal of Neuropathology & Experimental Neurology. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): A prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61–67. doi: 10.1136/jnnp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Luo L. The role of acrolein in spinal cord injury. Applied Neurology. 2006;2:22–27. [Google Scholar]
- Shi R, Luo J, Peasley MA. Acrolein inflicts axonal membrane disruption and conduction loss in isolated guinea pig spinal cord. Neuroscience. 2002;115:337–340. doi: 10.1016/s0306-4522(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Shi R, Rickett T, Sun W. Acrolein-mediated injury in nervous system trauma and diseases. Mol Nutr Food Res. 2011a;55:1320–1331. doi: 10.1002/mnfr.201100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sun W, McBride JJ, Cheng JX, Shi R. Acrolein induces myelin damage in mammalian spinal cord. J Neurochem. 2011b;117:554–564. doi: 10.1111/j.1471-4159.2011.07226.x. [DOI] [PubMed] [Google Scholar]
- Shibuta S, Varathan S, Mashimo T. Ketamine and thiopental sodium: individual and combined neuroprotective effects on cortical cultures exposed to NMDA or nitric oxide. Br J Anaesth. 2006;97:517–524. doi: 10.1093/bja/ael192. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clinical neuroscience research. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, Ouyang Z. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem. 2011;83:1197–1201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nature reviews. Drug discovery. 2005;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PL, Khan MA, Moskal JR, Todd KG, Tanay VA, Baker G. Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 2006;1122:184–190. doi: 10.1016/j.brainres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zheng L, Park J, Walls M, Tully M, Jannasch A, Cooper B, Shi R. Determination of Urine 3-HPMA, a Stable Acrolein Metabolite in a Rat Model of Spinal Cord Injury. J Neurotrauma. 2013;30:1334–1341. doi: 10.1089/neu.2013.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]