Abstract
Is forgetting in the short term due to decay with the mere passage of time, interference from other memoranda, or both? Past research on short-term memory has revealed some evidence for decay and a plethora of evidence showing that short-term memory is worsened by interference. However, none of these studies has directly contrasted decay and interference in short-term memory in a task that rules out the use of rehearsal processes. In this article the authors present a series of studies using a novel paradigm to address this problem directly, by interrogating the operation of decay and interference in short-term memory without rehearsal confounds. The results of these studies indicate that short-term memories are subject to very small decay effects with the mere passage of time but that interference plays a much larger role in their degradation. The authors discuss the implications of these results for existing models of memory decay and interference.
Keywords: short-term memory, decay, interference, recognition, verbal working memory
Why do we forget when the information to be remembered is modest in amount and the retention interval is short? That is, what causes forgetting of information in short-term memory? This is a question that has engaged psychology for over a century, and yet its answer remains elusive.
One theory that has a long history in accounting for forgetting is decay. The claim of this theory is that as time passes, information in memory erodes and is therefore less available for later retrieval. Decay has been a popular concept with respect to short-term memory, especially with the emergence and influence of Baddeley’s short-term memory architecture (Baddeley, 2000; Baddeley & Hitch, 1974). However, the concept of decay is not without problems. For one, the concept does not make much sense without elaboration. After all, the mere passage of time alone cannot cause forgetting. For a decay theory to be of value, it must lay claim to some process or processes that occur more and more as time passes.
Finding the mechanism or process of decay is one problem, but finding empirical evidence for decay is an even greater problem. In principle, it seems relatively straightforward to conduct an experiment to examine whether decay is a cause of forgetting: Provide a participant with some material to memorize, allow a varying short period of time during which the material must be maintained in memory, and then probe the participant to determine how much information was retained. If decay is operating, then as the length of the retention interval increases, there should be worse retrieval of the retained information. Although this experiment is in principle straightforward, in practice it is difficult to execute convincingly in a way that rules out alternative accounts.
Consider the classic study of Peterson and Peterson (1959), originally thought to provide strong evidence for decay. In this experiment, participants were given a letter trigram to store, followed by a retention interval that varied from 3 to 18 s. During the retention interval, participants were required to count backward by threes to prevent rehearsal of the memorandum. Following the retention interval, participants recalled the item in memory. Peterson and Peterson found that performance declined as retention intervals increased, and the authors attributed this decline to increasing decay of the memory trace with increasing time. The attribution of this effect to a decay mechanism is, however, suspect.
First, Peterson and Peterson argued that counting backward could not be a source of interference because their secondary-task materials differed sufficiently from the item to be stored in memory (letters vs. numbers). Yet, it is surely the case that the counting task requires short-term retention of material, just as does the main memory task (e.g., you have to remember the number 743 to do a subtraction of 3 from it to yield the next number in the series). So, retroactive interference is a likely contributor in this task. Also, others have shown that interference can be produced by other verbalizable items that are not similar to the to-be-remembered material (Postle, D’Esposito, & Corkin, 2005; Wixted, 2005), blunting Peterson and Peterson’s interference argument. Therefore, Peterson and Peterson’s claim that the materials are sufficiently distinct to avoid interference may not be appropriate.
Second, Keppel and Underwood (1962) showed that on the very first trial of an experiment like that of Peterson and Peterson (1959), there is little or no forgetting as a function of retention interval even though there is such forgetting on later trials. Keppel and Underwood interpreted this contrast between first and later trials as evidence that proactive interference plays a major role in the experiment and worsens memory performance. These findings substantially question whether a decay mechanism needs to be trotted out to account for any forgetting in this sort of experiment (Nairne, 2002). In short, proactive and retroactive interference accounts may provide a better explanation of the forgetting phenomenon that Peterson and Peterson attributed to decay.
Another important problem in assessing the role of decay on short-term memories for verbal material is the habitual tendency of people to rehearse material that they are to retain. This is evident in the laboratory and in everyday life. When we look up a phone number in the directory and then walk over to the phone, we rehearse the now memorized number until it is dialed. This happens so habitually that it is often not noticed and is difficult to disengage. The technique that investigators have used most often to prevent rehearsal (so that they could get an accurate gauge of whether decay was exerting an effect on memory) is to have subjects engage in a secondary task that prevents rehearsal.
Peterson and Peterson (1959) used counting backward as their secondary task, but we have already seen that this task, in itself, requires short-term retention, and so it does more than just prevent rehearsal; it produces interference. Others have tried different methods, such as tone detection, as a secondary task to prevent rehearsal. The idea here is to find a task that is taxing of mental capacity and therefore prevents rehearsal but does not tap short-term retention; and it must use items sufficiently dissimilar from the memoranda to render interference immaterial. Although early evidence from such experiments suggested that under these conditions there was no forgetting of primary material, and hence no influence of decay (Reitman, 1971; Shiffrin, 1973), later research discovered that the early work may not have taxed processing capacity sufficiently (Reitman, 1974). Indeed, a careful analysis of these studies by Roediger, Knight, and Kantowitz (1977) makes one wonder whether the use of a secondary task is appropriate to prevent rehearsal at all. They compared conditions in which a retention interval was filled by nothing, by a relatively easy task, or by a relatively difficult one. Both conditions with a filled interval led to worse memory performance, but the difficulty of the intervening task had no effect. Roediger et al. concluded that the primary memory task and the interpolated task, although demanding, used different processing pools of resources, and hence the interpolated tasks may not have been effective in preventing rehearsal. So, they argued, this sort of secondary-task technique may not prevent rehearsal and may not allow for a convincing test of a decay hypothesis.
Posner and Rossman (1965) explored the difficulty of interpolated tasks on memory performance and did find that the more difficult the interpolated task, the more forgetting ensued. However, in their experiments the interpolated tasks operated on the actual memoranda. More importantly, though, like Roediger et al. (1977), Posner and Rossman did find increases in memory errors even for simple interpolated tasks, suggesting that these tasks produce interference also. These data indicate that secondary tasks fail on two counts: by not eliminating rehearsal and by producing interference.
Other potential evidence for decay comes from studies of serial recall accuracy, which is better for words that have shorter articulatory durations compared with longer durations (known as the word-length effect; Baddeley, Thomson, & Buchanan, 1975; Mueller, Seymour, Kieras, & Meyer, 2003; Schweickert & Boruff, 1986). The word-length effect, however, is not without criticism. In a review by Lewandowsky and Oberauer (2008), the authors explained that the word-length effect is inherently correlational, dependent on specific stimulus materials and subject to other nonverbal rehearsal strategies such as refreshing (Raye, Johnson, Mitchell, Greene, & Johnson, 2007). In addition, the number of times that items are rehearsed in these studies is not controlled, so items with shorter articulatory durations may be rehearsed more often than those of longer durations, which may lead to stronger memory representations independent of decay. All these lines of evidence eliminate the word-length effect as viable evidence supporting decay.
More recently, research on serial recall has shown no evidence of time-based decay in verbal short-term memory. Lewandowsky, Duncan, and Brown (2004) have shown that altering recall speeds (by either speeding or slowing recall) had no impact on serial recall performance. This would not be predicted by decay models of short-term memory, which would hypothesize worse serial recall accuracy with slower recall speeds. The authors also eliminated rehearsal with articulatory suppression (e.g., having participants repeat a non-memory word aloud to eliminate the ability to rehearse memoranda) during the delays between stimulus presentations, which eliminates rehearsal confounds. In addition, the authors modeled their data and found that adding a time-weighting parameter did not improve the fits, as output interference alone could model the behavioral data (Lewandowsky, Duncan, & Brown, 2004).
It appears, then, that standard behavioral paradigms have not provided compelling evidence for the role of decay in forgetting of intentionally stored verbal material. Are there other approaches to the study of decay that may be more convincing?
One move is to examine the role of decay in the forgetting of nonverbal material, under the rationale that if the nonverbal material is not itself easily subject to a verbal code, participants will not be able to engage in rehearsal as a technique to maintain memory. This is a slippery route to take. First, there are many sorts of nonverbal materials that are themselves subject to verbal coding. For example, research by Meudell (1977) used 4 × 4 matrices, four of whose cells were filled, with the filled cells being the memoranda in the experiment. These sorts of stimuli seem quite susceptible to verbal coding. This problem can be avoided, however, as indicated by Harris (1952), who used auditory pitches as memoranda that differed subtly in frequency, so subtly that an effective verbal code would have been difficult to create. Harris varied the retention interval between a target tone and a probe tone from 0.1 to 25 s and found an orderly decline in performance in decisions about whether the tones matched with increasing retention intervals. A study of this sort seems more convincing about the value of decay as a mechanism of forgetting, at least on the face of it.
Even this study, however, may be subject to the interpretation that during the otherwise quiet retention interval, participants were engaged in some sort of thinking that made use of short-term retention processes and so exerted a retroactive interference effect on the experiment. Cowan, Saults, and Nugent (1997) also showed evidence of decay in a tone-matching task (i.e., worse performance with increased time between tones). However, these results are open to reinterpretation. In their experiment, Cowan et al. varied two intervals, the time between tones to be judged (interstimulus interval; ISI) and the time between tone pairs (interpair interval; IPI). The authors found that even when the ratio of IPI:ISI was controlled, increased forgetting ensued, with increased ISI thus supporting decay (Cowan et al., 1997). However, when the authors reanalyzed these data and considered the IPI from the previous trial and the ISI from the previous trial, different conclusions were drawn. For example, on trials where the previous trial’s IPI and ISI were long (24 s and 12 s, respectively) and the current IPI was long (24 s), no forgetting ensued across the current trial’s ISI, which varied from 1.5 to 12 s, thereby not supporting decay (Cowan, Saults, & Nugent, 2001). These results can be interpreted in terms of tones from the current trial being more distinct from one another and from previous tones at these longer time scales (Cowan et al., 2001), thereby mitigating proactive interference from past tones.
Additionally, Brown, Neath, and Chater (2007) simulated Cowan et al.’s (1997) original decay findings with their SIMPLE model, which is not dependent on time-based decay. The intuition behind this model is the following. When the ISI between the current pair of tones is longer, these tones are more susceptible to proactive interference from previous tones, even when the current IPI is increased to account for the longer current trial’s ISI. As such, the model successfully simulated the results from Cowan et al. (1997), leading the authors to conclude that the apparent effect of decay with increasing ISI may in fact be due to increased proactive interference from past tones with increased ISI (Brown, Neath, & Chater, 2007).
Another move to study decay is to encase the study of this mechanism in a task that does not overtly require memory, such as an incidental or implicit memory task. This is an important point because even with the compelling evidence against decay by Lewandowsky et al. (2004), participants were still required to recall all presented stimuli and thus could have performed more covert forms of rehearsal, such as refreshing (Raye et al., 2007), that could mask potential decay effects. In addition, articulatory suppression may not prevent such refreshing processes (Hudjetz & Oberauer, 2007; Raye et al., 2007). Because the task required repeating back all presented items, such refreshing strategies would be advantageous and would lead to better serial recall. More recently, however, Oberauer and Lewandowsky (2008) and Lewandowsky, Geiger, and Oberauer (2008) blocked refreshing with a choice reaction time task and found no forgetting in serial recall at long delays versus short delays, again showing that memory does not decay with the mere passage of time.
In all these studies that we have cited exploring decay, the participants were aware that they had to remember stimulus items on which they were to be tested at some later time. Although many researchers have been careful to prevent rehearsal and refreshing, which may have masked decay phenomena, it would be better if participants had no motivation to rehearse or refresh memoranda. This requires moving to a paradigm that tests memory more implicitly, thereby removing the motivation to rehearse memoranda. McKone (1995, 1998) tested decay in such a paradigm that explored decay in implicit short-term memory by varying the time between successive repetitions of an item in a lexical decision task. The issue was whether there was a savings in decision time, with more savings related to less time between item repetitions. What is most interesting about this experiment is that there was no overt memory task involved, so there was no reason for subjects to rehearse each item after a trial had been completed. McKone (1998) found that when the amount of time between repeated items increased (the lag interval varied from 2 to 16 s in increments of 2 s),1 lexical decision time increased, suggesting the decay of these short-term memory representations. McKone (1995, 1998) also varied the number of intervening items between repetitions, which also increased lexical decision time of the repetitions, and interestingly, this interference effect was stronger than the decay effect.
In our view, McKone’s (1998) study provides good evidence for decay: The paradigm provides no encouragement for rehearsal, and decay and interference were independently manipulated. Of course, one may argue that the technique used by McKone does not tap the role of decay in explicit short-term memory in that her measure of memory depended on the facilitation of a lexical decision. Therefore, these results may be tangentially related to the exploration of decay in short-term memory, because it could be argued that McKone’s stimuli never entered the explicit focus of attention (i.e., they were never maintained or retrieved) and rather were processed without an intentional memory component. Nevertheless, this technique is an effective one for controlling for other issues, as we argue, and so it bears further exploration.
Taken together, the evidence supporting decay is equivocal. Studies of explicit memory provide some substance to the notion that decay is a source of forgetting, but these results are often difficult to interpret for two reasons. First, participants have a habitual tendency to rehearse during unfilled intervals, and second, preventing rehearsal with a secondary task has the potential to interfere with memory performance in the primary task. We now describe a new paradigm intended to avoid both problems.
Exploring Decay and Interference in Explicit Short-Term Memory
To contrast decay and interference as causes of forgetting in short-term memory, we used a recent-probes task that is a variant of the item recognition task introduced by Sternberg (1966; see also Monsell, 1978). As we describe below, this task has the virtues of testing explicit short-term memory, avoiding any encouragement for rehearsal, and supporting precise and orthogonal manipulations of retention intervals and item-based interference.
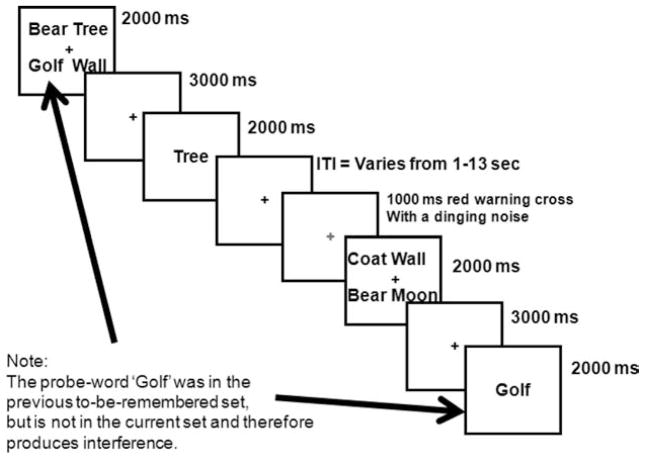
In this task the participant is shown four target words to remember for a brief retention interval of several seconds. A probe word is then presented, and the participant is instructed to respond affirmatively if the probe is one of the words in the stimulus set or negatively if it is not. The manipulation of interest has to do with pairs of trials in which the probe does not match any member of the current target set but does match a member of the set shown on the previous trial. On these trials, participants are delayed in responding “no” to the probe compared with a novel probe that has not appeared recently. This delay in responding is due to the high familiarity of the recent probe, it having been presented on the previous trial. These two no-response trial types (recent and nonrecent) are the trials of interest in this paradigm and are portrayed in Figures 1 and 2. The extra time taken to negate a recently presented no-probe (recent negative [RN] trial) is typically 50–100 ms more than for a nonrecent no-probe (nonrecent negative [NRN] trial). This effect is highly reliable in both response time and accuracy, but is typically more robust in response time because of high accuracy overall in this paradigm. The effect has been replicated many times, and there are neuroimaging data localizing the brain mechanisms that are engaged by the interference produced by the recent-probes task (Badre & Wagner, 2005; Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; D’Esposito, Postle, Jonides, Smith, & Lease, 1999; Jonides & Nee, 2006; Jonides, Smith, Marshuetz, & Koeppe, 1998; Mecklinger, Weber, Gunter, & Engle, 2003; Nee, Jonides, & Berman, 2007; Nelson, Reuter-Lorenz, Sylvester, Jonides, & Smith, 2003). In summary, the recent-probes task provides robust interference effects of previously seen items affecting recognition performance, both behaviorally and neurally.
Figure 1.
Interference trial (recent negative; RN) from the recent-probes task. ITI = intertrial interval.
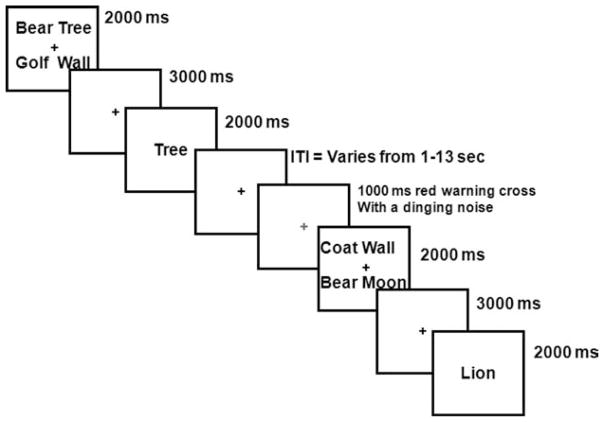
Figure 2.
Noninterference trial (nonrecent negative; NRN) from the recent-probes task. ITI = intertrial interval.
The epoch of time in the recent-probes task that interests us is the intertrial interval (ITI). We seek to examine whether variations in the length of this interval or in the insertion of other tasks during this interval has an effect on the size of the recent-probes effect. This task is ideal for investigating causes of forgetting because once any trial has ended in this task, participants have little reason to rehearse items on that trial or any previous trials. Therefore, this task avoids the problem of having rehearsal occur during an interval (the ITI) when a representation may be decaying.
Exploring Decay in Short-Term Memory
The aim of these experiments was to document whether short-term memories show evidence of decay in the recent-probes task. We varied the ITI that separated adjacent trials; if memories decay with the mere passage of time, then RN probes taken from trials that had longer preceding ITIs should not be as interfering compared with RN probes that were taken from previous trials that had shorter ITIs. Here we measure the effect of time from the end of the previous trials (i.e., from the previous trial’s probe) when the previous trial’s items were last refreshed. Therefore, with ITIs of 1, 5, 9, and 13 s, the total time from the previous trial could be 7, 11, 15, and 19 s. These timelines are outlined in Table 1 for all our experiments.
Table 1.
The Delay Time Values (in Seconds) for Experiments 1–5 and 7
| Experiment | Delay time lines
|
||||
|---|---|---|---|---|---|
| ITI | Warning cross | Stimulus set | Retention | Probe | |
| 1: Low proactive interference | |||||
| Duration | 1, 5, 9, or 13 | 1 | 2 | 3 | Terminates with response |
| Onset | 0 | 1.0–13.0 | 2.0–14.0 | 4.0–16.0 | 7.0–19.0 |
| 2: Lowest proactive interference | |||||
| Duration | 1, 5, 9, or 13 | 1 | 2 | 3 | Terminates with response |
| Onset | 0 | 1.0–13.0 | 2.0–14.0 | 4.0–16.0 | 7.0–19.0 |
| 3: Fast ITI | |||||
| Duration | 0.5, 2, 3.5, or 5 | 0.5 | 2 | 1 | 2 |
| Onset | 0 | 0.5–5.0 | 1.0–5.5 | 3.0–7.5 | 4.0–8.5 |
| 4: Fastest ITI | |||||
| Duration | 0.3, 0.8, 1.3, or 1.8 | No warning | 2 | 1 | 2 |
| Onset | 0 | n/a | 0.3–1.8 | 2.3–3.8 | 3.3–4.8 |
| 5: No articulatory suppression | |||||
| Duration | 1, 5, 9, or 13 | 1 | 2 | 3 | Terminates with response |
| Onset | 0 | 1.0–13.0 | 2.0–14.0 | 4.0–16.0 | 7.0–19.0 |
| 5: Articulatory suppression | |||||
| Duration | 1, 5, 9, or 13 | 1 | 2 | 3 | Terminates with response |
| Onset | 0 | 1.0–13.0 | 2.0–14.0 | 4.0–16.0 | 7.0–19.0 |
| 7: Blank ITI versus filled | |||||
| Duration | 1 or 10a | 1 | 2 | 3 | Terminates with response |
| Onset | 0 | 1.0–10.0 | 2.0–11.0 | 4.0–13.0 | 7.0–16.0 |
Note. The delay times are broken down by different durations of the different components of a recent-probes trial. ITI = intertrial interval; n/a = not applicable.
On some trials there was an entire trial that separated trials that lasted for 10 s rather than a blank 10-s delay. This trial was equated in total time with the blank 10-s interval. On these trials the recent negative probe was taken from the two-back set.
Experiment 1
Method
Twenty participants (18 women, 2 men; mean age = 25.2 years) were recruited from the University of Michigan to participate in the study. All participants gave informed consent as reviewed by the university’s Institutional Review Board. Participants were paid $10 per hour for their participation plus bonuses for fast and accurate responding throughout the experiment. Bonus scores were calculated on a trial-by-trial basis and were calculated with the following equation:
where probe accuracy (ACC) is a binary variable, 1 if correct and 0 if incorrect, and RT is response time. Individual trial scores were summed together to yield a total score. Participants were paid a penny for each point of their total score.
Procedure
We used the recent-probes task to assess decay by varying the ITI between adjacent trials. There were four ITI values: 1 s, 5 s, 9 s, and 13 s. On each trial the participant was shown four target words for 2 s. Following a 3-s blank delay (retention interval), the participant was shown one of four possible probe words (which defined the trial-types variable that we analyzed): a nonrecent positive (NRP) probe that was a member of the current stimulus set but was not a member of the past stimulus set, a recent positive (RP) probe that was a member of the current set and the previous set, an NRN probe that was not a member of the current target set and was novel (i.e., never seen in the experiment), and an RN probe that was not a member of the current set but was a member of the previous trial’s set. For each target set, two words overlapped with the previous set so that recency of appearance could not be used to predict the type of trial that would be encountered (a positive or negative trial). There were 192 trials total, with 48 RN, 48 NRN, 48 RP, and 48 NRP trials. Of the 48 trials in each trial type, 12 were from each of the different ITI values. Trials were presented in random order, and an equal number of each trial type was presented in each block of the experiment. There were four blocks total.
Materials
We used 440 words in this experiment. Words ranged from four to six letters and from one to two syllables with a mean frequency of 118.96 per million (SD = 109.042).
Design and analysis
In these studies we were interested in only three dependent measures: NRN response time, RN response time, and the effect of contrasting RN and NRN response time. We report positive trial accuracy only to show that participants took the task seriously; positive trial performance was not important theoretically. In addition, overall accuracy for this task is near ceiling; therefore accuracy data are not explored in great detail. A repeated measures analysis of variance (ANOVA) 4 (time intervals) × 1 (trial type) design was used in this experiment. There were three dependent variables: NRN response time, RN response time, and the RN–NRN contrast. Of most interest was whether the response time to RN trials and the RN–NRN contrast decreased with increasing time. In the analysis of response time, only the means of correct trials were used. Planned comparison paired t tests were later performed to test contrasts of interest as well as linear contrasts to test linear response time decreases as a function of increasing ITI.
Results and Discussion
In this experiment we found no evidence for decay in short-term memory. Time did not reliably alter RN response time, F(3, 57) = 0.626, ns; the RN–NRN contrast, F(3, 57) = 2.469, p = .07, or NRN response time, F(3, 57) = 2.744, p = .051. However, the RN–NRN contrast showed a borderline reliable effect that seemed to be driven by increases in NRN response time with increasing delay time. As can be seen from Figure 3, response time to RN trials does not decrease with increasing delay time and stays rather constant at 670 ms—a finding that does not support decay. In addition, not one of the linear contrasts was reliable, which tested a linear decline in response time with increasing ITI (though there was a borderline reliable increase in NRN response time). Moreover, there were no effects on accuracy, and accuracy for all trial types, including positive trials, was above 94%. Lastly, with paired t tests we found that the RN–NRN contrast was highly reliable at all time intervals. In sum, Experiment 1 yielded little to no evidence for decay. Had decay played a role, response time should have decreased with increasing time for RN trials and the RN–NRN contrast. The results from Experiment 1 can be seen in Figure 3 and in Tables 2–4.
Figure 3.
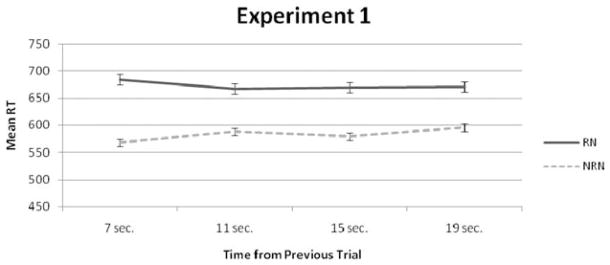
Results from Experiment 1 displaying recent negative (RN) and nonrecent negative (NRN) response time (RT) by intertrial interval. The 95% confidence intervals of this plot were based on formulas from Loftus and Masson (1994).
Table 2.
Mean Correct Response Time (in Milliseconds) for Experiments 1–5
| Experiment | Total delay time | |||
|---|---|---|---|---|
| 1: Low proactive interference | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 568 (18) | 588 (24) | 579 (23) | 596 (24) |
| RN | 684 (26) | 667 (32) | 670 (27) | 670 (27) |
| RN–NRN | 116 (12) | 79 (15) | 91 (12) | 74 (14) |
| 2: Lowest proactive interference | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 586 (20) | 615 (23) | 609 (22) | 610 (23) |
| RN | 686 (27) | 662 (24) | 669 (26) | 680 (21) |
| RN–NRN | 100 (18) | 48 (14) | 60 (13) | 70 (11) |
| 3: Fast ITI | ||||
| Trial type | 4 s | 5.5 s | 7.0 s | 8.5 s |
| NRN | 533 (18) | 544 (16) | 537 (19) | 541 (17) |
| RN | 615 (19) | 637 (22) | 600 (23) | 612 (25) |
| RN–NRN | 81 (8) | 93 (14) | 63 (14) | 71 (17) |
| 4: Fastest ITI | ||||
| Trial type | 3.3 s | 3.8 s | 4.3 s | 4.8 s |
| NRN | 571 (38) | 572 (41) | 561 (41) | 563 (34) |
| RN | 632 (44) | 640 (40) | 632 (46) | 658 (53) |
| RN–NRN | 61 (17) | 68 (14) | 71 (11) | 95 (23) |
| 5: No articulatory suppression | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 613 (38) | 625 (46) | 651 (56) | 647 (50) |
| RN | 711 (47) | 687 (41) | 707 (62) | 694 (56) |
| RN–NRN | 97 (34) | 62 (22) | 56 (16) | 47 (17) |
| 5: Articulatory suppression | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 720 (75) | 725 (73) | 739 (78) | 730 (79) |
| RN | 832 (89) | 833 (80) | 815 (91) | 803 (87) |
| RN–NRN | 112 (38) | 108 (18) | 76 (34) | 73 (29) |
Note. Standard errors in parentheses. ITI = intertrial interval; NRN = nonrecent negative; RN = recent negative.
Table 4.
Analysis of Variance Results on Response Time for Experiments 1–3
| Experiment | Univariate tests
|
|||||
|---|---|---|---|---|---|---|
| Measure
|
Linear contrasts
|
|||||
| NRN | RN | RN–NRN | NRN | RN | RN–NRN | |
| 1: Low PI | ||||||
| F | 2.74 | 0.63 | 2.47 | 4.20 | 0.65 | 3.76 |
| Significance | 0.05 | 0.60 | 0.07 | 0.05 | 0.43 | 0.07 |
| 0.13 | 0.03 | 0.12 | 0.18 | 0.03 | 0.17 | |
| Observed power | 0.63 | 0.17 | 0.58 | 0.49 | 0.12 | 0.45 |
| 2: Lowest PI | ||||||
| F | 5.47 | 0.91 | 3.05 | 7.19 | 0.05 | 1.99 |
| Significance | 0.00 | 0.44 | 0.04 | 0.01 | 0.82 | 0.17 |
| 0.21 | 0.04 | 0.13 | 0.26 | 0.00 | 0.09 | |
| Observed power | 0.92 | 0.24 | 0.69 | 0.72 | 0.06 | 0.27 |
| 3: Fast ITI | ||||||
| F | 0.84 | 1.60 | 1.15 | 0.28 | 0.78 | 1.51 |
| Significance | 0.48 | 0.21 | 0.34 | 0.61 | 0.40 | 0.25 |
| 0.07 | 0.13 | 0.09 | 0.02 | 0.07 | 0.12 | |
| Observed power | 0.21 | 0.38 | 0.28 | 0.08 | 0.13 | 0.20 |
Note. Univariate tests test the reliability of intertrial interval (ITI) as a predictor of response time for the different dependent variables. Linear contrasts test the linear contrast or monotonic decrease of response time as a function of ITI. NRN = nonrecent negative; RN = recent negative; PI = proactive interference.
There is one additional point to consider from Experiment 1. In this experiment there were occasions when an RN probe could have been seen repeatedly on many previous sets because the RN probe was chosen randomly from the previous set. This repetition occurred on roughly 50% of the RN trials. This repetitive stimulus presentation could have raised the familiarity of RN items, which may have prevented them from decaying as quickly with time if the traces had stronger activation from the beginning. When we explored post hoc (with a repeated measures ANOVA) those RN trials in which the probe was from the previous trial only, we found that response time for RN trials, F(3, 57) = 0.827, ns; NRN trials, F(3, 57) = 1.721, ns; and the RN–NRN contrast, F(3, 57) = 1.578, ns, did not change with increasing time. Accuracy also did not change with time for these trials, as accuracy for RN trials, F(3, 57) = 1.290, ns; NRN trials, F(3, 57) = 1.260, ns; and the RN–NRN contrast, F(3, 57) = 1.815, ns, did not change with increasing delay times. Therefore, when we analyzed the trials of Experiment 1 with the lowest familiarity levels, we still found no evidence for decay with the mere passage of time. Although this analysis yielded no evidence of decay for the purest trials in Experiment 1, we thought it wise to control this variable experimentally. This issue motivated Experiment 2.
Experiment 2: Lower Proactive Interference
In Experiment 2 we ensured that all RN probes were presented only in the immediately previous set2 and were not members of many previous target sets consecutively. In addition, we ensured that RN probes were not probed items on the previous set. We felt that this arrangement would reduce ambient proactive interference levels even lower than in Experiment 1. We still maintained the same hypothesis as in the previous study that response time to RN trials would not vary with increasing time between trials.
Method
Twenty-two participants (17 women, 5 men; mean age = 20.3 years) were recruited from the University of Michigan to participate in the study. One participant was excluded for having very low accuracy (below 50% on some trial types). Other than removing repeated probes, this experiment was the same in all respects as that of Experiment 1.
Results and Discussion
Experiment 2 replicated the findings of Experiment 1; there were no changes in response time and accuracy with increasing delay time, again suggesting that short-term memories in this paradigm do not decay with the passage of time. Delay time did not reliably alter RN response time, F(3, 60) = 0.911, ns, but the RN–NRN contrast did vary reliably with time, F(3, 60) = 3.048, p < .05. However, this change in the contrast was due to idiosyncratic changes in NRN response time with changes in delay time, F(3, 60) = 5.471, p < .001. When we explored this effect further, by separating participants according to working memory spans (as measured with operation span;3 Turner & Engle, 1989; Unsworth, Heitz, Schrock, & Engle, 2005), we found that only the low-span participants showed these reliable changes in NRN response time and RN response time.4 The suggestion is that they may not have been as vigilant and may have had more task-unrelated thoughts throughout the study, especially during long ITIs (Kane et al., 2007).
In addition, not one of the linear contrasts was reliable, except for NRN response time. This is important because the reliable changes did not produce any systematic effects with increased time; rather the changes were more idiosyncratic and would not be predicted by decay theories (that pattern was nonmonotonic). Moreover, there were no effects on accuracy; accuracy for all trial types, including positive trials, was above 95%. Lastly, with paired t tests we found that the RN–NRN contrast was highly reliable at all delay intervals. In sum, Experiment 2 yielded little evidence for decay. Had decay played a role, response time should have decreased monotonically with increasing delay time for RN response time and the RN–NRN contrast. The changes in the RN–NRN contrast were due to idiosyncratic changes in NRN response time with increasing time. These reliable NRN response time changes with increasing delay time concerned us and motivated Experiments 3 and 4. The results from Experiment 2 can be seen in Tables 2–4.
Experiments 3 and 4: Shorter ITIs
Experiments 1 and 2 showed that short-term memories do not decay reliably with the mere passage of time. However, concerns arose regarding participants’ vigilance at longer delays for NRN trials, which produced borderline reliable changes in the RN–NRN effect for Experiment 1 and reliable changes for Experiment 2. In addition, as shown in Table 1, these changes in the RN–NRN effect seemed to be driven by delay times between 7 and 11 s. These concerns led us to quicken the pace of the experiment and focus on delay values that were near 7 and 11 s. This achieves two goals. First, a quicker pace to the experiment and shorter ITI values should eliminate any vigilance problems that may have arisen in Experiments 1 and 2. Such vigilance problems may have been related to task-unrelated thoughts that could have produced interference at longer time delays. Second, exploring decay at shorter delay times allowed us to examine whether decay processes happen quite early in the delay interval and may have been largely completed by the time we began measurements at the shortest time delay of 7 s in our earlier experiments.
Experiment 3
Method
Twelve participants (7 women, 5 men; mean age = 20.8 years) were recruited from the University of Michigan. All subject procedures were the same as in Experiments 1 and 2.
The procedure for this experiment was similar to that of Experiment 2 except for two changes. First, the retention interval between the stimulus display and the probe word was shortened from 3 s to 1 s. This was done to reduce the total time that separated contiguous trials so that decay could be explored at shorter intervals. Second, the ITIs that were used were shortened to 500 ms, 2,000 ms, 3,500 ms, and 5,000 ms. In addition, there was a 500-ms warning that alerted participants that the next trial was approaching. Therefore our total delay times in this experiment were 4 s, 5.5 s, 7 s, and 8.5 s, which can be seen in Table 1. Lastly, the probe in this experiment remained on the screen for 2,000 ms independent of the participant’s response time. Other than these changes, this experiment was the same in all respects to that of Experiments 1 and 2.
Results and Discussion
Experiment 3 replicated the key findings of Experiments 1 and 2 (modulo the changes in NRN response time from those studies): There were no changes in response time with increasing delay, again strongly suggesting that short-term memories in this paradigm do not decay with the mere passage of time. Delay time did not reliably alter RN response time, F(3, 33) = 1.605, ns; the RN–NRN contrast, F(3, 33) = 1.150, ns; or NRN response time, F(3, 33) = 0.844, ns. In addition, none of the linear contrasts was reliable, and no effects were found on accuracy, as accuracy for all trial types was above 93%. Lastly, with paired t tests we found that the RN–NRN contrast was highly reliable at all ITI intervals. Therefore Experiment 3 replicated the findings of Experiments 1 and 2 but did so in two important ways. First, by shortening the delay intervals, we removed potential vigilance effects. Second, we verified an absence of decay around the shorter time delays of Experiments 1 and 2 (i.e., 7 and 11 s) by sampling more delay time points around those delay time values.
Experiment 4
In Experiment 4 we shortened the delay times even further than in Experiment 3 to explore decay at even shorter intervals. It may have been that at longer delay intervals we missed opportunities to find decay, especially if decay in short-term memory exists on a much shorter time scale. Experiment 4 was designed to explore this issue.
Method
Twelve participants (8 women, 4 men; mean age = 21.4 years) were recruited from the University of Michigan to participate in the study. All subject procedures were the same as in the previous experiments.
The procedure for this experiment was similar to that of Experiment 3, the only difference being the shortening of the ITIs even further. In this study the ITIs that were used were 300 ms, 800 ms, 1,300 ms, and 1,800 ms, which translated into delay times of 3.3 s, 3.8 s, 4.3 s, and 4.8 s, which can be seen in Table 1. For this study there was no warning fixation cross indicating that the next trial was approaching as it was unnecessary with such short ITIs. All other aspects of this experiment were the same as those of Experiment 3.
Results and Discussion
Experiment 4 replicated the findings of the previous three experiments as there were no changes in response time with increasing ITI as shown in Tables 2, 3, and 5. Delay time did not reliably alter RN response time, F(3, 33) = 1.124, ns; the RN–NRN contrast, F(3, 33) = 0.954, ns; or NRN response time, F(3, 33) = 0.316, ns. In addition, none of the linear contrasts was reliable, and no effects were found on accuracy, as accuracy for all trial types was above 92%. Lastly, with paired t tests we found that the RN–NRN contrast was highly reliable at all delay time intervals. Therefore, Experiment 4 replicated the findings of Experiments 1–3, and did so by eliminating vigilance effects and by testing decay at much shorter time intervals, where decay may have had a better chance to exist.
Table 3.
Accuracy Values for Experiments 1–5
| Experiment | Total delay time | |||
|---|---|---|---|---|
| 1: Low proactive interference | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 99.6% (0.4) | 100.0% (0.0) | 98.8% (0.7) | 99.2% (0.6) |
| RN | 96.8% (0.9) | 94.7% (1.3) | 95.1% (1.6) | 95.5% (1.9) |
| RN–NRN | −2.8% (0.9) | −5.3% (1.3) | −3.7% (1.8) | −3.7% (2.1) |
| 2: Lowest proactive interference | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 98.1% (0.7) | 99.2% (0.5) | 99.6% (0.4) | 98.8% (0.9) |
| RN | 98.3% (0.8) | 96.1% (1.3) | 97.5% (1.0) | 97.0% (1.0) |
| RN–NRN | 0.2% (1.2) | −3.1% (1.5) | −2.1% (1.0) | −1.8% (1.0) |
| 3: Fast ITI | ||||
| Trial type | 4 s | 5.5 s | 7.0 s | 8.5 s |
| NRN | 99.3% (0.7) | 97.9% (2.1) | 100.0% (0.0) | 99.3% (0.7) |
| RN | 98.7% (0.9) | 98.7% (0.9) | 97.3% (1.6) | 97.3% (1.1) |
| RN–NRN | −0.7% (1.2) | 0.8% (2.4) | −2.8% (1.6) | −2.0% (1.0) |
| 4: Fastest ITI | ||||
| Trial type | 3.3 s | 3.8 s | 4.3 s | 4.8 s |
| NRN | 97.9% (1.5) | 99.3% (0.7) | 99.3% (0.7) | 99.3% (0.7) |
| RN | 93.1% (3.0) | 91.8% (2.9) | 95.2% (1.9) | 93.1% (2.5) |
| RN–NRN | −4.8% (2.2) | −7.6% (2.8) | −4.2% (1.6) | −6.3% (2.3) |
| 5: No articulatory suppression | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 96.7% (2.6) | 96.3% (3.9) | 96.7% (2.6) | 96.3% (3.9) |
| RN | 91.8% (3.9) | 93.0% (2.9) | 92.0% (3.1) | 94.4% (4.1) |
| RN–NRN | −4.9% (1.7) | −3.3% (2.8) | −4.7% (3.0) | −1.9% (2.0) |
| 5: Articulatory suppression | ||||
| Trial type | 7 s | 11 s | 15 s | 19 s |
| NRN | 99.1% (0.9) | 98.1% (1.9) | 98.2% (1.2) | 100.0% (0.0) |
| RN | 96.3% (2.0) | 94.4% (2.4) | 98.2% (1.2) | 99.1% (0.9) |
| RN–NRN | −2.8% (2.4) | −3.7% (3.5) | 0.0% (1.3) | −0.9% (0.9) |
Note. Standard errors in parentheses. ITI = intertrial interval; NRN = nonrecent negative; RN = recent negative.
Table 5.
Analysis of Variance Results on Response Time for Experiments 4 and 5
| Experiment | Univariate tests
|
|||||
|---|---|---|---|---|---|---|
| Measure
|
Linear contrasts
|
|||||
| NRN | RN | RN–NRN | NRN | RN | RN–NRN | |
| 4: Fastest ITI | ||||||
| F | 0.32 | 1.12 | 0.95 | 0.43 | 1.35 | 1.99 |
| Significance | 0.81 | 0.35 | 0.43 | 0.53 | 0.27 | 0.19 |
| 0.03 | 0.09 | 0.08 | 0.04 | 0.11 | 0.15 | |
| Observed power | 0.10 | 0.27 | 0.24 | 0.09 | 0.19 | 0.25 |
| 5: No AS | ||||||
| F | 1.24 | 0.34 | 1.07 | 2.28 | 0.09 | 1.54 |
| Significance | 0.32 | 0.80 | 0.38 | 0.17 | 0.78 | 0.25 |
| 0.13 | 0.04 | 0.12 | 0.22 | 0.01 | 0.16 | |
| Observed power | 0.29 | 0.11 | 0.25 | 0.27 | 0.06 | 0.19 |
| 5: AS | ||||||
| F | 0.50 | 0.49 | 0.65 | 1.10 | 2.27 | 2.49 |
| Significance | 0.69 | 0.69 | 0.59 | 0.32 | 0.17 | 0.15 |
| 0.06 | 0.06 | 0.08 | 0.12 | 0.22 | 0.24 | |
| Observed power | 0.14 | 0.14 | 0.17 | 0.15 | 0.26 | 0.29 |
Note. Univariate tests test the reliability of intertrial interval (ITI) as a predictor of response time for the different dependent variables. Linear contrasts test the linear contrast or monotonic decrease of response time as a function of ITI. NRN = nonrecent negative; RN = recent negative; AS = articulatory suppression.
Experiment 5: Preventing Potential Covert Rehearsal
Experiments 1–4 were built around the rationale that the recent-probes task is a good platform to examine the influence of decay because the task does not just discourage rehearsal during the critical delay interval; there was no reason at all for subjects to rehearse past trial items. Nonetheless, although there was no reason for participants to rehearse the items from the previous trial during the ITI, it could be that participants covertly rehearsed these items anyway. If this were the case, of course, our paradigm would not be the ideal platform to test decay as a theory of forgetting that we have billed it to be. To address this issue, in Experiment 5 we had participants perform articulatory suppression during the ITI to prevent covert rehearsal. If participants were covertly rehearsing during the ITI, then those who engaged in articulatory suppression should be more susceptible to decay in short-term memory than those who did not have articulatory suppression during the ITI.
Method
Twenty participants (12 women, 8 men; mean age = 21.65 years) were recruited from the University of Michigan to participate in the study. Two participants were removed: 1 for having inadvertently been a participant previously and another for having extremely low accuracy scores (33% on some trial types). All subject procedures were the same as in the previous experiments.
Procedure
The procedure for this experiment was similar to that of Experiment 1, as the same ITIs were used. However, half the participants were randomly chosen to be in the articulatory suppression condition, where participants had to count aloud “1, 2, 3” repeatedly during the ITI. The other participants performed the task in its original form. Experimenters were within earshot to ensure that the participants were performing the articulatory suppression task aloud.
Design and analysis
Our design and analysis were similar to those of Experiments 1–4 except that we added a between-subjects variable for whether the participant engaged in articulatory suppression.
Results and Discussion
Experiment 5 replicated the findings of the previous four experiments, as there were no changes in response time and accuracy with increasing delay time. This was true both for participants who replicated the procedure of Experiment 1 and for those who engaged in articulatory suppression. In short, the addition of articulatory suppression had no effect in revealing evidence for the operation of decay.
In Tables 2, 3, and 5 one can see that delay time played no role in altering response time for any of the dependent variables for both the articulatory suppression and nonarticulatory suppression conditions. Of most interest was whether articulatory suppression interacted with delay time. We found that it did not, as RN response time did not change with increasing delay time depending on the articulatory suppression condition, F(3, 48) = 0.400, ns; nor did the RN–NRN contrast, F(3, 48) = 0.161, ns, or NRN response time, F(3, 48) = 0.295, ns. As expected, articulatory suppression did slow participants overall, because articulatory suppression may hinder participants from being as prepared to encode upcoming stimulus sets and because having to engage in articulatory suppression essentially makes this a task-switching paradigm. Additionally, no effects were found in accuracy, and accuracy for all trial types was above 93%. Thus, Experiment 5 replicated the findings of Experiments 1–4 even when any possible covert rehearsal of the previous trial’s items was mitigated by articulatory suppression.
Experiment 6: Testing the Effects of Executive or Conscious Control
With Experiments 1–5 we have shown no evidence of short-term memory degradation with the mere passage of time. With Experiment 5 we showed that participants were not rehearsing previous items during the ITI, because articulatory suppression during the ITI had no influence. However, there have been recent proposals for refreshing processes that are not based on articulatory rehearsal, and these could potentially be used to reactivate past items (Raye et al., 2007). Such refreshing may allow participants to tag past items strategically with a context code (i.e., the current probe word was a member of the previous stimulus set), and therefore reactivating them could potentially help participants determine the correct negative response to recent negative foils (i.e., thereby counteracting familiarity of the recently seen items). Experiment 6 was aimed at manipulating such conscious strategies by instructing participants to ignore past lists once a trial had ended. If participants have some executive control over this effect, we would expect to see a change in the RN–NRN effect for participants who were instructed to ignore past sets versus those who were not. As stated above, such instructions could mitigate the RN–NRN effect if participants are able to tag past items as foils. However, such instructions could also increase the RN–NRN effect if these instructions make past items more salient or familiar and therefore more interfering. In addition, the instructions may change the effect differentially from subject to subject, which would be uncovered by an increase in variance in the RN–NRN effect.
Method
Forty participants (24 women, 16 men; mean age = 21 years) were recruited from the University of Michigan to participate in the study.
Procedure
The procedure for this experiment was similar to that of Experiment 1, but only the 5,000-ms ITI was used. However, half the participants were in the instruction condition, in which participants were warned to ignore previous sets. The other participants performed the task with its original instructions.5
Materials
A subset of 30 words from Experiment 1 was used in this experiment.
Design and analysis
A between-subjects ANOVA was conducted comparing RN, NRN and RN–NRN response times for subjects who were and were not instructed to ignore previous stimulus sets.
Results and Discussion
We found that instructing participants to ignore past sets had no impact on RN response time, F(1, 38) = 0.158, ns; NRN response time, F(1, 38) = 0.062, ns; or the RN–NRN contrast, F(1, 38) = 0.032, ns, as corroborated with a between-subjects ANOVA (see Figure 4). These data indicate that participants may not be able to consciously remove past sets from mind to mitigate the interference that past items produce on current trials. For example, one may hypothesize that participants could tag past sets as being from an episodic context different from the current set, which could dampen the interfering ability of past items. However, our data suggest that the recent-probes effect is not subject to strategic executive control, making it unlikely that some of the participants in earlier experiments were engaged in refreshing or item tagging. Additionally, instructing participants to ignore past sets did not increase the RN–NRN effect (by potentially making RN items more salient); nor did it increase the variance of the effect compared with the no-instruction condition.
Figure 4.
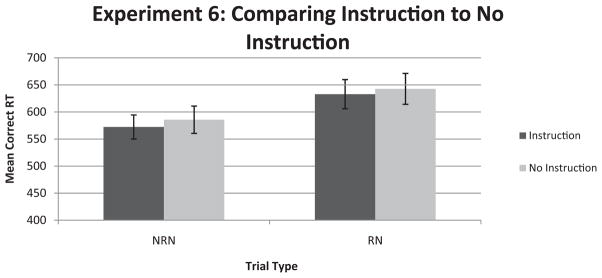
Mean correct response time (RT) results for Experiment 6 in which we either instructed participants to ignore past sets or did not provide any instructions. NRN = nonrecent negative; RN = recent negative. Error bars represent standard error of the mean.
Experiment 7: Direct Comparison of Decay and Interference
What we have in our first five experiments is null results, replicated over and over. It is these null results that have caused us to argue that decay plays little role in accounting for forgetting of the familiarity of information that underlies the recent-probes effect. With that said, there were some unreliable trends that may have implicated some time-based decay. Of course, null results have to be taken with caution, but we have been cautious in various ways. We explored decay over various time intervals, we impeded rehearsal as a covert process, and we explored whether the effect could be mitigated by instructing participants to ignore past sets. Even with this cautious attitude, we are left with a consistent finding: Variations in the delay interval in our task left the magnitude of the interference effect undiminished. This leads us to conclude that time-based decay has little effect on this short-term memory task.
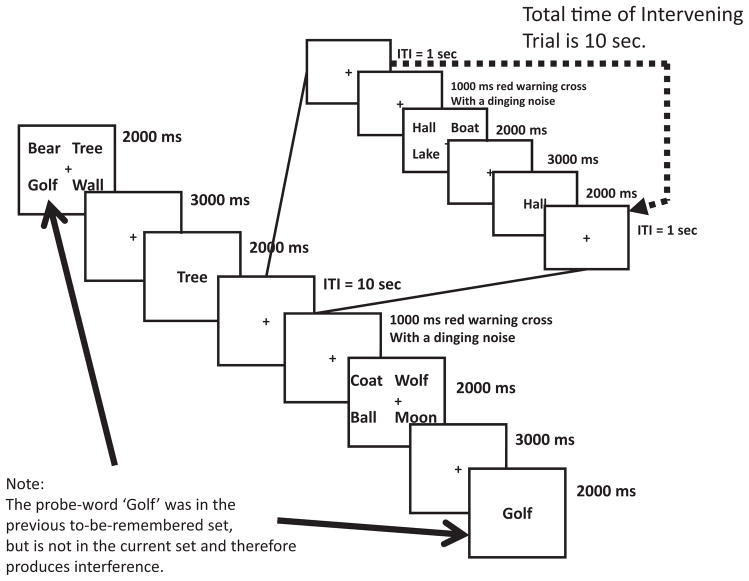
With this in mind, we turned to interference as the key account of forgetting in this paradigm. To compare the effect of interference with the effect of the passage of time, we constructed an experiment that pitted interference against decay in short-term memory. Again, the recent-probes task was used, with one major variation: There were three types of RN trials. One third of the RN trials had probes that were taken from the two-back set and therefore had one intervening trial that separated the two-back set from the current set. Another third of the RN trials were taken from the one-back set but had an ITI that was equated to the length of a single trial (10 s). These two RN trials are shown in Figure 5. Finally, one third of the RN trials had an ITI of 1 s (i.e., the canonical RN trial). With these RN trial types, we could directly compare interference versus decay by comparing response time and accuracy to the various RN trial types. To test the effects of interference, we could compare RN two-back trials versus RN one-back trials with an ITI of 10 s. To test the effects of time-based decay, we could compare response time and accuracy of RN one-back trials with an ITI of 10 s versus RN one-back trials with an ITI of 1 s. We predicted that RN two-back trials would have faster response times compared with the other RN trials on the basis that interference plays a stronger role in accounting for forgetting in this paradigm compared with decay.6
Figure 5.
Schematic of two recent negative trials from Experiment 7. Notice that the intertrial interval (ITI) separating the two trials on the left can be a blank 10-s ITI or filled with another trial that lasts for 10 s. In that case, the word golf would be taken from the two-back set. In addition, there were trials when recent negative trials had only a 1-s blank ITI preceding them.
Method
Twelve participants (7 women, 5 men; mean age = 21.5 years) were recruited from the University of Michigan. All subject procedures were the same as in the previous experiments.
Procedure
In this study there were seven trial types: two NRP trials7 (one with an ITI of 1 s and one with an ITI of 10 s), two NRN trials (one with an ITI of 1 s and one with an ITI of 10 s), and three RN trials (one with an ITI of 1 s, one with an ITI of 10 s, and one in which the probe word was taken from the two-back set with each of the two previous trials having an ITI of 1 s, leading to a total delay time of 10 s). Therefore the total delay times from the past set were 7 s in the case of the 1-s ITI and 16 s in the case of the 10-s ITI. Half the trials were negative, and half the trials were positive; there were 192 trials in total (48 of each NRP trial type, 24 of each RN trial type, and 10 of each NRN trial type). One additional change we made for this task was that stimulus sets had no overlapping words, so that each set was composed of a new set of words that had not been seen for at least three trials. This was done to eliminate RP trials to reduce the length of the experiment overall. The retention interval in this study was 3 s, as it was for Experiments 1, 2, 5, and 6.
Design and analysis
A repeated measures ANOVA with one predictor, interval type, was used in this design. The three intervals were a blank 1-s interval, a filled interval with an intervening trial, and a blank 10-s interval. In addition, there were two measures of interest, RN response time and the RN–NRN contrasts. In the response time analysis, only the means of correct trials were used. With this design we could explore how the different intervals affected these two measures. Planned comparison t tests were also performed on comparisons of interest.
Results and Discussion
We found that interference played a large role in forgetting in short-term memory, and we found no evidence for decay, which replicated our previous findings. RN probes taken from two-back stimulus sets were easier to reject than those taken from the one-back set. In addition, longer delay times did not significantly alter performance, which confirmed that the mere passage of time does not cause forgetting in short-term memory. The results from Experiment 7 are shown in Figure 6.
Figure 6.
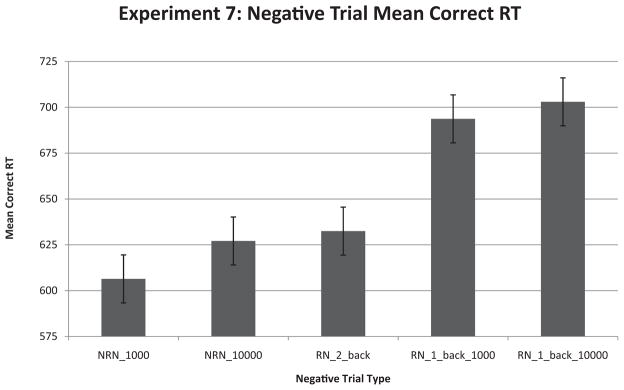
Results from recent-probes task pitting decay against interference. Here we show the results from all the negative trial types. The _1000 or _10000 suffix designates a blank intertrial interval of that length in milliseconds. The two-back designation indicates that the probe word was taken from the two-back set. RT = response time; NRN = nonrecent negative; RN = recent negative. Error bars represent standard error of the mean.
With our repeated measures ANOVA we found that interval type was a significant predictor of RN response time, F(2, 22) = 6.725, p < .01, and the RN–NRN contrast, F(2, 22) = 4.450, p < .05. These reliable effects were driven by the two-back condition, as RN probes taken from the two-back stimulus set were easier to reject.
With planned paired t tests, RN two-back trials had significantly lower response times than one-back RN probes at ITIs of 1 s (Mdiff = 61.21 ms), t(11) = 5.15, p < .001, and had significantly lower response times than one-back RN probes at ITIs of 10 s (Mdiff = 70.51 ms), t(11) = 2.97, p < .02. However, there were no significant differences between one-back RN probes at ITIs of 1 s and one-back RN probes at ITIs of 10 s (Mdiff = 9.3 ms), t(11) = 0.37, ns, showing that short-term memories do not decay with the passage of time. In addition, the RN–NRN difference was reliably smaller when RN probes were taken from the two-back set compared with a blank 1-s ITI (Mdiff = 82 ms), t(11) = 6.18, p < .001, and a blank 10-s ITI (Mdiff = 70.51 ms), t(11) = 2.97, p < .02.8 However, no differences were found between RN–NRN for long and short ITIs (Mdiff = −11 ms), t(11) = 0.46, ns. These data provide strong evidence showing that forgetting in short-term memory is due more to interference than to decay with time. There were no effects on accuracy, as participants’ accuracy for each trial type was approximately 98%.
Joint Analysis
After completing these seven experiments, we thought it appropriate to aggregate the data from these studies together to explore decay further. We averaged the RN–NRN effects across the various time intervals from our experiment and calculated delay time as the time from the previous trial’s probe word. Our time range was 3.3–19 s. We then regressed the RN–NRN effect against time to see whether time was a reliable predictor of the RN–NRN effect. In addition to this regression, we compared these aggregated data to those of Experiment 7 to compare the effect of decay to the effect of interference for RN trials and the RN–NRN contrast.
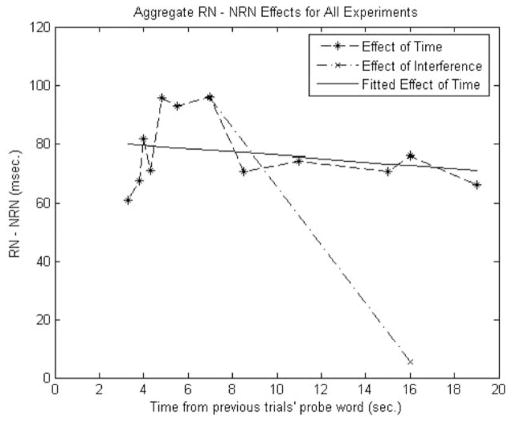
From this regression analysis we found a slight trend of decay with time, with the RN–NRN contrast decreasing by 1.225 ms/s of additional delay time. This regression was borderline reliable, F(1, 24) = 3.288, p = .082. We then normalized the RN–NRN difference by dividing this effect by RN + NRN to remove some potential scaling effects from the different studies (i.e., Experiments 3 and 4 had overall faster response time, and the articulatory suppression participants in Experiment 5 were slower overall). With this new regression we found that the time variable was more reliable in predicting the normalized effect, F(1, 24) = 9.127, p = .006, where the beta for the regression was −.001356 normalized effect units/s. Utilizing the average RN + NRN effect of 1,337 ms, this slope converts to a decrease of 1.814 ms in the RN–NRN contrast with every additional second of delay time. Therefore we suspect that there is, in fact, a small but reliable decay effect when we aggregate our data across experiments. These results can be seen in Figure 7.
Figure 7.
Aggregated data from all experiments. The dashed line with asterisks represents the aggregated data according to delay time across all our studies. The solid line is the linear fit of the effect of delay time on the recent negative–nonrecent negative (RN–NRN) contrast. The dashed line with Xs represents the effect of interference (i.e., taking the two-back probe as the RN probe on the current trial). From the figure one can see the stronger effect of interference compared with time-based decay.
There are a few important points to make. First, what are we to make of the small decay effect that emerges after aggregating across experiments? Exploring our decay function in Figure 7, we see an initial increase in the RN–NRN effect with increasing time delay, followed by a drop in the effect with a long plateau. It seems that existing decay theories would have difficulty modeling these data with their existing smooth exponential functions. To make matters worse, what if there was steep decay from 0 s of delay to 3.3 s (intervals we could not test in our paradigm)? This would suggest a kind of step function, also inconsistent with current decay models. Second, in our experiments that explored decay at shorter time scales (Experiments 3 and 4), we found no evidence for decay with time and in fact found a slight but unreliable increase. This suggests to us that at longer delays participants might be engaged in some mental activity, such as mind wandering, that would actually produce interference during these longer delays. Lastly, it is important to compare these effects of time with the known effects of interference. Figure 7 graphically shows the effect of time-based decay (the shallow sloped line) together with the effect of interference (the steep line). It is clear that the effect of interference swamps the small effect of decay. On the basis of estimates from our simple regression analysis, it would take a delay of 78 s for time-based decay to reduce the RN–NRN effect to zero. For interference, this only required taking an RN probe from the two-back trial. In sum, there appears to be a small but reliable effect of time in our data. However, this effect may not be easily predicted by existing decay theories (a topic to which we return in more detail below), it is confounded with potentially increasing mental activity at longer delays, and, most importantly, it was overshadowed by the effects of interference.
General Discussion
In this article we explored an important and prominent topic in short-term memory research: Does forgetting in short-term memory occur due to decay with time, interference from other material, or both? With six experiments we have shown that decay with time does not produce much, if any, forgetting in short-term memory (modulo the small effect found from the aggregate analysis) and that interference plays a much more prominent role. Recent research has also corroborated this finding (Lewandowsky et al., 2004, 2008; Lewandowsky & Oberauer, 2008; Nairne, 2002; Oberauer & Lewandowsky, 2008). However, an advantage of our experimental task over past research is that we have taken a different approach to tackling the rehearsal problem; not by preventing it but by rendering it counterproductive to participants’ intentions. Therefore, we feel that our paradigm and results add important evidence to the growing consensus that time-based decay plays little role in causing forgetting in short-term memory.
There are still some remaining questions that need to be addressed. One concerns the sensitivity of the present experiments: Perhaps we did not find decay in the individual experiments because they lacked sufficient power. It is always possible, in principle, to construct a specific decay function with a quantitative form that is not detectable by any given experiment, so ruling out the entire class of decay theories is not possible (as evidenced by the small but reliable effect of time that was shown with our aggregated data). But we can also inquire about the ability of our data set to detect decay effects comparable to other empirical findings and inquire about its ability to provide evidence against existing theoretical proposals for decay. We briefly consider these questions next, followed by a sketch of a candidate theory that we believe provides a promising account of the mechanisms underlying the phenomena surrounding the recent-probes task.
Effect Size and Power Relative to Other Empirical Findings
One way to calculate an expected decay effect size is to use the effect size of McKone (1998). From 3- to 7-s delays, McKone found roughly a 35-ms reduction in repetition priming, which can be used as an assay of decay. Our ability to detect such a reduction in the RN–NRN response time, given our sample size and our observed variance, is .52, and we found no such effect (our power here is smaller, as we only had 12 subjects at the 3.3-s interval and needed to perform a between-subjects analysis, as the same subjects were not tested at the 3.3-s interval and the 7-s interval). In fact, from Figure 7, one can see an opposite trend from 3.3 to 7 s. To detect our small but reliable effect of decay, we needed to aggregate 96 subjects’ worth of data. Additionally, such small decay effects are not clearly predicted by existing decay theories. In sum, we do not believe that our inability to find decay is due to a lack of power.
We must also consider whether we are exploring decay at the proper time scale. In our paradigm, the shortest time scale with which we explored decay was that in Experiment 4. If we measure decay from the time when the probe from the last set was presented, when those items were reactivated by retrieval (and were last rehearsed), we explored decay between 3.3 and 5.1 s. At these intervals, McKone did find decay, whereas we did not.9 Therefore, we believe that we explored decay at sensible time intervals, ones that previous research had shown to be within the operating window of a decay mechanism. Lastly, if there is decay at very short intervals (less than 3.3 s), decay theories would need to be adjusted and may reflect more of a step function, of steep decay at short intervals followed by a paucity of decay at longer intervals. In summary, although our paradigm cannot examine decay at very short time scales, our timing parameters are still sufficient to question what an overall decay function would look like (i.e., it may not be a smooth exponential).
Consistency With Existing Models of Decay
A number of prominent existing models of memory include well-specified decay components that might be inconsistent with the data presented here. These include the Page and Norris (1998) primacy model and its associated exponential decay equation. An examination of the form of this equation and the specific parameter values reported in Page and Norris suggests that it should predict a decline in response time with increasing delay times, if one assumes that the interference of a distractor is a function of its activation strength. But as we discuss now, the application of such theories may not be this straightforward.
Another prominent decay theory is the base-level activation equation of the ACT-R architecture (Anderson, 2007; Anderson et al., 2004), which posits that the activation levels of items in declarative memory follow a nonlinear, negatively accelerated form. This equation (with its associated fixed decay parameter of 0.5) is considered one of the most robust and successful components of ACT-R (Anderson, 2007). ACT-R’s memory theory has a further advantage of being integrated with a more general theory of cognitive and motor control. We now consider briefly what is required to test that theory given our data set, not only because ACT-R is a prominent decay theory but also because such consideration yields lessons for testing any decay theory.
It is tempting for present purposes simply to plug in appropriate time values into the decay and retrieval latency equations and generate estimates of the effects of decay. But this approach skips a fundamental step in applying an architectural theory: specifying the task strategy. Effects of the basic architectural mechanisms are expressed through strategies that organize the mechanisms in service of task goals. These strategies can modulate—and sometimes even obscure—the effects of the underlying mechanisms, both quantitatively and qualitatively (the problem of strategic variation is a difficult one; see, e.g., Meyer & Kieras, 1997; Newell, 1990). In fact, in our initial attempts to develop detailed ACT-R models of the probe task using an existing published strategy (Anderson & Lebiere, 1998), we observed a surprising degree of this modulation. In general, directly testing the fixed memory mechanisms is a significant theoretical challenge, even in simple tasks. What is required is a combination of testing the theory against multiple kinds of data sets (as advocated most persuasively by Newell, 1990) and adopting both modeling and empirical approaches (such as our rehearsal manipulations) that greatly constrain the choice of strategy as a theoretical degree of freedom (e.g., Howes, Lewis, & Vera, 2008). We note that such methodological challenges are not restricted to applying general theories of cognitive architecture, such as ACT-R. Any theory of some aspect of the fixed cognitive system, such as the nature of memory decay, faces these challenges, because any given posited fixed cognitive mechanism expresses itself only through selected task strategies.
Given these considerations, a more circumspect view of our results suggests that they represent a new set of quantitative regularities that should provide important constraints on any detailed theory of memory that makes precise assertions about decay mechanisms, but that this empirical constraint will be felt most sharply when joined with the broad set of other growing results, from other tasks, also showing flat effects of time (Lewandowsky et al., 2004, 2008; Lewandowsky & Oberauer, 2008; Nairne, 2002; Oberauer & Lewandowsky, 2008). There is a major opportunity to use computational modeling to test extant theories of decay and interference, but we think such an exercise would be most profitable if it takes into account a wide range of empirical effects and uses modeling techniques that help control for effects of strategic variation.
With these caveats in mind, our results do seem to align more straightforwardly with more recent models of short-term memory that do not implicate decay, including models such as SIMPLE (Brown et al., 2007) and SOB (Farrell & Lewandowsky, 2002). In SIMPLE, attention can (optionally) be directed away from time, whereas SOB is necessarily completely free of any effect of time and depends only on interference mechanisms. We now sketch our own approach to understanding the recent-probes task that is consistent with such models that explain forgetting in terms of interference alone.
Possible Mechanisms of the Recent-Probes Task
It is important to consider the mechanisms involved in the recent-probes task and compare those mechanisms to processes involved in other tasks that found decay, such as shown by McKone (1998). First, let us consider different neural mechanisms that are involved in repetition priming compared with explicit item recognition (Berry, Henson & Shanks, 2006). Many authors have reported double dissociations both neurally and behaviorally between priming and recognition memory (Gabrieli, Fleischman, Keane, Reminger, & Morrell, 1995; Hamann & Squire, 1997a, 1997b; Keane, Gabrieli, Mapstone, Johnson, & Corkin, 1995), with priming being dependent on the occipital lobe, suggesting a strong perceptual component (Fiebach, Gruber, & Supp, 2005). In contrast our task robustly activates the left ventrolateral prefrontal cortex10 for the RN–NRN contrast (Badre & Wagner, 2005; Bunge et al., 2001; D’Esposito et al., 1999; Jonides et al., 1998; Mecklinger et al., 2003; Nee et al., 2007; Nelson et al., 2003). In addition, McKone and Dennis (2000) found that phonological representations were less susceptible to time-based decay compared with orthographic representations in a similar repetition priming study. In sum, differences in task demands, underlying neural processes, and the nature of the encoding of the stimuli could explain why decay was found in the repetition priming task but not in our recent-probes task.
We must also consider what the recent-probes task has allowed us to measure. Beyond the manipulation of ITI, which allowed us to assess the effects of delay, we were able to further investigate whether participants rehearse past items during the blank ITIs in this paradigm. As Experiments 5 and 6 showed, articulatory suppression did not modulate the effect, nor did instructing participants to ignore past sets. Therefore we argue not only that there is no incentive for subjects to rehearse in the task but that regardless of whether they had any incentive to rehearse, they did not.
To understand better what causes the recent-probes effect that is at the heart of the paradigm we have used, we turn to a theoretical interpretation of the effect provided by Jonides and Nee (2006). In their review, the authors subscribed to the biased-competition model (Desimone & Duncan, 1995; Kan & Thompson-Schill, 2004) as a theoretical model to explain the recent-probes effect. According to this model, when an RN probe is shown, it activates attributes or features that are associated with the RN probe word, such as its familiarity (which is high), context (seen on the previous trial), and semantic representation. The important features here are familiarity and item context. The high familiarity of the item will bias one to respond affirmatively when, in fact, the correct response is negative. At the same time, the context of the RN probe does not match that of the current item’s context, and this contextual mismatch will bias one to respond negatively, which is the correct response. Therefore there are competing tendencies for RN items, and these competing tendencies slow participants compared to NRN items that have very low item familiarity, owing to greater retroactive interference (Jonides & Nee, 2006). This model also correctly predicts that RP probes will yield faster responses than NRP probes (a facilitation effect), which is sometimes found in this paradigm.
For a decay theory to accommodate these data, it would need to hypothesize that the two opponent features (i.e., item familiarity and item context) decay at the same rate, thus hiding any effects of decay, as these two attributes seem to counteract each other. Here we appeal to Occam’s razor. An interference account need not rely on two opponent processes balancing each other out to explain our data; rather an interference account can explain our data with one feature, namely, the presence or absence of interference. Therefore we subscribe to an interference account on the basis of its simplicity.11 In addition, the likelihood that two opponent processes would balance each other perfectly seems small, especially when one considers the research done showing the dissociation between processes reliant on item context versus item familiarity (Jacoby, 1991) and their different time courses (Yonelinas & Jacoby, 1994).
Conclusion
In conclusion, although null results such as these cannot completely rule out the possibility of some effect of decay, the consistent pattern of results across these six experiments, coupled with the extremely small effects observed, provides strong evidence against decay as a mechanism for forgetting in short-term memory. We argue that the small effect of time detected in the aggregate analysis might be a result of interference playing a role at longer time delays, with participants performing some mental activity (e.g., mind wandering) that could be interfering. Furthermore, considerations of the sensitivity of the paradigm with respect to the best existing empirical evidence for decay suggest that our experiments did have sufficient power to detect canonical decay effects at reasonable delay intervals where decay had been shown to exist (McKone, 1998). Our data show a persistence of short-term memory that may question the shape of existing decay functions, especially if there is rapid decay at shorter time delays. Finally, we found clear evidence of interference as a mechanism of forgetting, which overshadowed any effect of decay in our paradigm.
Acknowledgments
This research was supported in part by National Science Foundation (NSF) Grant 0520992 and National Institute of Mental Health Grant MH60655 and in part by an NSF Graduate Research Fellowship to Marc G. Berman. We would like to thank John Meixner, Katie Rattray, and Courtney Behnke for help in data collection.
Footnotes
In McKone’s study all the lag intervals varied by 2 s, except for the last lag, which jumped from 10 to 16 s.
On each trial of the experiment, two words overlapped from the previous set, which meant that each RN probe was actually taken from the past set and the one before it. Keeping this overlap prevented us from being able to take the probe word from the past set only.
In the operation span task that was used was the automated operation span task (Unsworth et al., 2005). Here subjects needed to remember words while simultaneously solving math problems. We defined high- and low-span participants by performing a median split on their operation span scores.
Low-span participants showed a reliable difference in RN response time when comparing the 1-s ITI to the 5-s ITI, t(10) = 2.29, p < .05.
The subjects who ran on the instruction version had ITIs of only 5,000 ms. Those who ran on the no-instruction condition had all four ITI conditions, but only the 5,000-ms ITI was analyzed. Accuracies for all other trial types were above 95%, and the mean correct response time values for the ITI values of 1, 5, 9, and 13 s for NRN trials were 579, 586, 585, and 576 and for RN trials 638, 643, 655, and 646, respectively. here were no reliable differences found between these ITI values.
Of course, some of our previous experiments can be analyzed this way as well. Indeed, we analyzed Experiment 1 and another experiment that used the recent-probes task (Nee et al., 2007), where we looked at the impact of the number of intervening trials that separated the source trial from which the negative probe word was taken. We found a decreasing linear relationship between response time and the number of intervening trials (i.e., the more intervening trials, the easier it was to reject the RN probe).
For this study there were no RP trials because stimulus sets did not have any overlapping words.
This is the same as comparing the RN conditions directly, as the same NRN baseline was used from the blank 10-s ITI.
McKone (1998) also explored decay at slightly shorter delay intervals of 2 s.
Nee et al. (2007) reported activation in the occipital cortex for the RN–NRN contrast, but the other six neuroimaging studies of our task that we cite did not report occipital cortex activation. In addition, we found increased activation in the occipital cortex for the RN–NRN contrast, which is the reverse finding from repetition priming studies that found decreased activation for repeated items compared with nonrepeated items. Therefore we do not believe that this task recruits the same visual perceptual processing that is found in priming studies and is less reliant on perceptual processing overall compared to repetition priming.
We would like to thank Stephan Lewandowsky for helping to conceive this line of reasoning.
References
- Anderson JR. How can the human mind occur in the physical universe? New York: Oxford University Press; 2007. [Google Scholar]
- Anderson JR, Bothell D, Byrne MD, Douglass S, Lebiere C, Qin Y. An integrated theory of the mind. Psychological Review. 2004;111:1036–1060. doi: 10.1037/0033-295X.111.4.1036. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Lebiere C. The atomic components of thoughts. Mahwah, NJ: Erlbaum; 1998. [Google Scholar]
- Baddeley AD. The episodic buffer: A new component of working memory? Trends in Cognitive Science. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baddeley AD, Thomson N, Buchanan M. Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior. 1975;14(6):575–589. [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cerebral Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Berry CJ, Henson RNA, Shanks DR. On the relationship between repetition priming and recognition memory: Insights from a computational model. Journal of Memory and Language. 2006;55(4):515–533. [Google Scholar]
- Brown GDA, Neath I, Chater N. A temporal ratio model of memory. Psychological Review. 2007;114(3):539–576. doi: 10.1037/0033-295X.114.3.539. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Cowan N, Saults S, Nugent L. The role of absolute and relative amounts of time in forgetting within immediate memory: The case of tone-pitch comparisons. Psychonomic Bulletin & Review. 1997;4(3):393–397. [Google Scholar]
- Cowan N, Saults S, Nugent L. The ravages of absolute and relative amounts of time on memory. In: Roediger HL III, Nairne JS, Neath I, Surprenant A, editors. The nature of remembering: Essays in honor of Robert G. Crowder. Washington, DC: American Psychological Association; 2001. pp. 315–330. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE, Lease J. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related fMRI. Proceedings of the National Academy of Sciences, USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell S, Lewandowsky S. An endogenous distributed model of ordering in serial recall. Psychonomic Bulletin & Review. 2002;9(1):59–79. doi: 10.3758/bf03196257. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: Spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2005;25(13):3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE, Fleischman DA, Keane MM, Reminger SL, Morrell F. Double dissociation between memory systems underlying explicit and implicit memory in the human brain. Psychological Science. 1995;6(2):76–82. [Google Scholar]
- Hamann SB, Squire LR. Intact perceptual memory in the absence of conscious memory. Behavioral Neuroscience. 1997a;111:850–854. doi: 10.1037//0735-7044.111.4.850. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Squire LR. Intact priming for novel perceptual representations in amnesia. Journal of Cognitive Neuroscience. 1997b;9:699–713. doi: 10.1162/jocn.1997.9.6.699. [DOI] [PubMed] [Google Scholar]
- Harris JD. The decline of pitch discrimination with time. Journal of Experimental Psychology. 1952;43(2):96–99. doi: 10.1037/h0057373. [DOI] [PubMed] [Google Scholar]
- Howes A, Lewis RL, Vera A. Cognitively bounded rationality. 2008 Manuscript submitted for publication. [Google Scholar]
- Hudjetz A, Oberauer K. The effects of processing time and processing rate on forgetting in working memory: Testing four models of the complex span paradigm. Memory & Cognition. 2007;35(7):1675–1684. doi: 10.3758/bf03193501. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30(5):513–541. [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annual Review of Psychology. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences, USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(4):466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18(7):614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Keane MM, Gabrieli JDE, Mapstone HC, Johnson KA, Corkin S. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal lobe lesions. Brain. 1995;118:1129–1148. doi: 10.1093/brain/118.5.1129. [DOI] [PubMed] [Google Scholar]
- Keppel G, Underwood BJ. Proactive inhibition in short-term retention of single items. Journal of Verbal Learning and Verbal Behavior. 1962;1(3):153–161. [Google Scholar]
- Lewandowsky S, Duncan M, Brown GDA. Time does not cause forgetting in short-term serial recall. Psychonomic Bulletin & Review. 2004;11(5):771–790. doi: 10.3758/bf03196705. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, Geiger SM, Oberauer K. Interference-based forgetting in verbal short-term memory. Journal of Memory and Language. 2008;59(2):200–222. [Google Scholar]
- Lewandowsky S, Oberauer K. The word length effect provides no evidence for decay in short-term memory. Psychonomic Bulletin & Review. 2008;15(5):875–888. doi: 10.3758/PBR.15.5.875. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1(4):476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- McKone E. Short-term implicit memory for words and nonwords. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21(5):1108–1126. [Google Scholar]
- McKone E. The decay of short-term implicit memory: Unpacking lag. Memory & Cognition. 1998;26(6):1173–1186. doi: 10.3758/bf03201193. [DOI] [PubMed] [Google Scholar]
- McKone E, Dennis C. Short-term implicit memory: Visual, auditory, and cross-modality priming. Psychonomic Bulletin & Review. 2000;7(2):341–346. doi: 10.3758/bf03212991. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Weber K, Gunter TC, Engle RW. Dissociable brain mechanisms for inhibitory control: Effects of interference content and working memory capacity. Cognitive Brain Research. 2003;18:28–38. doi: 10.1016/j.cogbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Meudell PR. Effects of length of retention interval on proactive interference in short-term visual memory. Journal of Experimental Psychology: Human Learning and Memory. 1977;3(3):264–269. [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 1. Basic mechanisms. Psychological Review. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Monsell S. Recency, immediate recognition memory, and reaction time. Cognitive Psychology. 1978;10(4):465–501. [Google Scholar]
- Mueller ST, Seymour TL, Kieras DE, Meyer DE. Theoretical implications of articulatory duration, phonological similarity, and phonological complexity in verbal working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(6):1353–1380. doi: 10.1037/0278-7393.29.6.1353. [DOI] [PubMed] [Google Scholar]
- Nairne JS. Remembering over the short-term: The case against the standard model. Annual Review of Psychology. 2002;53:53–81. doi: 10.1146/annurev.psych.53.100901.135131. [DOI] [PubMed] [Google Scholar]
- Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. NeuroImage. 2007;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CYC, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proceedings of the National Academy of Sciences, USA. 2003;100:1171–1175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A. Unified theories of cognition. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- Oberauer K, Lewandowsky S. Forgetting in immediate serial recall: Decay, temporal distinctiveness, or interference? Psychological Review. 2008;115(3):544–576. doi: 10.1037/0033-295X.115.3.544. [DOI] [PubMed] [Google Scholar]
- Page MPA, Norris D. The primacy model: A new model of immediate serial recall. Psychological Review. 1998;105(4):761–781. doi: 10.1037/0033-295x.105.4.761-781. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58(3):193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rossman E. Effect of size and location of informational transforms upon short-term retention. Journal of Experimental Psychology. 1965;70(5):496–505. doi: 10.1037/h0022545. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M, Corkin S. Effects of verbal and nonverbal interference on spatial and object visual working memory. Memory & Cognition. 2005;33(2):203–212. doi: 10.3758/bf03195309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Greene EJ, Johnson MR. Refreshing: A minimal executive function. Cortex. 2007;43(1):135–145. doi: 10.1016/s0010-9452(08)70451-9. [DOI] [PubMed] [Google Scholar]
- Reitman JS. Mechanisms of forgetting in short-term memory. Cognitive Psychology. 1971;2(2):185–195. [Google Scholar]
- Reitman JS. Without surreptitious rehearsal, information in short-term memory decays. Journal of Verbal Learning and Verbal Behavior. 1974;13(4):365–377. [Google Scholar]
- Roediger HL, Knight JL, Kantowitz BH. Inferring decay in short-term memory: Issue of capacity. Memory & Cognition. 1977;5(2):167–176. doi: 10.3758/BF03197359. [DOI] [PubMed] [Google Scholar]
- Schweickert R, Boruff B. Short-term memory capacity: Magic number or magic spell? Journal of Experimental Psychology: Learning, Memory, and Cognition. 1986;12(3):419–425. doi: 10.1037//0278-7393.12.3.419. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM. Information persistence in short-term memory. Journal of Experimental Psychology. 1973;100(1):39–49. [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966 Aug 5;153(3736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? Journal of Memory and Language. 1989;28(2):127–154. [Google Scholar]
- Unsworth N, Heitz RR, Schrock JC, Engle RW. An automated version of the operation span task. Behavior Research Methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Wixted JT. A theory about why we forget what we once knew. Current Directions in Psychological Science. 2005;14(1):6–9. [Google Scholar]
- Yonelinas AP, Jacoby LL. Dissociations of processes in recognition memory: Effects of interference and of response speed. Canadian Journal of Experimental Psychology. 1994;48(4):516–535. doi: 10.1037/1196-1961.48.4.516. [DOI] [PubMed] [Google Scholar]