Abstract
OBJECTIVE
To determine the cellular architecture of the inflammatory infiltrate in adipose tissue from obese mice, and identify the source of inflammatory cytokines in adipose tissue at a single cell level.
DESIGN AND METHODS
Adipose tissue from diet-induced obese mice was digested by collagenase treatment and fractionated by density centrifugation to obtain an adipocyte floating layer and a pellet of stromal vascular cells. The cellular architecture of the adipocyte-macrophage interaction in both intact white adipose tissue and the separated density gradient floating layer fraction was analyzed by confocal immunohistochemistry. Cytokine expression was detected by semi-quantitative real time PCR and immunohistochemical analysis.
RESULTS
Three dimensional image analysis of white adipose tissue and the separated ‘adipocyte’ floating layer revealed lipid-engorged macrophages, macrophages in contact with lipid droplets and sheath-like assemblies of macrophages surrounding adipocytes. The macrophages immunostained for TNFα and to a lesser extent for the immunoregulatory cytokine IL-10. TNFα staining was associated only with macrophages indicating that macrophages and not adipocytes are the source of TNFα expression in the adipocyte floating layer.
CONCLUSION
Macrophages form assemblies that tightly adhere to and cover adipocytes and lipid droplets. TNFα found in low density adipocyte preparations is due to contamination with macrophages.
Keywords: adipocyte, inflammation, obesity, macrophage, TNFα, IL–10
Introduction
In obesity, adipose tissue is in a state of chronic low-grade inflammation (1, 2) with elevated numbers of immune cells, including macrophages and T cells, and dysregulated expression of cytokines/chemokines (reviewed in (3, 4)). The inflammatory components have direct and indirect effects on metabolic pathways and insulin sensitivity. Of note, TNFα causes insulin resistance, at least in part by blunting insulin signaling (5). Thus, it is important to understand the cellular architecture and interactions within this specific microenvironment.
The cellular infiltrate in adipose tissue from obese animals is organized in cellular assemblies known as crown-like structures (CLS) and contains a large number of macrophages (6). Crown-like structure macrophages are found predominantly around dying adipocytes, and appear to engulf adipocyte-derived lipid fragmented into small droplets (7). Mechanistic details of these events are still unknown as are the signals that orchestrate these events. To address these questions further, more information is required about the architecture of the cellular infiltrate surrounding adipocytes.
In an attempt to identify the cellular source of TNFα, Hotamisligil et al. (5) separated adipocytes from non-adipocytes based on buoyancy and found that the floating cells, presumed to contain adipocytes, expressed more TNFα mRNA than stromal vascular cells found in the pellet. Based on a carefully prepared adipocyte fraction, Fain et al. (8) concluded that non-adipocytes, most likely macrophages, were the major source of TNFα in human adipose tissue. Although the majority of studies suggest that macrophages are the main source of TNFα in adipose tissue, adipocytes are still considered candidate producers of TNFα (6, 9). A definitive identification of the source of TNFα and other cytokines requires an analysis at the single cell level and a more detailed analysis of the cellular architecture of the adipose tissue inflammatory infiltrate.
In the present study, whole adipose tissue as well as density fractionated tissue preparations (10) from obese mice were analyzed by confocal immunohistochemistry to generate 3D images of the adipose tissue inflammatory infiltrate. This shows that buoyancy alone does not separate adipocytes from other types of cells. Adipocyte-associated macrophages, and not the adipocytes themselves, stain positive for TNFα. Previous reports, reviewed in (11), of separated adipocytes producing TNFα may be indicative of the inability to separate adipocytes from macrophages even after digestion and separation into single adipocytes.
Methods and Procedures
Mice and diets
Ceacam1 null mice (Cc1−/−), genetically predisposed to developing obesity and metabolic syndrome, were propagated on a C57BL/6 genetic background (12, 13). C57BL/6 (The Jackson Laboratory, Bar Harbor, ME) and Cc1−/− male mice were bred in house and used throughout the study. Mice had free access to water and one of three diets; regular diet (RD) (Lab Diets #5001), high-fat diet (HF), or Western high-fat/high carbohydrate diet (HF/HC) (Research Diets Inc., New Brunswick, NJ, USA #D12451 and D12079B, respectively). Feeding began at two months of age and continued for 1 to 4.5 months. Weight measurements were taken at the initiation of feeding and prior to sacrifice and tissue harvesting. Visceral adiposity was calculated as the percentage of peri-gonadal visceral white adipose tissue mass relative to body weight after overnight fasting. Both strains of mice were considered together for analysis and divided into: non-obese mice fed a RD diet with less than 3.5% visceral adiposity and obese mice fed a HF or HF/HC diet with 5–7% visceral adiposity. All procedures were approved by the Institutional Animal Care and Use Committee.
Tissue histology and adipocyte cell measurement
Small pieces of adipose tissue were removed and fixed for greater than 24 hours in Z-fix buffered zinc formalin fixative (Anatech, Battle Creek, MI). Tissue was processed, paraffin-embedded, and cut into 5µm sections and H&E stained.
RNA isolation and semi-quantitative real-time PCR analysis
Adipose tissue and isolated cell populations were homogenized using one-time-use generators and a Powergen 125 tissue homogenizer (ThermoFisher Scientific, Pittsburg, PA). Frozen tissue samples (30–50 mg) were stored in RNAlater (Qiagen, Valencia, CA). Messenger RNA (mRNA) was isolated and purified using RNeasy mini kits (Qiagen) according to the manufacturer's protocol. cDNA was synthesized using M-MLV Reverse Transcriptase (ThermoFisher Scientific) and was used in triplicate semi-quantitative real-time PCR reactions (qRT-PCR) to measure the relative amount of mRNAs. Real time amplification was obtained via Absolute qRT-PCR SYBR mastermix and a CFX96 system Thermocycler (Bio-Rad, Hercules, CA) used per manufacturer's instructions. Forward and reverse primers were designed using the Primer Express 1.5 software (Applied Biosystems, Foster City, CA, USA) or identified using PrimerBank (http://pga.mgh.harvard.edu/primerbank/Harvard Medical School). Glyceraldehyde-3 phosphate dehydrogenase (Gapdh) was used as an endogenous control to normalize the amount of starting cDNA. The delta Ct quantification method was employed. Mouse primer sequences (forward/reverse) were as follows:
Gapdh: CCAGGTTGTCTCCTGCGACT/ATACCAGGAAATGAGCTTGACAAAGT;
TNFα:CATCTTCTCAAAATTCGAGTGACAA/TGGGAGTAGACAAGGTACAACCC;
IL-10: GGTTGCCAAGCCTTATCGGA/ACCTGCTCCACTGCCTTGCT;
F4/80: CTTTGGCTATGGGCTTCCAGTC/CAAGGAGGACAGAGTTTATCGTG;
Adiponectin: AGCCGCTTATATGTATCGCTCA/TGCCGTCATAATGATTCTGTTGG;
CD68: CTTCCCACAGGCAGCACAG/ AATGATGAGAGGCAGCAAGAGG
Adipose tissue separation
Stromal vascular cells (SVC) and adipocyte fractions were isolated from adipose tissue following a method modified from Lumeng et al. (10). One gram of visceral adipose tissue from each mouse was pooled, finely minced, and suspended in PBS containing magnesium and calcium, 1.5% fatty acid and endotoxin free bovine serum albumin (BSA), 5mM glucose, and 100 Units of penicillin and 100µg streptomycin. Minced tissue was centrifuged at 500rcf for 5 minutes to pellet red blood cells (RBC). Collagenase (≥125U/mg, Sigma, St. Louis MO) was added to a final concentration of 1.0mg/ml, and the tissue was routinely digested in a shaking water bath (200Hz) for 45–50 minutes at 37°C. Further digestion time resulted in lysed adipocytes. Undigested material was removed by straining through a 100µm sieve (BD Biosciences, San Jose, CA, USA) and SVC were pelleted by centrifugation at 500rcf for 5 minutes. Remaining RBCs in the SVC fraction were lysed with erythrocyte lysing buffer (Lonza, Walkersville, MD, USA) for 5 min at room temperature. Floating adipocytes were removed and washed with PBS three times. Purified cell fractions were either lysed for mRNA isolation and further analyzed by qRT-PCR, or analyzed by fluorescence activated cell sorter (FACS) or laser scanning confocal microscopy (LSCM).
Magnetic separation of adipose tissue macrophages from SVC
Macrophages within the SVC pellet were purified using anti-F4/80-PE antibody 2.0µg/ml (eBioscience, San Diego, CA) and EasySep PE selection kit (Stemcell Technologies, Vancouver, BC, Canada) per manufacturer’s protocol. Purity of the separated cell population was >95% as confirmed by FACS analysis of F4/80+ cells vs. total SVC using a BD FACScan and CELLquest software (BD Biosciences, San Jose, CA) (FACS data not shown).
Fixation and antibody staining of visceral white adipose tissue (WAT) and floating layer adipocytes
Adipose tissue for microscopy was harvested in 2–3mm pieces and fixed using Z-fix formalin (Anatech) for 12 hours before being stored in PBS at 4°C. Adipose tissue and floating layer cells were permeabilized in 1% Triton X-100 for 10 minutes. For simple staining, fixed adipose tissue and floating layer cells were stained with rat anti-mouse F4/80 (Invitrogen, Carlsbad, CA) to mark macrophages and detected with donkey anti-rat IgG conjugated to AlexaFluor488 (Invitrogen) or IL-10 was stained with anti-mouse IL-10 biotin conjugate (Invitrogen) and an Alexa647 conjugated streptavidin (Invitrogen). Tissues were incubated with primary antibodies overnight then washed 3× with staining buffer before application of secondary stains. Secondary staining reagents were applied for 2 hours then washed 3 times in PBS before imaging. For complex 5-color staining, fixed tissue and floating layer adipocytes were stained utilizing directly conjugated fluorescent anti-mouse monoclonal antibodies: anti-TNFα conjugated FITC (eBioscience), anti-IL-10 conjugated PE (BD Bioscience) and anti-F4/80 conjugated AlexaFluor647 (Invitrogen) in PBS staining buffer with 1% BSA. Antibodies were incubated with samples overnight at room temperature with rocking. All stained samples were then counterstained for 15 minutes at room temperature with 5µM BODIPY 558/568 (Molecular Probes, Inc. Eugene, OR) to visualize lipid and/or 40µM Hoechst 33342 (Invitrogen) to visualize nuclei. All samples were washed three times with PBS before visualization. Adipose tissue samples were placed directly on a coverslip with buffer and visualized. For the floating layer samples, the aqueous layer was removed down to 20ul of sample which was then mixed with 50ul Fluormount-G (Southern Biotech, Birmingham, AL) and mounted using a coverslip.
Laser scanning confocal microscopy (LSCM)
Samples were imaged using a Leica TCS SP5 laser scanning microscope (Leica Microsystems, Bannockburn, IL) equipped with conventional solid state and a Ti-sapphire tunable multi-photon laser (Coherent, Santa Clara, CA). Images were acquired in the 3D XYZ plane in 1–5µm steps with a 20× objective (NA 0.70) or 63× objective (NA 1.40) using the sequential scan mode to eliminate any spectral overlap in the individual fluorophores. Specifically, FITC was excited at 488nm with collection at 500–558nm. The BODIPY 558/568 dye (Molecular Probes, Inc.) was excited at 561nm and collected at 567–609nm. AlexaFluor647 (Invitrogen) was excited at 633nm and collected at 644–713nm. PE was excited at 561nm and collected at 567–609nm. The Hoechst dye was excited with the multi-photon (MP) laser tuned to 790nm with collection at 420–500nm. Selected images are a 2D representation of the 3D LSCM image stack as labeled.
Statistical analysis
The 2-way ANOVA comparisons and Bonferroni post-tests were performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Additionally, direct comparisons were made utilizing a T-test via Microsoft Excel (Microsoft Corp., Redmond, WA).
Results
Macrophages appear in crown-like structures with obesity and take up lipid
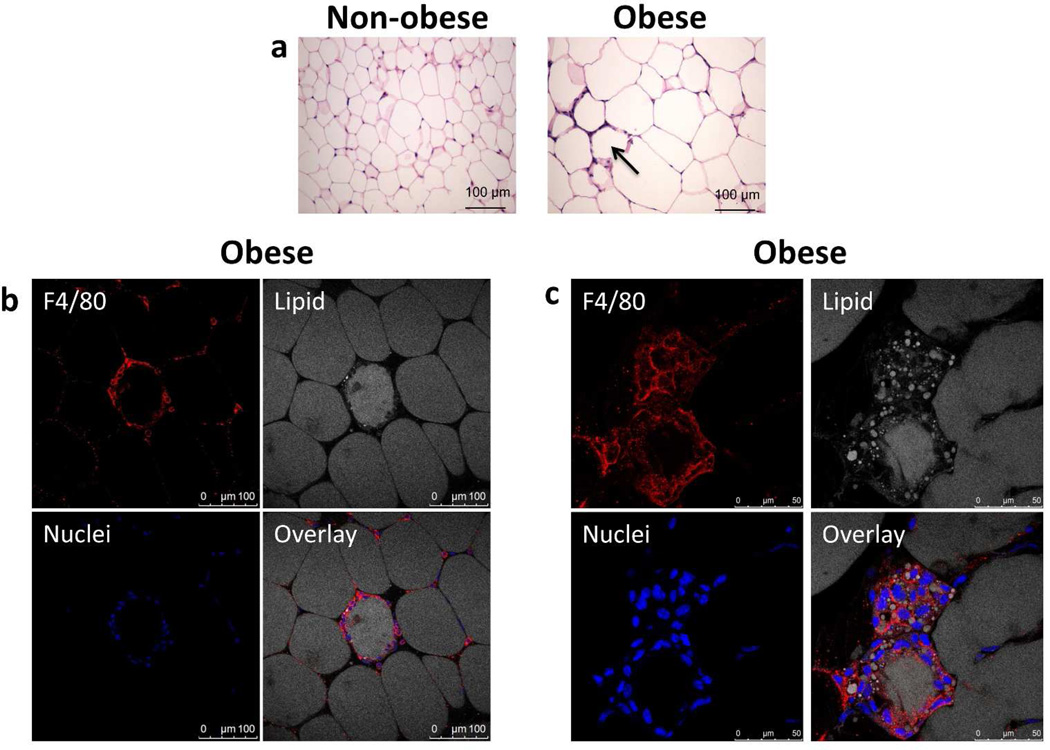
Hematoxylin and eosin staining of visceral WAT from obese mice displayed cell accumulation around adipocytes known as crown-like structures (CLS) (Figure 1a), while non-obese mice remained CLS-free. Adipocyte size increased in obese mice compared to non-obese mice (Figure 1a) consistent with previous observations (14).
Figure 1.
Obese mice have a higher prevalence of crown-like structures (CLS) and macrophage infiltration. (a) Figure shown compares white adipose tissue from a non-obese mouse (<3.5% adiposity) with an obese mouse (>5% adiposity). Examples of classical crown–like structures surrounding individual adipocytes are indicated by arrow. Images were obtained with a 20× objective after hematoxylin and eosin staining. (b,c) Fixed adipose tissue from obese mice was stained for F4/80 (red) to denote macrophages, nuclei stained with Hoechst dye (blue), and lipid stained with BODIPY 558/568 (gray). Adipose tissue was analyzed by laser scanning confocal microscopy (LSCM) and a 1µm sections are presented. (b) Macrophages represent the major population comprised in the CLS. Images collected with a 20× objective. (c) Macrophages within CLS uptake lipid. Lipid can be observed within the macrophages surrounding adipocytes in the CLS. Images collected with a 63× objective.
Laser scanning confocal microscopy (LSCM) analysis of the adipose tissue from obese animals showed that macrophages are a major component of the CLS as determined by F4/80 staining (Figure 1b) consistent with previous publications (15, 16). The lipid staining inside some of the CLS cells clearly demonstrated that macrophages have lipid in their cytoplasm (Figure 1c) Structures such as these can be seen in Nishimura et al, but with CD68 staining (16).
White adipose tissue from obese mice contained macrophages, but not adipocytes, that expressed TNFα
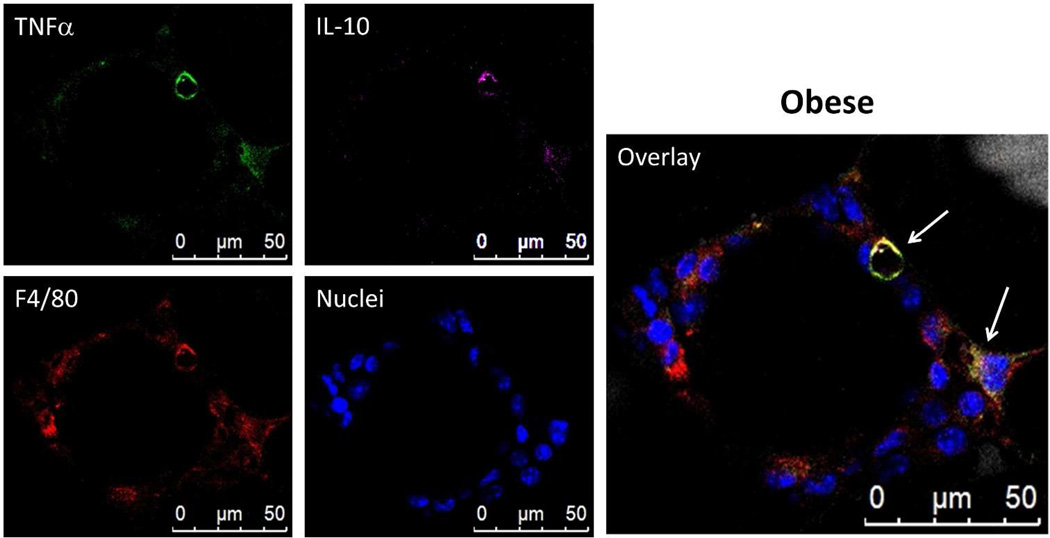
Figure 2 shows a CLS containing macrophages. Some macrophages stained only with F4/80, without TNFα or IL-10 (Figure 2; compare F4/80 with overlay). The majority of F4/80 positive macrophages however, also stained for TNFα (Figure 2; compare F4/80 with TNFα and overlay) and occasionally macrophage areas gave distinctive staining for F4/80 and TNFα as well as IL-10 (Figure 2 overlay; arrows). Overall, most macrophages (>11) expressed TNFα while a minority (~2) expressed both TNFα and IL-10. None of the macrophages, within the CLS shown in Figure 2, produced IL-10 alone.
Figure 2.
Most CLS macrophages express TNFα while a few express both TNFα and IL-10. Images presented are a LSCM 2D projection of a 3D image stack. Adipose tissue from obese mice that was fixed and stained with BODIPY 558/568 for lipid (gray), Hoechst for nuclei (blue), anti-F4/80 for macrophages (red), anti-TNFα (green), and anti-IL-10 (magenta). The panel shows a four micron 2D projection captured with a 63× objective. The majority of CLS macrophages produce TNFα and some appear to produce both TNFα and IL-10 (arrows) while others have no cytokine expression (F4/80 only).
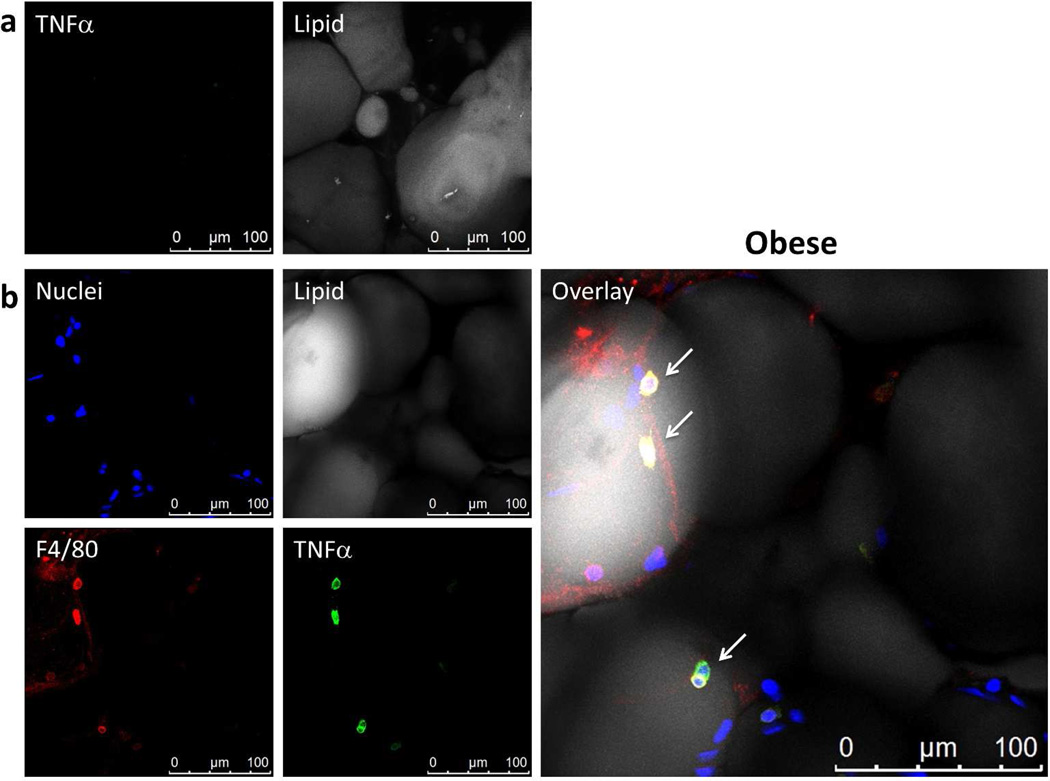
Previous reports have suggested that adipocytes are a source of TNFα (6, 9, 11, 17). Figures 3a and 3b demonstrate that adipocytes did not express TNFα even under obese conditions. The only TNFα expression is associated with F4/80 positive macrophages as shown by co-localization (Figure 3b overlay; arrows).
Figure 3.
Adipocytes do not express TNFα. Whole adipose tissue was stained with BODIPY 558/568 for lipid (gray), Hoechst for nuclei (blue), anti-F4/80 for macrophages (red), anti-TNFα (green). All images were captured using LSCM with a 63× objective and are 2D projections of a 3D image stack. (a) Negative TNFα staining throughout the adipocytes visualized. Nuclei not shown. (b) TNFα staining was associated only with macrophages (arrows). No adipocytes stain for TNFα. Yellow in the overlay image indicates co-localization of TNFα and F4/80.
Separated floating layer “adipocytes” from obese mice were persistently associated with macrophages markers
We sought to further clarify the cellular origin of TNFα and IL-10 cytokines from obese visceral adipose tissue by utilizing adipose tissue digestion and cellular separation. Adipose tissue was separated as described (10, 14) into a floating adipocyte layer and a pelleted SVC layer known to contain multiple types of cells, including macrophages (18).
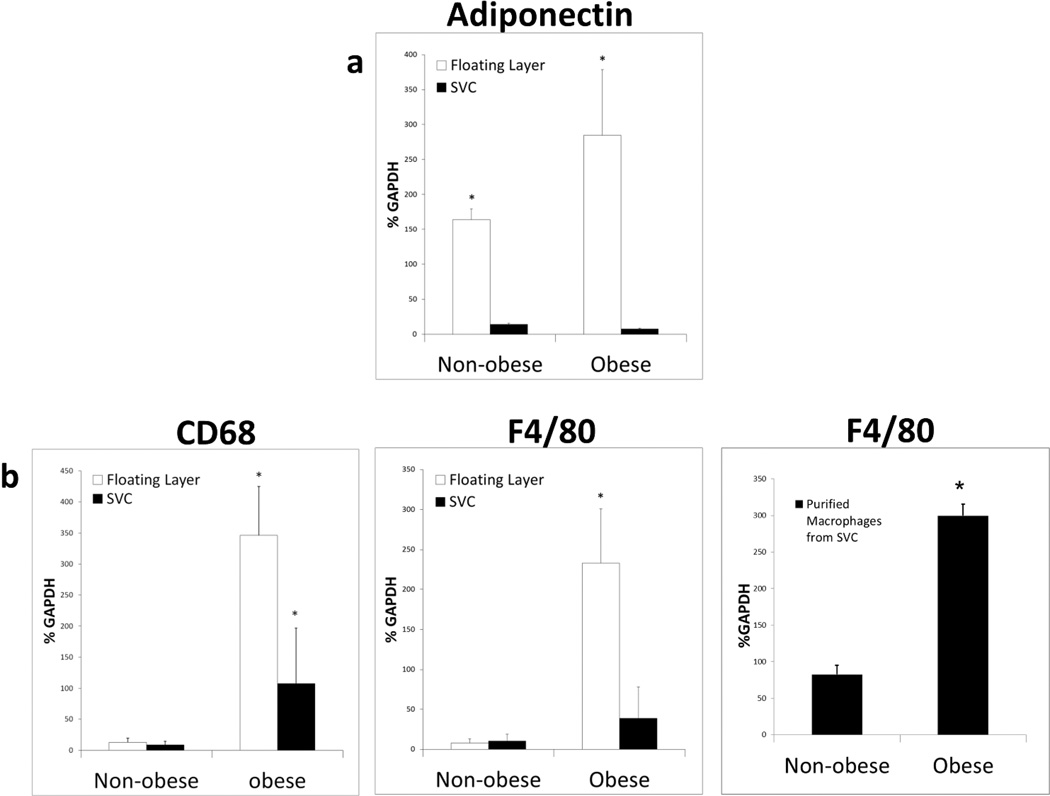
Only the floating adipocyte layer in both non-obese and obese mice showed expression of the adipocyte marker adiponectin (19, 20), whereas the SVC fraction was essentially negative (Figure 4a). Surprisingly, expression of the macrophage markers F4/80 and CD68 was significantly higher in the adipocyte floating layer compared to the SVC layer of separated obese adipose tissue (Figure 4b). This is the opposite of what is expected if the SVC layer in obese mice contained all the macrophages. There was little expression of either F4/80 or CD68 in the non-obese adipocyte floating layer or SVC cell preparations (Figure 4b). When macrophages were purified from the SVC fraction there was a significant increase of F4/80 expression in obese mice compared to non-obese mice (Figure 4b). This is unlikely to be caused by adipocyte expression of F4/80, as mature cultured 3T3-L1 adipocytes were tested by qRT-PCR and were found not to express F4/80 or other macrophage markers (data not shown). In the floating layer and in the SVC fraction, the expression of F4/80 and CD68 was higher in obese compared to the non-obese mice. Even after collagenase digestion and washing, a significant number of macrophages remained in the floating layer prepared from obese mice as determined by F4/80 and CD68 markers.
Figure 4.
After collagenase digestion and purification, the floating layer contains adipocytes, but also macrophages in the obese condition. Floating layer cells and stromal vascular cells (SVC) were collected and mRNA was extracted and reverse transcribed. qRT-PCR was performed using indicated primer pairs and relative expression levels were determined after normalizing to GAPDH. (a) Adiponectin, the marker for intact adipocytes, is detected in the floating layer, but not in the SVC layer indicating that the floating layer contains the adipocytes. (b) The macrophage markers CD68 and F4/80 indicate a significant increase in macrophage presence in the SVC layer and the floating layer of obese animals. Macrophages were also purified from SVC using magnetic bead separation linked to anti-F4/80 and mRNA for F4/80 quantitated.
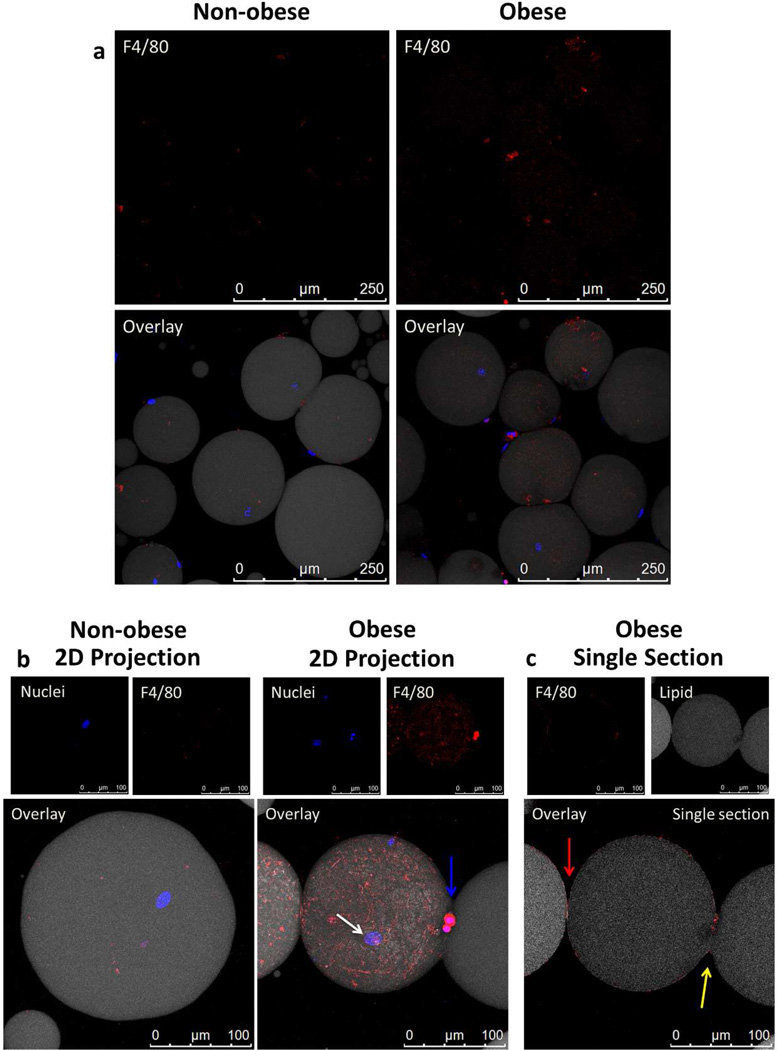
Macrophages remained adherent to single adipocytes or lipid droplets in the ‘adipocyte’ floating layer
The qRT-PCR experiments described above suggested that the floating adipocyte layer contained a significant number of macrophages. To determine how macrophages interact with adipocytes we stained the floating layer cells and analyzed them by LSCM. Adipocytes in the adipocyte floating layer were digested to single adipocytes (Figure 5a). Macrophages, as indicated by F4/80 staining, remained adherent to and even appear to surround several isolated single adipocyte cells obtained from obese mice (Figure 5a). This was not observed in non-obese mice. Using 63× magnification the distinctive oval shaped adipocyte nucleus is highly visible as indicated by white arrows (Figure 5b). The two macrophage nuclei stain more densely and are indicated by the blue arrow (Hoechst dye). In Figure 5b (obese panel), the F4/80 stains very brightly close to the macrophage nuclei but becomes fainter as the macrophage “spreads out” over the adipocyte. The adipocyte nucleus is visible underneath the macrophage cover. The obese adipocyte with attached macrophages was analyzed further in Figure 5c. This single section in the z-stack displays the F4/80 and lipid staining and shows two separate cells one to the left (the full cell is not shown) and the one in the center. The cell in the center is segmented with one segment covered by macrophages and being pinched off from the remaining segment not covered by macrophages. The segmented structure is clearly a single cell since only one adipocyte nucleus is seen. Both segments are connected by a bridge of cytoplasm indicated by the yellow arrow in Figure 5c. In contrast, the cell to the left is clearly separate and surrounded by F4/80+ macrophages (red arrow). The macrophages surrounding the cell in the center appear to be pinching off part of the adipocyte.
Figure 5.
Adipocytes in the floating layer were fully liberated from adipose tissue, yet macrophages remained bound to the single cell adipocytes, especially adipocytes recovered from obese mice. Floating layer cells were stained with anti-F4/80 (red) to stain macrophages, BODIPY 558/568 (grey) to stain lipid and Hoechst dye (blue) to stain nuclei. Anti-F4/80 primary antibody was visualized using anti-rat IgG Alexa647 conjugated secondary antibody. A sample from obese mice was also stained using the secondary antibody only and showed no background staining (data not shown) (a) Images show liberated, intact adipocytes after collagenase digestion and washing from non-obese and obese mice. The top panels show increased F4/80 covering adipocytes from obese mice when compared to non-obese mice. Images were collected with 20× objective. (b) Individual adipocyte images from obese and non-obese mice collected with 63× objective. In the obese condition, macrophages are seen covering individual intact adipocytes. An intact adipocyte was confirmed by the presence of an adipocyte nucleus (white arrow), while macrophage nuclei are morphologically different and stain more densely (blue arrow). (c) A one micron section taken from the middle of the 3D z-stack of the macrophage covered adipocyte in Figure 5b reveals that the large, single adipocyte is effectively being pinched in half by the macrophages surrounding it. This can be seen by the continuation of lipid (gray) between the attached circular bodies and the break in F4/80 staining on the right-hand side of the frame (yellow arrow) in contrast to the distinct border (red arrow) of macrophages covering the separate adipocytes on the left-hand side of the frame.
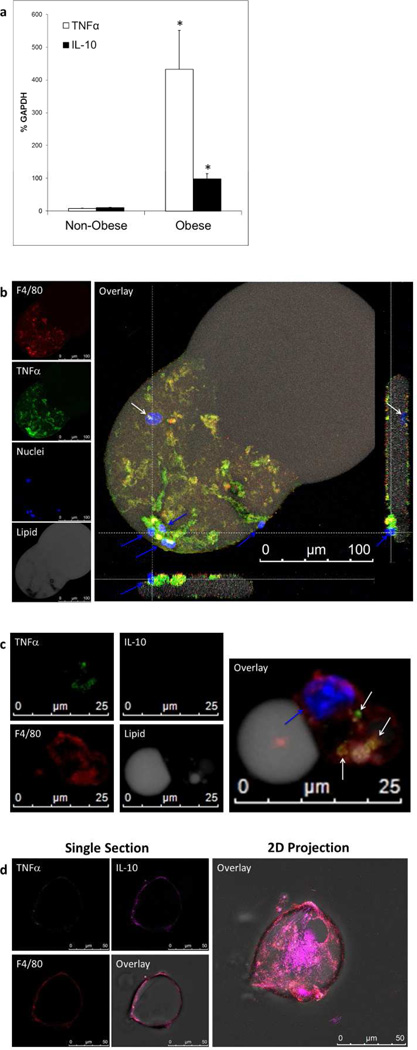
Similar structures can be seen in Figure 6b. As the 2D projection of macrophage-adipocyte interactions shows (Figure 6b), an oval nucleus belonging to the adipocyte is under a sheath of macrophages, which partially cover the adipocyte (Figure 6b; white arrows in the image stack overlay and single horizontal section right hand side). The adipocyte appears deformed, indicating that macrophages adhere to each other and exert a compressive force on the adipocyte. Several comma shaped dense macrophage nuclei appear on the surface (Figure 6b blue arrows). Despite the segmentation of the adipocytes, a single adipocyte nucleus was observed within a continuous cytoplasm in both cases. The segmented lipid seen in Figure 6c is most likely also due to this process. This intimate interaction between adipocytes and macrophages occurs in “adipocyte” floating layer cells obtained from obese mice.
Figure 6.
The adipocyte floating layer has high expression of TNFα and IL-10 in obese mice compared to non-obese mice. (a) qRT-PCR of floating layer cells showing relative amounts of TNFα compared to IL-10 mRNA normalized to GAPDH. Obese mice had a 40-fold increase in TNFα (open boxes) while only inducing a 10-fold increase in IL-10 production (black boxes). * p<0.05 in all figures. (b) Floating layer adipocyte with attached macrophages. Floating layer cells stained with BODIPY (gray) for lipid to denote adipocytes, F4/80 (red) for macrophages, TNFα (green), IL-10 (magenta), and nuclei (blue). There was no IL-10 expressed in either cell type in this example (field not shown). TNFα can clearly be seen to be associated with only the macrophages, but not the adipocyte (F4/80 vs. TNFα panels and overlay). The adipocyte nucleus is indicated by the white arrow in the overlay and right side view, while macrophage nuclei are indicated by the blue arrows. The figure is an LSCM 2D projection of a 3D image stack using a 63× objective. The side views are 1um thick sections imaged from the corresponding dotted white lines parallel to the section.(c) Macrophages in the floating adipocyte layer ingest lipid and produce TNFα. At high magnification using LSCM and 63× objective a 2D projection shows a macrophage (red) with a single nucleus (blue arrow) remained attached to and ingested lipid (gray) in the floating layer. TNFα (green) expression was associated with the macrophage (white arrows) and not the attached or ingested lipid. No IL-10 is produced. (d) Floating layer macrophage(s) with engulfed lipid produced IL-10 to a lesser extent. Individual panels (left) show a one micron section of a LSCM image using a 63× objective. The floating layer cells were stained for F4/80 (red), TNFα (green) and IL-10 (magenta) and the overlay depicts all of these fields plus a brightfield image. The macrophage, shown with engulfed lipid, is expressing mostly IL-10, but there is some expression of TNFα. A 2D projection of the LSCM image shows the extent of IL-10 production in the macrophage surrounding the lipid.
Macrophages in the adipocyte floating layer are responsible for TNFα expression
Floating layer preparations from non-obese and obese mice were analyzed for TNFα and IL-10 expression by qRT-PCR. There was little expression of either TNFα or IL-10 in the non-obese mice. IL-10 increased significantly by 10-fold and TNFα increased significantly by 40-fold in floating layer cells obtained from obese mice. The amount of TNFα was significantly greater than that of IL-10.
Macrophages and adipocytes in the floating layer from obese mice were analyzed by LSCM in order to determine which cells were expressing TNFα. TNFα staining consistently coincided with F4/80 macrophage staining (Figure 6b) and there was no TNFα stain associated with the adipocyte itself. In multiple experiments at different time points of feeding, we never observed floating adipocytes that stained positive for TNFα. There is a loss of grey lipid mirroring the heaviest areas of TNFα staining leaving black clear tracks (Figure 6b, lipid panel). IL-10 staining was negative for both the macrophages and the adipocyte shown.
In addition to macrophages adherent to adipocytes, isolated macrophages were observed as well in the floating layer. Invariably, such macrophages had either engulfed significant amounts of lipid or they were in contact with lipid droplets, which would explain why they were found in the low-density fraction. Figure 6c illustrates macrophages stained with F4/80 in the floating adipocyte layer that are surrounding, or are engorged with lipid. The lipid areas shown in Figure 6c are too small (25–50µm) to be full adipocytes (100–300µm, see scale in Figures 5a,5b,5c, and Figure 6b overlay) and lack an adipocyte nucleus. High-resolution confocal analysis of the adipocyte floating layer revealed repeated z-stacks of macrophages with engulfed lipid. The single macrophage (single blue comma shaped nucleus indicated by the blue arrow) depicted In Figure 6c is in tight contact with a lipid droplet (grey lipid covered faintly by F4/80 red stain and flattened contact interface). There are smaller areas of lipid inside the body of the macrophage. The macrophage is expressing TNFα (Figure 6c), but not IL-10. The pattern of TNFα staining (Figure 6c) did not coincide with lipid staining (Figure 6c) indicating that the TNFα is only associated with the macrophage.
Figure 6d illustrates another example of a macrophage having surrounded or engulfed lipid. In this case, the macrophage is clearly expressing IL-10, but also a small amount of TNFα as shown in the single section views. IL-10 secreting macrophages in the floating layer as seen in Figure 6d did not occur as frequently as macrophages expressing TNFα alone, reflecting the qRT-PCR results.
Discussion
The confocal immunohistochemical analysis in this study has revealed novel insights into the cellular architecture of the inflammatory infiltrate of obese adipose tissue and the interaction between macrophages and adipocytes and adipocyte-derived lipid. The data show that macrophages are in tight contact with adipocytes or lipid droplets, an interaction that survives tissue disruption, collagenase digestion and density gradient centrifugation. This suggests that macrophages are not simply trapped by surrounding adipocytes, but are retained in the floating layer, possibly by physical attachment to low density adipocytes or lipid droplets. Tight contact with adipocytes is consistent with a role for macrophages in clearing dying adipocytes (7).
In addition to adhering to adipocytes or lipid droplets, macrophages were observed to form contacts with each other when surrounding the much larger adipocytes. Although reminiscent of multinuclear giant cells found in granulomatous disorders (21), the sheath-like structures partially covering adipocytes have not been observed previously. Sheaths of macrophages impinge on the adipocyte and the macrophage-macrophage interaction appears to have sufficient strength to compress the adipocyte in the contact area. Most likely, the compressed adipocytes in Figures 5c and 6b, visible here in 3D, represents crown-like structures detected in histological sections with 6b representing an earlier time point in the progression of coverage.
Macrophages in contact with lipid structures that are smaller than an adipocyte were observed as well. Most likely, these are lipid droplets or lipid-filled fragments of adipocytes. Although it is not clear whether macrophages can break up lipid droplets, ingest and ultimately degrade ingested lipid, our data, like others (7), clearly show that macrophages do have mechanisms to take up lipid in adipose tissue. In the present study, lipid-laden macrophages in CLS were observed by LSCM imaging of white adipose tissue. Lipid uptake may constitute another mechanism, in addition to contact with low-density lipid structures that reduces the density of macrophages to allow them to float during density gradient centrifugation. In a previous study, density gradient centrifugation was used to isolate lipid-laden macrophages (22). The mechanism of lipid uptake and transport that operates in adipose tissue is currently unclear. Cinti et al. (7) suggested that the residual lipid, in necrotic adipocytes, fragmented into smaller lipid droplets which can be phagocytosed by crown-like structure macrophages.
It has been suggested that adipocytes can grow only up to a threshold above which they will generate a stress signal or die (23). In fact, increased adipocyte cell death has been observed in obese mice and humans (7, 24). Cinti et al. determined that the adipocytes which were surrounded by macrophages in adipose tissue forming CLS were in fact dead (7). In the experiments described here, this condition occurred in obese mice with 5–7% adiposity. This suggests that CLS are a physiologic response to obesity exceeding a threshold.
The 3D analysis of the cellular architecture of the inflammatory infiltrate allowed unequivocal assignment of TNFα to macrophages. In all the experiments performed (1–4.5 month feeding of HF or HF/HC diets to generate obese mice), we did not observe a single adipocyte expressing TNFα in intact white adipose tissue or in the separated floating layer. All TNFα staining was associated with macrophages. In white adipose tissue macrophages expressed TNFα, and the vast majority of the macrophages in the separated floating layer expressed TNFα. IL-10 expression was observed to a lesser extent, but again was associated with macrophages. A high ratio of TNFα to IL-10 expression was reflected in cytokine mRNA levels. There are reports of simultaneously elevated inflammatory TNFα (25, 26) and immunoregulatory IL-10 (27, 28) in obese individuals. Co-existence of TNFα and IL-10 with an imbalance in favor of TNFα are hallmarks of chronic inflammatory conditions (6).
In vitro, TNFα is cytotoxic to both cultured adipocytes and pre-adipocytes (29, 30, 31, 32, 33). TNFα has also been shown to activate lipolysis in adipocytes (reviewed in (34)) via the TNF receptor I (TNFR1) (35, 36). Indeed, in the present study, lipid staining of adipocytes was visibly diminished adjacent to sites where macrophage expression of TNFα was heaviest. The lipotoxic and lipolytic activity of macrophage products and the macrophage activating activity of responding adipocytes establish a self-reinforcing vicious cycle maintaining chronic adipose tissue inflammation (18).
As observed by others previously, crown-like structures contain activated macrophages (15, 16). The majority of macrophages in the floating layer were proinflammatory expressing TNFα. Activated macrophages express adhesion molecules allowing for tight adherence (37, 38). In order to deform adipocytes as shown in the present study, macrophages need to tightly adhere to each other as well as the adipocyte target. The tight contact of macrophages with each other and with adipocytes has implications for future studies of inflamed adipose tissue (39). The only procedures that would recover adipocytes from inflamed areas of the tissue would require disrupting macrophage/macrophage and macrophage/adipocyte interactions. There is currently no known procedure to achieve this and allow the preservation of cell integrity. The molecular nature of the tight adherence of macrophages to other macrophages and to adipocytes warrants further investigation.
What is already known about this subject
Inflammatory macrophages are present in crown-like structures in adipose tissue under obese conditions.
Contaminating non-adipocytes, most likely macrophages are the source of TNFα in adipocyte preparations based on buoyancy.
Macrophages engulf lipid in inflamed adipose tissue.
What this study adds
Three dimensional immunohistochemistry of the adipocyte-macrophage interaction at the single cell level in the floating layer of separated adipose tissue preparations.
Macrophages remain tightly bound to adipocytes even after tissue digestion and density gradient centrifugation, occasionally forming multi-cellular assemblies covering adipocytes.
Macrophages, and not adipocytes, are the source of TNFα expression in the adipocyte floating layer based on 3D image analysis at the single cell level.
Acknowledgement
The authors would like to thank Dr William Gunning for use of his microscope and facilities to obtain the histology image (Figure 1b). This work was supported by a grant from the USDA/NIFA (2010-38903-20740) to MFM and SMN and by the Wolfe Innovation Fund (University of Toledo Foundation). The work was also supported by grants from the NIH; R01 DK054254, R01 DK083850, and R01 HL112248 to SMN. L. A. Ebke, B. D. Slotterbeck, and A. G. Al-Dieri researched, analyzed data, and contributed to the drafting of the manuscript. A. Nestor-Kalinoski provided image collection and helped analyze laser scanning confocal microscopy images. S. Ghosh-Lester and Lucia Russo provided adipose tissue from 4.5 and 4 month-feeding experiments respectively. S. M. Najjar provided the use of CeDER facilities, breeding pairs of Cc1−/− mice, design of feeding experiments, contributed to scientific discussion, and edited/reviewed the manuscript. H. von Grafenstein contributed to scientific discussion, data interpretation, and manuscript writing/review. M. F. McInerney oversaw the work, including its conception and study design, analyzed data, led scientific discussions and wrote/reviewed/edited the manuscript. All authors have read and approved the manuscript.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflict of interest.
References
- 1.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 3.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Molecular Aspects of Medicine. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Molecular and cellular endocrinology. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, NY) 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 6.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of Lipid Research. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Fain JN, Bahouth SW, Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28:616–622. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- 9.Murakami K, Bujo H, Unoki H, Saito Y. High fat intake induces a population of adipocytes to coexpress TLR2 and TNFalpha in mice with insulin resistance. Biochemical and biophysical research communications. 2007;354:727–734. doi: 10.1016/j.bbrc.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Lee SJ, et al. Carcinoembryonic antigenrelated cell adhesion molecule 1 - A link between insulin and lipid metabolism. Diabetes. 2008;57:2296–2303. doi: 10.2337/db08-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu E, Dubois M-J, Leung N, Charbonneau A, Turbide C, Avramoglu RK, et al. Targeted Disruption of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 Promotes Diet-Induced Hepatic Steatosis and Insulin Resistance. Endocrinology. 2009;150:3503–3512. doi: 10.1210/en.2008-1439. [DOI] [PubMed] [Google Scholar]
- 14.Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, et al. Deletion of the Angiotensin Type 2 Receptor (AT2R) Reduces Adipose Cell Size and Protects From Diet-Induced Obesity and Insulin Resistance. Diabetes. 2005;54:991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 15.Lumeng C, DelProposto J, Westcott D, Saltiel A. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil G, Spiegelman B. Tumor necrosis factor alpha: a key component of the obesitydiabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 18.Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocrine journal. 2012;59:849–857. doi: 10.1507/endocrj.ej12-0271. [DOI] [PubMed] [Google Scholar]
- 19.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitamins and Hormones. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 20.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. The Journal of Clinical Investigation. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto H, Mizuno K, Horio T. Monocyte-derived multinucleated giant cells and sarcoidosis. Journal of dermatological science. 2003;31:119–128. doi: 10.1016/s0923-1811(02)00148-2. [DOI] [PubMed] [Google Scholar]
- 22.Chase AJ, Bond M, Crook MF, Newby AC. Role of nuclear factor-kappa B activation in metalloproteinase-1, -3, and-9 secretion by human macrophages in vitro and rabbit foam cells produced in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:765–771. doi: 10.1161/01.atv.0000015078.09208.92. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 24.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. The Journal of biological chemistry. 2010;285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. Journal of Clinical Investigation. 1995;95:2111. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotamisligil G, Arner P, Caro J, Atkinson R, Spiegelman B. Increased adipose tissue expression of tumor necrosis factor-a in human obesity and insulin resistance. Journal of Clinical Investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of Low Interleukin-10 Levels with the Metabolic Syndrome in Obese Women. Journal of Clinical Endocrinology & Metabolism. 2003;88:1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 28.Juge-Aubry CE, Somm E, Pernin A, egrave, Alizadeh N, Giusti V, et al. Adipose tissue is a regulated source of interleukin-10. Cytokine. 2005;29:270–274. doi: 10.1016/j.cyto.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Prins JB, Walker NI, Winterford CM, Cameron DP. Apoptosis of human adipocytes in vitro. Biochemical and biophysical research communications. 1994;201:500–507. doi: 10.1006/bbrc.1994.1730. [DOI] [PubMed] [Google Scholar]
- 30.Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O'Rahilly S, et al. Tumor necrosis factoralpha induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- 31.Heller RA, Song K, Fan N, Chang DJ. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992;70:47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- 32.Wallach D. Cell death induction by TNF: a matter of self control. Trends in biochemical sciences. 1997;22:107–109. doi: 10.1016/s0968-0004(97)01015-3. [DOI] [PubMed] [Google Scholar]
- 33.Declercq W, Denecker G, Fiers W, Vandenabeele P. Cooperation of both TNF receptors in inducing apoptosis: involvement of the TNF receptor-associated factor binding domain of the TNF receptor 75. Journal of immunology. 1998;161:390–399. [PubMed] [Google Scholar]
- 34.Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Seminars in cell & developmental biology. 1999;10:19–29. doi: 10.1006/scdb.1998.0273. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Soriano J, Llovera M, Carbo N, Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Lipid metabolism in tumour-bearing mice: studies with knockout mice for tumour necrosis factor receptor 1 protein. Molecular and cellular endocrinology. 1997;132:93–99. doi: 10.1016/s0303-7207(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 36.Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptorspecific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS letters. 2000;469:77–82. doi: 10.1016/s0014-5793(00)01250-3. [DOI] [PubMed] [Google Scholar]
- 37.Gurlo T, Kawamura K, von Grafenstein H. Role of inflammatory infiltrate in activation and effector function of cloned islet reactive nonobese diabetic CD8+ T cells: involvement of a nitric oxide-dependent pathway. Journal of immunology. 1999;163:5770–5780. [PubMed] [Google Scholar]
- 38.Gurlo T, von Grafenstein H. Antigen-independent cross-talk between macrophages and CD8+ T cells facilitates their cooperation during target destruction. Int Immunol. 2003;15:1063–1071. doi: 10.1093/intimm/dxg106. [DOI] [PubMed] [Google Scholar]
- 39.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell metabolism. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]