Abstract
Previous investigations found that a subset of children with autism spectrum disorder (ASD) in California possessed plasma autoantibodies that reacted intensely with brain interneurons or other neural profiles. Moreover, for several cohorts of American women, maternal autoantibody reactivity to specific fetal brain proteins was highly specific to mothers of children with ASD. We sought to determine whether children and their mothers from a regionally specific cohort from the Basque Country of Spain demonstrated similar reactivity. Some children’s plasma reacted to interneurons, beaded axons or other neural profiles with no difference in the occurrence of these antibodies in children with or without ASD. Findings on the maternal antibodies confirmed previous research; plasma reactivity to fetal brain a combination of proteins at 37 and 73 kDa or 39 and 73 kDa was found exclusively in mothers of children with ASD.
Keywords: Autism spectrum disorders, Autoantibody, Brain, International
Introduction
Autism spectrum disorder (ASD) is diagnosed based on an individual displaying a combination of behaviors in the three core areas of social impairment, communication impairment and the presence of repetitive or stereotyped behaviors. Among individuals with ASD, however, much heterogeneity exists both across these core areas, as well as with other features that are commonly associated with ASD such as intellectual disability, seizures or gastrointestinal disturbances (Geschwind and Levitt 2007).
Numerous studies demonstrate that ASD is diagnosed in countries across the globe, although methodological differences between studies prohibit direct comparison of rates (ADDM 2012; Baron-Cohen et al. 2009; Garciía-Primo et al. 2011; Gillberg et al. 2006; Kadesjo et al. 1999). Given the heterogeneity that exists among individuals with a diagnosis of ASD, it is important to study children with ASD across multiple geographical, cultural and ethnic cohorts to determine which features truly generalize to the group of children affected by ASD as a whole.
Several immune system differences have been observed among some individuals with ASD, including increased activation of brain microglia (Morgan et al. 2010; Vargas et al. 2005) and increased levels of certain cytokines including macrophage chemoattractant protein-1, tumor growth factor-beta 1, tumor necrosis factor alpha, interleukin-6, granulocyte–macrophage colony-stimulating factor, and interferon gamma in brain tissue and cerebrospinal fluid (Li et al. 2009; Vargas et al. 2005). Western blot analysis and immunohistochemical studies have found evidence for the presence of antibodies in the plasma of some children with ASD that bind brain tissue (Cabanlit et al. 2007; Connolly et al. 1999; Dalton et al. 2003; Singer et al. 2006; Singh et al. 1997; Wills et al. 2009). Some mothers of children with ASD also demonstrate antibodies to fetal brain tissue (Braunschweig et al. 2008, 2012; Croen et al. 2008; Singer et al. 2008; Zimmerman et al. 2007). Brain-reactive antibodies have also been observed in individuals with other disorders including attention-deficit/hyperactivity disorder (ADHD) and Down syndrome (Rout et al. 2012; Talja et al. 2009), but the significance and etiology of these brain-reactive antibodies is not yet well understood.
In a group of children between 3 and 14 years of age in California, intense reactivity to Golgi cells, interneurons located between the molecular and granule cell layers in the cerebellum that function to inhibit granule cells (D’Angelo 2008), was found in 21 % of plasma samples from children with ASD. Intense reactivity to these cells was not observed with plasma samples from typically developing children (Wills et al. 2009). The plasma that reacted intensely to Golgi cells also reacted to interneurons in numerous other brain regions including the cerebral cortex, hippocampal formation, and the amygdaloid complex (Wills et al. 2011).
Children enrolled in the Autism Phenome Project (APP), a large-scale multidisciplinary study at the UC Davis MIND Institute, were subsequently evaluated for plasma immunoreactivity to interneurons. These children comprised a younger group (2–6 years of age, median age = 3 years) than those that were included in the Wills et al. study (3–14 years of age, median age = 5½ years). Among the children in the APP cohort, several showed the same pattern of plasma immunoreactivity to Golgi cells and other inter-neurons. While 10 % of children with ASD showed inter-neuron staining, so did 9 % of typically developing subjects (Rossi et al. 2011). Several other patterns of plasma reactivity to brain tissue were also observed in this study. For example, some plasma samples reacted with neuronal cell bodies (not restricted to interneurons) throughout the brain and others reacted to neuronal nuclei. Plasma reactivity to interneurons or to other profiles within the brain was found to occur with the same frequency in subjects with ASD and in typically developing subjects (42 % in both cases). While these findings decreased the likelihood that the neural immunoreactivity was a useful biomarker for ASD, Rossi et al. (2011) did observe that subjects (both ASD and typical) whose plasma reacted to brain tissue had scores on the Child Behavior Checklist indicating a greater frequency of behavioral and emotional problems.
Western blot analyses in which children’s plasma was reacted with cerebellar proteins has revealed that reactivity to cerebellar proteins at 45 kDa and 62 kDa are associated with a diagnosis of autism and autism spectrum disorder, respectively (Goines et al. 2011). In addition, those children with the 45 kDa autoantibodies had lower cognitive and executive function, irrespective of diagnosis. The presence of maternal autoantibodies to fetal brain proteins has also been examined extensively by western blot. A subset of mothers of children with ASD were found to possess a combination of antibodies reacting to proteins at 37 and 73 kDa, and this combination has not been observed in the plasma of mothers of TD children (Braunschweig et al. 2008, 2012). Furthermore, a combination of antibodies reacting to proteins at 39 and 73 kDa is also highly specific to mothers of children with ASD (Braunschweig et al. 2012; Croen et al. 2008).
In the current study, we tested for brain-reactive antibodies in the plasma of children from the Gipuzkoa region of the Basque Country of Spain, whose ages matched closely with those from the Wills et al. (2009) cohort, to determine whether similar brain-reactivity is found in children across countries. Further, we asked whether reactivity of maternal plasma to proteins at 37 and 73 kDa or 39 and 73 kDa is observed specifically in mothers of children with ASD in Gipuzkoa.
Methods
The protocol was approved by the Bioethical Research Committee of the Gipuzkoa Health Area authority and by the IRB of the University of California, and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Researchers from Spain completed the electronic IRB course from the United States’ National Institutes of Health. Parents of each subject provided written informed consent for their child to participate, and mothers gave informed consent to participate in the study.
Participants
Thirty-seven subjects with ASD and 37 typically developing subjects were recruited from Gautena, the regional program for autism spectrum disorders in the Gipuzkoa province of Spain, and from local schools. Children suspected of meeting diagnostic criteria for ASD were clinically evaluated using the Autism Diagnostic Observation Schedule-Generic (ADOS-G) and the Autism Diagnostic Interview-Revised (ADI-R) by a practitioner trained to reliability in these measures. Control subjects from local schools were screened with the Social Communication Questionnaire (SCQ), and any subject scoring above 15 was not included and were offered follow up services (this occurred for one subject). Subjects were matched for age and gender, such that the ASD and the typically developing control groups each included 35 males and 2 females between the ages of 3 and 13 years, with a median age of 7 years. The biological mothers of each of these children also participated, and were screened for autistic traits by completing the Autism-Spectrum Quotient (AQ) questionnaire.
Immunohistochemical Analysis
Plasma samples were processed and results were evaluated and documented in an identical manner to that described in Rossi et al. (2011). Brain tissue from an 8-year old male rhesus macaque (Macaca mulatta) perfused as described in Rossi et al. (2011) served as the substrate for immunohistochemical analysis. Prior to analyzing new samples with the macaque brain used in the current paper, positive control samples known to react to interneurons in the cerebellum and throughout the cerebral cortex of other mature rhesus brains were reacted with the tissue and the same pattern of reactivity was observed. All results were scored blind to the diagnosis of the individual, and results from the ASD group were compared to those from the TD group using the Fisher’s exact test (appropriate for analyses in which the number of subjects in one or more cells is less than or equal to five).
Brain Protein Preparation
Rhesus macaque cerebellum protein medleys were used to probe child plasma samples for anti-brain IgG reactivity. Though other brain regions are known to be a target of IgG in children with autism, we opted to use cerebellar proteins because they provide the most consistently reliable target protein preparation in children with an autism spectrum disorder (Cabanlit et al. 2007; Wills et al. 2009). Rhesus macaque fetal brain protein medleys were utilized to probe maternal plasma for anti-brain IgG reactivity based upon independent studies by our laboratory and others that demonstrated a high degree of specificity for maternal antibodies to fetal brain in autism (Braunschweig et al. 2008; Zimmerman et al. 2007). Monkey brain specimens were acquired through the University of California, Davis Primate Center and prepared in our laboratory. Whole cerebellum was obtained from two healthy adult male Rhesus monkeys, and a whole fetal brain was obtained from a gestational day 152 Rhesus macaque. To prepare the protein medleys, 1.0 g of fresh brain tissue was suspended in 10 mL of 20 mM HEPES-OH, pH 7.5, containing 320 mM sucrose, 1 mM EDTA, 5 mM DTE, protease inhibitors (1 mM PMSF and Roche Complete™protease inhibitor), and phosphatase inhibitors (0.2 mM Na2VO3 and 1 mM NaF). The suspension was homogenized using a Teflon/potter homogenizer and centrifuged at 800×g for 10 min to remove nuclei and undissolved material. Protein medleys were then diluted ten-fold with 50 mM Tris–HCl, pH 6.8, containing 25 % glycerol and 1 % lithium dodecyl sulfate (LDS). The final protein products were reconcentrated to 12.5 mg/mL using Amicon® Ultra-4 centrifugal filter devices (Millipore, Billerica, MA).
Western Blotting
Plasma IgG reactivity to brain proteins was measured using western blot technology. 300 μg/ml of brain extracts and 5 μl of Magic Mark protein standard (Invitrogen, Carlsbad, CA) were loaded into 4–12 % gradient prep-well Nu-PAGE Bis–Tris gels (Invitrogen, Carlsbad, CA) and electrophoresed at 200 volts for 1 h. After gel electrophoresis, proteins were transferred at 50 V for 16 h to a nitrocellu-lose membrane. The membranes were then blocked with casein in PBS (Thermo Scientific, Rockford, IL) for 30 min at room temperature. Membranes were cut into vertical strips, and each strip was incubated with a sample of maternal or child plasma diluted 1:400 in 5 % casein in PBS plus 0.05 %Tween (PBST) for 2 h at room temperature. Strips were washed 5 times for 5 min durations with PBST, followed by a 30-minute incubation with horse-radish peroxidase-conjugated goat anti-human IgG secondary (Zymed, San Francisco, CA) diluted 1:20,000. After washing, the signal was developed using a 5-min incubation with SuperSignal Chemiluminescent Substate (Pierce, Rockford, IL). Bands were visualized using a FluorChem 8900 imager and AlphaEaseFC imaging software (Alpha Innotech Corporation, San Leandro, CA). Positive and negative control reference standards were run on each blot.
Results
Immunoreactivity of Samples from Children to Adult Brain Tissue
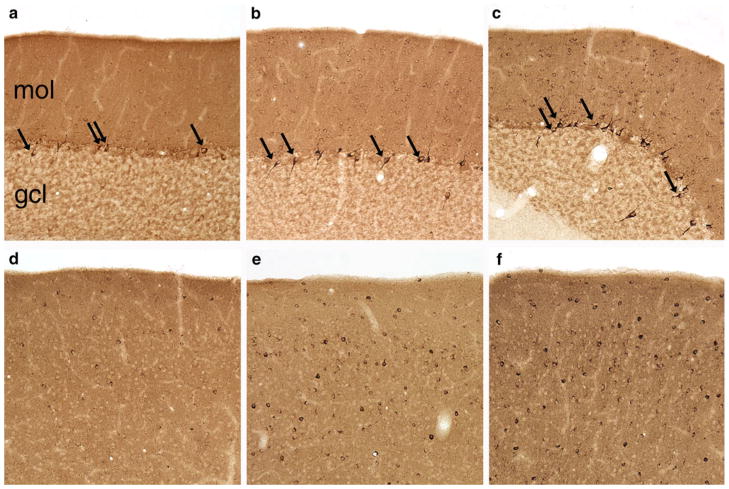
Immunoreactivity to Golgi cells of the cerebellum and a subset of other interneurons throughout the brain was observed in 15 samples from the current study (Fig. 1). The pattern of immunoreactivity was identical to what was observed in previous studies (Fig. 1 a, d). Golgi neurons and other interneurons in the cerebellum were clearly labeled (Fig. 1 b, c) and interneurons were also stained in the cerebral cortex (Fig. 1 e, f). As in the Rossi et al. (2011) paper, anti-GABA neuron immunoreactivity was seen with plasma from both ASD and typically developing children. Plasma from 8 subjects with ASD (22 %) and 7 TD subjects (19 %) reacted to Golgi cells and to other interneurons, a difference that was not statistically significant (p = 0.65, Fisher’s exact test).
Fig. 1.
Some plasma samples reacted to cerebellar Golgi neurons and other interneurons throughout the brain in an identical manner to interneuron-reactive plasma from previous studies. Each sample was reacted with one section through the rhesus monkey cerebellum (a–c) and one section through one hemisphere that included somatosensory cortex (d–f). As a positive control, plasma known to react with interneurons (a, d) was processed in parallel with plasma from the current study. Several samples from the ASD group reacted to Golgi cells (b) and a subset of cortical interneurons (e), as did several samples from the TD group (c, f)
As in our previous studies, we rated the intensity of staining for each sample that reacted to interneurons. Samples were considered intensely labeled (‘‘++’’) if Golgi cells and numerous cortical interneurons were consistently and darkly labeled, moderately labeled (‘‘+’’) if intense labeling was less consistent across the hemispheric or cerebellar section, and ‘‘somewhat labeled’’ (‘‘−/+’’) if labeling was weaker and/or less consistent. Among the subjects with ASD whose plasma reacted to brain inter-neurons, one sample was rated ‘‘++’’ and 7 samples were rated ‘‘−/+’’. Among TD subjects whose plasma reacted to interneurons, 2 samples were rated ‘‘++’’, 2 were rated ‘‘+’’, and 3 were rated ‘‘−/+’’. No significant difference was observed in the frequency of subjects with (n = 1, 3 %) and without (n = 2, 5 %) ASD whose plasma displayed intense reactivity to interneurons.
Two plasma samples reacted to neuronal nuclei throughout the brain including cells within the cerebral hemisphere, as previously described in Rossi et al. (2011). One sample was from a subject with ASD and one was from a TD subject (2.7 % of samples from each group).
Plasma from one individual reacted specifically to axons within the brain that had a beaded morphology evidenced by variations in the diameter of each axon along its length; this pattern of reactivity was found in two TD children and one subject with ASD in the APP cohort (Rossi et al. 2011). This plasma was from an individual in the ASD group (2.7 %); no plasma from subjects in the TD group displayed this type of immunoreactivity.
In total, plasma from 10 subjects with ASD (27 % of subjects) displayed reactivity to some component of brain tissue whereas plasma from 8 subjects in the TD group (22 % of subjects) reacted to brain tissue (Table 1). The occurrence of any type of reactivity to brain tissue did not differ between these two groups (p = 0.59, Fisher’s exact test).
Table 1.
Comparison of plasma immunoreactivity in children in the current study (Gipuzkoa) and in the previous two cohorts (Wills et al. 2009; Rossi et al. 2011)
| Wills et al. 2009 | APP | Gipuzkoa (Basque Country, Spain) | |
|---|---|---|---|
| Interneurons: ++ intensity | |||
| ASD | 7 (21 %) | 3 (3 %) | 1 (3 %) |
| TD | 0 (0 %) | 2 (5 %) | 2 (5 %) |
| p value | (not reported) | 1.00 | 1.00 |
| Interneurons: Any intensity | |||
| ASD | 22 (65 %) | 9 (10 %) | 8 (22 %) |
| TD | 3 (13 %) | 4 (9 %) | 7 (19 %) |
| p value | <.01 | 1.00 | .772 |
| Any immunoreactivity | |||
| ASD | (not reported) | 36 (42 %) | 10 (27 %) |
| TD | (not reported) | 18 (42 %) | 8 (22 %) |
| p value | (not reported) | 1.00 | .588 |
We also reacted plasma from children with ASD and TD control children with homogenized cerebellar proteins in western blot experiments. Plasma from 16 % of subjects with ASD and 8 % of TD subjects showed reactivity to cerebellar proteins at 45 kDa. Plasma from 8 % of subjects with ASD and 8 % of TD subjects showed reactivity at 62 kDa. The frequency with which reactivity at 45 or 62 kDa was observed did not differ significantly between the three groups (Table 2).
Table 2.
Summary of western blot results from children’s samples
| Subjects with 45 kDa band | Subjects with 62 kDa band | |
|---|---|---|
| ASD (n = 37) | 6 (16 %) | 3 (8 %) |
| TD (n = 37) | 3 (8 %) | 3 (8 %) |
The number of samples reacting to cerebellar proteins at 45 and 62 kDa, with the percentage of subjects reacting positively in parentheses
Immunoreactivity of Plasma Samples from Mothers to Fetal Brain Protein
We assayed the plasma samples collected from mothers of the study children for the presence of antibodies to fetal brain proteins at 37, 39, and 73 kDa, as combinations of reactivity at 37 and 73 kDa or 39 and 73 kDa were previously shown to be highly specific to mothers with ASD. Plasma reacting at both 37 kDa and 73 kDa was exclusively observed in mothers of children with ASD, and occurred in 5 % of samples. Similarly, reactivity at both 39 and 73 kDa was only observed in the group of mothers from the ASD group (n = 1, 3 %) and was not observed in any mothers from the TD group (Table 3). Given the relatively small sample size, the finding that reactivity to fetal proteins at 37 and 73 kDa or 39 and 73 kDa occurred exclusively in mothers of children with ASD was not statistically significant (p = 0.493 and 1.00 respectively).
Table 3.
Summary of western blot results for maternal samples
| Subjects with 37 kDa band | Subjects with 39 kDa band | Subjects with 73 kDa band | Subjects with both 37 and 73 kDa band | Subjects with both 39 and 73 kDa band | |
|---|---|---|---|---|---|
| ASD (n = 37) | 5 (13.5 %) | 11 (29.7 %) | 5 (13.5 %) | 2 (5.4 %) | 1 (2.7 %) |
| TD (n = 37) | 1 (2.7 %) | 11 (29.7 %) | 6 (16.2 %) | 0 (0 %) | 0 (0 %) |
The number of maternal samples reacting to fetal brain proteins at 37, 39, and 73 kDa, with the percentage of subjects reacting positively in parentheses
Discussion
The aim of this study was to determine whether reactivity to brain tissue, previously characterized in children’s and maternal plasma samples originating from California, also occurs for children and mothers in a cohort from the Gipuzkoa region of Spain. Given the heterogeneity that has been observed for subjects with ASD (Geschwind and Levitt 2007), it is crucial to evaluate empirical findings across multiple geographical, cultural, and ethnic populations, and to report which of these generalize to the group of children affected by ASD.
Our results indicate that plasma from 27 % of ASD subjects and 22 % of TD subjects displayed some type of brain reactivity, including reactivity to a subset of inter-neurons, nuclei, or beaded axons. Strikingly, the frequency with which intense immunoreactivity of plasma to brain interneurons found in the current study, 3 % of samples in the ASD group and 5 % of samples in the TD group, was identical to that observed in the Autism Phenome Project cohort. These results suggest that the particular pattern of immunoreactivity to brain interneurons that we have reported previously is not exclusive to children in California. Children whose plasma reacted to brain tissue in a previous study (Rossi et al. 2011) were shown to have significantly increased measures of behavioral and emotional problems as reported by parents on the Child Behavior Checklist (CBCL). It is not possible to determine whether the Basque-Spanish children identified with brain immunoreactivity are more likely to have similar findings, since the CBCL was not administered to them. It is interesting that despite genetic, environmental, and cultural differences, identical patterns of reactivity to interneurons were found across all three groups of children tested to date. The specific antigen present in the interneurons to which the plasma is reacting, and the timing and stimulus for the production of these antibodies, has yet to be identified. A recent study found antibodies against an enzyme that catalyzes the conversion of glutamic acid to the inhibitory neurotransmitter GABA, in 15 % of children with autism (mean age 11 years) (Rout et al. 2012). The pattern of cerebellar staining in the Rout et al. study, in which Purkinje cells were predominantly labeled, differed from that observed in the current study, in which Golgi cells were labeled. However, the similarity between the two studies, namely the observation of specific plasma reactivity to a subset of cerebellar interneurons, is noteworthy in that together these findings provide two examples of interneuron-specific plasma autoantibodies in children.
The functional significance of the antibodies detected in children’s plasma is unknown. In general, circulating antibodies are not thought to have access to antigens in the brain due to tight restriction by the blood–brain barrier (BBB). However, it has been shown that immune system cells including antibody-producing B cells can cross the BBB during inflammatory conditions such as those that occur during infection (Hickey 2001; Miller 1999). In addition, peripherally circulating antibodies are able to reach the brain in cases where the BBB is temporarily breached, as has been demonstrated with administration of lipopolysaccharide (Kowal et al. 2004). Thus, mechanisms exist whereby the peripheral autoantibodies detected in the present study could reach the brain. Whether this occurred in the subjects we studied has not been determined.
Our examination of maternal plasma revealed that 5 % of samples from mothers of children with ASD reacted to fetal brain proteins at 37 and 73 kDa, and 3 % of samples from the same group reacted to proteins at 39 and 73 kDa. These combinations did not occur in samples from mothers of children in the TD group. These results suggest that the patterns of reactivity previously found to be highly specific to mothers of children with autism (Braunschweig et al. 2008, 2012; Croen et al. 2008) occurs across cultures and genetic backgrounds. As in previous studies, these two specific combinations of reactivity were exclusive to mothers of children in the ASD group. In a previous study, maternal samples from mothers of children with developmental delay without autism have been analysed and reactivity to proteins at 37 and 73 or 39 and 73 kDa were not observed, suggesting this reactivity is specific to ASD (Braunschweig et al. 2008). Another study found that reactivity at 39 and 73 kDa can be detected in maternal plasma during mid-pregnancy (15–19 weeks) (Croen et al. 2008), suggesting the potential to use maternal plasma screening as a predictive tool in determining whether an increased likelihood exists for a child developing ASD in a subset of cases.
Conclusion
This international replication study contributes importantly to our understanding of a role for plasma brain-reactivity in ASD. The current findings, in combination with those from Rossi et al. (2011) suggest that there is not consistent evidence to indicate that reactivity of plasma from children to neural profiles in the brain is related to an ASD diagnosis. A comparison of the current findings with several previous studies with regard to maternal plasma immunoreactivity, however, suggests that two combinations of reactivity—to proteins at 37 and 73 kDa or 39 and 73 kDa—are specific to mothers of children with ASD across different populations. Studies such as this that compare biological features in children with ASD from different geographic locations will provide important insights into which factors are consistently observed in individuals diagnosed with ASD.
Acknowledgments
The generous collaboration of staff and families from the Gautena Autism regional program and from the community schools (San Benito Ikastola from Lazkao and Ekintza Ikastola from Donostia/San Sebastián) is acknowledged. Partial funding for this project came from the Policlinica Gipuzkoa—Dr. Carlos Elósegui Research Foundation. Dr. Fuentes has received research support from, has served as speaker for, or has served on the advisory boards of Eli Lilly and Co., Janssen, and Shire.
Contributor Information
Christy C. Rossi, Department of Psychiatry and Behavioral Sciences, University of California, Davis, Sacramento, CA, USA, The MIND Institute, University of California, Davis, 2825 50th Street, Sacramento, CA 95817, USA, Department of Psychology, University of Denver, Denver, CO, USA
Joaquin Fuentes, Child and Adolescent Psychiatry Unit, Policlinica Guipuzkoa and Gautena Autism Society, Donostia/San Sebastián, Spain.
Judy Van de Water, The MIND Institute, University of California, Davis, 2825 50th Street, Sacramento, CA 95817, USA, Division of Rheumatology, Allergy and Clinical Immunology, University of California, Davis, Sacramento, CA, USA, NIEHS Center for Children’s Environmental Health, University of California, Davis, Sacramento, CA, USA.
David G. Amaral, Email: dgamaral@ucdavis.edu, Department of Psychiatry and Behavioral Sciences, University of California, Davis, Sacramento, CA, USA, The MIND Institute, University of California, Davis, 2825 50th Street, Sacramento, CA 95817, USA, Center for Neuroscience and California National Primate Research Center, University of California, Davis, Sacramento, CA, USA
References
- ADDM. Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveillance Summaries. 2012;61:1–19. [PubMed] [Google Scholar]
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism-spectrum conditions: UK school-based population study. British Journal of Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: Maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, et al. Behavioral correlates of maternal antibody status among children with autism. Journal of Autism and Development Disorders. 2012;42(7):1435–1445. doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Annals of the New York Academy of Sciences. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. Journal of Pediatrics. 1999;134:607–613. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: The early markers for autism study. Biological Psychiatry. 2008;64:583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Annals of Neurology. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- D’Angelo E. The critical role of Golgi cells in regulating spatio-temporal integration and plasticity at the cerebellum input stage. Frontiers in Neuroscience. 2008;2:35–46. doi: 10.3389/neuro.01.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Primo P, Canal-Bedia R, Martín Cilleros MV, Guisuraga Fernández Z, Herráez-García L, Herraez García MM, et al. Population-based autism screening program using the M-CHAT in Spain. International meeting for autism research; San Diego, California. 2011. [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Cederlund M, Lamberg K, Zeijlon L. Brief report: ‘‘The autism epidemic’’. The registered prevalence of autism in a Swedish urban area. Journal of Autism and Developmental Disorders. 2006;36:429–435. doi: 10.1007/s10803-006-0081-6. [DOI] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain, Behavior, and Immunity. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Kadesjo B, Gillberg C, Hagberg B. Brief report: Autism and Asperger syndrome in seven-year-old children: A total population study. Journal of Autism and Developmental Disorders. 1999;29:327–331. doi: 10.1023/a:1022115520317. [DOI] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. Journal of Neuroimmunology. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW. Immunobiology of the blood-brain barrier. Journal of Neurovirology. 1999;5:570–578. doi: 10.3109/13550289909021286. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Rossi CC, Van de Water J, Rogers SJ, Amaral DG. Detection of plasma autoantibodies to brain tissue in young children with and without autism spectrum disorders. Brain, Behavior, and Immunity. 2011;25:1123–1135. doi: 10.1016/j.bbi.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout UK, Mungan NK, Dhossche DM. Presence of GAD65 autoantibodies in the serum of children with autism or ADHD. European Child and Adolescent Psychiatry. 2012;21(3):141–147. doi: 10.1007/s00787-012-0245-1. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. Journal of Neuroimmu-nology. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. Journal of Neuroim-munology. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatric Neurology. 1997;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Talja I, Reimand T, Uibo O, Reimand K, Aun S, Talvik T, et al. Antibodies to neurofilaments. Annals of the New York Academy of Sciences. 2009;1173:130–136. doi: 10.1111/j.1749-6632.2009.04624.x. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain, Behavior, and Immunity. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Rossi CC, Bennett J, Cerdeno VM, Ashwood P, Amaral DG, et al. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Molecular Autism. 2011;2:5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain, Behavior, and Immunity. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]