Abstract
Studies of plasma amyloid-β levels as potential biomarkers for incident Alzheimer’s disease (AD) have yielded contradictory results. We explored the associations between plasma Aβ40, Aβ42, truncated amyloid-β levels and prognosis of dementia in participants of the prospective 3C-Study. 120 aged individuals diagnosed with 2-year incident dementia were followed up for 7 years. The associations between amyloid-β plasma levels and baseline cognitive score, cognitive decline and death were examined. A higher level of baseline plasma amyloid-β was associated with worse cognitive status 2 years prior to incident dementia diagnosis. In incident AD patients, the association was only significant for Aβ40 and Aβn-42. In the fast cognitive decliners group-especially in AD cases-a higher level of 5pg/ml of baseline Aβ42, Aβn-42,Aβn-42/Aβn-40 and Aβ42/Aβ40 ratios were associated with a lower risk of fast cognitive decline based on the IST score. There was no association between peptide levels and mortality in demented subjects. When assayed at prodromal stage, plasma amyloid-β levels may be potentially useful markers of fast cognitive decline in individuals who subsequently become demented.
Keywords: Aged; Aged, 80 and over; Alzheimer Disease; blood; mortality; Amyloid beta-Peptides; blood; Biological Markers; blood; Dementia; blood; mortality; Disease Progression; Female; Follow-Up Studies; France; epidemiology; Humans; Incidence; Male; Mild Cognitive Impairment; blood; mortality; Multivariate Analysis; Prognosis; Proportional Hazards Models; Prospective Studies; Risk Factors; Urban Population; statistics & numerical data
INTRODUCTION
It has been known for several years that low cerebrospinal fluid (CSF) levels of the 42-amino-acid amyloid-β fragment (Aβ42) are strongly associated with current or future Alzheimer’s disease (AD) in patients with mild cognitive impairment (MCI) [1]. However, since CSF sampling is an invasive, time-consuming procedure, there has been increasing interest in establishing whether the plasma amyloid-β levels could also be relevant [2]. In this context, we and others have reported that a low plasma amyloid-β Aβ42/Aβ40 ratio is associated with an increased risk of dementia in two large, longitudinal studies [3, 4]. Even though the latter observation is still controversial and calls for further investigation, several lines of evidence suggest that plasma amyloid-β levels could be a marker of the short-term dementia risk. In particular: (i) the association of a low plasma Aβ42/Aβ40 ratio with AD risk is restricted to individuals diagnosed during the two years following the assay [3, 4]; (ii) Amyloid-β plasma levels are associated with the risk of conversion to dementia in MCI [5]; (iii) conversion to AD is accompanied by a concomitant decrease in the plasma Aβ42/Aβ40 ratio [6]. These various observations suggest that in addition to their potential as an indicator of short-term dementia risk, amyloid-β plasma levels may also be markers of disease progression and may therefore be useful in terms of prognosis. Even though the prognosis for incident dementia has already been linked to CSF Aβ42 patterns, there are no data on the potential value of plasma amyloid-β levels as prognosis biomarkers. Hence, we decided to determine whether or not plasma amyloid-β levels are associated with the prognosis in dementia, measured as cognitive decline and mortality in incident demented cases from the French Three-City (3C) prospective cohort study of men and women aged 65 and over.
MATERIALS AND METHODS
The 3C study
The 3C Study is a population-based, prospective study of the relationship between vascular factors and dementia. It has been carried out in three French cities: Bordeaux, Montpellier and Dijon. Between January 1999 and March 2001, 9,294 non-institutionalized subjects aged 65 or over agreed to participate in the study. At inclusion, blood samples were obtained from 8,682 individuals. The baseline data collection included socio-demographic and lifestyle characteristics, symptoms and complaints, main chronic conditions, medication use and neuropsychological testing. These data were updated at each follow-up examination, performed in 2001–2003, 2003–2005, 2006–2008. Information on vital status and date of death was available until 2009 (Figure 1). The study protocol has been described in detail elsewhere [4, 7] and was approved by the Institutional Review Board at Kremlin-Bicêtre University Medical Center. All participants gave their written, informed consent to participation.
Figure 1.
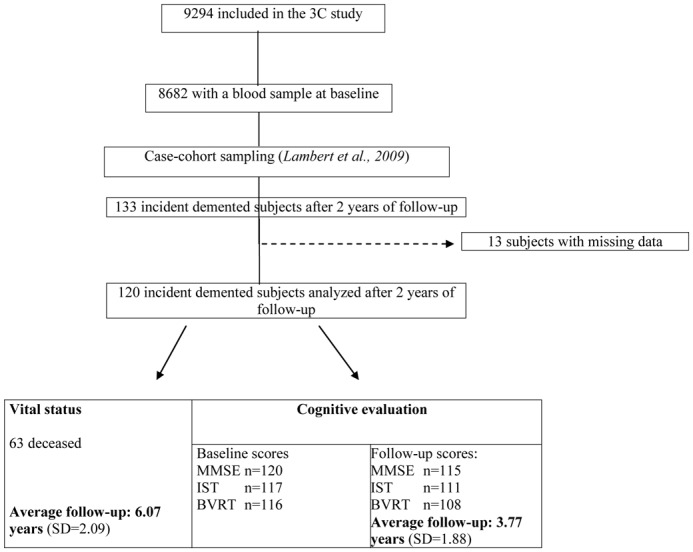
Participant disposition for the Three City (3C) Study.
Evaluation of dementia
At baseline and at each follow-up examination, participants were screened for dementia in a three-step procedure. First, specially trained psychologists administered a battery of neuropsychological tests. Second, patients who screened positive for dementia on the basis of neuropsychological tests were examined by a neurologist in Dijon. In Montpellier and Bordeaux, all participants were examined at baseline. During follow-up, participants with suspected incident dementia (on the basis of their neuropsychological test results) were all examined by a neurologist. Lastly, an independent committee of neurologists reviewed all potential prevalent and incident cases of dementia in order to obtain a consensus on the condition’s diagnosis and etiology, according to the criteria given in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). Dementia was classified according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria for AD and the National Institute of Neurologic Disorders and Stroke criteria for vascular dementia [8, 9]. Subjects with a typical history of AD (progressive worsening of memory or other cognitive functions) and documented stroke were classified as having mixed dementia. Our analysis included incident dementia cases at the 2-year examination. None of the participants was demented at the start of the study, when the plasma samples were taken.
Amyloid-β peptide assays
Non-fasting plasma samples were collected at baseline in tubes containing sodium EDTA as an anticoagulant. Following centrifugation, plasma samples were aliquoted into polypropylene tubes, stored at −80°C and only thawed immediately prior to amyloid-β quantification. The analyses were performed in a single centralized laboratory in Lille (SS, LB). Plasma amyloid-β peptide levels were measured blind to cognitive status. The plasma amyloid-β peptide assay was performed using the INNO-BIA kit (Innogenetics, Ghent, Belgium), based on a multiplex xMAP technique with a LABScan-100 system (Luminex BV, The Netherlands). Aβ40 and Aβ42 (kit format A) and Aβn-40 and Aβn-42 (kit format B) were respectively determined. Amyloid-β peptide levels from each blood draw were measured in duplicate. The same plasma sample was measured in each assay and we obtained analogous coefficients of variations (CVs): 7% for Aβ40 and 6% for Aβ42. At this level, it is important to note that the affinities of antibodies used in formats A and B (3D6 and 4G8, respectively) for Aβ are different and this makes difficult the comparison of the absolute levels of the full-length forms with those of the truncated forms.
Cognitive functions
At baseline and during follow-up, the following cognitive tests were administered by trained staff: the Mini Mental State Examination (MMSE; theoretical score: 0–30), the Isaacs Set Test (IST, for testing overall fluency; total score in 4 categories at 15 seconds) and the Benton Visual Retention Test (BVRT, testing recall of previously presented designs; theoretical score: 0–15). The MCI status of each participant was defined at baseline of the recruitment. In the whole 3C population (n=9,294), 43.05% were defined as MCI (n = 3831 and 396 subjects with available data). The characteristics of the MCI subjects have been described elsewhere by our team [10]. The MCI subjects were defined according to the revised MCI criteria (MCI-R criteria) [11]. These criteria are those proposed by the Stockholm consensus group [12]. They stipulate: (i) presence of a cognitive complaint from either the subject and/or a family member; (ii) absence of dementia; (iii) change from normal functioning; (iv) decline in any area of cognitive functioning; (v) preserved overall general functioning but possibly with increasing difficulty in the performance of activities of daily living.
Statistical methods
Amyloid-β variables (Aβ40, Aβ42, Aβn-40, Aβn-42, Aβ42/Aβ40 and Aβn-42/Aβn-40) were examined as quantitative parameters. The multiple regression analyses used to probe associations between amyloid-β variables and cognitive score, have tested various known potential confounders: age (in years), gender, educational level (no schooling or primary school vs. secondary or university degree), study center, Apo E (presence or absence of the APOE ε4 allele), marital status (living alone, No/Yes), body mass index (BMI; normal value BMI<25 kg/m2 vs. overweight BMI≥25 kg/m2), alcohol consumption (No/Yes), smoking status (never-smoker vs. former or current smoker), self-reported history of vascular disease, hypertension (systolic/diastolic blood pressure ≥ 160 mmHg/≥95 mmHg, respectively, or current antihypertensive medication), hypercholesterolemia (fasting blood cholesterol >6.2 mmol/L or use of lipid-lowering medication), diabetes (fasting blood glucose ≥ 7.0 mmol/L or use of anti-diabetes treatment), depression (Center for Epidemiologic Studies Depression Scale (CES-D) score over 16 or current treatment for depression) and limitations in at least one of the Instrumental Activities of Daily Living (IADL).
In order to consider the longest cognitive follow-up period for each subject (Figure 1), the definition of cognitive decline was based on the difference between the last score noted during follow-up and the baseline score, divided by the length of follow-up (mean: 3.77 years, SD=1.88). Subjects were classified as “fast decliners” when they presented a decline equal to or greater than the upper quartile of the population as a whole (i.e. ≥ 2.13 points per year of follow-up for the MMSE score; ≥ 1.48 points/year for the BVRT score and ≥ 2.43 points/year for the IST score). Logistic regression models were used to determine the association between amyloid-β concentrations and the risk of fast cognitive decline, while controlling for potential confounders and the baseline cognitive score.
Associations between amyloid-β levels and the risk of mortality were determined by Cox proportional hazards regression, with age (in years) used for the time axis and left truncation at age of study entry.
Multivariate adjusted models included covariates that were associated with cognitive tests (MMSE, BVRT or IST) based cognitive decline or mortality (p< 0.15 in the univariate model). All analyses were performed first on the whole dementia population and then on probable or possible AD cases. The analyses were performed using SAS software (version 9.2, SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the subjects
During the first 2–year follow-up period, incident dementia was diagnosed in 133 subjects with available baseline plasma amyloid-β concentration data (Figure 1). Four individuals had aberrant plasma amyloid-β concentration value and 9 had missing data about confounding factors in the multivariate analysis. After exclusion of these 13 participants, the 120 analyzed cases of dementia were classified as follows: 81 cases of AD (38 possible and 43 probable), 23 cases of mixed/vascular dementia, 7 cases of dementia with Parkinson’s disease and 9 with other types of dementia (Lewy body dementia: n=4; dementia associated with multiple sclerosis, alcoholism, etc.: n=4; undefined dementia: n=1). The mean follow-up was 6.07 years (SD=2.09). Baseline cognitive tests scores of MMSE, IST and BVRT were available for 120, 117 and 116 subjects respectively. The mean follow-up concerning cognitive scores was 3.77 years (SD=1.88). During follow-up, 63 subjects deceased. The incident dementia population’s main baseline characteristics are summarized in Table 1. Mean age at baseline was 78.4 (SD=5.7) and 68 of the patients were females (56.7%). A primary school educational level was reported by 42.5% of subjects. High school and University educational level were noted in 17.5% of cases each. A history of CVD was reported in 37.5%. More than 50% of the subjects presented hypercholesterolemia (51.3%) and hypertension (57.5%). Overweight was observed in 36.2% and diabetes in 13.3%. The mean MMSE score was 25.6 (SD=1.99) at the baseline visit and 22.7 (SD=2.9) two years later (at time of diagnosis). The mean IST score was 24.8 (SD=5.7) at baseline and fell to 22.5 (SD=6.2) at the time of diagnosis. The BVRT score was 9.8 (SD=2.72) at baseline. 72.6% of the subjects were classified at baseline as having MCI and 26.7% had an IADL limitation. The mean values for all the amyloid-β levels are presented in Table 1. Levels were not correlated with age nor with sex in this sample (data not shown). Additionally, due to the link between albumin and plasma proteins levels, we evaluated the association between concentration of albumin and the risk of cognitive decline (IST decline (n=118): p=0.48, MMSE decline (n=122): p=0.84, Benton decline (n=115): p=0.23) and also between albumin and plasma amyloidβ-β (ββ40: p=0.8; Aβ42: p=0.2; Aβn-40: p=0.37; Aβn-42: p=0.86). No association was underlined. The total plasma proteins concentration was not available in our study.
Table 1.
Description of the 120 incident cases of dementia.
| Dementia | ||||
|---|---|---|---|---|
|
| ||||
| n | (%) | m (SD) | ||
| Gender | Female | 68 | 56.7 | |
| Age at baseline | (years) | 120 | 78.4 (5.7) | |
| Education | Primary | 51 | 42.5 | |
| Middle school | 27 | 22.5 | ||
| High school | 21 | 17.5 | ||
| University | 21 | 17.5 | ||
| Smoking status | Current or Former | 44 | 36.7 | |
| Body Mass Index | Normal | 63 | 54.3 | |
| Overweight | 42 | 36.2 | ||
| Obese | 11 | 9.5 | ||
| Hypercholesterolemia | 61 | 51.3 | ||
| Hypertension | 69 | 57.5 | ||
| History of CVD | 45 | 37.5 | ||
| Diabetes | 16 | 13.3 | ||
| Alcohol (g/day) | <1 | 22 | 18.3 | |
| 1–36 g | 83 | 69.2 | ||
| >36 g | 15 | 12.5 | ||
| Living status | Alone | 47 | 39.2 | |
| Apo E | At least 1 Apoε4 allele | 44 | 36.7 | |
| IADL | At least 1 | 32 | 26.7 | |
| Depression | CES-D score <16 or antidepressants | 53 | 44.2 | |
| MCI status | 85 | 72.6 | ||
| MMSE* (n=120) | 120 | 25.6 (1.99) | ||
| BVRT* (n=116) | 116 | 9.8 (2.72) | ||
| IST* (n=117) | 117 | 24.8 (5.7) | ||
| Plasma Aβ levels | ||||
| Aβ40 | pg/ml | 120 | 246.0 (56.39) | |
| Aβ42 | pg/ml | 120 | 39.8 (10.55) | |
| Aβn-40 | pg/ml | 120 | 269.4 (62.33) | |
| Aβn-42 | pg/ml | 120 | 27.7 (8.32) | |
| Aβ42/Aβ40 ratio | 120 | 0.2 (0.05) | ||
| Aβn-42/Aβn-40 ratio | 120 | 0.1 (0.03) | ||
Data are presented for the 120 demented subjects, except for the BVRT (n=116) and the IST (n=117).
SD: Standard Deviation; CVD: Cardiovascular Disease; Apo E: Apo lipoprotein E; IADL: Instrumental Activities of Daily Living; CES-D: Center for Epidemiologic Studies Depression Scale; MCI: Mild Cognitive Impairment; MMSE: Mini Mental State Examination; BVRT: Benton Visual Retention Test; IST: Isaacs Set Test.
The ApoEε4+/ε4+ status was found on 3 of the 120 patients (2.5% of the total sample).
Cognition
Cognitive impairment (Table 2)
Table 2.
Plasma amyloid-β levels (for higher level of 5 pg/ml) and cognitive scores at baseline in incident dementia cases and Alzheimer’s disease patients. A multiple linear regression model including gender, age, educational level, center, alcohol consumption, hypertension, IADL, depression and a history of vascular disease.
| All dementia | Alzheimer | |||
|---|---|---|---|---|
| β | p | β | p | |
| IST score | ||||
| Aβ40 | −0.13 | 0.005 | −0.13 | 0.04 |
| Aβ42 | −0.60 | 0,03 | −0.45 | 0.18 |
| Aβn-40 | −0.09 | 0.03 | −0.07 | 0.19 |
| Aβn-42 | −0.79 | 0.02 | −1.03 | 0.01 |
| Aβ42/Aβ40 | 1.68 | 0.18 | 1.64 | 0.30 |
| Aβn-42/Aβn-40 | −1.01 | 0.55 | −3.18 | 0.16 |
| BVRT score | ||||
| Aβ40 | −0.01 | 0.53 | −0.02 | 0.39 |
| Aβ42 | 0.16 | 0.20 | 0.04 | 0.79 |
| Aβn-40 | −0.002 | 0.90 | 0.01 | 0.57 |
| Aβn-42 | 0.001 | 0.99 | −0.17 | 0.33 |
| Aβ42/Aβ40 | 0.97 | 0.10 | 0.66 | 0.30 |
| Aβn-42/Aβn-40 | −0.11 | 0.89 | −1.54 | 0.09 |
| MMSE score | ||||
| Aβ40 | 0.02 | 0.32 | 0.03 | 0.27 |
| Aβ42 | 0.06 | 0.49 | 0.17 | 0.15 |
| Aβn-40 | −0.001 | 0.96 | 0.00003 | 1.00 |
| Aβn-42 | −0.01 | 0.95 | 0.10 | 0.51 |
| Aβ42/Aβ40 | −0.15 | 0.73 | 0.11 | 0.84 |
| Aβn-42/Aβn-40 | −0.10 | 0.89 | 0.36 | 0.66 |
|
| ||||
| IST | All dementia (n=117) | AD cases (n=80) | ||
| BVRT | All dementia (n=114) | AD cases (n=77) | ||
| MMSE | All dementia (n=117) | AD cases (n=80) | ||
In the whole demented population (“All dementia” in the Table 2), high levels of Aβ40 (β=−0.13; p=0.005), Aβ42 (β=−0.60; p=0.03), Aβn-40 (β= −0.09; p=0.03) and Aβn-42 (β= −0.79; p=0.02) were all associated with a lower IST verbal fluency score at baseline (i.e. 2 years before incident dementia diagnosis), according to a multiple linear regression model that included gender, age, educational level, center, alcohol consumption, IADL limitations, hypertension, depression and a history of vascular disease (Table 2). None of the associations between plasma levels and the baseline MMSE score or BVRT score reached statistical significance in the whole demented population.
For AD patients (“Alzheimer” in Table 2), the same multiple linear regression model revealed that high levels of Aβ40 and Aβn-42 were also correlated with a low IST score. In AD patients, there was no obvious relationship between the IST score on the one hand and the Aβ42 level or the two peptide ratios on the other. None of the associations between plasma levels and the baseline MMSE score or BVRT score reached statistical significance in AD cases (Table 2).
Faster cognitive decline (Table 3)
Table 3.
The risk of fast cognitive decline in all dementia cases and in AD cases: the crude and adjusted odds ratios (ORs) and 95% confidence intervals (CI) associated with each 5 pg/ml increase in the various plasma amyloid-β peptide levels. The adjusting factors were gender, age, educational level, center, alcohol consumption, hypertension, IADL, depression, cognitive score at baseline and a history of vascular disease.
| All | dementia | AD | ||||
|---|---|---|---|---|---|---|
| Adjusted OR | 95%CI | p | Adjusted OR | 95%CI | p | |
|
Isaac Set Test
a
| ||||||
| Aβ40 | 0.97 | 0.92–1.03 | 0.32 | 1.02 | 0.92–1.13 | 0.76 |
| Aβ42 | 0.70 | 0.51–0.96 | 0.03 | 0.35 | 0.15–0.81 | 0.01 |
| Aβn-40 | 0.96 | 0.92–1.01 | 0.11 | 0.96 | 0.90–1.03 | 0.23 |
| Aβn-42 | 0.82 | 0.56–1.20 | 0.31 | 0.29 | 0.11–0.75 | 0.01 |
| Aβ42/Aβ40 | 0.36 | 0.08–1.54 | 0.17 | 0.002 | 0.001–0.18 | 0.008 |
| Aβn-42/Aβn-40 | 1.43 | 0.24–8.53 | 0.70 | 0.02 | 0.001–1.01 | 0.05 |
|
| ||||||
|
Benton Visual Retention Test
b
| ||||||
| Aβ40 | 0.95 | 0.90–1.01 | 0.09 | 0.94 | 0.85–1.05 | 0.27 |
| Aβ42 | 0.72 | 0.54–0.97 | 0.03 | 0.76 | 0.47–1.21 | 0.25 |
| Aβn-40 | 0.99 | 0.95–1.04 | 0.68 | 1.02 | 0.95–1.10 | 0.59 |
| Aβn-42 | 0.84 | 0.58–1.21 | 0.35 | 0.95 | 0.53–1.71 | 0.86 |
| Aβ42/Aβ40 | 0.48 | 0.12–1.98 | 0.31 | 0.57 | 0.07–4.42 | 0.59 |
| Aβn-42/Aβn-40 | 0.46 | 0.06–3.56 | 0.46 | 0.33 | 0.01–11.49 | 0.54 |
|
| ||||||
|
MMSE
c
| ||||||
| Aβ40 | 1.01 | 0.97–1.06 | 0.51 | 1.03 | 0.97–1.10 | 0.36 |
| Aβ42 | 1.06 | 0.83–1.34 | 0.66 | 1.27 | 0.89–1.81 | 0.18 |
| Aβn-40 | 1.01 | 0.97–1.05 | 0.51 | 1.02 | 0.96–1.07 | 0.56 |
| Aβn-42 | 1.04 | 0.77–1.40 | 0.81 | 1.23 | 0.80–1.88 | 0.34 |
| Aβ42/Aβ40 | 1.11 | 0.35–3.53 | 0.86 | 0.96 | 0.19–4.96 | 0.96 |
| Aβn-42/Aβn-40 | 0.64 | 0.13–3.13 | 0.58 | 0.95 | 0.10–9.15 | 0.97 |
111 all dementia cases, including 28 fast decliners; 76 AD cases, including 14 fast decliners.
108 all dementia cases, including 27 fast decliners; 73 AD cases, including 12 fast decliners.
111 all dementia cases, including 29 fast decliners; 75 AD cases, including 15 fast decliners.
Of the demented subjects (“All dementia”) with cognitive follow-up data, 29, 28 and 27 were defined as fast decliners according to the MMSE, the IST and the BVRT scores, respectively. In a multivariate analysis, a higher level of 5pg/ml of baseline Aβ42 level was associated with a decreased risk of cognitive decline for both the IST (odds ratio (OR) =0.70; 95% confidence interval (CI) [0.51–0.96], p=0.03) and BVRT scores (OR=0.72; 95%CI [0.54–0.97], p=0.03) (Table 3). None of the associations between plasma levels and faster cognitive decline according to the MMSE score reached statistical significance in the “all dementia” group.
For the 76 AD cases (including 15, 14 and 12 fast decliners, according to the MMSE, IST and BVRT scores, respectively), the risk of cognitive decline according to the IST decreased significantly with a higher level of 5 pg/ml of plasma Aβ42 at baseline (OR= 0.35; 95%CI 0.15–0.81], p=0.01), Aβn-42 (OR= 0.29; 95%CI [0.11–0.75], p=0.01), and with a higher level of the two ratios Aβ42/ Aβ40 (OR= 0.002; 95%CI [0.001–0.18], p=0.008) and Aβn-42/ Aβn-40 (OR= 0.02; 95%CI [0.001–1.01], p=0.05) (Table 3). Associations with BVRT were not confirmed in AD patients. None of the associations between plasma levels and cognitive decline according to the MMSE score reached statistical significance in AD cases.
Mortality
During the follow-up period (mean: 6.07 years, SD=2.09), 63 demented patients (including 35 AD cases) died. In neither univariate nor multivariate analyses was the risk of death associated with plasma amyloid-β levels in the dementia patients or in AD cases (Supplementary Table 1).
DISCUSSION
To the best of our knowledge, this is the first report to show that plasma levels of some forms of truncated or non-truncated amyloid-β peptides and their ratios are associated with faster cognitive decline in non-demented subjects converting to dementia during a 2-year follow-up. We also found an association between an elevated plasma amyloid-β level and poorer cognitive status two years before the dementia diagnosis.
Our findings raise issues concerning the role of blood-based biomarkers in AD and prompt reexamination of the ways in which measuring amyloid-β and its truncated forms might be helpful, especially in terms of prognosis. Most of the data stay controversial in this aspect and few studies have examined potential relationships between prognosis and plasma amyloid-β levels. Interestingly, we observed a decreased risk of faster cognitive decline with higher plasma levels of Aβ42, Aβn-42, and with higher Aβ42/Aβ40 and Aβn-42/Aβn-40 ratios in AD cases, whereas in the whole group of demented patients only Aβ42 was associated with a faster cognitive decline as measured by the IST and also by the BVRT score. It should be noted that the association between amyloid-β and fast cognitive decline was the strongest evidenced for verbal fluency, and not significant with the BVRT nor the MMSE, probably because the IST is one of the domains known to be affected early in the course of AD as illustrated in the PAQUID study [13]. Moreover, the MMSE as a global neuropsychological test is not relevant for early AD. The first analysis of the Northern Manhattan community cohort determined that high plasma Aβ42 levels were associated with faster cognitive decline-primarily in memory-in non-demented elders [14]. In line with our results Schupf et al. (2008) showed that declines in Aβ42 levels or in the Aβ42/Aβ40 ratio are sensitive indicators of recent conversion to AD [5]. A similar association between the Aβ42/Aβ40 ratio and an imminent risk of cognitive decline was reported in the Mayo Rochester AD patient registry [15]. Our results are also in agreement with a very recent report from Yaffe et al., (2011) in which low plasma Aβ42/Aβ40 ratio in dementia-free, community-dwelling older adults appears to predict more rapid cognitive decline than in subjects with a high ratio [16].
The advantage of our study is that our design enabled us to examine plasma Aβ42 and Aβ40 levels and their truncated forms in the 2-year interval preceding diagnosis of dementia, which can be considered as corresponding (at least in part) to prodromal AD. With reference to Jack’s dynamic model, amyloid-β peptides may be pathological early in the course of the disease [17]. We found an association between an elevated plasma amyloid-β level and poorer cognitive status two years before the dementia diagnosis. Poorer verbal fluency was correlated with higher plasma levels of all forms of amyloid-β in incident dementia cases and only with Aβ40 and truncated Aβn-42 levels in AD patients. These results were independent of various demographic, lifestyle and vascular factors. Cross-sectional [18–20] and longitudinal studies of plasma Aβ42 levels in normal or MCI patients [1, 6] have generated conflicting results [21]. In the Northern Manhattan community cohort with repeated assays, authors indicated that plasma levels of Aβ42 began to decrease soon after diagnosis of AD [22]. Although a higher risk of progression towards AD was observed in MCI patients with low levels of plasma Aβ42 and a low Aβ42/Aβ40 ratio [3, 6, 18], the mean plasma Aβ42 and Aβ40 levels and amyloid-β peptide ratios have not been found to have predictive value in this context. Some articles have reported slightly higher Aβ42 or Aβ40 plasma levels in patients with AD than in healthy age-matched controls and that high level of plasma Aβ42 and a large Aβ42 /Aβ40 ratio are risk factors for future AD [3, 4, 22–24].
There are several possible disparities between literature findings and our present results; they include different sample collecting, processing and plasma amyloid-β assay methods, lower amyloid-β levels in plasma than in CSF, circadian fluctuations in amyloid-β levels [25], and peripheral confounding factors (platelets, the binding of Aβ with albumin, cholesterol content and other proteins) [26–29]. First, due to the population-based study design, we do not have CSF or functional neuroimaging biomarkers such as Pittsburgh Compound B (PIB) or florbetapir positron emission tomography brain neuro-imaging to compare to plasma amyloid-β results. The prognosis in dementia has been linked to patterns of CSF biomarkers [30–33]. Although functional neuroimaging data in a pre-prodromal stage of the disease are still a matter of debate, the correlation between plasma Aβ isoforms and fibrillar brain amyloid-β load as measured by PIB retention is underlined [34]. Aβ42 as well as the Aβ42/Aβ40 ratio in plasma were significantly lower in AD patients and inversely correlated with in vivo measures of fibrillar brain Aβ load as measured by PIB [35]. Concerning confounding factors, except for albumin which does not show any correlation in our study, the role of platelets cannot be ignored. Platelets represent a source of circulating Aβ and AβPP [27] and mostly contain Aβ ending at residue 40, with a small amount of Aβ42, in both the quiescent and activated states [29]. It is known that, in the brain, Aβ peptides exist both within defined deposits (plaques and vascular) and as a diverse array of other forms including soluble, membrane associated and intracellular species that may play far more significant roles in the production of dementia than the molecules sequestered in extracellular plaques. Aβ peptides are generated outside of the central nervous system in appreciable quantities by the skeletal muscle, platelets and vascular walls [26, 36, 37] and in other non-neural tissues [38, 39]. These distinct reservoirs may allow for an active and dynamic interchange of Aβ peptides between the brain and periphery. Even if the efforts to validate plasma Aβ peptides as dementia biomarkers have been fraught with frustration, the critical role that circulating Aβ peptides play in AD pathology cannot be ignored. Moreover, our method is solid and we think that peripheral Aβ could be sufficient to define a model for disease progression as described also in previous studies [40] and as a marker of incipient AD. Further investigations are needed about brain Aβ turnover and metabolism and exact relationship between plasma and brain Aβ.
The analysis of the Aβ truncated forms has been insufficiently studied by other groups despite the important literature on the matter. The potential relevance of truncated amyloid-β peptides in AD has been underlined by Buée et al., who showed in early stages of AD, through post-mortem analysis of brain insoluble fractions, that these species represent more than 60% of all amyloid-β species in Alzheimer’s pathology [41]. These truncated species have been shown to induce learning impairments and neuronal apoptosis when injected in murine models [42]. Moreover, they are detectable in CSF and it has been suggested that their levels are relevant in the diagnosis of AD [43]. However, little is known about their relationship with cognitive decline; especially fast cognitive decline, giving to our study a major impact. Interestingly, distinctive non-linear relationships with cognitive scores and plasma biomarkers have been underlined in our study as previously reported with CSF biomarkers [30–32, 44–49]. The weight of CSF biomarkers is not the same in all stages of AD, probably because of the kinetics of CSF biomarkers during the course of cognitive impairments. CSF Aβ40 levels showed a biphasic curve, whereas CSF Aβ42 levels showed a monotonic but non-linear dependence on cognitive scores, with major variations up to the change point and then mild or no variations [44, 50].
We did not find any associations with mortality - another indicator of prognosis or more aggressive evolution of the disease but our sample size limits the power of this analysis. An association between high levels of Aβ42 and mortality has been reported in AD patients [22] and Down’s syndrome patients [51].
Although our results concern a small number of incident cases and a single measurement of amyloid-β levels, their relevance is reinforced by the large size of the 3C study database. We controlled for factors known to be associated with the AD prognosis and/or plasma amyloid-β levels, such as age [18–20, 22] and cardiovascular factors [52]. Even though our cognitive evaluation was limited in comparison with the extensive battery used in clinical research, the IST score is known to be impacted early in the course of the disease. It is also easy to apply in routine clinical daily (especially by primary care physicians) and could be used to develop a preventive approach to dementia.
When assayed at a prodromal stage, plasma amyloid-β levels may be potentially useful markers of fast cognitive decline in individuals who subsequently become demented. Current results also lend to the potential utility of plasma amyloid-β as an indicator of disease progression. Our results need to be replicated in larger populations and with several assays over time per subject before any clinical recommendation can be made. Nevertheless, we believe that our present data raise intriguing questions about peripheral biomarkers of brain lesions in AD and how they relate to current and future cognitive status.
References
- 1.Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, Wallin A, Minthon L, Blennow K. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Shoji M. Biomarkers of the dementia. Int J Alzheimers Dis. 2011;2011:564321. doi: 10.4061/2011/564321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 4.Lambert JC, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, Dartigues JF, Tzourio C, Alperovitch A, Buee L, Amouyel P. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009;73:847–853. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- 5.Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P, Ravetch J, Mayeux R. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fei M, Jianghua W, Rujuan M, Wei Z, Qian W. The relationship of plasma Abeta levels to dementia in aging individuals with mild cognitive impairment. J Neurol Sci. 2011;305:92–96. doi: 10.1016/j.jns.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–325. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 8.Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease : report of the NINCDS-ADRDA Work Group under the auspices of Department of Health Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. 939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 10.Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–984. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 11.Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008 doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 12.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 13.Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 14.Cosentino SA, Stern Y, Sokolov E, Scarmeas N, Manly JJ, Tang MX, Schupf N, Mayeux RP. Plasma ss-amyloid and cognitive decline. Arch Neurol. 2010;67:1485–1490. doi: 10.1001/archneurol.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of Low Plasma Abeta42/Abeta40 Ratios With Increased Imminent Risk for Mild Cognitive Impairment and Alzheimer Disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, Younkin LH, Kuller L, Ayonayon HN, Ding J, Harris TB. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui JK, Laws SM, Li QX, Villemagne VL, Ames D, Brown B, Bush AI, De Ruyck K, Dromey J, Ellis KA, Faux NG, Foster J, Fowler C, Gupta V, Hudson P, Laughton K, Masters CL, Pertile K, Rembach A, Rimajova M, Rodrigues M, Rowe CC, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Ward V, Martins RN, Group AR. Plasma amyloid-beta as a biomarker in Alzheimer’s disease: the AIBL study of aging. J Alzheimers Dis. 2010;20:1233–1242. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- 19.Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 20.Le Bastard N, Leurs J, Blomme W, De D, Pp, Engelborghs S. Plasma Amyloid-beta Forms in Alzheimer’s Disease and Non-Alzheimer’s Disease Patients. J Alzheimers Dis. 2010 doi: 10.3233/JAD-2010-091501. [DOI] [PubMed] [Google Scholar]
- 21.Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma Amyloid-beta as a Predictor of Dementia and Cognitive Decline: A Systematic Review and Meta-analysis. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma A [beta]40 and A [beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 23.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 24.Pomara N, Willoughby LM, Sidtis JJ, Mehta PD. Selective reductions in plasma Abeta 1–42 in healthy elderly subjects during longitudinal follow-up: a preliminary report. Am J Geriatr Psychiatry. 2005;13:914–917. doi: 10.1176/appi.ajgp.13.10.914. [DOI] [PubMed] [Google Scholar]
- 25.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL. Secretion of Alzheimer’s disease Abeta amyloid peptide by activated human platelets. Lab Invest. 1998;78:461–469. [PubMed] [Google Scholar]
- 27.Li QX, Berndt MC, Bush AI, Rumble B, Mackenzie I, Friedhuber A, Beyreuther K, Masters CL. Membrane-associated forms of the beta A4 amyloid protein precursor of Alzheimer’s disease in human platelet and brain: surface expression on the activated human platelet. Blood. 1994;84:133–142. [PubMed] [Google Scholar]
- 28.Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer’s disease beta A4 amyloid precursor protein in human platelets. J Biol Chem. 1995;270:14140–14147. doi: 10.1074/jbc.270.23.14140. [DOI] [PubMed] [Google Scholar]
- 29.Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallin AK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O. CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology. 2010;74:1531–1537. doi: 10.1212/WNL.0b013e3181dd4dd8. [DOI] [PubMed] [Google Scholar]
- 31.Kester MI, van der Vlies AE, Blankenstein MA, Pijnenburg YA, van Elk EJ, Scheltens P, van der Flier WM. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1353–1358. doi: 10.1212/WNL.0b013e3181bd8271. [DOI] [PubMed] [Google Scholar]
- 32.van der Vlies AE, Verwey NA, Bouwman FH, Blankenstein MA, Klein M, Scheltens P, van der Flier WM. CSF biomarkers in relationship to cognitive profiles in Alzheimer disease. Neurology. 2009;72:1056–1061. doi: 10.1212/01.wnl.0000345014.48839.71. [DOI] [PubMed] [Google Scholar]
- 33.Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 34.Degerman Gunnarsson M, Lindau M, Wall A, Blennow K, Darreh-Shori T, Basu S, Nordberg A, Larsson A, Lannfelt L, Basun H, Kilander L. Pittsburgh compound-B and Alzheimer’s disease biomarkers in CSF, plasma and urine: An exploratory study. Dement Geriatr Cogn Disord. 2010;29:204–212. doi: 10.1159/000281832. [DOI] [PubMed] [Google Scholar]
- 35.Devanand DP, Schupf N, Stern Y, Parsey R, Pelton GH, Mehta P, Mayeux R. Plasma Abeta and PET PiB binding are inversely related in mild cognitive impairment. Neurology. 2011;77:125–131. doi: 10.1212/WNL.0b013e318224afb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, Kalback WM, Emmerling MR, Beach TG, Roher AE. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol. 2000;156:797–805. doi: 10.1016/s0002-9440(10)64947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Nostrand WE, Melchor JP. Disruption of pathologic amyloid beta-protein fibril assembly on the surface of cultured human cerebrovascular smooth muscle cells. Amyloid. 2001;8(Suppl 1):20–27. [PubMed] [Google Scholar]
- 38.Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 39.Catteruccia N, Willingale-Theune J, Bunke D, Prior R, Masters CL, Crisanti A, Beyreuther K. Ultrastructural localization of the putative precursors of the A4 amyloid protein associated with Alzheimer’s disease. Am J Pathol. 1990;137:19–26. [PMC free article] [PubMed] [Google Scholar]
- 40.Gustaw-Rothenberg KA, Siedlak SL, Bonda DJ, Lerner A, Tabaton M, Perry G, Smith MA. Dissociated amyloid-beta antibody levels as a serum biomarker for the progression of Alzheimer’s disease: a population-based study. Exp Gerontol. 2010;45:47–52. doi: 10.1016/j.exger.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sergeant N, Bombois S, Ghestem A, Drobecq H, Kostanjevecki V, Missiaen C, Wattez A, David JP, Vanmechelen E, Sergheraert C, Delacourte A. Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J Neurochem. 2003;85:1581–1591. doi: 10.1046/j.1471-4159.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- 42.Youssef I, Florent-Bechard S, Malaplate-Armand C, Koziel V, Bihain B, Olivier JL, Leininger-Muller B, Kriem B, Oster T, Pillot T. N-truncated amyloid-beta oligomers induce learning impairment and neuronal apoptosis. Neurobiol Aging. 2008;29:1319–1333. doi: 10.1016/j.neurobiolaging.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Vanderstichele H, De Meyer G, Andreasen N, Kostanjevecki V, Wallin A, Olsson A, Blennow K, Vanmechelen E. Amino-truncated beta-amyloid42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin Chem. 2005;51:1650–1660. doi: 10.1373/clinchem.2005.051201. [DOI] [PubMed] [Google Scholar]
- 44.Haldenwanger A, Eling P, Kastrup A, Hildebrandt H. Correlation between cognitive impairment and CSF biomarkers in amnesic MCI, non-amnesic MCI, and Alzheimer’s disease. J Alzheimers Dis. 2010;22:971–980. doi: 10.3233/JAD-2010-101203. [DOI] [PubMed] [Google Scholar]
- 45.Blom ES, Giedraitis V, Zetterberg H, Fukumoto H, Blennow K, Hyman BT, Irizarry MC, Wahlund LO, Lannfelt L, Ingelsson M. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27:458–464. doi: 10.1159/000216841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okonkwo OC, Alosco ML, Griffith HR, Mielke MM, Shaw LM, Trojanowski JQ, Tremont G. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 2010;67:688–696. doi: 10.1001/archneurol.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- 49.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 50.Williams JH, Wilcock GK, Seeburger J, Dallob A, Laterza O, Potter W, Smith AD. Non-linear relationships of cerebrospinal fluid biomarker levels with cognitive function: an observational study. Alzheimers Res Ther. 2011;3:5. doi: 10.1186/alzrt64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schupf N, Patel B, Pang D, Zigman WB, Silverman W, Mehta PD, Mayeux R. Elevated plasma beta-amyloid peptide Abeta(42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol. 2007;64:1007–1013. doi: 10.1001/archneur.64.7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seppala TT, Herukka SK, Hanninen T, Tervo S, Hallikainen M, Soininen H, Pirttila T. Plasma Abeta42 and Abeta40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. 2010;81:1123–1127. doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]


