Abstract
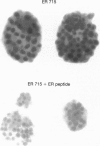
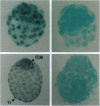
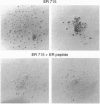
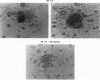
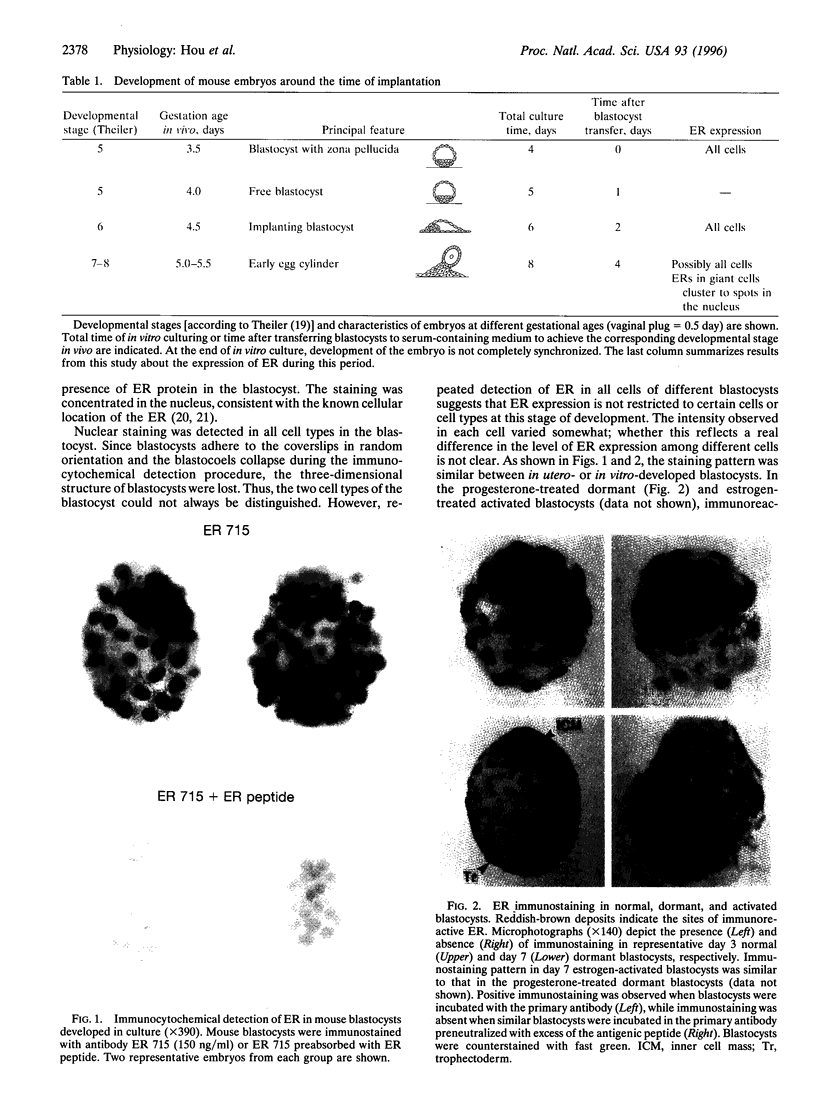
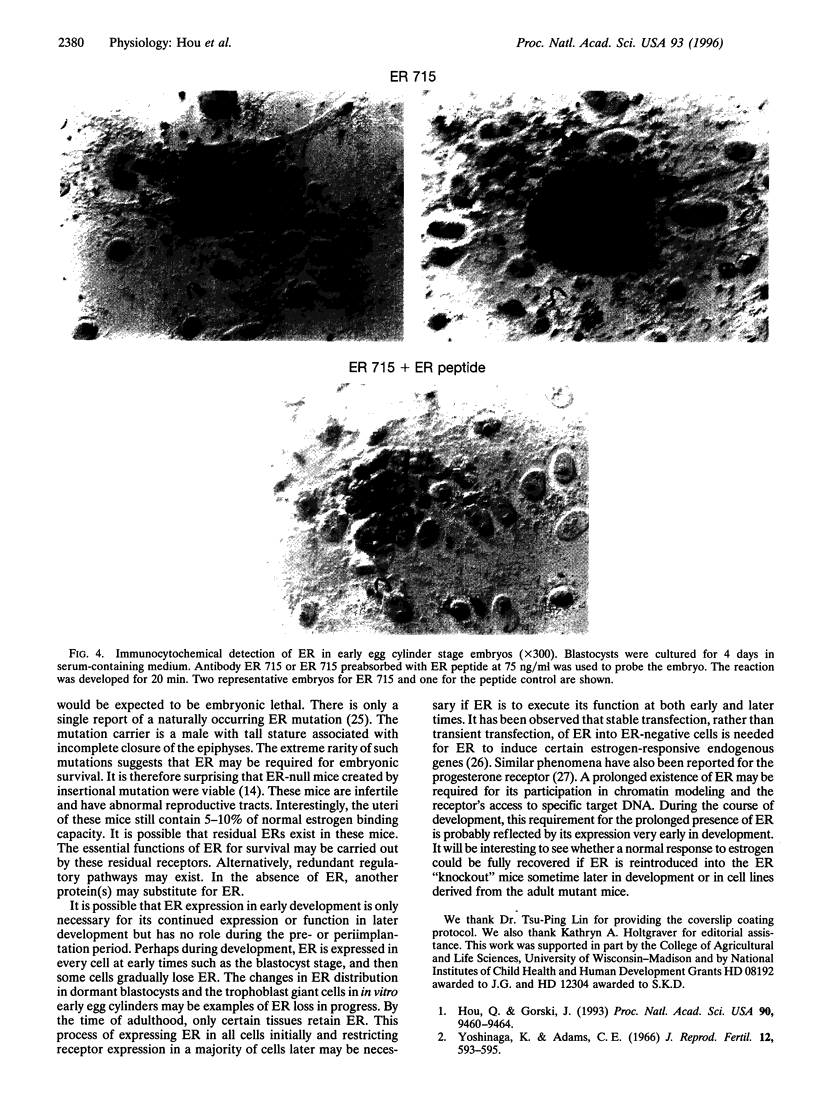
We previously showed that estrogen receptor (ER) mRNA is present in preimplantation mouse embryos. The apparent synthesis of ER mRNA by the blastocyst at the time of implantation when estrogen is required was of special interest. A demonstration of the presence of ER protein would support the idea that estrogen can act directly on the embryo. The mouse embryo at the blastocyst stage is differentiated into two cell types, the trophectoderm and the inner cell mass. To determine whether ER mRNA is translated into ER protein and its cell-specific distribution, immunocytochemical analyses were performed in mouse blastocysts. ER protein was detected in all cell types of the normal, dormant, or activated blastocyst. To trace the fate of ER in these cell types, immunocytochemistry was performed in implanting blastocysts and early egg cylinder stage embryos developed in culture. Again, ER was detected in all cells of the implanting blastocyst. At the early egg cylinder stage, continued expression of ER was observed in cells derived from the inner cell mass or the trophoblast. In trophoblast giant cells, ER was concentrated in small regions of the nucleus, possibly the nucleoli, which was similar to that observed in dormant and activated blastocysts. The embryonic expression of ER at such early stages in a broad array of cells suggests that ER may have a general role during early development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Furlow J. D., Ahrens H., Mueller G. C., Gorski J. Antisera to a synthetic peptide recognize native and denatured rat estrogen receptors. Endocrinology. 1990 Sep;127(3):1028–1032. doi: 10.1210/endo-127-3-1028. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Hsu Y. C. Correlative scanning electron, transmission electron, and light microscopic studies of the in vitro development of mouse embryos on a plastic substrate at the implantation stage. J Embryol Exp Morphol. 1980 Apr;56:23–39. [PubMed] [Google Scholar]
- Holmes P. V., Dickson A. D. Estrogen-induced surface coat and enzyme changes in the implanting mouse blastocyst. J Embryol Exp Morphol. 1973 Jun;29(3):639–645. [PubMed] [Google Scholar]
- Hou Q., Gorski J. Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9460–9464. doi: 10.1073/pnas.90.20.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. C., Baskar J., Stevens L. C., Rash J. E. Development in vitro of mouse embryos from the two-cell egg stage to the early somite stage. J Embryol Exp Morphol. 1974 Jan;31(1):235–245. [PubMed] [Google Scholar]
- Kaneko K. J., Gélinas C., Gorski J. Activation of the silent progesterone receptor gene by ectopic expression of estrogen receptors in a rat fibroblast cell line. Biochemistry. 1993 Aug 17;32(32):8348–8359. doi: 10.1021/bi00083a039. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Moyer J. S., Golding T. S., Couse J. F., Korach K. S., Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. P., Carter N. B. Transcriptional activity of blastomeres in mouse embryos during delayed implantation and after oestradiol benzoate-induced resumption of development. Cell Differ. 1984 Apr;14(1):19–23. doi: 10.1016/0045-6039(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Nieder G. L., Weitlauf H. M., Suda-Hartman M. Synthesis and secretion of stage-specific proteins by peri-implantation mouse embryos. Biol Reprod. 1987 Apr;36(3):687–699. doi: 10.1095/biolreprod36.3.687. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Das S. K., Andrews G. K., Dey S. K. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria B. C., Jones K. L., Flanders K. C., Dey S. K. Localization and binding of transforming growth factor-beta isoforms in mouse preimplantation embryos and in delayed and activated blastocysts. Dev Biol. 1992 May;151(1):91–104. doi: 10.1016/0012-1606(92)90216-4. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Archer T. K., Hamlin-Green G., Hager G. L. Newly expressed progesterone receptor cannot activate stable, replicated mouse mammary tumor virus templates but acquires transactivation potential upon continuous expression. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11202–11206. doi: 10.1073/pnas.90.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. P., Boyd J., Frank G. R., Takahashi H., Cohen R. M., Specker B., Williams T. C., Lubahn D. B., Korach K. S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994 Oct 20;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Torbit C. A., Weitlauf H. M. The effect of oestrogen and progesterone on CO2 production by 'delayed implanting' mouse embryos. J Reprod Fertil. 1974 Aug;39(2):379–382. doi: 10.1530/jrf.0.0390379. [DOI] [PubMed] [Google Scholar]
- Weitlauf H. M. A comparison of the rates of accumulation of nonpolyadenylated and polyadenylated RNA in normal and delayed implanting mouse embryos. Dev Biol. 1982 Sep;93(1):266–271. doi: 10.1016/0012-1606(82)90258-5. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Wan Y. J., Damjanov I. Positioning of inner cell mass determines the development of mouse blastocysts in vitro. J Embryol Exp Morphol. 1981 Oct;65:105–117. [PubMed] [Google Scholar]
- Wu T. C., Wang L., Wan Y. J. Expression of estrogen receptor gene in mouse oocyte and during embryogenesis. Mol Reprod Dev. 1992 Dec;33(4):407–412. doi: 10.1002/mrd.1080330406. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Adams C. E. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966 Dec;12(3):593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]