Abstract
Objectives
Lapse to opiate use after initiation of buprenorphine treatment is common and is a strong predictor of poor treatment retention and increased risk of chronic opiate use. Drug-cues and situations or events associated with distress are known to provoke craving and increase risk for lapse. The current study evaluated the predictive validity of a behavioral index of persistence during a stress-challenge among opiate users identified as affectively vulnerable to lapse risk due to elevated depressive symptoms.
Methods
Patients from on ongoing clinical trial (n=48) completed a stress-challenge task prior to receiving their first dose of buprenorphine.
Results
After controlling for levels of craving on their induction day, persistence on the stress-challenge task prior to initiating buprenorphine treatment was associated with successful transition to early abstinence, and lower rates of opiate use during the initial three months of buprenorphine treatment across antidepressant and placebo groups.
Conclusions
Results from this preliminary study suggest the promise of laboratory-based behavioral paradigms in facilitating understanding of important mechanisms of early lapse. Identifying individual behavioral responses to drug- and stress-cues prior to attempts at abstinence may facilitate delivery of adjunctive behavioral treatments to prevent early lapse.
1. Introduction
Identifying patients at risk for early lapse to opiate use during outpatient treatment with buprenorphine is a priority in efforts to combat the substantial medical and psychosocial consequences associated with opiate dependence. Buprenorphine, a long-acting partial opioid agonist, offered as office-based maintenance treatment for opioid dependence, has demonstrated efficacy in reducing opiate cravings, ameliorating withdrawal discomfort, and increasing periods of abstinence from illicit drug use. However, lapse to opiate use after initiation of buprenorphine treatment is common (Marsch, Bickel, Badger, & Jacobs, 2005) and is a strong predictor of poor treatment retention and increased risk of chronic opiate use (Stein, Cioe, & Friedmann, 2005; Stein et al. 2010).
Risk for lapse despite adequate pharmacologic treatment with opiate agonists implicates the substantial role for events or situations that increase craving and motivate drug-seeking behavior (Goldstein & Volkow, 2002; Lubman, Yucel, & Pantelis, 2004; Robinson & Berridge, 2001). Situations and events directly linked to drug use behavior, and those associated with emotional distress both reliably induce similar levels of craving among pharmacologically treated opiate users in early recovery (Hyman, Fox, Hong, Doebrick, & Sinha, 2007). While craving may represent a final common pathway to relapse risk, situations and events associated with distress are differentiated by increases in accompanying negative affect. Vulnerability to persistent craving and early lapse may arise both from difficulty tolerating provocations from drug-cues and the negative affect generated by distressing events. Avoidance of early lapse may hinge in part on indiviudal differences in the capacity to persist in abstinence-focused behavior and inhibit drug-seeking behavior when challenged by negative affect and associated cravings during early abstinence.
The unwillingness or inability to persist when experiencing emotional distress and somatic discomfort has long been posited as an important mechanism underlying many forms of psychopathology (Eyesenck, 1947; Linehan, 1993; Ryans, 1939). Distress tolerance is the behavioral tendency to continue to pursue a goal, such as sustained abstinence, despite encountering emotional distress. From this perspective, it is not simply the affective distress that relates to relapse to drug use, but how one responds to affective distress that may be critical to determining substance use outcomes (for review see (Richards, Daughters, Bornovalova, Brown, & Lejuez, 2010)). Risk for early lapse due to low distress tolerance may be particularly apparent among those with affective vulnerabilities (Leyro, Zvolensky, & Bernstein, 2010). Opiate users who lapse early during attempts at abstinence may differ from those who do not on several affective dimensions associated with the ability to tolerate distress. First, early lapsers may have vulnerability to react strongly to stressors, reacting with high levels of negative affect (Hyman et al., 2007; Sinha, 2001). Early lapsing illicit drug users have been found to be more reactive to stressors both physiologically (Back, Payne, Simpson, & Brady, 2010) and affectively (Hyman et al., 2007). Stronger affective reactions to stressors and difficulty regulating negative affect may combine to produce stronger motivations for relief provided by drug use and may help explain the previously described relationship between affective vulnerabilty seen with depression and relapse to opiates (Brewer, Catalano, Haggerty, Gainey, & Fleming, 1998). Individuals seeking treatment with buprenorphine commonly present with elevated depressive symptoms and often receive antidepressant medication (Stein et al., 2010). Antidepressants have the potential to decrease risk for early lapse by reducing negative affect and associated cravings and/or reactions to stressors (Dichter, Tomarken, Freid, Addington, & Shelton, 2005; Tomarken, Dichter, Freid, Addington, & Shelton, 2004; Wichers et al., 2009).
Individual differences in reactivity to stressors and tolerance of accompanying distress have been examined in relation to early lapse in smoking cessation where controlled laboratory studies have employed behavioral measures of persistence during distress tolerance tasks to predict affective reactions and lapse to smoking during early periods of abstinence (Abrantes et al., 2009; Brandon et al., 2003; Brown, Lejuez, Kahler, & Strong, 2002; Brown et al., 2009). Behavioral measures of persistence during distress tolerance tasks also have been shown to predict dropout from residential substance use treatment (Daughters et al., 2005). Difficulty persisting in cognitively demanding stress-challenge tasks may provide an analogue for identifying those at risk for early lapse to opiates when faced with increased negative affect and craving.
Among persons inititating buprenorphine treatment, we predict that low distress tolerance, as evidenced by less persistence on stress-challenge tasks, will be associated with: a) greater affective vulnerability as evidenced by patient self-report measures of increased depressive symptoms, lower tolerance of discomfort, and greater avoidance of discomfort; b) greater craving and negative affect reactivity to behavioral stress-challenge tasks; c) poor treatment outcomes as evidenced by early lapse to opiate use. Finally, we expect that the antidepressant escitalopram, compared to placebo, will be associated with less volatile craving and fewer depressive symptoms during buprenorphine treatment, particularly among those with low distress tolerance.
2. Methods
2.1. Overall Study Design
The goal of the parent study was to determine whether treatment of depressive symptoms with escitalopram (Lexapro) during buprenorphine treatment for opioid dependence would improve treatment retention compared to placebo in a 12-week, randomized, double-blind trial. Research assistants conducting assessments were blind to treatment assignments.
2.2. Participants
One hundred forty seven persons with scores on the Modified Hamilton Depression Revised Scale greater than 14 (Miller, Norman, & Bishop, 1985) were enrolled between 11/06 and 5/09 (see Stein et al., 2010). This secondary analysis includes the final forty-eight study participants who participated in a laboratory-based behavioral assessment along with standard study procedures prior to treatment allocation. All study procedures were approved by the Butler Hospital Instutional Review Board.
2.3 Measures
Time Line Follow Back
(TLFB: (Sobell & Sobell, 1996)). This calendar-based interview provided day-to-day summaries of opiate use in the 90-days preceding the baseline interview.
Modified Penn Craving Scale
(PCS: (Flannery, Volpicelli, & Pettinati, 1999)). This 7-item measure was originally developed for alcohol-craving and was adapted for tracking the strength of craving for opiate use in the past-week. Each item was rated using one of seven detailed response options. Sample mean was 4.93 (SD = 1.08), internal consistency coefficient was 0.88 in the current sample.
Beck Depression Inventory
(BDI, (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961)). This standard 21-item inventory measured past-week depressive symptoms. The sample mean was 28.43 (SD=9.46) and internal consistency coefficient was 0.88 in the current sample.
Discomfort Intolerance Scale
(DIS, (Schmidt, Richey, & Fitzpatrick, 2006)). This five item face-valid scale indexes physical discomfort tolerance with two items (i.e. I have a high pain threshold) and discomfort avoidance with three items (i.e. I take extreme measures to avoid feeling physically uncomfortable). Each item had anchors at the low (‘Not like me’), mid-point (‘Moderately Like Me’), and high (‘Extremely Like Me’) points of the seven-point scale. Internal consistency for the 5-item combined scale has been reported to be 0.74 and was 0.61 in the current sample.
2.4. Behavioral assessment during stress-challenge
On the morning of buprenorphine induction, patients were told to arrive at the research site having not used opioids for at least 12 hours. Time since last dose of illicit opioid varied depending on drug of choice, but all participants had symptoms of withdrawal based on the Clinical Opiate Withdrawal Scale (Wesson & Ling). After completing baseline interviews, while still in withdrawal, participants were escorted to a room dedicated to laboratory assessments and equipped with a respondent computer and monitor, ceiling-mounted video camera and audio capture equipment. Research assistants oriented participants to procedures, ensured familiarity with the computer, and described the task using a standardized script. A stress-challenge task, Paced Auditory Serial Addition Task (PASAT; (Lejuez, Kahler, & Brown, 2007)), included visual analogue assessment of affective distress and craving-to-use-opiates prior to and following the stress-challenge task. The PASAT has been used widely with substance use populations (Brown et al., 2009; Daughters et al., 2005; Vanderkaay & Patterson, 2006).
In brief, the PASAT challenge task requires participants to attend to isolated pairs of numbers presented successively on a computer screen, calculate their sum, and use the computer mouse to select the correct answer ranging from 1 to 20. Incorrect responses resulted in explosion sounds while correct responses generated no auditory feedback and increased a score-counter visible to participants. The PASAT challenge-task was divided into two phases with the same procedures to succesively provide assessment of reactions to sustained exposure to task demands and then to evaluate the time to task termination, a primary index of persistence. Each phase was preceded by a self-report of current affective distress (irritability, difficulty concentrating, frustration, anxiety, physical discomfort) and craving to use opiates. Participants were asked to ‘continue for as long as you can but do not continue past the point at which you feel uncomfortable’ and to press the ‘end this math task’ during the second phase when they wanted to stop.
2.5. Treatment and follow-up procedures
Escitalopram (Lexapro) 10 mg was begun five days (up to seven days for those with scheduling problems or arriving for induction without being in opiate withdrawal) prior to the buprenorphine induction day at a time when participants were still using their opiate of choice (Stein et al., 2010). Buprenorphine induction was performed under the treating physician's supervision, as previously described (Stein et al., 2005) following the PASAT in these 48 participants. In general, buprenorphine doses ranging from 12-16 mg/day were required for stabilization. Participants returned one week later and then to biweekly follow-up appointments (which coincided with research interviews), where they were provided with exactly enough of the study medication (escitalopram or placebo, one pill daily) and buprenorphine to last until the next appointment. Participants were not discharged for continued use of drugs, nor was their frequency of follow-up medical visits changed by positive toxicological results.
2.6. Primary Outcomes
Opiate Use
Outcomes. At each interview, participants were asked to provide a urine specimen to test for urine toxicology. The Screeners® Dip Drug Test with the Integrated Screeners® Autosplit® KO12B™ Test Cup was used. Opiate use was evaluated though the 11th week of the 12-week treatment as some participants elected to taper off of the study buprenorphine in the final week.
Early lapse
was defined as an opiate-positive urine toxicology at the week 1 visit. We also assessed continued opiate use by evaluating all urine toxicologies during follow-up.
3. Results
3.1. Baseline Differences Between Placebo (PBO) and Escitalopram (ESC) Conditions
As can be seen in Table 1, there were no significant demographic, opiate use, or depressive symptom differences between participants randomized to either PBO or ESC. Concurrent use of other substances was common with 79% reporting use of other drugs in the last 30 days. Preferences for types of opiates were 58% heroin, 21% oxycontin, 21% other opioid (vicodin, percocet, etc.). No differences were observed for self-report DIS scores across conditions.
Table 1.
Baseline characteristics and performance on behavioral tasks across treatment Placebo and Escitalopram groups.
| Placebo | Escitalopram | |||||
|---|---|---|---|---|---|---|
| (n=26) | (n=22) | |||||
|
|
|
|||||
| Variables | Mean | SD | Mean | SD | p | |
| 1 | % Female | 69% | -- | 55% | -- | 0.45 |
| 2 | Age | 34.54 | 10.25 | 33.95 | 10.47 | 0.85 |
| 3 | Years of Education | 12.50 | 1.85 | 11.90 | 1.63 | 0.63 |
| 4 | % Days of opiate use in past month | 91% | 0.15 | 83% | 0.23 | 0.15 |
| 5 | % Used Heroin in past month | 55% | -- | 40% | -- | 0.53 |
| 6 | % Used other drugs in past month a | 85% | -- | 73% | -- | 0.51 |
| 7 | Penn Craving Scale | 4.94 | 0.96 | 4.92 | 1.22 | 0.95 |
| 8 | Beck Depression | 28.65 | 10.35 | 31.59 | 9.96 | 0.32 |
| 9 | Discomfort Tolerance | 4.35 | 4.14 | 5.68 | 3.83 | 0.25 |
| 10 | Distress Avoidance | 11.54 | 3.31 | 12.57 | 3.54 | 0.31 |
| 11 | PASAT persistence | 234.69 | 171.93 | 201.45 | 163.29 | 0.50 |
= Other drugs include marijuana, cocaine, amphetamines, and benzodiazapines
3.2. Affective vulnerability and reactivity to the stress-challenge
We used separate linear mixed effects regression models to estimate increases in affective distress and craving to use opiates in reaction to the PASAT, with control for corresponding pre-task levels and covariates including percentage of opiate use days, gender, age, and level of craving and depressive symptoms. In support of the internal validity of the PASAT as a stress-challenge, we observed a significant increase in affective distress (B = 15.51, SE = 1.99, df = 47, d = 0.79 , p = 0.000) and craving to use opiates (B = 6.45, SE = 2.72, df = 47, d = 0.27, p =0.022) after phase one of the PASAT.
Baseline level of depressive symptoms (BDI) was related to higher affective distress during the PASAT (B = 1.01, SE = 0.34, df = 47, p = 0.005) but did not relate to differential change in affective distress after phase one of the task (BDI X Time interactions: p's > 0.05). Other proposed affective vulnerability measures, including the DIS, were not related significantly to levels of affective distress or cravings to use opiates after phase one of the PASAT (p's > 0.10).
3.3. Persistence on Stress-Challenge Task
Average persistence times during phase two of the PASAT are presented in Table 1. We included planned covariates as above using percentage of opiate use days, gender, age, and level of craving and depressive symptoms in robust linear regression (Rousseeuw & Yohai, 1984) when predicting non-normally distributed persistence times during phase two of the PASAT. We evaluated whether self-reports on the DIS scale prior to the PASAT, and affective and craving levels immediately prior to and following phase one of the stress-challenge tasks, were related to persistence on the PASAT. In a covariate adjusted model, only lower levels of craving (B =-3.02, SE = 0.93, p = 0.002) prior to the PASAT and greater increases in cravings (B =2.45, SE = 1.03, p = 0.02) after phase one of the PASAT were predictive of longer persistence time during phase two of this stress-challenge task. Individuals with greater change in craving had lower craving prior to the stress-challenge task while those with high craving prior to the task sustained high craving levels. DIS ratings prior to the PASAT and changes in negative affect after phase one of the the PASAT was not related to persistence during phase 2 (p's > 0.10).
3.4. Persistence and Lapse to Opiate Use
Generalized linear mixed models for binary outcomes (Pinhero & Bates, 2000) were used to assess positive opiate toxicology results assessed on the day of the first dose of buprenorphine through 1-, 3-, 7-, 9-, and 11-weeks after initiating buprenorphine treatment. Likelihood ratio testing of unconditional models (i.e. no covariates or predictors) with varying random effects (i.e. random intercept, linear and quadratic time) supported the use of quadratic effect for time (log Likelihood=-163, X2=15.73; df=4; p=0.003). Lapses to opiate use were most frequent early after initiating buprenorphine and then stabilized over subsequent weeks. Table 2 lists model estimates predicting positive opiate toxicologies. Controlling for levels of depressive symptoms (p = 0.12), frequency of opiate use (p = 0.04), level of craving (p = 0.94) and receipt of antidepressant medication (p = 0.98), lower persistence time on the PASAT was related to higher odds of a positive opiate toxicology over the 11-week assessment period (p = 0.04). Lower persistence time remained a significant predictor of lapse risk (B= -0.005, SE= 0.002, p= 0.02) after adjusting for DIS (B= -0.02, SE= 0.06, p= 0.76) or changes in affect (B= -0.02, SE= 0.02, p= 0.50) or craving (B= 0.001, SE= 0.02, p= 0.98) during the PASAT. Although 46/48 participants provided at least 2 opiate toxicologies during follow-ups and were included in models, we repeated the models after substituting positive opiate results for missing values. Missing opiate toxicologies were 4%, 12%, 10%, 23%, 29%, and 38% at the first dose, 1-, 3-, 7-, 9-, and 11-week assessments. The effect of the PASAT in predicting opiate use during buprenorphine treatment was attenuated and fell below traditional values for statistical significance (B = -0.003, SE = 0.002, p = 0.07).
Table 2.
Results from generalized linear mixed models evaluating opiate use along with changes in craving and depressive symptoms assessed on the day of the first dose of buprenorphine and 1-, 3-, 7-, 9-, and 11-weeks after initiating buprenorphine treatment.
| Positive Opiate Tests | Craving | Depression | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| B | SE | p | B | SE | p | B | SE | p | |
| Base model terms | |||||||||
| (Intercept) | -3.50 | 2.32 | 0.13 | 2.13 | 0.92 | 0.02 | 3.97 | 5.95 | 0.51 |
| Linear Time | -0.62 | 0.21 | 0.00 | -0.16 | 0.02 | 0.00 | -3.01 | 0.47 | 0.00 |
| Quadratic Time | 0.05 | 0.02 | 0.01 | -- | -- | -- | 0.23 | 0.04 | 0.00 |
| Planned Baseline Covariates | |||||||||
| Age | 0.01 | 0.03 | 0.78 | -0.01 | 0.29 | 0.91 | 0.04 | 0.09 | 0.66 |
| Gender | 0.06 | 0.77 | 0.98 | -0.03 | 0.01 | 0.49 | -0.81 | 1.83 | 0.66 |
| Opiate use days | 4.92 | 2.37 | 0.04 | 2.22 | 0.96 | 0.01 | 6.64 | 5.30 | 0.22 |
| Craving | 0.03 | 0.39 | 0.94 | 0.12 | 0.15 | 0.41 | -0.30 | 0.95 | 0.75 |
| Beck Depression | -0.06 | 0.04 | 0.12 | -0.04 | 0.01 | 0.02 | 0.31 | 0.09 | 0.00 |
| Treatment Effect | |||||||||
| Escitalopram vs. Placebo | -0.02 | 0.74 | 0.98 | 0.27 | 0.28 | 0.34 | 4.77 | 1.76 | 0.01 |
| Behavioral Stress Challenge | |||||||||
| PASAT | -0.004 | 0.002 | 0.04 | 0.000 | 0.001 | 0.90 | 0.006 | 0.005 | 0.24 |
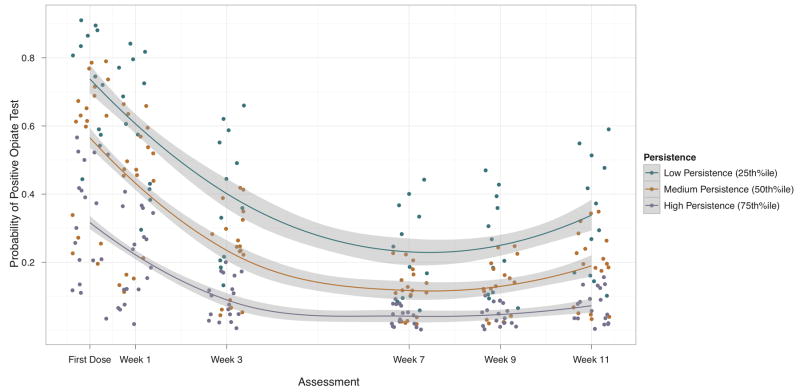
Model predicted probabilities of a positive opiate toxicology at each assessment are presented for participants along with regression lines and 95% confidence bands for Low, Medium, and High persistence respondents corresponding to the 25th, 50th, and 75th percentiles of PASAT persistence scores (see Figure 1). Participants in PBO and ESC were charaterized by high levels of other substance use at baseline 85% and 73%, respectively. High rates of substance use continued during follow-up with 62% of participants reporting other substance use during the follow-up assessment period.
Figure 1.
Predicted probabilities of a positive opiate test assessed during buprenorphine treatment. Although analyzed continuously, we present lines and confidence bands for groupings of Low (25th percentile), Medium (50th percentile), and High (75th percentile) levels of PASAT persistence times. Lower persistence scores were associated with increased probability of positive opiate tests, particularly early after the first dose of buprenorphine and at the end of scheduled treatment when titration strategies were initiated.
3.5. Persistence relationships with Craving and Depressive Symptoms During Treatment
We used linear mixed effects models to examine levels of craving and depressive symptoms assessed on the day of the first dose of buprenorphine though 1-, 3-,7-,9-, and 11-weeks after initiating buprenorphine treatment. Model estimates, standard errors, and significance values are listed in Table 2.
Does persistence during stress-challenge predict greater craving?
Evaluation of unconditional models did not support the inclusion of random effects for individual differences in changes in craving over time (X2 = 2.34, p = 0.31). On average, craving scores decreased significantly during treatment (p = 0.00). Using planned covariates including baseline level of craving (p = 0.34), depressive symptoms (p = 0.02), frequency of opiate use (p = 0.01), and the effect of antidepressant medication (p = 0.34), lower persistence time on phase two of the PASAT (p = 0.90) was not related to levels of craving during treatment. In a subsequent model we tested the interactive effects of antidepressant medication and level of persistence on levels of craving during treatement. Effect of antidepressant treatment on craving trajectories was not significantly different across levels of persistence on phase two of the PASAT (p > 0.05).
Does persistence during stress-challenge predict greater depressive symptoms?
Evaluation of unconditional models supported the inclusion of both linear and quadratic terms (X2 = 44.14; p<.0001) to describe the rapid reduction in depressive symtpoms following the first buprenorphine dose and the relative leveling off of symptoms throughout treatment. With control for planned covariates including baseline level of craving (p = 0.75), depressive symptoms (p = 0.00), frequency of opiate use (p = 0.22), and the effect of antidepressant medication (p = 0.01), lower persistence time on phase two of the PASAT (p = 0.24) was not related to levels of depressive symptoms during treatment. In a subsequent model we tested the interactive effects of antidepressant medication and level of persistence on levels of depressive symptoms during treatment. Effect of antidepressant treatment on depressive symptom trajectories was not significantly different across levels of persistence on phase two of the PASAT (p > 0.05).
4. Discussion
The current study evaluated the predictive validity of a behavioral index of persistence during a stress-challenge among opiate users identified as affectively vulnerable to lapse risk due to elevated depressive symtpoms. Patients randomized to receive either escitalopram or placebo completed a stress-challenge task prior to receiving their first dose of buprenorphine. Persistence on the stress-challenge task prior to initiating buprenorphine treatment predicted successful transition to early abstinence, and lower rates of opiate use during the initial three months of buprenorphine treatment across antidepressant and placebo groups.
We did not find support for hypotheses that lower levels of behavioral persistence during a stress-challenge task would be associated with concurrent self-report measures of affective vulnerability or negative affective reactions to the stress-challenge. Increases in negative affect during the task were uniformly high and were not predictive of persistence. This behavioral index of distress tolerance was not related to other domains of affective functioning including the intensity of depressive symptoms at baseline or volatility in symptoms during treatment. Limits in the range of depressive symptoms, timing of assessments, or strength of relationships between distress tolerance within more acutely depressed respondents may have obscured relationships. Alternatively, distress tolerance may be related primarily to reactions to negative affect and be less sensitive to severity of depressive symptoms during treatment.
Persistence during the stress-challenge was lowest among those with the highest pre-challenge levels of craving. Levels of craving on the first day of abstinence had the strongest relationship to peristence during the stress-challenge task suggesting that tolerance of distress may be impaired by concurrent craving. One interpetation of these results may be that the critical factor in predicting opiate use is craving, and not persistence. However, pre-challenge levels of craving were not associated with lapse risk and persistence remained associated with lapse-risk even after control for pre-challenge levels of craving. Further, the higher lapse risk among those with lower distress tolerance remained after adjusting for individual differences in craving reactions during the challenge. This pattern of results links concurrent craving to opiate use and suggests that distress tolerance may be more than a craving reaction. Our theoretical model would suggest that rather than severity of craving alone, poor distress tolerance (low persistence during the stress-challenge) is thought to convey risk for lapse due to a failure to inhibit drug seeking behavior when provoked by craving or negative affect. During periods of abstinence, reports of day-to-day craving levels among pharmacologically-treated opiate dependent patients have been shown to be associated with greater attentional bias and greater engagement of cognitive resources during controlled presentation of drug-cues (Lubman et al., 2004). Situational stressors may further tax cognitive resources to manage persistent craving and increase motivations to avoid the distress associated with such cues. Vulnerability for lapse may arise from a confluence of increased attentional pull towards salient cues, greater inefficiency in regulating craving provocations, and decreased tolerance of distress. Neurobiological models of relapse have highlighted the role of hypoactivity of the orbitofrontal-infralimbic cortex system (Volkow, Fowler, Wang, & Goldstein, 2002) in adversely shaping the stress response during withdrawal (Koob, 2008) and may provide insights into the mechanisms underlying this behavioral phenotype. Additionally, a broadening of assessments using other behavioral indices of distress tolerance (e.g., breath-holding, mirror tracing, anagrams, carbon dioxide-enriched air challenge) might be included to refine understanding of behavioral resposes to stress-challenges. Since distress tolerance may be amenable to assessment prior to buprenorphine treatment, this behavioral phenotype may have significant clinical implications. These vulnerabilities may escape identification with self-report and suggest the importance of behavioral assessments.
This study had several limitations. The exploration of distress tolerance in persons with elevated depressive symptoms may limit the ability to uncover relationships with other indices of affective vulnerability. We were not able to fully characterize the range of comorbid psychopathology with diagnostic information which may furhter limit generalization of these findings from this exploratory study. Participants were evaluated after being randomized to escitalopram or placebo and thus individual performance on the behavioral stress-challenge task was influenced by their treatment assignment. We wanted to assess behavioral persistence during a stress-challenge in the context of withdrawal. Assessing persistence during withdrawal increases ecological validity albeit at the expense of isolating withdrawal and persistence effects. Our statistical control for levels of craving during the behavioral stress-challenge task only partially accounts for this potential design issue. Although studies using this task in other substances did not show differential performance across abstinent and satiated states (Brown, et al, 1999), additional studies are needed to evalaute the impact of these associations prior to entering opiate withdrawal. We conducted this exploratory study using a design not specifically powered statistically for hypotheses and the current results serve as preliminary support for the potential of behavioral measures of persistence to predict treatment outcomes. The reduced effects of persistence on lapse risk when presuming all missing toxicological results were opioid positive was likely impacted by the small sample size. The current findings are in need of replication. Finally, although we argue for the importance of behavioral indices of distress tolerance, we confined our test of self-report of distress tolerance to a single measure with margnial internal consistency estimates in this sample with elevated depressive symptoms; other self-report indices of distress tolerance may be sensitive to relapse risk.
Early lapse to opiate use after effective pharmaocologic treament with buprenorphine is common and often leads to full relapse and treatment drop-out (Stein et al., 2010). Research on early lapse to opiates implicates the significance of both drug-cues and situations or events associated with distress in provoked craving and increased risk for lapse. Encouraging evidence suggests behavioral interventions facilitating negative affect regulation may weaken the link between rises in craving, negative affect, and risk for lapse in substance use treatment (Witkiewitz & Bowen, 2010). Behavioral interventions that target skills to improve regulating negative affect and physical discomfort associated with withdrawal may decrease the likelihood of craving and drug use in response to experienced negative affect. Identifying individual behavioral responses to drug- and stress-cues prior to attempts at abstinence may facilitate delivery of adjunctive behavioral treatments to prevent early lapse.
Acknowledgments
This study was funded by the National Institute on Drug Abuse DA022207, Clinical Trial NCT# 00475878. Dr. Stein is a recipient of a NIDA Mid-Career Award DA 000512.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrantes AM, Strong DR, Cohn A, Cameron AY, Greenberg BD, Mancebo MC, Brown RA. Acute changes in obsessions and compulsions following moderate-intensity aerobic exercise among patients with obsessive-compulsive disorder. J Anxiety Disord. 2009;23(7):923–927. doi: 10.1016/j.janxdis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35(11):1001–1007. doi: 10.1016/j.addbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. J Abnorm Psychol. 2003;112(3):448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93(1):73–92. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111(1):180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine Tob Res. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, Brown RA. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. J Abnorm Psychol. 2005;114(4):729–734. doi: 10.1037/0021-843X.114.4.729. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Freid CM, Addington S, Shelton RC. Do venlafaxine XR and paroxetine equally influence negative and positive affect? J Affect Disord. 2005;85(3):333–339. doi: 10.1016/j.jad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Eyesenck H. Dimensions of personality. London: Routledge & Kegan Paul; 1947. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcoholism, clinical and experimental research. 1999;23(8):1289–1295. [Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol. 2007;15(2):134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor: Implications for behavioral assessment. The Behavior Therapist 2007 [Google Scholar]
- Leyro TM, Zvolensky MJ, Bernstein A. Distress tolerance and psychopathological symptoms and disorders: a review of the empirical literature among adults. Psychol Bull. 2010;136(4):576–600. doi: 10.1037/a0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: The Guilford Press; 1993. [Google Scholar]
- Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99(12):1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Jacobs EA. Buprenorphine treatment for opioid dependence: the relative efficacy of daily, twice and thrice weekly dosing. Drug Alcohol Depend. 2005;77(2):195–204. doi: 10.1016/j.drugalcdep.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Miller I, Norman W, Bishop W. The modified Hamilton Rating Scale for Depression. Psychiatry Research. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Richards JM, Daughters SB, Bornovalova MA, Brown RA, Lejuez CW. Substance use disorders. In: Zvolensky MJ, Bernstein A, Vujanovic AA, editors. Distress tolerance. New York: Guilford Press; 2010. [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Ryans DG. The measurement of persistence: an historical review. Psychological Bulletin. 1939;36(9):715–739. [Google Scholar]
- Schmidt NB, Richey JA, Fitzpatrick KK. Discomfort intolerance: Development of a construct and measure relevant to panic disorder. Journal of anxiety disorders. 2006;20(3):263–280. doi: 10.1016/j.janxdis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Toronto, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. Journal of general internal medicine. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Herman DS, Kettavong M, Cioe PA, Friedmann PD, Tellioglu T, Anderson BJ. Antidepressant treatment does not improve buprenorphine retention among opioid-dependent persons. J Subst Abuse Treat. 2010;39(2):157–166. doi: 10.1016/j.jsat.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC. Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord. 2004;78(3):235–241. doi: 10.1016/S0165-0327(02)00306-3. [DOI] [PubMed] [Google Scholar]
- Vanderkaay MM, Patterson SM. Nicotine and acute stress: effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biological psychology. 2006;71(2):191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Barge-Schaapveld DQ, Nicolson NA, Peeters F, de Vries M, Mengelers R, van Os J. Reduced stress-sensitivity or increased reward experience: the psychological mechanism of response to antidepressant medication. Neuropsychopharmacology. 2009;34(4):923–931. doi: 10.1038/npp.2008.66. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of consulting and clinical psychology. 2010;78(3):362–374. doi: 10.1037/a0019172. [DOI] [PMC free article] [PubMed] [Google Scholar]