Abstract
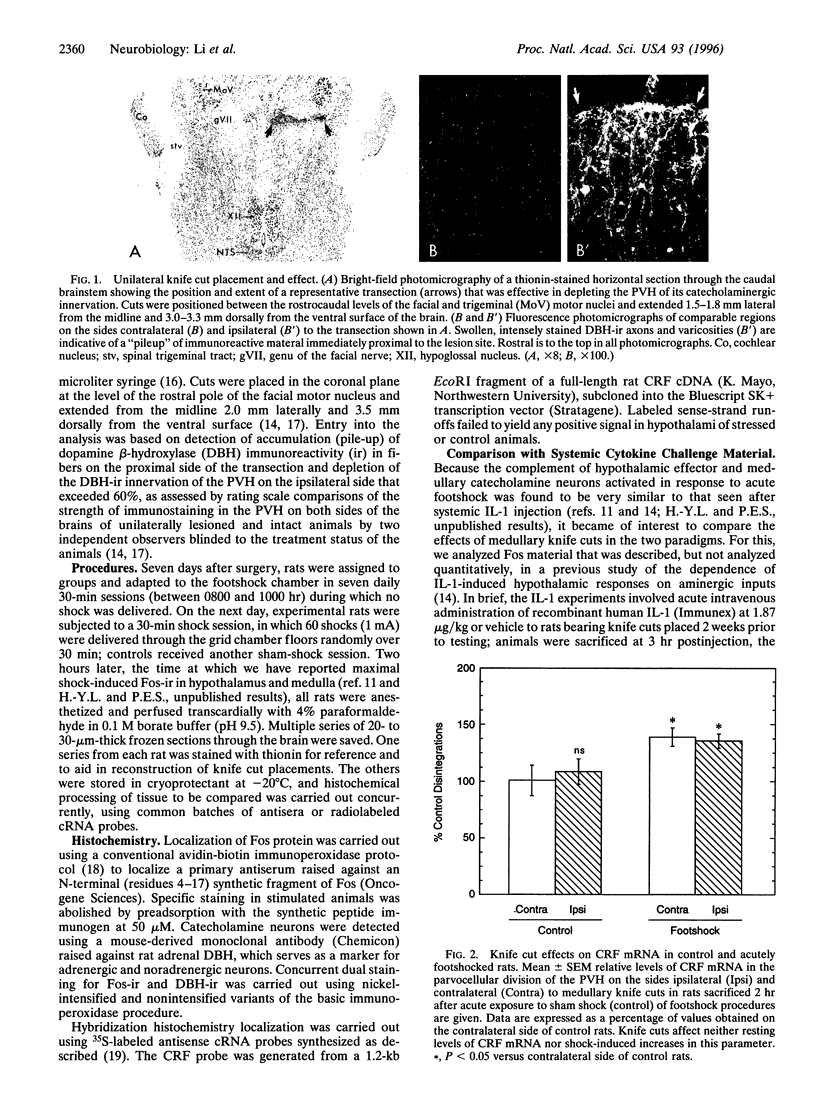
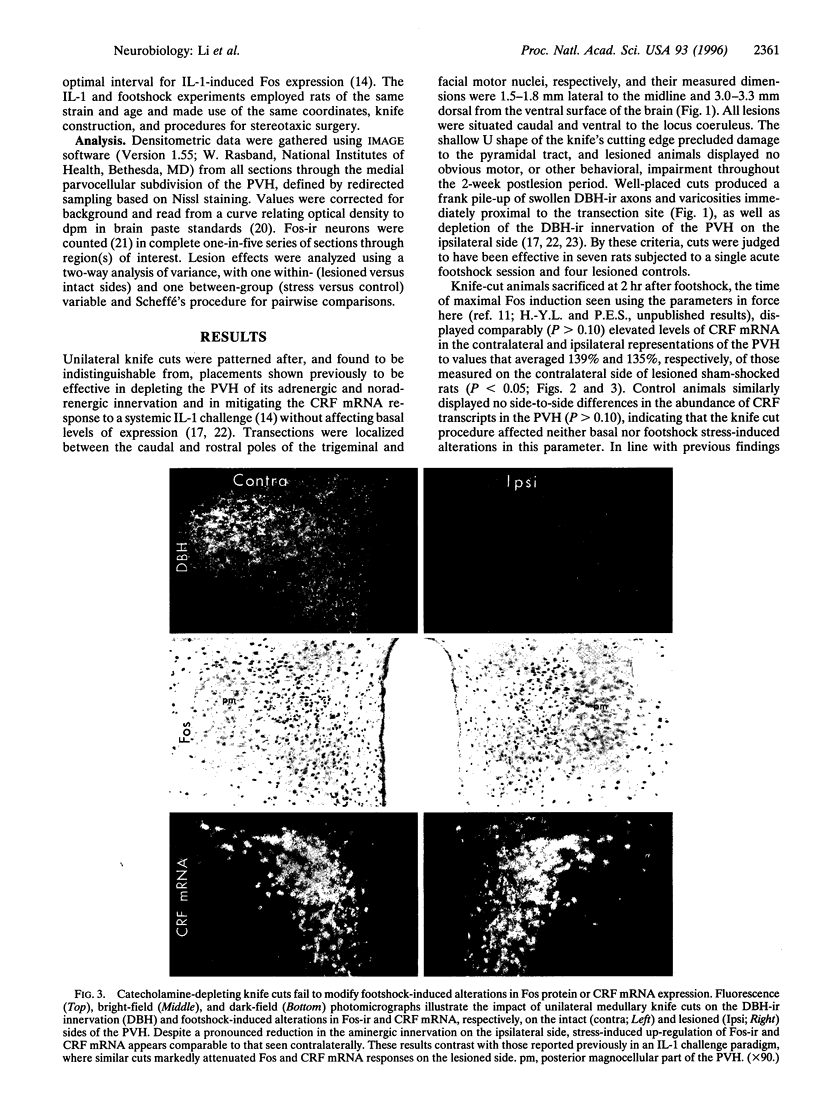
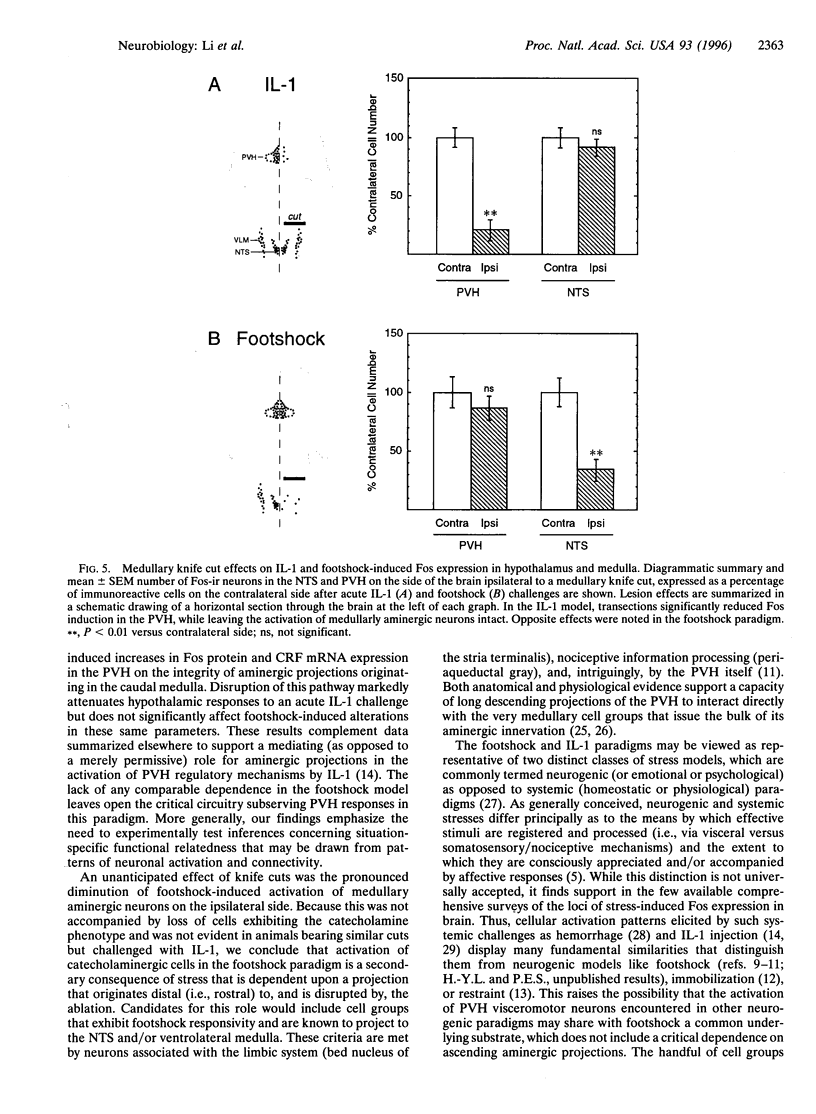
Intermittent electrical footshock induces c-fos expression in parvocellular neurosecretory neurons expressing corticotropin-releasing factor and in other visceromotor cell types of the paraventricular hypothalamic nucleus (PVH). Since catecholaminergic neurons of the nucleus of the solitary tract and ventrolateral medulla make up the dominant loci of footshock-responsive cells that project to the PVH, these were evaluated as candidate afferent mediators of hypothalamic neuroendocrine responses. Rats bearing discrete unilateral transections of this projection system were exposed to a single 30-min footshock session and sacrificed 2 hr later. Despite depletion of the aminergic innervation on the ipsilateral side, shock-induced up-regulation of Fos protein and corticotropin-releasing factor mRNA were comparable in strength and distribution in the PVH on both sides of the brain. This lesion did, however, result in a substantial reduction of Fos expression in medullary aminergic neurons on the ipsilateral side. These results contrast diametrically with those obtained in a systemic cytokine (interleukin 1) challenge paradigm, where similar cuts ablated the Fos response in the ipsilateral PVH but left intact the induction seen in the ipsilateral medulla. We conclude that (i) footshock-induced activation of medullary aminergic neurons is a secondary consequence of stress, mediated via a descending projection transected by our ablation, (ii) stress-induced activation of medullary aminergic neurons is not necessarily predictive of an involvement of these cell groups in driving hypothalamic visceromotor responses to a given stressor, and (iii) despite striking similarities in the complement of hypothalamic effector neurons and their afferents that may be activated by stresses of different types, distinct mechanisms may underlie adaptive hypothalamic responses in each.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady L. S., Lynn A. B., Herkenham M., Gottesfeld Z. Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci. 1994 Aug;14(8):4951–4964. doi: 10.1523/JNEUROSCI.14-08-04951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs R. M., Van der Beek E. M., Renaud L. P., Day T. A., Jhamandas J. H. Oxytocin localization and function in the A1 noradrenergic cell group: ultrastructural and electrophysiological studies. Neuroscience. 1990;39(3):717–725. doi: 10.1016/0306-4522(90)90255-3. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Brown E. R., Ericsson A., Kovács K. J., Sawchenko P. E. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993 Dec;13(12):5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W. E., Herman J. P., Battaglia D. F., Akil H., Watson S. J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995 Jan;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, Bohn M. C., Sawchenko P. E. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990 Feb 22;292(4):651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, Sawchenko P. E. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988 Aug 1;274(1):60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Ericsson A., Kovács K. J., Sawchenko P. E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994 Feb;14(2):897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R. M., Kapatos G., Carey R. J. A retracting wire knife for stereotaxic brain surgery made from a microliter syringe. Physiol Behav. 1973 Apr;10(4):813–815. doi: 10.1016/0031-9384(73)90168-6. [DOI] [PubMed] [Google Scholar]
- Hoffman G. E., Smith M. S., Verbalis J. G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993 Jul;14(3):173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Li Y. W., Dampney R. A. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994 Aug;61(3):613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Mezey E., Skirboll L. R., Hökfelt T. Adrenergic projections from the lower brainstem to the hypothalamic paraventricular nucleus, the lateral hypothalamic area and the central nucleus of the amygdala in rats. J Chem Neuroanat. 1992 Sep-Oct;5(5):407–415. doi: 10.1016/0891-0618(92)90057-w. [DOI] [PubMed] [Google Scholar]
- Pezzone M. A., Lee W. S., Hoffman G. E., Pezzone K. M., Rabin B. S. Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain Res. 1993 Apr 16;608(2):310–318. doi: 10.1016/0006-8993(93)91472-5. [DOI] [PubMed] [Google Scholar]
- Pezzone M. A., Lee W. S., Hoffman G. E., Rabin B. S. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res. 1992 Nov 27;597(1):41–50. doi: 10.1016/0006-8993(92)91503-7. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Arias C. A., Mortrud M. T. Local tetrodotoxin blocks chronic stress effects on corticotropin-releasing factor and vasopressin messenger ribonucleic acids in hypophysiotropic neurons. J Neuroendocrinol. 1993 Aug;5(4):341–348. doi: 10.1111/j.1365-2826.1993.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E. Effects of catecholamine-depleting medullary knife cuts on corticotropin-releasing factor and vasopressin immunoreactivity in the hypothalamus of normal and steroid-manipulated rats. Neuroendocrinology. 1988 Nov;48(5):459–470. doi: 10.1159/000125050. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982 Nov;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Senba E., Umemoto S., Kawai Y., Noguchi K. Differential expression of fos family and jun family mRNAs in the rat hypothalamo-pituitary-adrenal axis after immobilization stress. Brain Res Mol Brain Res. 1994 Jul;24(1-4):283–294. doi: 10.1016/0169-328x(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Sladek J. R., Jr, Sladek C. D. Anatomical reciprocity between magnocellular peptides and noradrenaline in putative cardiovascular pathways. Prog Brain Res. 1983;60:437–443. doi: 10.1016/S0079-6123(08)64410-6. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jul 22;285(4):413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]