Abstract
There is an urgent need to develop predictive indicators of the effect of species loss on ecosystem functioning. Body size is often considered as a good indicator because of its relationship to extinction risk and several functional traits. Here, we examined the predictive capacity of species body size in marine and freshwater multitrophic systems. We found a significant, but weak, effect of body size on functioning. The effect was much stronger when considering the effect of body size within trophic position levels. Compared to extinctions ordered by body size, random extinction sequences had lower multiple species loss effects on functioning. Our study is the first to show experimentally, in multitrophic systems, a more negative impact of ordered extinction sequences on ecosystem functioning than random losses. Our results suggest apparent ease in predicting species loss effect on functioning based on easily measured ecological traits that are body size and trophic position.
The effects of biodiversity loss on the functioning of natural ecosystems are not easily predictable1, especially for complex food webs2. They might depend on the competitive dominance or the trophic position of species lost, interaction strengths between species, functional traits of both species lost and those remaining in the system, and relative control of both biotic and abiotic factors over ecosystem properties3. A major challenge is to derive predictive and easily measurable indicators of biodiversity effects on functioning accounting for their complexity. Recent studies suggested that the effect of single species loss could be predicted without complete investigations of system interactions4.
Central to the metabolic theory of ecology, body size is linked to multiple biological rates such as growth, reproduction and mortality5,6. Measuring body size is an easy way to collapse co-varying traits over a single dimension, with no need to observe these traits directly7. In nature, species removal rarely happens randomly, contrary to simulations performed in past studies8. Larger species are often considered more vulnerable to extinction9 than smaller ones because of low population densities, slow growth, high energetic requirements and overexploitation10. Simulations also showed that losses of larger species would have a greater effect on ecosystem functions, such as bioturbation, than smaller species9.
We experimentally tested the predictability of body size for ecosystem functioning. We conducted experimental extinctions in multitrophic systems of up to 10 taxa in marine and freshwater mesocosms and assessed subsequent effects on ecosystem function. We predicted that 1) the effect of removing a taxon on the system will increase with its body size. Then, we compared the effect of random and non-random multi species sequential extinctions. We predicted that 2) non-random sequential extinctions (ordered by body size) will increase the rate of change in ecosystem functions compared to random extinction sequences.
Results
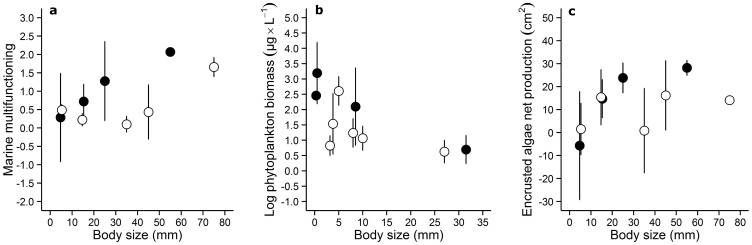
We found a significant positive effect of body size on marine multifunctioning (1st PCA axis only; adj. R2 = 0.14, Fig. 1, Table 1). Body size had a negative effect on phytoplankton biomass (adj. R2 = 0.30) in the freshwater ecosystem (Table 2). We also found a significant effect of body size on encrusted net production in the marine ecosystem once controlling for trophic position (adj. R2 = 0.14). The addition of trophic position also increased the proportion of variance explained by body size in marine multifonctioning (adj. R2 = 0.33; ΔAIC = 6.06) and phytoplankton biomass (adj. R2 = 0.44; ΔAIC = 5.86).
Figure 1. Effects of body size on ecosystem functioning.
The proxies of ecosystem functioning illustrated here are (a) marine multifunctioning, (b) freshwater phytoplankton biomass and (c) marine encrusted algae net production.  indicate grazers and
indicate grazers and  indicate non-grazers. The y-axis refer to the effect size between values of functioning recorded in mesocoms with removals and values of functioning recorded in reference mesocoms (no removal).
indicate non-grazers. The y-axis refer to the effect size between values of functioning recorded in mesocoms with removals and values of functioning recorded in reference mesocoms (no removal).
Table 1. Statistical results for the marine system. a) linear regression, b) ANCOVA with trophic position as predictor and c) asymmetrical two-way crossed ANOVA for the marine system with Number of taxa removed (no) and Sequence as main factors. The variability of the factor Sequence was then divided between 1) random versus non-random extinction sequence (R vs NR) and 2) differences among random extinctions sequences (among random). P values in bold correspond to significant results.
| Marine system | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sources of variation | Periphyton dry mass | Macroalgae net production | Encrusted algae net production | Multifunctioning (1st axis) | Multifunctioning (2nd axis) | |||||||
| F | p | F | p | F | p | F | p | F | p | |||
| (a) | Adjusted R2 | 0.0432 | 0.0000 | 0.0985 | 0.1383 | 0.0000 | ||||||
| Body size | 2.1745 | 0.1528 | 0.5843 | 0.4518 | 3.8396 | 0.0613 | 5.1726 | 0.0318 | 0.4449 | 0.5109 | ||
| (b) | Adjusted R2 | 0.0568 | 0.3093 | 0.1373 | 0.3341 | 0.1640 | ||||||
| Body size | 1.4243 | 0.2444 | 2.8514 | 0.1042 | 5.1714 | 0.0322 | 9.9722 | 0.0043 | 0.0250 | 0.8758 | ||
| Trophic role | 1.3587 | 0.2552 | 12.7837 | 0.0015 | 2.1259 | 0.1578 | 8.3510 | 0.0081 | 6.5560 | 0.0172 | ||
| (c) | Adjusted R2 | 0.2569 | 0.4206 | 0.4158 | 0.5570 | 0.4422 | ||||||
| No. taxa removed | 5.4311 | 0.0143 | 8.6657 | 0.0023 | 0.6311 | 0.5434 | 5.6244 | 0.0127 | 1.1036 | 0.3531 | ||
| Sequence | ||||||||||||
| R vs NR | 8.7416 | 0.0044 | 0.1243 | 0.7256 | 1.1647 | 0.2848 | 6.2287 | 0.0153 | 11.8585 | 0.0011 | ||
| Among random | 2.0768 | 0.0522 | 3.5819 | 0.0019 | 4.3657 | 0.0003 | 5.0189 | 0.0001 | 4.8956 | 0.0001 | ||
| No. × Sequence | ||||||||||||
| No. × (R vs NR) | 1.5384 | 0.2231 | 0.3216 | 0.7262 | 0.1004 | 0.9046 | 0.3542 | 0.7032 | 1.8618 | 0.1643 | ||
| No. × (Among random) | 1.1490 | 0.3343 | 2.0241 | 0.0257 | 3.2733 | 0.0004 | 3.5915 | 0.0002 | 2.4692 | 0.0060 | ||
Table 2. Statistical results for the freshwater system. a) linear regression, b) ANCOVA with trophic position as predictor and c) asymmetrical two-way crossed ANOVA for the freshwater system with Number of taxa removed (no) and Sequence as main factors. The variability of the factor Sequence was then divided between 1) random versus non-random extinction sequence (R vs NR) and 2) differences among random extinctions sequences (among random). P values in bold correspond to significant results.
| Sources of variation | Freshwater system | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phytoplankton biomass | Periphyton dry mass | Bacterioplankton abundance | [Total nitrogen] | [Total phosphorus] | Multifunctioning (1st axis) | Multifunctioning (2nd axis) | |||||||||
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | ||
| (a) | Adjusted R2 | 0.2999 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0165 | |||||||
| Body size | 13.4256 | 0.0010 | 0.2444 | 0.6249 | 0.4730 | 0.4972 | 0.1384 | 0.7127 | 0.0109 | 0.9175 | 0.2447 | 0.6247 | 1.4866 | 0.2329 | |
| (b) | Adjusted R2 | 0.4413 | 0.0000 | 0.0335 | 0.0000 | 0.0000 | 0.0703 | 0.1022 | |||||||
| Body size | 17.5880 | 0.0003 | 0.2159 | 0.6459 | 0.5749 | 0.4549 | 0.1317 | 0.7195 | 0.0178 | 0.8950 | 0.3427 | 0.5631 | 1.7931 | 0.1917 | |
| Trophic role | 8.0858 | 0.0084 | 0.6096 | 0.4417 | 2.5059 | 0.1251 | 0.0045 | 0.9471 | 0.7745 | 0.3866 | 3.9235 | 0.0579 | 3.6714 | 0.0660 | |
| (c) | Adjusted R2 | 0.4609 | 0.2022 | 0.0156 | 0.0000 | 0.0000 | 0.2563 | 0.0000 | |||||||
| No. taxa removed | 3.6046 | 0.0460 | 0.1042 | 0.9015 | 0.3314 | 0.7218 | 0.9490 | 0.4039 | 0.5676 | 0.5758 | 0.1468 | 0.8644 | 0.3430 | 0.7137 | |
| Sequence | |||||||||||||||
| R vs NR | 10.3540 | 0.0020 | 0.3367 | 0.5637 | 0.1100 | 0.7412 | 0.0269 | 0.8702 | 2.6849 | 0.1061 | 0.3198 | 0.5736 | 0.2252 | 0.6367 | |
| Among random | 4.6910 | 0.0001 | 2.4851 | 0.0165 | 1.4601 | 0.1815 | 1.0383 | 0.4197 | 1.4285 | 0.1942 | 3.3077 | 0.0022 | 1.4527 | 0.1844 | |
| No. × Sequence | |||||||||||||||
| No. × (R vs NR) | 0.4688 | 0.6278 | 1.7206 | 0.1869 | 0.2273 | 0.7973 | 0.2713 | 0.7632 | 0.2107 | 0.8106 | 0.5428 | 0.5837 | 0.1102 | 0.8958 | |
| No. × (Among random) | 2.5285 | 0.0033 | 1.6856 | 0.0648 | 1.0667 | 0.4038 | 0.7946 | 0.6991 | 0.5916 | 0.8932 | 1.8936 | 0.0318 | 0.9900 | 0.4819 | |
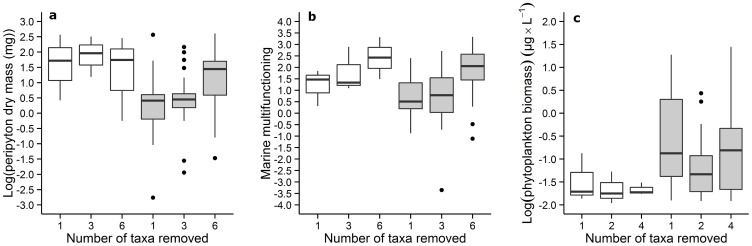
Asymmetrical ANOVA showed that the effect sizes of periphyton dry mass and marine multifunctioning were greater for extinction sequences ordered by body size, and the phytoplankton biomass lower, compared to random extinction sequences (Fig. 2). In fact, the effect of the first taxon removed (the intercept) was greater for ordered sequences in both systems, but then the effect of subsequent removal converged and the slope of the extinction-functioning relationship was not different between the two sequences. We found no significant effect of the number of taxa removed on other functions.
Figure 2. Effects of taxa removal on ecosystem functioning according to random and non-random extinction sequences.
The proxies of ecosystem functioning illustrated here are (a) periphyton dry mass (b) marine multifunctioning and (c) freshwater phytoplankton biomass. White boxplots indicate non-random extinction sequences and grey boxplots indicate random extinction sequences. The y-axis refer to the effect size between values of functioning recorded in mesocoms with removals and values of functioning recorded in reference mesocosms (no removal).
Discussion
The experiment, performed with two different aquatic ecosystems, showed a stronger effect of large taxa extinctions on ecosystem functions (supportive of prediction #1). The removal of smaller taxa from the marine ecosystem, such as Littorina spp. and T. testudinalis benefited encrusted algae net production, while macroalgae net production increased when larger taxa such as S. droebachiensis were removed. Conversely, the removal of large taxa in the freshwater ecosystem decreased phytoplankton biomass. It is noteworthy that body size had predominantly affected functions directly related to consumption. This result might be explained by increasing strength of per capita trophic interactions with body size4.
Although body size can be viewed as a measure aggregating several traits influencing ecosystem functioning7,9,11, it was not sufficient to explain alone the variability observed in functioning. The addition of the trophic position as a second explanatory variable revealed a stronger relationship between grazers body size and functioning than for the other taxa. The predictive capacity of the body size was therefore much more convincing when combined to other traits related to trophic position. Extinction risk and its subsequent effects on functioning might therefore depend on the trophic position, with greater effects associated with the loss of large consumers12,13.
We also found contrasting effects of random and non-random multi-taxa extinction sequences, in agreement with simulation studies9,14,15,16. In the marine ecosystem, both sequences overall led to higher periphyton dry mass and multifunctioning, but non-random extinction sequences impacted more on the functioning. Conversely, the decrease in phytoplankton biomass following non-random extinctions occurred at lower values of biomass in the freshwater ecosystem. For both systems, the effect of the first taxon removal was stronger for the ordered than the random sequences, but then the slope of the extinction-functioning relationship was the same.
As mentioned above, larger taxa have greater risks to get extinct9,10. Here, our results suggest that in addition, the consequences of losing these larger taxa might be more significant for the ecosystem functioning. The conjugation of these two factors indicates that we should be more concerned by the non-random character of species extinctions. To our knowledge, our study is the first to show experimentally the negative effects of ordered extinctions sequences in multitrophic systems. However, the loss or gain of a particular function, as the number of taxa removed increases, did not occur more rapidly following a non-random extinction, contrary to our second hypothesis. It was previously shown that the complexity of interactions within ecological food webs makes biodiversity-ecosystem functioning relationships idiosyncratic and almost unpredictable2,17. We indeed observed a much larger variability among random sequences. The analysis of identity effects in this system revealed that species interactions in food webs might balance each other and therefore prevent the observation of any general relationship between biodiversity and ecosystem functioning17.
Our experiment reports the immediate effect of removing the largest organisms on ecosystem functioning. Our hypothesis was based on the idea that larger organisms have higher absolute energy consumption. However, metabolic theory also predicts that the efficiency of energy consumption should increase with species body size6. Consequently, over the long-term, we should expect that the loss of large species would be compensated by less efficient smaller ones. It was not possible to test for this second mechanism due to the short time-scale of the experiment. Our experimental design captured a fraction of the potential effects of losing larger taxa on the functioning. We should expect that compensatory growth over the long-term would result in altered productivity and nutrient cycling. Further long-term investigations testing specifically for the consequences of changes in energy use with the loss of larger species would be necessary before the application of the current findings to broader realistic extinction scenarios.
Global warming and overexploitation of resources are likely to induce a shift toward reduced species body size in aquatic ecosystems18,19, and will ultimately affect ecosystem functioning20. Studies linking directly body size to ecosystem functioning are essential to assess and predict subsequent effects of changes in size spectra on ecosystem dynamics. Our experimental study conducted in parallel in two aquatic systems showed that predictions of species loss effects, in multitrophic food webs, might not be efficiently achieved when based simply on body size, but showed great potential when combined with other easily measured ecological traits such as the trophic position.
Methods
Removal sequences
We ran two complementary experiments in parallel, with the same experimental design, both in marine and freshwater mesocosms (Supplementary Fig. S1). Starting from a regional pool of 9 marine and 10 freshwater taxa, we assembled the different mesocosm communities by removing 0, 1, 3, 6 and 0, 1, 2, 4 taxa from the regional pool respectively in the marine and freshwater systems, according to two sequences: random and non-random. A non-random removal sequence denotes removals ordered by body size, from the largest to smallest. In the random sequences, all taxa were randomly removed from the regional pool such that each unique taxa was removed an equal number of times at each level in order to evenly distribute the effect of removing each taxa across the experiment (further details in ref. 17). All combinations were replicated three times, for a total of 93 marine and 102 freshwater mesocosms. We did not perform any compensatory readjustment of biomass following taxa removal because the organisms we studied are discrete and have low population size, and it would have required too many assumptions about the redistribution of biomass among remaining groups (see discussion on this issue in ref. 17). The short duration of the experiment also prevented a population response to the removal of taxa. The experiment therefore has to be interpreted as a report of immediate effects of taxa removal on ecosystem functioning, before any compensatory growth takes place.
Marine mesocosms
Marine mesocosms (21 L) were maintained during 6 weeks with a supply of filtered surface water (~50 μm) from the Lower St.Lawrence estuary. Taxa consisted of 9 representative species of the neighbouring sublittoral: Cancer irroratus (70–80 mm), Strongylocentrotus droebachiensis (50–60 mm), Mytilus edulis (40–50 mm), Nucella lapillus (30–40 mm), Littorina littorea (20–30 mm), Gammarus spp. (10–20 mm), Testudinalia testudinalis (10–20 mm), Semibalanus balanoides (0–10 mm) and a mixture of Littorina saxatilis/obtusata (0–10 mm) (Supplementary Fig. S2). We chose length over body mass as potential measure of body size. Both approaches would induce biases by overestimating the metabolically active mass among organisms with heavy shells, compared with species of similar mass but having thinner shell, if any. In this experiment, length was preferred over body mass for technical issues, particularly in the freshwater system in which organisms exhibited very small body mass. Densities were adjusted to recorded densities where organisms were collected. We measured changes in periphyton dry mass, macroalgae and encrusted algae net production as proxies of ecosystem function. Periphyton dry mass was assessed from a 1 cm × 1 cm sample of pre-incubated Hester-dendy plate (dry mass; 24 hours at 60°C). We estimated macroalgae net production by weighing the residual biomass from 100 g of the brown algae Fucus evanescens, placed in each of the mesocosms at the beginning of the experiment. We determined encrusted algae net production (Ralfsia verrucosa) in calculating changes in the algae cover present on a single rock placed in each mesocosm at the day 0, with image processing program ImageJ (National Institute of Health, USA).
Freshwater mesocosms
Freshwater mesocosms (60 L) were filled with 40 L of filtered freshwater (20 μm nylon mesh) and maintained outdoors for 8 weeks. Taxa consisted of small zooplankton (0.063–0.5 mm), large zooplankton >0.5 mm), Hyalellidae (3–4 mm), Dystiscidae (3–4 mm), Corixidae (5 mm), Coenagrionidae (6–14 mm), Planorbidae (6–11 mm), Gerridae (7–9 mm), Cyprinidae (22–32 mm) and Lymnaeidae (27–36 mm). We considered five ecosystem properties as proxies of ecosystem function: phytoplankton biomass, periphyton dry mass, bacterioplankton abundance, total nitrogen and total phosphorus concentration. Phytoplankton biomass was determined from 150 ml water samples filtered onto Whatman GF/F filters and extracted for 24 hours in 90% acetone, at 5°C in the dark21. Chlorophyll a and phaeopigment concentrations were calculated after measuring fluorescence before and after acidification (HCl 1 M) with a 10-AU fluorometer (Turner Designs, Sunnyvale, USA)22. Periphyton dry mass was assessed as for the marine ecosystem. Bacterioplankton abundance was measured using standard flow cytometric analysis. Samples for bacteria abundance determination were fixed with glutaraldehyde 0.1% final concentration and stored at −80°C until flow cytometry analysis23. Total nitrogen (TN) and total phosphorus (TP) were measured using the copper-cadmium standard reduction method for autoanalyzer after alkaline persulfate digestion24.
Statistics
In all analyses, for the marine and the freshwater ecosystems, we considered respectively 3 and 5 measures of ecosystem functioning and a measure of multifunctioning. For multifunctioning, we conducted a PCA and used scores of the two axes as the dependent variable. The first and second axes explained respectively 46.3% and 31.9% of the variation in the marine ecosystem (Supplementary Table S1). In the freshwater ecosystem, these axes explained respectively 25.3% and 22.5% of the variation. In the marine ecosystem, positive scores on the first axis were mostly linked to encrusted algae net production and negative scores to macroalgae net production. In the freshwater ecosystem, positive scores were attributable mostly to periphyton dry mass and negative scores to the combination of the four others functions measured.
The effect of the body size of single taxon extinction on ecosystem functioning was tested with linear regression (Hypothesis 1). We also controlled with ANCOVA for the effect of the trophic position (grazers vs non-grazers) as a categorical variable. The difference between the effect of random and non-random extinction sequences was tested with an asymmetrical crossed two-way ANOVA, with Number of taxa removed and Sequence as main factors. The variability of the factor Sequence was then divided between: 1) random versus non-random extinction sequences and 2) among random extinctions sequences (Hypothesis 2, Supplementary Table S2). Normality was assessed using the Shapiro-Wilk test and homogeneity of variance was assessed using explanatory checks of plots of residuals against predicted values.
Author Contributions
A.S., E.H., P.A., C.N. and D.G. designed the study; A.S. and E.H. collected the organisms, conducted the experiment and performed the lab analyses; A.S., E.H., P.A., C.N. and D.G. contributed to the data collection; A.S. performed the statistical analyses and drafted the manuscript, A.S. and D.G. analysed the data; A.S., E.H., P.A., C.N. and D.G. discussed the results and commented on the manuscript.
Supplementary Material
Supplementary figures and tables
Acknowledgments
This research was supported by NSERC, CHONe, Québec-Ocean and the Canada Research Chair Program. We thank Michèle Rousseau, Aline Carrier, Renaud McKinnon, Marie-France Lavoie, Vanessa Pencalet, Nathalie Morin, Jean Lambert and Isabelle Côté.
References
- Naeem S. Ecosystem consequences of biodiversity loss: the evolution of a paradigm. Ecology 83, 1537–1552 (2002). [Google Scholar]
- Poisot T., Mouquet N. & Gravel D. Trophic complementarity drives the biodiversity-ecosystem functioning relationship in food webs. Ecol. Lett. 16, 853–861 (2013). [DOI] [PubMed] [Google Scholar]
- Hooper D. U. et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–36 (2005). [Google Scholar]
- Berlow E. L. et al. Simple prediction of interaction strengths in complex food webs. Proc. Nat. Acad. Sci. USA 106, 187–191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. H. in The Ecological Implications of Body Size (Cambridge, Cambridge University Press, 1983).
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M. & West G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- Woodward G. et al. Body size in ecological networks. Trends Ecol. Evol. 20, 402–409 (2005). [DOI] [PubMed] [Google Scholar]
- Raffaelli D. How extinction patterns affect ecosystems. Science 306, 1141–1142 (2004). [DOI] [PubMed] [Google Scholar]
- Solan M. et al. Extinction and ecosystem function in the marine benthos. Science 306, 1177–1180 (2004). [DOI] [PubMed] [Google Scholar]
- Cardillo M. et al. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005). [DOI] [PubMed] [Google Scholar]
- Finke D. L. & Denno R. F. Predator diversity dampens trophic cascades. Nature 429, 407–410 (2004). [DOI] [PubMed] [Google Scholar]
- Duffy J. E. Biodiversity loss, trophic skew and ecosystem functioning. Ecol. Lett. 6, 680–687 (2003). [Google Scholar]
- Petchey O. L. et al. Species loss and the structure and functioning of multitrophic systems. Oikos 104, 467–478 (2004). [Google Scholar]
- Ives A. R. & Cardinale B. J. Food-web interactions govern the resistance of communities after non-random extinctions. Nature 429, 174–177 (2004). [DOI] [PubMed] [Google Scholar]
- Gross K. & Cardinale B. J. The functional consequences of random vs. ordered species extinctions. Ecol. Lett. 8, 409–418 (2005). [Google Scholar]
- Isbel F. et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl Acad. Sci. USA 110, 11911–11916 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey É., Séguin A., Nozais C., Archambault P. & Gravel D. Identity effects dominate the impacts of multiple species extinctions on the functioning of complex food webs. Ecology 94, 169–179 (2013). [DOI] [PubMed] [Google Scholar]
- Pauly D., Christensen V., Dalsgaard J., Froese R. & Torres Jr F. Fishing down marine food webs. Science 279, 860–863 (1998). [DOI] [PubMed] [Google Scholar]
- Daufresne M., Lengfellner K. & Sommer U. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106,12788–12793 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossena M. et al. Warming alters community size structure and ecosystem functioning. Proc. R. Soc. Lond. B 279, 3011–3019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. R. & Maita Y. L. in A Manual of Chemical and Biological Methods for Seawater Analysis (Toronto, Pergamon Press, 1984).
- Holm-Hansen O., Lorenzen C. J., Holmes R. W. & Strickland J. D. H. Fluorometric determination of chlorophyll. J. Conseil 30, 3–15 (1965). [Google Scholar]
- Belzile C., Brugel S., Nozais C., Gratton Y. & Demers S. Variations of the abundance and nucleic acid content of heterotrophic bacteria in Beaufort Shelf waters during winter and spring. J. Mar. Syst. 74, 946–956 (2008). [Google Scholar]
- Grasshoff K., Ehrhardt M. & Kremling K. in Methods of Seawater Analysis. (New York, Verlag Chemie, 1983).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables