Abstract
Matrix metalloproteinases (MMPs) have been implicated in a variety of pathophysiological conditions, of which MMP-7 is expressed by tumor cells of epithelial and mesenchymal origin. However, the function of MMP-7 in human lung adenocarcinoma (LAC) is unclear. In the present study the expression of MMP-7 in LAC was examined by immunohistochemical assay using a tissue microarray procedure. A loss-of-function experiment was performed to explore the effects and molecular mechanisms of lentiviral vector-mediated MMP-7 siRNA (siMMP-7) on cell proliferation and invasive potential in LAC A549 cells, measured by MTT and Transwell assays, respectively. It was found that, the expression of MMP-7 protein in LAC was significantly increased compared with that in adjacent non-cancerous tissues (ANCT) (76.0% vs 44.0%, P<0.001), and positively correlated with lymph node metastases of the tumor (P=0.014). Furthermore, targeted inhibition of cyclooxygenase-2 (COX-2) by siRNA downregulated the expression of MMP-7 and inhibited invasion of LAC cells, and knockdown of MMP-7 suppressed tumor proliferation and invasion in LAC cells. Taken together, our findings indicate that increased expression of MMP-7 is associated with lymph node metastasis and upregulated by COX-2, and promotes the tumorigenesis of LAC, suggesting that MMP-7 may be a potential therapeutic target for the treatment of cancer.
Key words: MMP-7, COX-2, lung adenocarcinoma
Introduction
Lung cancer is the most commonly diagnosed type of cancer and the primary cause of cancer-related deaths worldwide.1 The current best approach for treatment of cancer is complete surgical removal of the tumor and adjacent lymph nodes. However, the efficacy of this therapeutic approach alongside hormone, radio, and chemo-therapy are very limited.2 Tumor is also a genetic disease that develops from a multi-step process. Single or multiple mutations in genes related to growth control, invasion, and metastasis form the molecular genetic basis of malignant transformation and tumor progression.3 Therefore, identification of key genes or targets related to tumorigenesis is critical for prevention and treatment of cancer.
Matrix metalloproteinases (MMPs), produced by stromal fibroblast-like cells in the vicinity of various malignancies, have been shown to have a significant role in determining cancer cell behaviors, of which MMP-7 is identified to be overexpressed in non-small cell lung cancer (NSCLC).4-6 The expression of MMP-7 is higher in adenocarcinoma than in the epidermoid form of NSCLC,7 but not in normal epithelia.8 MMP-7 is also expressed in bronchiolization of alveoli (BCA), a precursor of lung cancer, promoting proliferation, migration, and attenuation of apoptosis.8 Overexpression of MMP-7 is associated with tumor proliferation and chemoresistance, and constitutes a prognostic factor in several solid tumors,5,9,10 suggesting an independent positive prognostic factor in NSCLC patients.11
However, some studies have shown that MMP-2, but not MMP-7 and MMP-9, may be implicated in early-stage tumor invasion, metastasis, and apoptosis inhibition in human lung adenocarcinoma (LAC).12-14 MMP-7 expression does not significantly correlate with the clinicopathological factors and unfavorable prognosis in NSCLC.4,15 Thus, in the present study, the expression of MMP-7 in LAC was examined by immunohistochemical assay using a tissue microarray procedure. A loss-of-function experiment was performed to explore the function of MMP-7 in LAC A549 cells. We hypothesized that MMP-7 might function as an oncogen in human LAC, and serve as a potential therapeutic target for the treatment of cancer.
In addition, COX-2 is highly expressed in LAC and represents an independent prognostic factor.16 COX-2 can mediate the tumor metastasis of breast cancer cells through EGFR-activated PI3K/Akt and MAPK pathways.17 Targeting COX-2 controls tumor growth, angiogenesis, lymphangiogenesis and lung metastasis in breast cancer, and selective COX-2 inhibitor celecoxib induces epithelial-mesenchymal transition in lung cancer via activating MEK-ERK signaling.18,19 But, whether COX-2 is involved in the tumorigenesis of LAC through regulation of MMP-7 expression is unknown. Our present study will clarify the regulation of COX-2 on MMP-7 as well as the function of MMP-7 in LAC cells.
Materials and Methods
Materials
The LAC A549 cell line used for experiments was obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China). Adenovirus-mediated small interference COX-2 (siCOX-2) and lentivirus-mediated MMP-7 siRNA (siMMP-7) vectors, negative control vectors, and virion-packaging elements were purchased from Genechem (Shanghai, China); COX-2 and MMP-7 primers were synthesized by ABI (Framingham, MA, USA). The tissue microarray of human LAC was purchased from the branch of Biomax (Xi’an, China). All antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Drugs and reagents
Dulbecco’s Modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA); TRIzol Reagent and Lipofectamine 2000 were obtained from Invitrogen (Carlsbad, CA, USA); M-MLV Reverse Transcriptase was purchased from Promega (Madison, WI, USA); SYBR Green Master Mix was obtained from Takara (Otsu, Japan); and the ECL Plus Kit was obtained from GE Healthcare (Piscataway, NJ, USA).
Clinical samples and data
A tissue microarray was prepared for the IHC test using a total of 50 consecutive cases of human LAC tissues and corresponding ANCT, which were collected from the Department of Medical Oncology affiliated with Shanghai Pulmonary Hospital ,from June 2004 and December 2010. The study was approved by the Medical Ethics Committee of Tongji University School of Medicine, and written informed consent was obtained from the patients or their parents before sample collection. All of the cases were reviewed by two pathologists.
Tissue microarrays
For each case, we selected the tumor foci for construction of the tissue microarrays during routine diagnosis by marking them on the hematoxylin-eosin-stained slide using a waterproof pencil. The Advanced Tissue Arrayer (ATA-100; Chemicon International, Tamecula, CA) was used to create holes in a recipient paraffin block and to acquire cylindrical core tissue biopsies with a diameter of 1 mm from specific areas of the donor block. The tissue core biopsies were transferred to the recipient paraffin block at defined array positions. The resulting tissue microarrays contained tissue samples from 50 formalin-fixed, paraffin-embedded cancer specimens with known diagnosis and correlated ANCT from patients.
The block was incubated in an oven at 45°C for 20 min to allow complete embedding of the grafted tissue cylinders in the paraffin of the recipient block and then stored at 4°C until microtome sectioning.
Immunohistochemical staining
Anti-MMP-7 and COX-2 antibodies were used for IHC detection of the expression of MMP-7 and COX-2 protein in tissue microar-rays. Tissue microarray sections were processed for IHC analysis of MMP-7 and COX-2 protein as follows. Immunohistochemical examinations were carried out on 3 mm thick sections. For anti- MMP-7 and COX-2 immunohistochemistry, unmasking was performed with 10 mM sodium citrate buffer, pH 6.0, at 90°C for 30 min. For anti-MMP-7 and COX-2 immunohistochemistry, antigen unmasking was not necessary. Sections were incubated in 0.03% hydrogen peroxide for 10 min at room temperature, to remove endogenous peroxidase activity, and then in blocking serum (0.04% bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China; and 0.5% normal goat serum X0907, Dako Corporation, Carpinteria, CA, USA, in PBS) for 30 min at room temperature. Anti- MMP-7 and COX-2 antibodies were used at a dilution of 1:200. The antibody was incubated overnight at 4°C. Sections were then washed three times for 5 min in PBS. Non-specific staining was blocked with 0.5% casein and 5% normal serum for 30 min at room temperature. Finally, staining was developed using diaminobenzidine substrate, and sections were counterstained with hematoxylin. Normal serum or PBS was used to replace anti-MMP-7 and COX-2 antibodies in negative controls.
Quantification of MMP-7 protein expression
MMP-7 expression was semiquantitatively estimated as the total MMP-7 immunostaining score, which was calculated as the product of a proportion score and an intensity score. The proportion score reflected the fraction of positively stained cells (score 0, <5%; score 1, 5%–10%; score 2, 10%–50%; score 3, 50%–75%; score 4, >75%). The intensity score represented the staining intensity (score 0, no staining signal; score 1, weak positive signal; score 2, moderate positive signal; score 3, strong positive signal). Finally, a total expression score was given, ranging from 0 to 12. The score 0 was regarded as negative, score 1-3 was regarded as +, score 4-6 was regarded as ++, score 7-9 was regarded as +++, and score 10-12 was regarded as ++++. Two observers estimated the total immunostaining score, independently and blindly. The total score reported was the average of two observers.
Cell culture and transfection
LAC A549 cells were cultured in DMEM medium supplemented with 10% heat-inactivated FBS, 100/mL of penicillin, and 100 μg/mL of streptomycin. Cells in this medium were placed in a humidified atmosphere containing 5% CO2 at 37°C. Cells were subcultured at a 1:5 dilution in medium containing 300 µg/mL G418 (an aminoglycoside antibody, commonly used stable transfection reagent in molecular genetic testing). On the day of transduction, LAC cells were replated at 5×104 cells/well in 24-well plates containing serum-free growth medium with polybrene (5 mg/mL). When reached 50% confluence, cells were transfected with recombinant experimental virus or control virus at the optimal MOI (multiplicity of infection) of 50, and cultured at 37°C and 5% CO2 for 4 h. Then supernatant was discarded and serum containing growth medium was added. At 2 days of post-transduction, transduction efficiency was measured by the frequency of green fluorescent protein (GFP)-positive cells under a fluorescence microscope. The transduction efficiency of lentivirus-mediated shMMP-7 was calculated according to the ratio of fluorescent cells and non-fluorescent cells, arriving at more than 70%. Positive and stable transfectants were selected and expanded for further study. The MMP-7 siRNA virus vector-infected clone, the negative control vector-infected cells, and LAC cells were named as siMMP-7 group, negative control (NC) group, and LAC control (CON) group, respectively.
Quantitative real-time PCR
To quantitatively determine the mRNA expression levels of COX-2 and MMP-7 in LAC A549 cell line, Real-time PCR was performed. Total RNA was extracted from each clone using TRIzol according to the manufacturer’s protocol. Reverse transcription was carried out using M-MLV and cDNA amplification was performed using the SYBR Green Master Mix kit according to the manufacturer’s guidelines. The MMP-7 gene was amplified using a specific oligonucleotide primer and the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control. Data were analyzed using the comparative Ct method (2-ΔΔCt). Three separate experiments were performed for each clone.
Western blot assay
LAC A549 cells were harvested and protein extracted using lysis buffer (Tris-HCl, SDS, mercaptoethanol, and glycerol). Cell extracts were boiled for 5 min in loading buffer, and then an equal amount of cell extracts was separated on 15% SDS-PAGE gels. Separated protein bands were transferred onto polyvinylidene fluoride (PVDF) membranes, which were subsequently blocked in 5% skim milk powder. Primary antibodies against COX-2, MMP-7 and PCNA were diluted according to the manufacturer’s instructions and incubated overnight at 4°C. Subsequently, horseradish peroxidase-linked secondary antibodies were added at a dilution of 1:1000 and incubated at room temperature for 2 h. The membranes were washed 3 times with PBS, and the immunoreactive bands were visualized using the ECL Plus Kit according to the manufacturer’s instructions. The relative protein levels in different cell lines were normalized to the concentration of β-actin. Three separate experiments were performed for each clone.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay. Briefly, cells infected with MMP-7 siRNA-virus were incubated in 96-well-plates at a density of 1×105 cells per well with DMEM medium supplemented with 10% FBS. Cells were treated with 20 µL of MTT dye at 0, 24, 48, 72, and subsequently incubated with 150 μL of DMSO for 5 min. The color reaction was measured at 570 nm using an Enzyme Immunoassay Analyzer (Bio-Rad, Hercules, CA, USA). The proliferation activity was calculated for each clone.
[3H]-thymidine incorporation
DNA synthesis was determined by measuring [3H]-thymidine incorporation. Cells were plated onto 24-well plates at a density of 1.0×105 cells/well in quadruplets. Cells were serum deprived for 24 h, and serum stimulated in culture media containing 1.5 μCi/mL tritiated thymidine ([3H]dT) (specific activity of 740 GBq/mmol) (Perkin-Elmer, Waltham, MA, USA) for 4 h. Cells were fixed and washed in ice-cold 10% trichloroacetic acid. DNA was solubilized in 0.1 mol/L NaOH for 1h at 37°C. [3H]dT incorporated into the DNA was measured using liquid scintillation counting.
Transwell invasion assay
Transwell filters were coated with Matrigel (3.9 mg/mµL; 60-80 µL) on the upper surface of a polycarbonate membrane (diameter, 6.5 mm; pore size, 8 µm). After incubating at 37°C for 30 min, the Matrigel solidified and served as the extracellular matrix for analysis of tumor cell invasion. Harvested cells (1×105) in 100 µL of serum-free DMEM were added into the upper compartment of the chamber. A total of 200 µL of conditioned medium derived from NIH3T3 cells was used as a source of chemoattractant, which was placed in the bottom compartment of the chamber. After 24 h of incubation at 37°C with 5% CO2, the medium was removed from the upper chamber. The non-invaded cells on the upper side of the chamber were scraped off with a cotton swab. Cells that had migrated from the Matrigel into the pores of the inserted filter were fixed with 100% methanol, stained with hematoxylin, then mounted and dried at 80°C for 30 min. The number of cells invading through the Matrigel was counted in 3 randomly selected visual fields from the central and peripheral portion of the filter by using an inverted microscope (200× magnification). Each assay was repeated 3 times.
Statistical analysis
SPSS 20.0 was used for statistical analyses. The Kruskal-Wallis H test, x2 test, and one-way analysis of variance (ANOVA) were employed to analyze the expression rate in all groups. The LSD method of multiple comparisons was used when the probability for ANOVA was statistically significant. Significance was defined as P<0.05.
Results
The expression of MMP-7 and COX-2 in LAC tissues
The expression of MMP-7 and COX-2 protein was evaluated using IHC staining in LAC tissues. As shown in Figure 1, different levels of positive expression of MMP-7 and COX-2 protein were examined in LAC tissues. Positive MMP-7 and COX-2 immunostaining was localized in the cytoplasm of cancer tissue cells. According to the MMP-7 immunoreactive intensity, the positive expression of MMP-7 and COX-2 was significantly increased in LAC tissues compared with those in ANCT (P<0.001) (Table 1).
Figure 1.
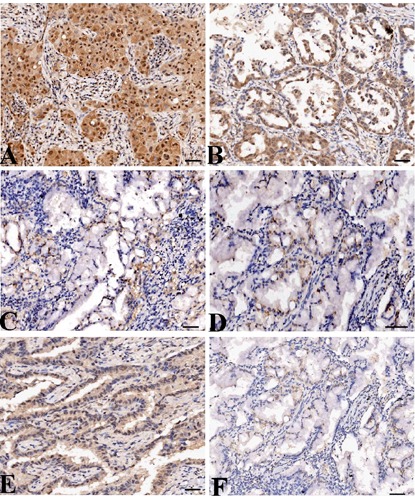
The expression of MMP-7 protein in LAC tissues (magnification: 200×). LAC tissues were immunohistochemically stained with an anti-MMP-7 and COX-2 antibodies and classified as positive expression (A) and negative expression (C). Adjacent non-cancer tissues were immunohistochemically stained with an anti-MMP-7 antibody and classified as positive expression (B) and negative expression (D). COX-2 was highly expressed in LAC tissues (E) and lowly expressed in ANCT (F). Positive immunostaining of MMP-7 and COX-2 was mainly localized in the cytoplasm of tumor and tissue cells. Scale bars: A-C, E,F) 75 µm; D) 150 µm.
Table 1.
The expression of MMP-7 protein in human lung adenocarcinoma.
| Target | Group | Total | Score | Positive rate | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|
| - | + | ++ | +++ | (%) | |||||
| MMP-7 | LAC | 50 | 12 | 11 | 21 | 6 | 76.0 | 16.328 | <0.001 |
| ANCT | 50 | 28 | 14 | 7 | 1 | 44.0 | |||
| COX-2 | LAC | 50 | 7 | 19 | 15 | 9 | 86.0 | 8.039 | 0.005 |
| ANCT | 50 | 21 | 14 | 11 | 4 | 58.0 | |||
LAC, lung adenocarcinoma; ANCT, adjacent non-cancerous tissues.
Association between MMP-7 expression and clinicopathologic characteristics
The relationship between MMP-7 expression and various clinical and pathologic characteristics of LAC patients was analyzed. As indicated in Table 2, no significant correlation was found between MMP-7 expression and age or gender. According to the pathological TNM staging, the cases were divided into two groups: stage I+II and stage III+IV. The group with early stage showed elevated rate of MMP-7 expression, but these two groups had no significant difference (P=0.175). Neither did the group T1+T2 and group T3+T4 based on tumor size (P=0.943). The cases were then divided into two groups: those with and those without lymph node metastases. The rate of MMP-7 expression was higher in 50.0% (25/50) of LAC with lymph node metastases than that in 26.0% (13/50) of LAC without lymph node metastases (P=0.014).
Table 2.
Correlation of MMP-7 expression with clinicopathologic characteristics of LAC patients.
| Variables | Cases (n) | MMP-7 | χ2 | P | |
|---|---|---|---|---|---|
| - | + | ||||
| Total | 50 | 12 | 38 | ||
| Age (years) | |||||
| <60 | 28 | 7 | 21 | 0.034 | 0.853 |
| ≥60 | 22 | 5 | 17 | ||
| Gender | |||||
| Male | 31 | 8 | 23 | 0.143 | 0.705 |
| Female | 19 | 4 | 15 | ||
| TNM staging | |||||
| I+II | 29 | 9 | 20 | 1.836 | 0.175 |
| III+IV | 21 | 3 | 18 | ||
| Tumor size | |||||
| T1+T2 | 42 | 10 | 32 | 0.005 | 0.943 |
| T3+T4 | 8 | 2 | 6 | ||
| Lymph node metastases | |||||
| No | 22 | 9 | 13 | 6.035 | 0.014 |
| Yes | 28 | 3 | 25 | ||
LAC, lung adenocarcinoma.
The effect of COX-2 on the expression of MMP-7 and cell invasion
According to our previous work,20 adeno -virus-mediated siCOX-2 was successfully constructed, and used to instantly transfect into LAC A549 cells. Then, the mRNA and protein expression levels of COX-2 and MMP-7 were detected by Real-time PCR (Figure 2 A,B) and Western blot assays (Figure 2 C-F). It was shown that the expression levels of COX-2 and MMP-7 were both decreased in siCOX-2 group compared to negative control group (NC) and untreated group (CON) (**P<0.01). To determine the effect of COX-2 on the invasive potential of LAC cells, the Transwell assay was carried out. It was found that the invasive potential of LAC cells was markedly decreased in siCOX-2 group compared to NC and CON groups (**P<0.01) (Figure 3 A,B).
Figure 2.
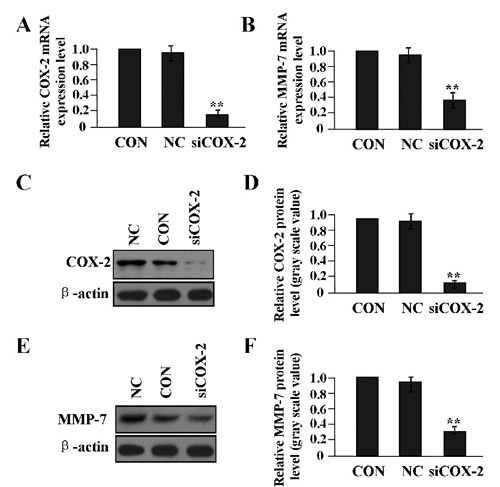
The effect of COX-2 on the expression of MMP-7. After LAC A549 cells were transfected with siCOX-2 adenovirus for 24 h, the expression levels of COX-2 and MMP-7 were detected by Real-time PCR (A, B) and Western blot assays (C-F). The expression of COX-2 and MMP-7 was significantly decreased in siCOX-2 group compared with the CON and NC groups (each **P<0.01), suggesting that COX-2 might upregulate the expression of MMP-7 in LAC cells.
Figure 3.
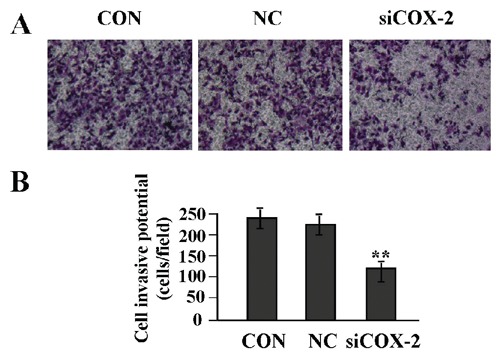
The effect of COX-2 knockdown on cell invasion. A,B) Transwell assay was used to determine cell invasion; cell invasive potential was markedly inhibited in siCOX-2 group compared with the CON and NC groups (**P<0.01), suggesting that targeted inhibition of COX-2 might block invasion of LAC cells. Scale bars: A) 75 µm.
Knockdown of MMP-7 expression in LAC A549 cells
After LAC A549 cells were stably transfected with lentivirus-mediated siMMP-7, the mRNA and protein expression level of MMP-7 were examined by Real-time PCR (Figure 4A) and Western blot assays (Figure 4 B,C). We found that the expression level of MMP-7 was significantly knocked down by siRNA in siMMP-7 group compared to NC and CON groups (**P<0.01).
Figure 4.
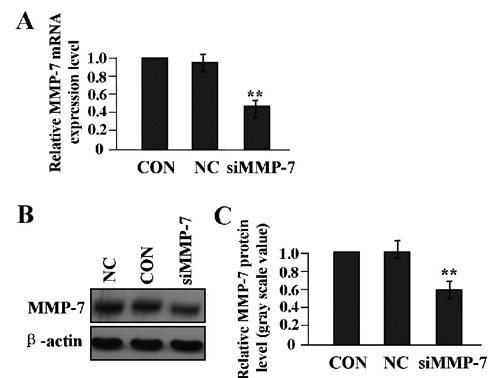
Knockdown of MMP-7 expression in LAC A549 cells. After LAC A549 cells were transfected with siMMP-7 lentivirus for 24 h, the expression level of MMP-7 was detected by real-time PCR (A) and Western blot assays (B,C), indicating that the expression of MMP-7 could be knocked down in siMMP-7 group compared with CON and NC groups (**P<0.01).
The effect of MMP-7 knockdown on cell proliferation
To confirm the effect of MMP-7 knockdown on tumor growth in LAC A549 cells, we evaluated the proliferative activities of LAC cells by MTT assay. We found that MMP-7 knockdown markedly suppressed the proliferative activities of LAC cells in a time-dependent manner compared to NC and CON groups (Figure 5A). Thus, the effect of MMP-7 knockdown on cell proliferation was further established by measuring [3H]dT incorporation following 4 h serum stimulation of cells deprived of serum for 24 h. The increase of [3H]dT incorporation induced by serum was higher in CON and NC groups than in siMMP-7 group, and the difference was statistically significant (**P<0.01) (Figure 5B). To understand molecular mechanisms of MMP-7 on cell proliferation, we examined the expression of PCNA in Lv-siMMP-7-transfected LAC cells by Western blot assay (Figure 5 C,D), indicating that the expression level of PCNA was significantly reduced in siMMP-7 group compared to NC and CON groups (**P<0.01).
Figure 5.
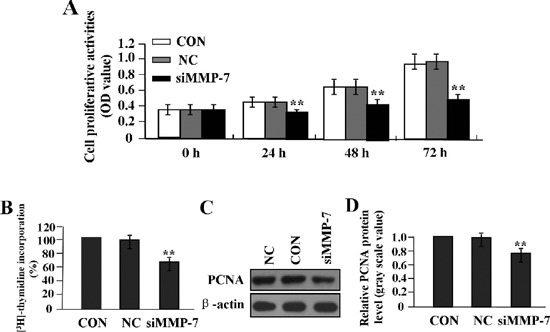
The effect of MMP-7 knockdown on cell proliferation. A) MTT assay was used to evaluate cell proliferative activity for consecutive 3 days; cell proliferative activity was remarkably diminished in a time-dependent manner in siMMP-7 group compared with the CON and NC groups (**P<0.01). B) [3H] thymi-dine incorporation into DNA was assessed by scintillation counting. Results are expressed as percentage of increase of [3H] thymi-dine incorporation in serum-stimulated cells over that of quiescent cells for each cell population, indicating that the increase of [3H]dT incorporation induced by serum was higher in CON and NC groups than in siMMP-7 group (**P<0.01). C,D) The expression level of PCNA protein, examined by Western blot assay, was downregulated in siMMP-7 group compared with the CON and NC groups (**P<0.01), suggesting that knockdown of MMP-7 might inhibit proliferation of LAC cells through downregulation of PCNA expression.
The effect of MMP-7 knockdown on cell invasion
To determine the effect of MMP-7 knockdown on the invasive potential of LAC cells, a Transwell assay was performed. The invasive potential of tumor cells in Transwell assay was determined by the ability of cells to invade a matrix barrier containing laminin and type IV collagen, the major components of the basement membrane. Representative micrographs of Transwell filters can be seen in Figure 6A. We found that the invasive potential of LAC cells was decreased in siMMP-7 group compared to NC and CON groups (**P<0.01) (Figure 6B).
Figure 6.
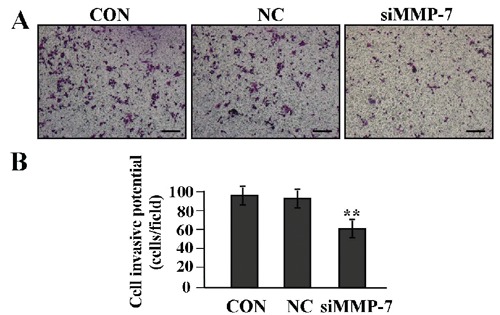
The effect of MMP-7 knockdown on cell invasion. A,B) Transwell assay was performed to determine cell invasion; cell invasive potential was markedly weakened in siMMP-7 group compared with the CON and NC groups (**P<0.01), suggesting that knockdown of MMP-7 might inhibit invasion of LAC cells. Scale bar: A) 75 µm.
Discussion
Matrix metalloproteinases (MMPs) play a crucial role in physiological and pathological matrix turnover. Some studies have shown that, the expression of MMP-1, -2, -7 and -10 is significantly increased in NSCLC, and can serve as independent prognostic factors for unfavorable outcome.21,22 Increased expression of MMP-1/-9 stimulates cell invasion and metastasis of lung cancer cells,23 while tissue inhibitors of MMPs inhibited cell invasion of LAC cells,24 suggesting that MMPs including MMP-7 may play an important role in the development of LAC. MMP-7, also known as matrilysin, is a minimal domain MMP that exhibits proteolytic activity against components of the extracellular matrix (ECM). MMP-7 is frequently overexpressed in human cancer tissues and plays an important role in cancer progression.25 Overexpression of MMP-7 is correlated with differentiation, lymph node metastasis and local invasiveness in some cancers.26,27 Moreover, it contributes to a poor prognosis in patients with colorectal cancer (CRC),28 intrahepatic cholangiocarcinoma29 and renal cell carcinoma,30 suggesting that MMP-7 can be used as a predictive marker of unfavorable prognosis in cancer patients. Considering the few studies of MMP-7 in LAC metastasis, in our present study, it was found that MMP-7 was highly expressed in the cytoplasm of LAC tissues compared to the ANCT, and correlated with lymph node metastases in LAC patients, indicating that cytoplasmic accumulation of MMP-7 might be involved in the development and progression of LAC.
Furthermore, some studies show that over-expression of MMP-7 promotes tumorigenesis through enhancing cell migration, invasion and cellular proliferation,25,31 while antisense oligonucleotide targeting MMP-7 inhibits tumor growth and invasion.32 In addition, inhibition of MMP-7 activity by its specific or nonspecific inhibitor suppresses tumor metastasis and angiogenesis in lung cancer.33,34 Inversely, Liu et al. have found that MMP-7 inhibits proliferation and modulates sensitivity of lung cancer cells to FasL-mediated apoptosis,35 indicating that MMP-7 may be multiple, multifarious, and multifaceted functions involving in tumorigenesis. Up to now, few reports have been reported about the function of MMP-7 in LAC cells. Our present study showed that knockdown of MMP-7 by RNA interference suppressed the expression of PCNA and proliferative activity of LAC cells. PCNA is a nuclear protein that is expressed in proliferating cells and may be required for maintaining cell proliferation, and used as a marker for cell proliferation of LAC.36 Thus, our data showed knockdown of MMP-7 might reduce cell proliferation of LAC cells through downregulation of PCNA expression. In addition, knockdown of MMP-7 could also weakened invasive potential of LAC cells, suggesting MMP-7 might act as an important therapeutic target for LAC.
Recently, how MMP-7 is regulated by upstream factors including COX-2 becomes the focus of many studies. Although multiple genetic alterations are necessary for lung cancer invasion and metastasis, COX-2 is involved in the progression of NSCLC and provides an insight into opportunities for targeted therapies in NSCLC.37,38 COX-2 has been reported to be associated with MMP-7 expression and COX-2 plus MMP-7 can serve as potential biomarkers for CRC screening.39 However, how COX-2 interacts with MMP-7 in lung cancer is unknown. In the present study, we found that knockdown of COX-2 gene could downregulate the expression of MMP-7 from transcriptional and translational levels, and inhibited cell invasion in LAC cells, suggesting that COX-2 might be involved in the tumorigenesis and progression of LAC through upregulation of MMP-7 expression.
In conclusion, our findings indicate that elevated expression of MMP-7 is associated with a higher incidence of metastases in human LAC; MMP-7 is upregulated by COX-2 and promotes growth and invasion of LAC cells, suggesting that MMP-7 may be a potential therapeutic target for the treatment of cancer.
Acknowledgments
This work was supported by the Shanghai Municipal Natural Science Foundation (No. 10ZR14249000).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30 [DOI] [PubMed] [Google Scholar]
- 2.William WN, Jr, Glisson BS. Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol 2011;8:611-9 [DOI] [PubMed] [Google Scholar]
- 3.Tajima Y, Yamazaki K, Makino R, Nishino N, Aoki S, Kato M, et al. Gastric and intestinal phenotypic marker expression in early differentiated-type tumors of the stomach: clinicopathologic significance and genetic background. Clin Cancer Res 2006;12:6469-79 [DOI] [PubMed] [Google Scholar]
- 4.Leinonen T, Pirinen R, Böhm J, Johansson R, Ropponen K, Kosma VM. Expression of matrix metalloproteinases 7 and 9 in non-small cell lung cancer. Relation to clinico-pathological factors, betacatenin and prognosis. Lung Cancer 2006;51:313-21 [DOI] [PubMed] [Google Scholar]
- 5.Kren L, Goncharuk VN, Krenová Z, Stratil D, Hermanová M, Skricková J, et al. Expression of matrix metalloproteinases 3, 10 and 11 (stromelysins 1, 2 and 3) and matrix metalloproteinase 7 (matrilysin) by cancer cells in non-small cell lung neoplasms. Clinicopathologic studies. Cesk Patol 2006;42:16-9 [PubMed] [Google Scholar]
- 6.Lin TS, Chiou SH, Wang LS, Huang HH, Chiang SF, Shih AY, et al. Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol Rep 2004;12:717-23 [PubMed] [Google Scholar]
- 7.Safranek J, Holubec L, Jr, Topolcan O, Pesta M, Klecka J, Vodicka J, et al. Expression of mRNA MMP-7 and mRNA TIMP-1 in non-small cell lung cancer. Anticancer Res 2007;27:2953-6 [PubMed] [Google Scholar]
- 8.Wang XY, Demelash A, Kim H, Jensen-Taubman S, Dakir el H, Ozbun L, et al. Matrilysin-1 mediates bronchiolization of alveoli, a potential premalignant change in lung cancer. Am J Pathol 2009;175:592-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Nakano J, Ishikawa S, Yokomise H, Ueno M, Kadota K, et al. Overexpression of matrix metalloproteinase-7 (MMP-7) correlates with tumor proliferation, and a poor prognosis in non-small cell lung cancer. Lung Cancer 2007;58:384-91 [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Zhang T, Li X, Huang J, Wu B, Huang X, et al. Predictive value of MMP-7 expression for response to chemotherapy and survival in patients with non-small cell lung cancer. Cancer Sci 2008; 99:2185-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenvold H, Donnem T, Andersen S, Al-Saad S, Al-Shibli K, Busund LT, et al. Overexpression of matrix metallopro-teinase-7 and -9 in NSCLC tumor and stromal cells: correlation with a favorable clinical outcome. Lung Cancer 2012;75:235-41 [DOI] [PubMed] [Google Scholar]
- 12.Weng Y, Cai M, Zhu J, Geng J, Zhu K, Jin X, et al. Matrix metalloproteinase activity in early-stage lung cancer. Onkologie 2013;36:256-9 [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Inagaki M, Takabe K, Kumagai J, Suzuki K. Rapid detection of matrix metal-loproteinase-7 and carcinoembryonic antigen mRNA expression in intraoperative pleural lavage samples using the reverse transcriptase loop-mediated isothermal amplification method. Acta Cytol 2009;53:283-91 [DOI] [PubMed] [Google Scholar]
- 14.Yang B, Gao J, Rao Z, Zhang B, Ouyang W, Yang C. Antisense oligonucleotide targeting matrix metalloproteinase-7 (MMP-7) changes the ultrastructure of human A549 lung adenocarcinoma cells. Ultrastruct Pathol 2011;35:256-9 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Oshima T, Yoshihara K, Nishi T, Arai H, Inui K, et al. Clinical significance of immunohistochemical expression of insulin-like growth factor-1 receptor and matrix metalloproteinase-7 in resected non-small cell lung cancer. Exp Ther Med 2012;3:797-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Wang J, Zhao W. Cox-2 in non-small cell lung cancer: a meta-analysis. Clin Chim Acta 2013;419:26-32 [DOI] [PubMed] [Google Scholar]
- 17.Kang JH, Song KH, Jeong KC, Kim S, Choi C, Lee CH, et al. Involvement of Cox-2 in the metastatic potential of chemotherapy-resistant breast cancer cells. BMC Cancer 2011; 11:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin X, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest 2012; 92:1115-28 [DOI] [PubMed] [Google Scholar]
- 19.Wang ZL, Fan ZQ, Jiang HD, Qu JM. Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal transition in human lung cancer cells via activating MEK-ERK signaling. Carcinogenesis 2013; 34:638-46 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Zhang QY, Fu YC, Wang T, Zhang J, Xu P, et al. Expression of p-Akt and COX-2 in gastric adenocarcinomas and aden-ovirus mediated Akt1 and COX-2 ShRNA suppresses SGC-7901 gastric adenocarcinoma and U251 glioma cell growth in vitro and in vivo. Technol Cancer Res Treat 2009;8:467-78 [DOI] [PubMed] [Google Scholar]
- 21.Lin TS, Chiou SH, Wang LS, Huang HH, Chiang SF, Shih AY, et al. Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol Rep 2004;12:717-23 [PubMed] [Google Scholar]
- 22.Moche M, Hui DS, Huse K, Chan KS, Choy DK, Scholz GH, et al. Matrix metalloproteinases and their inhibitors in lung cancer with malignant pleural effusion. Pneumologie 2005;59:523-8 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Tai HH. Increased expression of matrix metalloproteinases mediates thromboxane A2-induced invasion in lung cancer cells. Curr Cancer Drug Targets 2012;12:703-15 [DOI] [PubMed] [Google Scholar]
- 24.Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst 2008;100:59-69 [DOI] [PubMed] [Google Scholar]
- 25.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metallopro-teinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angio-genesis. Exp Biol Med (Maywood) 2006;231:20-7 [DOI] [PubMed] [Google Scholar]
- 26.Bi Z, Dong LD, Gu XM. Clinical significance of MMP-7 and PTEN expression in colorectal cancer. Hepatogastroenterology 2013;60:32-6 [DOI] [PubMed] [Google Scholar]
- 27.Liu XP, Kawauchi S, Oga A, Tsushimi K, Tsushimi M, Furuya T, et al. Prognostic significance of matrix metalloproteinase-7 (MMP-7) expression at the invasive front in gastric carcinoma. Jpn J Cancer Res 2002;93:291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Su K, Gao J, Rao Z. Expression and prognostic value of matrix metallopro-teinase-7 in colorectal cancer. Asian Pac J Cancer Prev 2012;13:1049-52 [DOI] [PubMed] [Google Scholar]
- 29.Hirashita T, Iwashita Y, Ohta M, Komori Y, Eguchi H, Yada K, et al. Expression of matrix metalloproteinase-7 is an unfavorable prognostic factor in intrahepatic cholangiocarcinoma. J Gastrointest Surg 2012;16:842-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Jung K. Plasma matrix metalloproteinase-7 as a metastatic marker and survival predictor in patients with renal cell carcinomas. Cancer Sci 2008;99:1188-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SK, Han YM, Yun J, Lee CW, Shin DS, Ha YR, et al. Phosphatase of regenerating liver-3 promotes migration and invasion by upregulating matrix metalloproteinases-7 in human colorectal cancer cells. Int J Cancer 2012;131:E190-203 [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Gao J, Rao Z, Zhang B, Ouyang W, Yang C. Antisense oligonucleotide targeting matrix metalloproteinase-7 (MMP-7) changes the ultrastructure of human A549 lung adenocarcinoma cells. Ultrastruct Pathol 2011;35:256-9 [DOI] [PubMed] [Google Scholar]
- 33.Rasheed SA, Efferth T, Asangani IA, Allgayer H. First evidence that the anti-malarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer 2010;127:1475-85 [DOI] [PubMed] [Google Scholar]
- 34.Naglich JG, Jure-Kunkel M, Gupta E, Fargnoli J, Henderson AJ, Lewin AC, et al. Inhibition of angiogenesis and metastasis in two murine models by the matrix metal-loproteinase inhibitor, BMS-275291. Cancer Res 2001;61:8480-5 [PubMed] [Google Scholar]
- 35.Liu H, Huang J, Wu B, Zhou Y, Zhu J, Zhang T. Matrilysin inhibits proliferation and modulates sensitivity of lung cancer cells to FasL-mediated apoptosis. Med Oncol 2008;25:419-30 [DOI] [PubMed] [Google Scholar]
- 36.Zhou B, Dai X, He A. [Molecular marker as an indicator in presaging prognosis of human lung adenocarcinoma]. [Article in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 1998;19:231-3 [PubMed] [Google Scholar]
- 37.Lee JM, Mao JT, Krysan K, Dubinett SM. Significance of cyclooxygenase-2 in prognosis, targeted therapy and chemoprevention of NSCLC. Future Oncol 2007;3:149-53 [DOI] [PubMed] [Google Scholar]
- 38.Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res 2004;10:4266s-9s [DOI] [PubMed] [Google Scholar]
- 39.Takai T, Kanaoka S, Yoshida K, Hamaya Y, Ikuma M, Miura N, et al. Fecal cyclooxygenase 2 plus matrix metalloproteinase 7 mRNA assays as a marker for colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 2009;18:1888-93 [DOI] [PubMed] [Google Scholar]


