Abstract
Pelizaeus-Merzbacher disease (PMD) is a severe hypomyelinating disease, characterized by ataxia, intellectual disability, epilepsy, and premature death. In the majority of cases, PMD is caused by duplication of PLP1 that is expressed in myelinating oligodendrocytes. Despite detailed knowledge of PLP1, there is presently no curative therapy for PMD. We used a Plp1 transgenic PMD mouse model to test the therapeutic effect of Lonaprisan, an antagonist of the nuclear progesterone receptor, in lowering Plp1 mRNA overexpression. We applied placebo-controlled Lonaprisan therapy to PMD mice for 10 weeks and performed the grid slip analysis to assess the clinical phenotype. Additionally, mRNA expression and protein accumulation as well as histological analysis of the central nervous system were performed. Although Plp1 mRNA levels are increased 1.8-fold in PMD mice compared to wild-type controls, daily Lonaprisan treatment reduced overexpression at the RNA level to about 1.5-fold, which was sufficient to significantly improve the poor motor phenotype. Electron microscopy confirmed a 25% increase in the number of myelinated axons in the corticospinal tract when compared to untreated PMD mice. Microarray analysis revealed the upregulation of proapoptotic genes in PMD mice that could be partially rescued by Lonaprisan treatment, which also reduced microgliosis, astrogliosis, and lymphocyte infiltration.
Introduction
Most individuals affected by Pelizaeus-Merzbacher disease (PMD [MIM 312080]) show a severe and progressive leukodystrophy of early onset. Infants develop nystagmus, poor head control, cerebellar dysfunction, spasticity of upper and lower extremities, and cognitive impairment.1–5 Onset and progression of PMD is clinically variable, however, and depends on the specific PLP1 mutation and, based on insight from bona fide PMD mouse models, on unknown modifier genes.6,7 Despite the detailed knowledge that has accumulated on the molecular genetic basis of the PMD,4,8 there is no curative therapy available.
Both proteolipid protein (PLP) and its smaller splice isoform DM20 are tetraspan membrane proteins in compacted myelin that account for nearly 20% of the total CNS myelin proteome.9 Mutations or dosage alterations of X-linked PLP1 cause PMD and spastic paraplegia type 2 (SPG2 [MIM 312920]) in human and clinically similar phenotypes in rodent models of PMD. Dysmyelination, secondary inflammation, and axonal damage contribute to severe motor impairment.4,5,10–13 The most common cause of PMD, accounting for approximately 60% of all cases, is duplication of entire PLP.8,14,15
It is thought that both Plp1 overexpression and point mutations in the gene exert a toxic gain-of-function effect in oligodendrocytes that is considerably more severe than Plp1 loss-of-function resulting from null mutations.5,16–21 In fact, gene deletions or other null mutations4,22 underlie a less severe disease, originally classified as SPG2 with degenerative changes of long axonal tracts.23–25 The ability of proteolipids to bind cholesterol is important for myelination,26 but the stoichiometry of PLP1 and cholesterol appears critical. In mice with Plp1 overexpression, PLP1/DM20 (lacking equivalent amounts of cholesterol) accumulates in the endo/lysosomal compartment.19,27 Indeed, treatment of these Plp1-overexpressing mice with dietary cholesterol dramatically ameliorates the mutant phenotype.28
Several Plp1 transgenic PMD models, defined by extra copies of wild-type Plp1, have been generated that exhibit a range of phenotypes, depending on gene dosage.29–33 In the present study, we used Plp1 transgenic homozygous mice in line #72, as described by Readhead et al.30 (referred to hereafter as PMD mice). In the CNS, the elevated Plp1 expression level causes dys- and demyelination, oligodendrocyte death, axonal loss in long fiber tracts, and microgliosis with lymphocyte infiltration. We note that transgenic mouse line #72 used here models the comparatively milder forms of PMD or SPG2, whereas line #66 models the severely affected “classical” PMD.21,28,30,32,34–36
Ligand-controlled transcription factors, such as the nuclear progesterone receptor, are promising targets for the therapy of myelin diseases caused by myelin gene overexpression.37 In the peripheral nervous system (PNS), progesterone promotes the synthesis of at least two myelin proteins, MPZ and PMP22, possibly by stimulating accumulation of two other transcription factors, EGR2 and SOX-10, in Schwann cells.38–43 For the CNS, there is only indirect evidence that progesterone serves a similar role as a “promyelinating” factor. In cultures of primary oligodendrocytes and organotypic slice cultures of cerebellum, progesterone increases the accumulation of MBP and 2′,3′-cyclic nucleotide 3′ phosphodiesterase (CNPase).44,45 Moreover, progesterone positively modulates remyelination after toxin-induced lesions of the cerebellar peduncle in aging rats.46 Additionally, progesterone contributes to oligodendrocyte precursor cell proliferation and differentiation.47,48 More recently, in a spinal injury model49 and in a Cuprizone model of demyelination,50 progesterone increased Plp1 mRNA expression. These observations provide proof of principle that the nuclear progesterone receptor acts upstream of Plp1 transcription in vivo and is therefore a plausible target to pharmacologically lower Plp1 expression in diseases such as PMD.
The present study aimed at testing the potential therapeutic effect of a newly developed progesterone antagonist, Lonaprisan (ZK230211), in a transgenic mouse model of PMD. We hypothesized that antagonists of steroid hormones can lower total Plp1 transcription in oligodendrocytes (and thus correct abnormal Plp1 mRNA and protein levels). This approach could lead to a rational therapy for the most common subgroup of PMD caused by PLP1 duplications.
Material and Methods
All experiments were conducted according to the Lower Saxony State regulations for animal experimentation in Germany, and animal studies were approved by the appropriate authority.
Transgenic Mice
Male mice in line #72 homozygous for an autosomal murine Plp1 transgene30 and maintained on the C57BL/6 background were used as bona fide models for human PMD. Plp1 overexpression was 1.8-fold (see Results) and motor symptoms in PMD mice were already measurable at study start at 3 weeks of age. Generally, untreated homozygous #72 mice fail to survive beyond 6 months of age.
Antiprogesterone Treatment
Lonaprisan (ZK230211), a nuclear progesterone antagonist, was kindly provided by Bayer.51 Male mice were assigned to treatment arms at random with respect to phenotype, weight, and parents. Lonaprisan or vehicle was administered by daily subcutaneous injections. Lonaprisan was dosed at 125 mg/kg body weight and suspended in a mixture of 90% sesame oil and 10% benzylbenzoate. In one control group, wild-type mice received vehicle without Lonaprisan. Treatment started at 3 weeks of age (time of weaning) and was performed for 10 weeks.
Brain Level Analysis of Lonaprisan
To examine the blood-brain-barrier passage of Lonaprisan, male wild-type mice were treated with Lonaprisan at 125 gm/kg body weight by subcutaneous injections on a daily basis for 10 days. At 1, 3, 9, and 24 hr after the last dose, the mice were sacrificed and perfused with HBSS in order to avoid interference with intravasal Lonaprisan, and brains were isolated. Lonaprisan levels were measured by mass spectrometry at Bayer.
Phenotype Analyses
Phenotypic analyses were performed by the same investigator blinded to genotype and therapy group. Motor phenotype was assessed by a limb-slipping test (grid test) in which mice were placed on a metal grid with 1 cm spacing between bars (bar diameter: 3 mm). Over a total walking distance of 2 m, the number of slips of both hindlimbs and forelimbs were assessed by the examiner. Disease severity was independently assessed with a clinical ranking scale from 1 to 5, with the following criteria: 1, normal phenotype (healthy); 2, ataxic gait; 3, tremor; 4, seizures; 5, death. Phenotype analyses were carried out at 3, 7, 10, and 13 weeks of age. Body weight was continuously recorded.
Histologic Analyses
Cervical spinal cord and total brain was fixed by immersion in 4% paraformaldehyde and 2.5% glutaraldehyde in cacodylate buffer (pH 7.2) for electron microscopy (EM) or in 4% paraformaldehyde for immunohistochemistry (IHC).
For IHC analyses, diaminobenzidine (DAB) stainings were used and the antibodies were diluted to the following concentrations: OLIG-2 (Dana-Farber Cancer Institute, rabbit polyclonal) 1:200; GFAP (Novocastra, murine monoclonal) 1:200; MAC-3 (PharMingen, rat monoclonal) 1:400; CD3 (Serotec, rat monoclonal) 1:150; BAX (Santa Cruz, mouse monoclonal) 1:100; Ki67 (DakoCytomation) 1:100; c-MYC (Santa Cruz, mouse monoclonal) 1:100. Immunostained cells were quantified in the corticospinal tract (CST, at cervical vertebral body 5) and in the ventral corpus callosus (CC).
For EM analyses, epoxy resin (Serva) embedding was performed after fixation in 1% OsO4.52 Ultrathin sections were contrasted by uranylacetate and lead citrate. Myelinated and unmyelinated axons were counted in the CST.
RNA Expression Analyses
RNA from total brain was isolated at study end from 13-week-old mice according to the manufacturer’s instructions (RNeasy lipid tissue minikit; QIAGEN) with TRIzol reagent (Invitrogen). Real-time quantitative PCR reactions (Taqman and SYBR Green assay) were carried out with the Lightcycler 480 (Roche). Samples ran in triplicate, each within 10 μl total volume according to a two-step protocol. Quantitation of PCR product was performed with the comparative ΔΔCt method as recommended by the manufacturer. Transgenic Plp1 mRNA overexpression was normalized against Ppia mRNA. For quantification of Lonaprisan effect on Plp1 expression, normalization was performed against the non-steroid-regulated, non-myelin-related, and ubiquitously expressed exon 1B transcript of Pmp22.39,53,54,55 Bax, Jun, and Casp7 mRNA expression was normalized against the mean of Rplp0, Ppia, and Atp5b mRNA expression. Primer sequences can be provided upon request.
Microarray Analysis
Total brain mRNA was taken from each three individual placebo- (n = 3) and Lonaprisan- (n = 3) treated PMD mice. Mouse Gene 1.0 ST tiling arrays (Affymetrix) were used for the measurements. Data analyses were performed by the integrated software packages Genomics Suite (Partek). The Gene Set Enrichment Analysis (GSEA) software was used to identify coordinated changes in a priori defined sets of functionally grouped genes.56,57
Sample Size Considerations
Sample sizes were calculated a priori for PMD mice by G∗Power software taking an alpha error of 5% and a power (1 – beta error) of 80% into account. Effect sizes and standard deviations were prespecified as follows: d = 1.2 and SD = 15% for the reduction of Plp1 mRNA fold-expression, d = 1.8 and SD = 10% for the preservation of myelinated axons/area, and d = 1.1 and SD = 20% for the reduction of number of slips.
Statistical Analysis
All values are expressed as mean ± SEM. We have tested all our data on Gaussian distribution and used parametric (Student’s t test) or nonparametric (Mann-Whitney U-test) testing, where applicable. Correlation analyses were performed by the Spearman’s rank correlation test. Statistica 7.0 (StatSoft) was used for statistical analyses.
Results
Lonaprisan Crosses the Blood-Brain Barrier and Downregulates Plp1 Overexpression
A pilot study was performed to examine whether Lonaprisan crosses the blood-brain barrier (Figure 1A). Lonaprisan levels were determined by mass spectroscopy in total brain lysates from wild-type mice, prepared 1, 3, 9, and 24 hr after the last subcutaneous application. Drug levels were detectable at 1 hr, increased up to 9 hr after the last dose, and were still detectable after 24 hr (1 hr: mean = 4.8 μmol/l, SEM = 1.6; 3 hr: mean = 5.6 μmol/l, SEM = 2.6; 9 hr: 10.8 μmol/l, SEM = 4.9; 24 hr: mean = 0.8 μmol/l, SEM = 0.2) (Figure 1B). In an attempt to establish the optimal dosage for Plp1 downregulation, we treated PMD mice over 10 days at three different dosages and examined Plp1 mRNA expression 24 hr after the last injection. Placebo-treated PMD mice showed a significant 1.8-fold Plp1 overexpression (n = 12, mean = 1.8, SEM = 0.1) compared to wild-type controls (n = 15, mean = 1.0, SEM = 0.04, p < 0.001) (Figure 1C). Lonaprisan dosages of 2 mg/kg (n = 8, mean = 1.9, SEM = 0.1) and 20 mg/kg (n = 8, mean = 1.7, SEM = 0.1) were not sufficient to reduce the Plp1 mRNA overexpression in PMD mice (n = 8, mean = 1.8, SEM = 0.1) after short-term treatment. However, Lonaprisan treatment in PMD mice at the dosage of 125 mg/kg significantly reduced Plp1 mRNA to 1.5-fold expression (n = 8, mean = 1.5, SEM = 0.1, p < 0.05) when compared to placebo-treated PMD mice (Figure 1D). This decrease is similar in extent to the therapeutic effect of a progesterone antagonist (Onapristone) in a transgenic rat model of CMT1A.37
Figure 1.
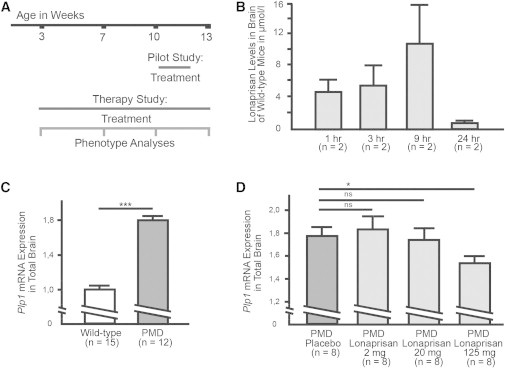
Pilot Studies in PMD Mice
(A) A short-term dosage study over 10 days and a long-term therapy study over 10 weeks were performed.
(B) Lonaprisan crossed the blood-brain barrier and was detectable 24 hr after the last subcutaneous dose.
(C) Plp1 mRNA expression was increased 1.8-fold when compared to wild-type controls.
(D) Dose-response curve demonstrating downregulation of Plp1 mRNA overexpression in a short-term pilot study.
ns indicates not significant, ∗p < 0.05, ∗∗∗p < 0.001, shown mean ± SEM.
Lonaprisan Long-Term Treatment Ameliorates the Clinical Phenotype of PMD Mice
After identifying the optimal dosage for Plp1 mRNA downregulation, Lonaprisan was subcutaneously applied to 3-week-old PMD mice for 10 consecutive weeks by daily injections at the dosage of 125 mg/kg, and placebo-treated wild-type and PMD mice served as controls (Figure 1A). Again, 10 weeks of Lonaprisan treatment reduced Plp1 overexpression at the mRNA level to 1.6-fold expression (n = 11, mean overexpression level = 1.6-fold, SEM = 0.1, p < 0.01) when compared to PMD controls (n = 12, mean overexpression level = 1.8-fold, SEM = 0.1) (Figure 2A), confirming data from the pilot experiments (Figure 1D).
Figure 2.
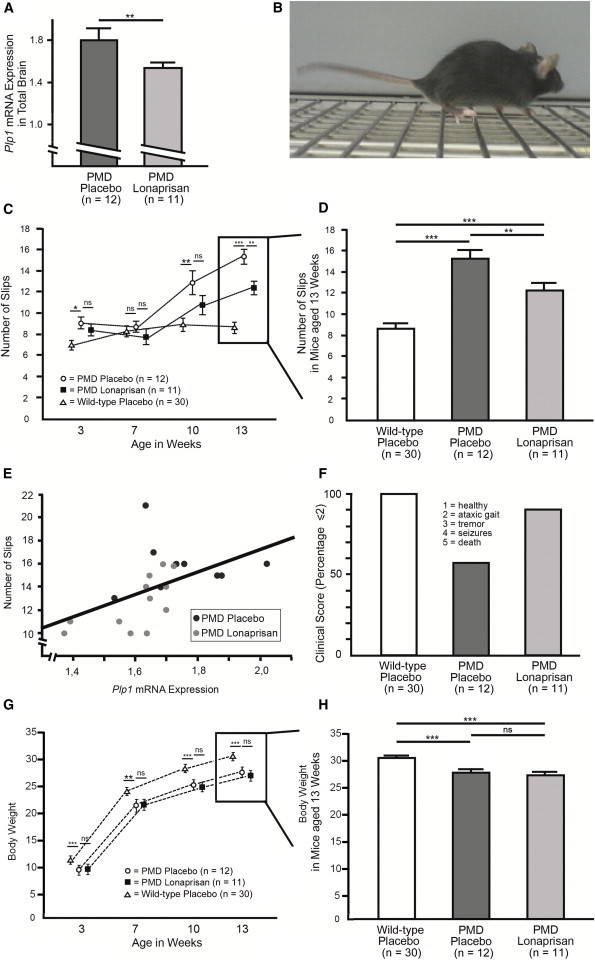
Behavioral Analysis of PMD Mice after Lonaprisan Therapy
(A) Lonaprisan long-term treatment downregulated Plp1 mRNA overexpression.
(B) Regular grid test analyses counting the number of slips were performed at the beginning at 3 weeks of age, at 7 and 10 weeks, and finally at the end at 13 weeks of age.
(C and D) Differences between Lonaprisan-treated PMD mice (n = 11) (filled squares) and placebo-treated control mice (n = 12) (circles) increased with age and became significant at 13 weeks of age at study end (C), which is illustrated in higher magnification of the final time point. Wild-type values (n = 30) are depicted for comparison (open bar) (D).
(E) Plp1 mRNA expression correlated with the grid test analysis, strongly suggesting a direct effect of Plp1 expression on the motor phenotype.
(F) By applying a clinical score in a descriptive analysis, PMD mice showed a pathologic phenotype and Lonaprisan increased the percentage of mice showing solely ataxic gait or even healthy condition.
(G and H) A significant body weight loss was already present in 3-week-old PMD mice (n = 12) compared to the wild-type situation (n = 30). This difference remained present throughout the study. In contrast, PMD placebo controls and Lonaprisan-treated PMD mice (n = 11) did not show any body weight differences at study start and Lonaprisan therapy did not alter the body weight by study end.
ns indicates not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, shown mean ± SEM.
Three-week-old PMD mice showed minor but significant motor deficiencies (i.e., more slips in the grid test) at the beginning of the trial (n = 12, mean = 9.0 slips, SEM = 0.6, p < 0.05) compared to wild-type controls (n = 30, mean = 6.9, SEM = 0.4), but there was no difference compared to PMD mice in the Lonaprisan treatment group (n = 11, mean = 8.4, SEM = 0.6) (Figures 2B and 2C). This confirmed a balanced stratification between the placebo and Lonaprisan PMD mice at therapy start. Seven weeks later, PMD groups had deteriorated in motor performance as expected. Moreover, Lonaprisan treatment (n = 11, mean = 10.7 slips, SEM = 0.9) did not reveal a significant difference in motor deficiencies in comparison to the performance of PMD mice receiving placebo (n = 12, mean = 12.8 slips, SEM = 1.1, not significant), although a clear trend was noticed (Figure 2C). However, after 10 weeks of Lonaprisan therapy, PMD mice showed significantly less progression (n = 11, mean = 12.4 slips, SEM = 0.6, p < 0.01) compared to placebo-treated PMD mice (n = 12, mean = 15.3 slips, SEM = 0.7) (Figure 2C), although the difference to wild-type mice remained obvious (n = 30, mean = 8.6 slips, SEM = 0.5) as shown in Figure 2D. Importantly, these grid test results could be directly correlated to the level of Plp1 mRNA overexpression in total brain (r2 = 0.2676, p < 0.05), strongly suggesting a direct Plp1-dosage effect on the motor phenotype (Figure 2E).
Finally, we applied a clinical score at the end of the trial in order to independently quantify Lonaprisan’s therapeutic effect. Here, Lonaprisan treatment increased the percentage of PMD mice with a low score of 2 (ataxic gait) or less (n = 11, 91%) when compared to placebo-treated PMD mice (n = 12, 58%). Wild-type controls always scored 1.0 (n = 30) (Figure 2F).
Body weight recordings revealed a significant body weight loss already present in 3-week-old PMD mice (n = 12, mean = 9.7 g, SEM = 0.2) compared to wild-type situation (n = 30, mean = 11.1 g, SEM = 0.2, p < 0.001). This difference remained present throughout the study at the ages of 7 (mean = 21.3 g, SEM = 0.8 and mean = 23.7 g, SEM = 0.3, p < 0.01), 10 (mean = 25.3 g, SEM = 0.5 and mean = 28.1 g, SEM = 0.3, p < 0.001), and 13 (mean = 27.8 g, SEM = 0.5 and mean = 30.4 g, SEM = 0.3, p < 0.001) weeks (Figure 2G). In contrast, PMD placebo controls and Lonaprisan-treated PMD mice did not show any body weight differences at study start (n = 11, mean = 10.2 g, SEM = 0.5). Also, Lonaprisan therapy in PMD mice did not alter the body weight in 7- (mean = 21.5 g, SEM = 0.6), 10- (mean = 24.7 g, SEM = 0.6), and 13- (mean = 26.8 g, SEM = 0.6) week-old mice at the study end (Figures 2G and 2H).
Preservation of Myelinated Axons
To investigate the therapeutic effect of Lonaprisan on dysmyelination and histopathology in the central nervous system, we examined a region that is severely affected in PMD mice (Figure 3A). The corticospinal tract (CST) of the cervical spinal cord was used for electron microscopy (EM) and morphometry. Whereas wild-type mice displayed on average 838 myelinated axons per 1,000 μm2 cross sectional area in the CST (n = 3, SEM = 49.6), PMD mice with severe dysmyelination showed only 198 myelinated axons per 1,000 μm2 (n = 10, SEM = 48.7, p < 0.001) (Figure 3A, quantification in 3B). Importantly, as judged by EM, the 10 week Lonaprisan therapy increased the number of myelinated axons by 25% to 254 in PMD mice (n = 11, SEM = 52.1, p < 0.05) (Figure 3B). Correspondingly, unmyelinated CST axons were numerous in PMD mice (n = 5, mean = 489/1,000 μm2, SEM = 91.6) but rare in wild-type mice (n = 3, mean = 7/1,000 μm2, SEM = 2.0, p < 0.01). However, Lonaprisan treatment did not alter the number of unmyelinated axons in PMD mice (n = 5, mean = 524/1,000 μm2, SEM = 77.6) (Figure 3C).
Figure 3.
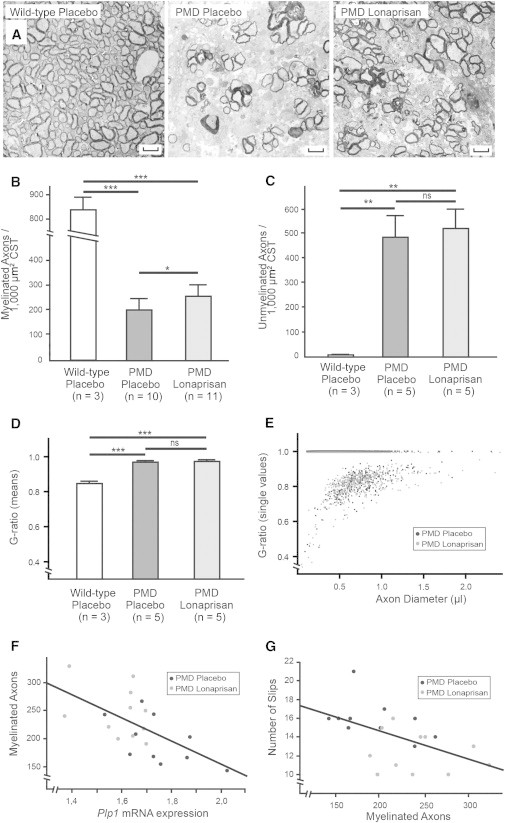
Electron Microscopic Analyses of the CNS after Lonaprisan Therapy in PMD Mice
(A) Electron microscopic graphs of the corticospinal tract showed preservation of myelinated axons after Lonaprisan treatment.
(B) Myelinated axons are reduced in PMD mice compared to wild-type controls, and after Lonaprisan therapy myelinated axons are increased.
(C) Nonmyelinated axon number was increased in the disease situation but was unaltered by Lonaprisan treatment.
(D and E) In PMD mice, Lonaprisan therapy did not increase the mean myelin sheath thickness (D) and corresponding myelin sheath thickness according to axonal size (E).
(F and G) Plp1 mRNA expression levels correlated to the myelinated axon number (F), and myelinated axon number correlated to the phenotype (G).
Scale bars represent 1.7 μm. CST indicates corticospinal tract, ns indicates not significant, ∗p < 0.05, ∗∗∗p < 0.001, shown mean ± SEM.
As expected, g-ratio analysis also revealed a significant hypomyelination in PMD mice (n = 5, mean = 0.97, SEM = 0.01) compared to wild-type controls (n = 3, mean = 0.84, SEM = 0.01, p < 0.001). Again, Lonaprisan therapy over 10 weeks did not influence the extent of hypomyelination in PMD mice (n = 5, mean = 0.97, SEM = 0.01, not significant) (Figure 3D). The g-ratio distribution as a function of axon size was also not different between placebo- and Lonaprisan-treated PMD mice (Figure 3E). When combined with the results above, we conclude that Lonaprisan acts on axonal preservation without any effects on myelination.
Correlation of Plp1 mRNA Expression, Myelinated Axons, and Phenotype
Plp1 mRNA expression levels correlated with the motor phenotype as measured by the grid test analysis in treated and untreated PMD mice (Figure 2E). Histological analysis showed that the number of myelinated axons correlated inversely with the Plp1 mRNA expression (r2 = 0.3693, p < 0.01) (Figure 3F), suggesting that Plp1 overexpression in oligodendrocytes (and abnormal gain of function) leads to the loss of myelinated axons. Likewise, the number of myelinated axons in the CST correlated with the motor phenotype (r2 = 0.2729, p < 0.05) (Figure 3G), demonstrating a strong link between Plp1 dosage, number of myelinated axons, and clinical phenotype in PMD mice.
Lonaprisan Therapy Modifies Expression of Apoptosis-Related Genes
To assess the molecular consequences of Lonaprisan treatment, we performed individual microarray analyses on brain cDNA preparations obtained from PMD mice treated with placebo (n = 3) and Lonaprisan (n = 3). When searching for functional groups, gene set enrichment analysis (GSEA) revealed a significant downregulation of genes involved in apoptosis in mouse brains after Lonaprisan therapy (p < 0.001, enrichment score = −0.38) (Figure 4A). The heatmap plot for apoptosis visualized 62 genes involved in the apoptosis pathway (Figure 4B). In order to validate the microarray data, we performed quantitative RT-PCR analyses on total brain cDNAs obtained from individual wild-type controls and PMD mice, treated either with placebo or Lonaprisan. For example, Bax mRNA expression was significantly increased in PMD mice (n = 7, mean = 2.2-fold, SEM = 0.3) compared to wild-type controls (n = 8, defined as 1.0, SEM = 0.2, p < 0.01). Here, Lonaprisan treatment significantly downregulated Bax overexpression when compared to placebo-treated PMD mice (n = 11, mean = 1.1-fold, SEM = 0.1, p < 0.001), reaching wild-type levels (Figure 4D). Jun mRNA expression was also increased in PMD mice (n = 7, mean = 4.2-fold, SEM = 0.7) compared to wild-type controls (n = 8, defined as 1.0, SEM = 0.5, p < 0.01) and Lonaprisan therapy normalized the mRNA overexpression to 1.3-fold (n = 11, mean = SEM = 0.3, p < 0.001), again approaching wild-type levels (Figure 4C). Finally, we measured Casp7 mRNA levels that were upregulated in PMD mice (n = 11, mean = 2.6-fold, SEM = 0.3) compared to wild-type controls (n = 8, defined as 1.0, SEM = 0.2, p < 0.001). Lonaprisan treatment over 10 weeks downregulated Casp7 overexpression in PMD mice to 1.6-fold (n = 11, SEM = 0.2, p < 0.05), but without reaching wild-type levels (p > 0.05) (Figure 4E).
Figure 4.
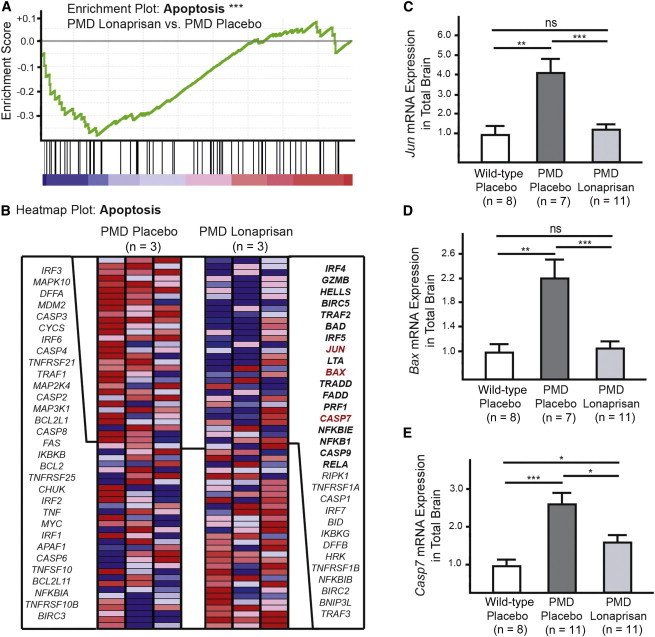
Microarray Analysis in PMD Mice Treated with Lonaprisan
(A and B) Microarray analysis revealed a downregulation of genes involved in apoptosis after Lonaprisan therapy (core enriched genes in bold).
(C–E) Jun, Casp7, and Bax were validated by quantitative RT-PCR.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, shown mean ± SEM.
Oligodendrocyte Loss, Microgliosis, and Infiltration of Lymphocytes
Next, we performed immunohistochemistry in the CST and corpus callosum (CC) in order to validate the effect of Lonaprisan treatment on the selected histopathological marker proteins OLIG-2, MAC-3, GFAP, and CD3 (Figure 5A). Counting OLIG-2-positive cells in the CST demonstrated more than 50% decrease of oligodendrocyte density in PMD mice (n = 6, mean = 7 cells per 30,000 μm2, SEM = 0.7, p < 0.001) when compared to wild-type controls (n = 3, mean = 16 cells per 30,000 μm2, SEM = 0.6). Here, 10 weeks of Lonaprisan treatment significantly prevented oligodendrocyte loss (n = 6, mean = 11 cells per 30,000 μm2, SEM = 1.4, p < 0.05), although wild-type values were not reached (Figure 5B). As expected, wild-type brains showed only a few microglia/macrophages within the CST as indicated by MAC-3-positive cells counts (n = 3, mean = 5 cells per 30,000 μm2, SEM = 1.2). In PMD mice, MAC-3-positive cells were several-fold increased, indicating active microgliosis (n = 6, mean = 92 cells per 30,000 μm2, SEM = 15.2, p < 0.01). Lonaprisan-treated PMD mice displayed fewer MAC-3-positive microglia and macrophages compared to untreated PMD mice (n = 6, mean = 31 cells per 30,000 μm2, SEM = 4.2, p < 0.01) but without approaching wild-type values (Figure 5C). Finally, GFAP cell counts in the CST revealed that Lonaprisan treatment also reduced astrogliosis (n = 6, mean = 8 cells per 30,000 μm2, SEM = 0.8) when compared to untreated PMD mice (n = 6, mean = 11 cells per 30,000 μm2, SEM = 1.1, p < 0.05). Moreover, wild-type values were not reached (n = 3, mean = 5 cells per 30,000 μm2, SEM = 1.5) (Figure 5D). In the CST area of wild-type mice, we never detected CD3-positive lymphocytes (n = 3, mean = 0 cells per 30,000 μm2, SEM = 0). However, we detected CD3-positive T cells in untreated PMD mice, suggesting that lymphocyte infiltration is stimulated by the disease (n = 6, mean = 3 cells per 30,000 μm2, SEM = 0.8, p < 0.05). In the Lonaprisan treatment group, the number of CD3-positive cells was significantly reduced (n = 6, mean = 0.7 cells per 30,000 μm2, SEM = 0.3, p < 0.05), statistically approaching the values found in wild-type mice (Figure 5E).
Figure 5.
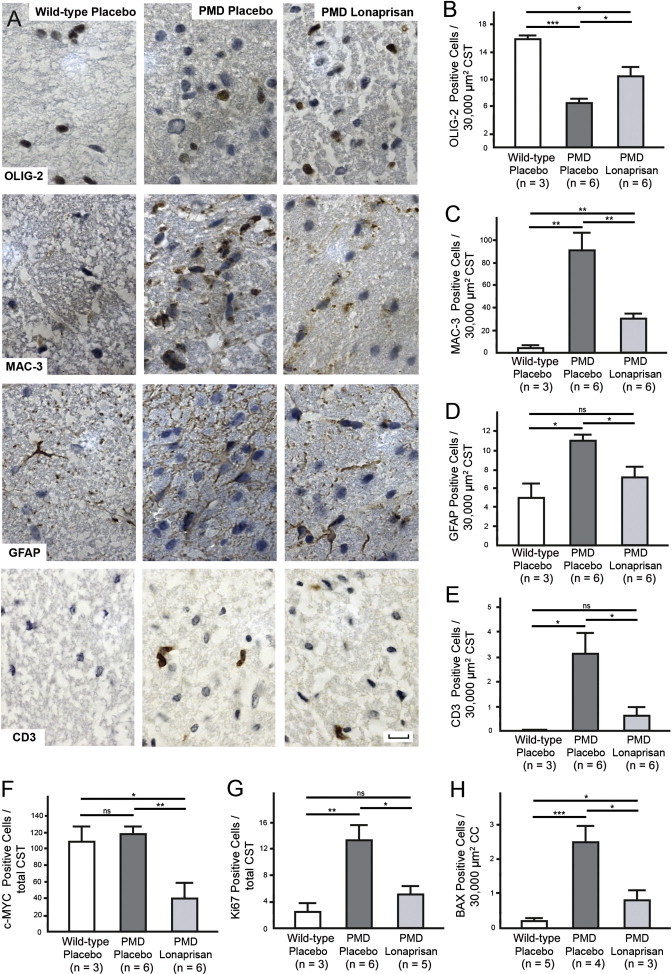
Immunohistochemistry in CNS after Treatment with Lonaprisan in PMD Mice
(A) Immunohistochemistry showing OLIG-2-, MAC-3-, GFAP-, and CD3-positive cells in wild-type and placebo- and Lonaprisan-treated PMD mice.
(B–E) Lonaprisan therapy reduced oligodendrocyte loss (B), microgliosis (C), astrogliosis (D), and lymphocyte infiltration (E).
(F–H) Further, cells positive for c-MYC, Ki67, and BAX were reduced after Lonaprisan therapy, indicating changes in cell cycle regulation resulting in reduced proliferation and apoptosis in PMD mice.
Scale bars represent 10 μm. CST indicates corticospinal tract, CC indicates corpus callosum, ns indicates not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, shown mean ± SEM.
Additionally, sections were analyzed for the expression of c-MYC (cell cycle regulator), Ki67 (proliferation marker), and BAX (apoptosis related marker). The density of c-MYC-positive cells in the CST (n = 6, mean = 119 cells per section, SEM = 9.5) was not different from wild-type controls (n = 3, mean 110 cells per section, SEM = 17.9). However, Lonaprisan therapy reduced this number to less than 50% in the CST of PMD mice (n = 6, mean = 41 cells per section, SEM = 19.4, p < 0.01) (Figure 5F). Further, Ki67-positive cells were found in significantly higher density in the CST of PMD mice (n = 6, mean = 14 cells per section, SEM = 2.3, p < 0.01) than in wild-type controls (n = 3, mean = 3 cells per section, SEM = 1.2). Ten weeks of Lonaprisan therapy reduced the density of Ki67-positive cells by more than 60% (n = 5, mean = 5 cells per section, SEM = 1.3, p < 0.05) without reaching wild-type values (Figure 5G). Interestingly and in line with transcriptional data (Figure 4D), the density of cells expressing the apoptosis-associated protein BAX in the corpus callosum was increased in PMD mice (n = 4, mean = 2.5 cells per 30,000 μm2, SEM = 0.5, p < 0.001) when compared to wild-type mice (n = 5, mean = 0.2 cells per 30,000 μm2, SEM = 0.1) and reduced after Lonaprisan therapy (n = 3, mean = 0.8 cells per 30,000 μm2, SEM = 0.3, p < 0.05) (Figure 5H).
Discussion
We tested the hypothesis that lowering toxic Plp1 overexpression in oligodendrocytes in a transgenic animal model of PMD via steroid antagonist would enable oligodendrocytes to better support axons. This may lead to a translatable therapy for a subgroup of individuals affected by PMD caused by PLP1 duplications.
A number of factors regulating Plp1 transcription in oligodendrocytes have been identified in vitro, such as MyT1,58 microRNA miR-20a,59 and the zinc finger protein YY1.60 Moreover, several potential transcription factor binding sites and enhancer elements have been identified in the 5′-flanking sequence61–63 and within intron 1 of mouse Plp1 (AP-1).64,65 One widely expressed transcription factor that is present in oligodendrocytes66 and can be pharmacologically targeted is the nuclear progesterone receptor, a ligand-activated Zinc-finger protein that is regulated by progesterone and inhibited by specific synthetic antagonists. The nuclear progesterone receptor is a member of a much larger steroid receptor superfamily that binds to palindromic response elements in the promoter regions of steroid-responsive genes.67 Progesterone is synthesized in the brain by neurons and glial cells68,69 and the progesterone receptor is expressed by neurons and oligodendrocytes.49,66,70–72 Furthermore, steroid-responsive elements including progesterone receptor binding sites73–75 can be predicted in PLP1 promoter (data not shown) and daily progesterone injections upregulate Plp1 mRNA in sciatic nerves of rats in vivo (Figure S1 available online).37 In the PNS, the activating effect of progesterone on Schwann cell differentiation and the transcriptional activation of myelin genes Pmp22 and Mpz are well known.76,77 The progesterone receptor antagonists Mifepristone (RU486), Onapristone (ZK98299), and the newly developed Lonaprisan (ZK230211), which was used in the present study, specifically inhibit the effect of the progesterone receptor on transcriptional activation.51 However, there are no data on the functional role of progesterone receptor on Plp1 transcription.
In the present study, we have chosen Lonaprisan because of its pure progesterone antagonistic activity without partial agonistic potential such as demonstrated by Mifepristone.78 Furthermore, Lonaprisan shows a more favorable safety profile than Onapristone, which may enable translation of our findings to humans.79
Lonaprisan was shown to cross the blood-brain barrier in wild-type mice and, in a dose-finding pilot study for 10 days in PMD mice, Lonaprisan daily treatment at 125 mg/kg body weight demonstrated potency to downregulate Plp1 1.8-fold mRNA overexpression to 1.5-fold expression. Importantly, after 10 weeks of daily Lonaprisan therapy, Plp1 mRNA overexpression was still reduced to 1.6-fold expression. Because PLP1 is most abundantly expressed in oligodendrocytes and Plp1 transgenic mice do not show ectopic PLP1 expression in the CNS,30 the reduction of Plp1 mRNA overexpression is predominantly restricted to oligodendrocytes. We have not quantified PLP1 protein because Plp1 dosage interferes with myelination itself.30 Therefore, any quantification of PLP1 proteins of PMD mice may yield a paradoxic result, because less (rather than more) PLP1 is detected, with PLP1 serving as a marker for myelin. Consequently, our attempts of pharmacologically reducing Plp1 transcription and rescuing myelination in the mouse model would consequently lead to more myelin and more myelin protein (including PLP1).
Lonaprisan therapy significantly prevented the loss of myelinated axons and improved the motor phenotype in PMD mice by approximately 50% toward wild-type level as shown with the grid test analysis. Applying a second phenotype measurement, the clinical score data revealed a higher percentage of PMD mice showing solely ataxic gait or even healthy condition after long-term therapy with Lonaprisan. Body weight loss as a sign for unspecific side effects was not observed in Lonaprisan-treated mice. However, Lonaprisan treatment did not show an effect on the number of unmyelinated axons nor on myelin sheath thickness. Importantly, Plp1 mRNA levels correlated inversely with the number of myelinated axons and with the phenotype of PMD mice. Further, myelinated axon numbers correlated with the clinical phenotype. These observations confirm the importance of tight Plp1 regulation and support the concept that the reduction of toxic Plp1 mRNA overexpression poses the key mechanism for the progesterone antagonistic effect. However, it is not known to what degree the progesterone receptor contributes to Plp1 mRNA expression.
In a randomized phase II study to investigate the efficacy, safety, and tolerability of Lonaprisan as second-line endocrine therapy for postmenopausal women with hormone receptor-positive metastatic breast cancer, maximum oral dosage of 100 mg was applied (ClinicalTrials.gov identifier NCT00555919). Therefore, when translating the current findings to human use, careful dose finding targeting Plp1 expression in individuals suffering from PMD is necessary.
PMD mice suffer from a lymphocyte infiltration into the CNS,36,80 microglia activation, and astrogliosis as well as described features.30,32,34,35 Lonaprisan therapy significantly decreased the number of lymphocytes present in the CNS, suggesting that there is reduced inflammation after long-term treatment with the progesterone antagonist. Because loss of progesterone receptor function mutants show proinflammatory effects,81 we interpret the reduced inflammation as secondary effect resulting from improved PMD pathology. Further effects of the long-term therapy with Lonaprisan included the prevention of OLIG-2-stained oligodendrocyte lineage cells and the reduction of MAC-3-stained microglia and GFAP-stained astrogliosis.
In order to address the mechanism by which Lonaprisan treatment can prevent axonal loss, we performed microarray analysis and applied the gene set enrichment analysis (GSEA). We found a significant downregulation of genes involved in apoptosis and validated this finding by quantitative RT-PCR at the level of single animals (n = 8–11) for three highly downregulated apoptotic genes: Jun, Casp7, and Bax. At the transcriptional level, the expression of all three genes was upregulated in PMD mice and downregulated after Lonaprisan therapy. By immunohistochemistry, PMD pathology was directly associated with higher apoptosis and proliferation marker expression when compared to wild-type littermates (BAX82 and Ki67,83 respectively). These observations are in line with an increase in apoptotic oligodendrocytes in PMD mice.32,35 Importantly, Lonaprisan therapy significantly reduced the number of cells overexpressing these apoptosis and proliferation markers. Furthermore, cell counts for c-MYC, a key regulator of cell cycle with proapoptotic and proproliferative actions,84 showed less c-MYC expression after Lonaprisan treatment.
Saher et al. recently reported a therapeutic strategy with high-cholesterol diet in PMD mice.28 This effect is mediated by the amelioration of intracellular PLP1 accumulation and increased PLP1 incorporation into myelin membranes that was accompanied by an upregulation of cholesterol-synthesizing enzymes (Hmgcr and Fdft1 mRNA) without regulatory effects on Plp1 mRNA overexpression itself. In contrast, Lonaprisan in PMD mice did not affect cholesterol-synthesizing-enzyme expression of Hmgcr, Fdft1, Mvd, or Lss mRNA (Figures S2A–S2D). Because cholesterol and Lonaprisan experimental therapies act independently, the combination may lead to synergistic effects.
Further more, a strong argument for therapeutic regulation of toxic overexpression of myelin proteins in the CNS is derived from a former therapy trial performed in a transgenic rat model for the most common inherited neuropathy, Charcot-Marie-Tooth disease (“CMT1A” rats).37,85,86 CMT1A is caused by an intrachromosomal duplication on chromosomal region 17p11.2 that leads to an increased gene dosage of the peripheral myelin protein of 22 kDa and consecutive demyelination of peripheral nerve axons.87 PMP22 is a small hydrophobic tetraspan protein expressed in myelinating glia of the PNS and shows a similar structure to PLP1. Daily administration of the pure progesterone antagonist Onapristone reduced Pmp22 overexpression, ameliorated axonal loss, and improved the clinical phenotype in Pmp22 transgenic CMT rats.37,88 In contrast to the correlations in the present study, CMT rats did not demonstrate stringent correlations of Pmp22 mRNA expression, axonal numbers, and the clinical phenotype upon antiprogesterone treatment, which may be explained by the missing consensus regions for the progesterone receptor in the Pmp22 promoter region. However, the observation of preserved axonal loss without any effects on myelin sheath thickness in the present study is consistent with the former progesterone therapy trial in CMT rats. In that study, we hypothesized that Pmp22 overexpression was reduced to a degree at which the axonal support function of Schwann cells was better maintained than myelination.89 We note that Plp1 loss-of-function mutants show no crude myelin abnormalities but demonstrate axonal pathology,5,22 implying that PLP1 is responsible for axonal support rather than myelination in the CNS. Because we do not assume that overexpression linearly contributes to PMD pathology and that oligodendrocytes tolerate increasing Plp1 mRNA levels only to a certain degree, the initial correction toward wild-type level may be considered as most important for axonal support.
In summary, we demonstrate proof of principle that lowering toxic Plp1 overexpression via a progesterone antagonist ameliorates axonal loss and the disease phenotype in Plp1 transgenic mice.
Acknowledgments
We thank Bayer for providing us with Lonaprisan. Electron microscopy was performed in the electron microscopic facility of the MPIEM headed by W. Möbius. This work was supported by grants of EU Health-2009-2.4.4.1 (LeukoTreat), DFG Research Center Molecular Physiology of the Brain (CMPB), and European Commission FP7-201535 (Ngidd) to K.-A.N. M.W.S. and R.F. were supported by the German Ministry of Education and Research (BMBF, FKZ: 01ES0812 to M.W.S.). M.W.S. was also supported by the Association Francaise contre Les Myopathies (AFM, Nr: 15037 to M.W.S.) and holds a Heisenberg Professorship. K.-A.N. holds an ERC Advanced Investigator Grant.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
ClinicalTrials.gov, http://clinicaltrials.gov
Gene Expression Omnibus (GEO), http://www.ncbi.nlm.nih.gov/geo/
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org/
Accession Numbers
Microarray data are available in the Gene Expression Omnibus database, accession number GSE55315.
References
- 1.Seitelberger F. Neuropathology and genetics of Pelizaeus-Merzbacher disease. Brain Pathol. 1995;5:267–273. doi: 10.1111/j.1750-3639.1995.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 2.Yool D.A., Edgar J.M., Montague P., Malcolm S. The proteolipid protein gene and myelin disorders in man and animal models. Hum. Mol. Genet. 2000;9:987–992. doi: 10.1093/hmg/9.6.987. [DOI] [PubMed] [Google Scholar]
- 3.Koeppen A.H., Robitaille Y. Pelizaeus-Merzbacher disease. J. Neuropathol. Exp. Neurol. 2002;61:747–759. doi: 10.1093/jnen/61.9.747. [DOI] [PubMed] [Google Scholar]
- 4.Garbern J.Y. Pelizaeus-Merzbacher disease: Genetic and cellular pathogenesis. Cell. Mol. Life Sci. 2007;64:50–65. doi: 10.1007/s00018-006-6182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruenenfelder F.I., Thomson G., Penderis J., Edgar J.M. Axon-glial interaction in the CNS: what we have learned from mouse models of Pelizaeus-Merzbacher disease. J. Anat. 2011;219:33–43. doi: 10.1111/j.1469-7580.2011.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cailloux F., Gauthier-Barichard F., Mimault C., Isabelle V., Courtois V., Giraud G., Dastugue B., Boespflug-Tanguy O., Clinical European Network on Brain Dysmyelinating Disease Genotype-phenotype correlation in inherited brain myelination defects due to proteolipid protein gene mutations. Eur. J. Hum. Genet. 2000;8:837–845. doi: 10.1038/sj.ejhg.5200537. [DOI] [PubMed] [Google Scholar]
- 7.Al-Saktawi K., McLaughlin M., Klugmann M., Schneider A., Barrie J.A., McCulloch M.C., Montague P., Kirkham D., Nave K.A., Griffiths I.R. Genetic background determines phenotypic severity of the Plp rumpshaker mutation. J. Neurosci. Res. 2003;72:12–24. doi: 10.1002/jnr.10561. [DOI] [PubMed] [Google Scholar]
- 8.Woodward K.J. The molecular and cellular defects underlying Pelizaeus-Merzbacher disease. Expert Rev. Mol. Med. 2008;10:e14. doi: 10.1017/S1462399408000677. [DOI] [PubMed] [Google Scholar]
- 9.Jahn O., Tenzer S., Werner H.B. Myelin proteomics: molecular anatomy of an insulating sheath. Mol. Neurobiol. 2009;40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saugier-Veber P., Munnich A., Bonneau D., Rozet J.M., Le Merrer M., Gil R., Boespflug-Tanguy O. X-linked spastic paraplegia and Pelizaeus-Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat. Genet. 1994;6:257–262. doi: 10.1038/ng0394-257. [DOI] [PubMed] [Google Scholar]
- 11.Hudson L.D. Pelizaeus-Merzbacher disease and spastic paraplegia type 2: two faces of myelin loss from mutations in the same gene. J. Child Neurol. 2003;18:616–624. doi: 10.1177/08830738030180090801. [DOI] [PubMed] [Google Scholar]
- 12.Nave K.-A., Griffiths I.R. Models of Pelizaeus–Merzbacher disease. In: Lazzarini R.A., Griffin J.W., Lassmann H., Nave K.-A., Miller R.H., Trapp B.D., editors. Myelin Biology and Disorders. Elsevier; Amsterdam: 2004. pp. 1125–1142. [Google Scholar]
- 13.Inoue K. PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics. 2005;6:1–16. doi: 10.1007/s10048-004-0207-y. [DOI] [PubMed] [Google Scholar]
- 14.Woodward K., Kendall E., Vetrie D., Malcolm S. Pelizaeus-Merzbacher disease: identification of Xq22 proteolipid-protein duplications and characterization of breakpoints by interphase FISH. Am. J. Hum. Genet. 1998;63:207–217. doi: 10.1086/301933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf N.I., Sistermans E.A., Cundall M., Hobson G.M., Davis-Williams A.P., Palmer R., Stubbs P., Davies S., Endziniene M., Wu Y. Three or more copies of the proteolipid protein gene PLP1 cause severe Pelizaeus-Merzbacher disease. Brain. 2005;128:743–751. doi: 10.1093/brain/awh409. [DOI] [PubMed] [Google Scholar]
- 16.Schneider A.M., Griffiths I.R., Readhead C., Nave K.A. Dominant-negative action of the jimpy mutation in mice complemented with an autosomal transgene for myelin proteolipid protein. Proc. Natl. Acad. Sci. USA. 1995;92:4447–4451. doi: 10.1073/pnas.92.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gow A., Lazzarini R.A. A cellular mechanism governing the severity of Pelizaeus-Merzbacher disease. Nat. Genet. 1996;13:422–428. doi: 10.1038/ng0896-422. [DOI] [PubMed] [Google Scholar]
- 18.Jung M., Sommer I., Schachner M., Nave K.A. Monoclonal antibody O10 defines a conformationally sensitive cell-surface epitope of proteolipid protein (PLP): evidence that PLP misfolding underlies dysmyelination in mutant mice. J. Neurosci. 1996;16:7920–7929. doi: 10.1523/JNEUROSCI.16-24-07920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simons M., Kramer E.M., Macchi P., Rathke-Hartlieb S., Trotter J., Nave K.A., Schulz J.B. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. J. Cell Biol. 2002;157:327–336. doi: 10.1083/jcb.200110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krämer-Albers E.M., Gehrig-Burger K., Thiele C., Trotter J., Nave K.A. Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia. J. Neurosci. 2006;26:11743–11752. doi: 10.1523/JNEUROSCI.3581-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karim S.A., Barrie J.A., McCulloch M.C., Montague P., Edgar J.M., Iden D.L., Anderson T.J., Nave K.A., Griffiths I.R., McLaughlin M. PLP/DM20 expression and turnover in a transgenic mouse model of Pelizaeus-Merzbacher disease. Glia. 2010;58:1727–1738. doi: 10.1002/glia.21043. [DOI] [PubMed] [Google Scholar]
- 22.Klugmann M., Schwab M.H., Pühlhofer A., Schneider A., Zimmermann F., Griffiths I.R., Nave K.A. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths I., Klugmann M., Anderson T., Yool D., Thomson C., Schwab M.H., Schneider A., Zimmermann F., McCulloch M., Nadon N., Nave K.A. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 24.Garbern J.Y., Cambi F., Lewis R., Shy M., Sima A., Kraft G., Vallat J.M., Bosch E.P., Hodes M.E., Dlouhy S. Peripheral neuropathy caused by proteolipid protein gene mutations. Ann. N Y Acad. Sci. 1999;883:351–365. [PubMed] [Google Scholar]
- 25.Edgar J.M., McLaughlin M., Yool D., Zhang S.C., Fowler J.H., Montague P., Barrie J.A., McCulloch M.C., Duncan I.D., Garbern J. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J. Cell Biol. 2004;166:121–131. doi: 10.1083/jcb.200312012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner H.B., Krämer-Albers E.M., Strenzke N., Saher G., Tenzer S., Ohno-Iwashita Y., De Monasterio-Schrader P., Möbius W., Moser T., Griffiths I.R., Nave K.A. A critical role for the cholesterol-associated proteolipids PLP and M6B in myelination of the central nervous system. Glia. 2013;61:567–586. doi: 10.1002/glia.22456. [DOI] [PubMed] [Google Scholar]
- 27.Karim S.A., Barrie J.A., McCulloch M.C., Montague P., Edgar J.M., Kirkham D., Anderson T.J., Nave K.A., Griffiths I.R., McLaughlin M. PLP overexpression perturbs myelin protein composition and myelination in a mouse model of Pelizaeus-Merzbacher disease. Glia. 2007;55:341–351. doi: 10.1002/glia.20465. [DOI] [PubMed] [Google Scholar]
- 28.Saher G., Rudolphi F., Corthals K., Ruhwedel T., Schmidt K.F., Löwel S., Dibaj P., Barrette B., Möbius W., Nave K.A. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat. Med. 2012;18:1130–1135. doi: 10.1038/nm.2833. [DOI] [PubMed] [Google Scholar]
- 29.Kagawa T., Ikenaka K., Inoue Y., Kuriyama S., Tsujii T., Nakao J., Nakajima K., Aruga J., Okano H., Mikoshiba K. Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron. 1994;13:427–442. doi: 10.1016/0896-6273(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 30.Readhead C., Schneider A., Griffiths I., Nave K.A. Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron. 1994;12:583–595. doi: 10.1016/0896-6273(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 31.Inoue Y., Kagawa T., Matsumura Y., Ikenaka K., Mikoshiba K. Cell death of oligodendrocytes or demyelination induced by overexpression of proteolipid protein depending on expressed gene dosage. Neurosci. Res. 1996;25:161–172. doi: 10.1016/0168-0102(96)01039-5. [DOI] [PubMed] [Google Scholar]
- 32.Anderson T.J., Klugmann M., Thomson C.E., Schneider A., Readhead C., Nave K.A., Griffiths I.R. Distinct phenotypes associated with increasing dosage of the PLP gene: implications for CMT1A due to PMP22 gene duplication. Ann. N Y Acad. Sci. 1999;883:234–246. [PubMed] [Google Scholar]
- 33.Bradl M., Bauer J., Inomata T., Zielasek J., Nave K.A., Toyka K., Lassmann H., Wekerle H. Transgenic Lewis rats overexpressing the proteolipid protein gene: myelin degeneration and its effect on T cell-mediated experimental autoimmune encephalomyelitis. Acta Neuropathol. 1999;97:595–606. doi: 10.1007/s004010051035. [DOI] [PubMed] [Google Scholar]
- 34.Anderson T.J., Schneider A., Barrie J.A., Klugmann M., McCulloch M.C., Kirkham D., Kyriakides E., Nave K.A., Griffiths I.R. Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. J. Comp. Neurol. 1998;394:506–519. doi: 10.1002/(sici)1096-9861(19980518)394:4<506::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Cerghet M., Bessert D.A., Nave K.A., Skoff R.P. Differential expression of apoptotic markers in jimpy and in Plp overexpressors: evidence for different apoptotic pathways. J. Neurocytol. 2001;30:841–855. doi: 10.1023/a:1019697506757. [DOI] [PubMed] [Google Scholar]
- 36.Edgar J.M., McCulloch M.C., Montague P., Brown A.M., Thilemann S., Pratola L., Gruenenfelder F.I., Griffiths I.R., Nave K.A. Demyelination and axonal preservation in a transgenic mouse model of Pelizaeus-Merzbacher disease. EMBO Mol. Med. 2010;2:42–50. doi: 10.1002/emmm.200900057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sereda M.W., Meyer zu Hörste G., Suter U., Uzma N., Nave K.A. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A) Nat. Med. 2003;9:1533–1537. doi: 10.1038/nm957. [DOI] [PubMed] [Google Scholar]
- 38.Koenig H.L., Schumacher M., Ferzaz B., Thi A.N., Ressouches A., Guennoun R., Jung-Testas I., Robel P., Akwa Y., Baulieu E.E. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 39.Désarnaud F., Do Thi A.N., Brown A.M., Lemke G., Suter U., Baulieu E.E., Schumacher M. Progesterone stimulates the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. J. Neurochem. 1998;71:1765–1768. doi: 10.1046/j.1471-4159.1998.71041765.x. [DOI] [PubMed] [Google Scholar]
- 40.Guennoun R., Benmessahel Y., Delespierre B., Gouézou M., Rajkowski K.M., Baulieu E.E., Schumacher M. Progesterone stimulates Krox-20 gene expression in Schwann cells. Brain Res. Mol. Brain Res. 2001;90:75–82. doi: 10.1016/s0169-328x(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 41.Magnaghi V., Ballabio M., Consoli A., Lambert J.J., Roglio I., Melcangi R.C. GABA receptor-mediated effects in the peripheral nervous system: A cross-interaction with neuroactive steroids. J. Mol. Neurosci. 2006;28:89–102. doi: 10.1385/jmn:28:1:89. [DOI] [PubMed] [Google Scholar]
- 42.Magnaghi V., Ballabio M., Roglio I., Melcangi R.C. Progesterone derivatives increase expression of Krox-20 and Sox-10 in rat Schwann cells. J. Mol. Neurosci. 2007;31:149–157. doi: 10.1385/jmn/31:02:149. [DOI] [PubMed] [Google Scholar]
- 43.Schumacher M., Hussain R., Gago N., Oudinet J.P., Mattern C., Ghoumari A.M. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front. Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung-Testas I., Schumacher M., Robel P., Baulieu E.E. The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell. Mol. Neurobiol. 1996;16:439–443. doi: 10.1007/BF02088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghoumari A.M., Ibanez C., El-Etr M., Leclerc P., Eychenne B., O’Malley B.W., Baulieu E.E., Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J. Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 46.Ibanez C., Shields S.A., El-Etr M., Baulieu E.E., Schumacher M., Franklin R.J. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol. Appl. Neurobiol. 2004;30:80–89. doi: 10.1046/j.0305-1846.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- 47.Gago N., El-Etr M., Sananès N., Cadepond F., Samuel D., Avellana-Adalid V., Baron-Van Evercooren A., Schumacher M. 3alpha,5alpha-Tetrahydroprogesterone (allopregnanolone) and gamma-aminobutyric acid: autocrine/paracrine interactions in the control of neonatal PSA-NCAM+ progenitor proliferation. J. Neurosci. Res. 2004;78:770–783. doi: 10.1002/jnr.20348. [DOI] [PubMed] [Google Scholar]
- 48.Ghoumari A.M., Baulieu E.E., Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135:47–58. doi: 10.1016/j.neuroscience.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Labombarda F., González S.L., Lima A., Roig P., Guennoun R., Schumacher M., de Nicola A.F. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 2009;57:884–897. doi: 10.1002/glia.20814. [DOI] [PubMed] [Google Scholar]
- 50.Acs P., Kipp M., Norkute A., Johann S., Clarner T., Braun A., Berente Z., Komoly S., Beyer C. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57:807–814. doi: 10.1002/glia.20806. [DOI] [PubMed] [Google Scholar]
- 51.Fuhrmann U., Hess-Stumpp H., Cleve A., Neef G., Schwede W., Hoffmann J., Fritzemeier K.H., Chwalisz K. Synthesis and biological activity of a novel, highly potent progesterone receptor antagonist. J. Med. Chem. 2000;43:5010–5016. doi: 10.1021/jm001000c. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson U., Schultz R.L. Fixation of the central nervous system from electron microscopy by aldehyde perfusion. I. Preservation with aldehyde perfusates versus direct perfusion with osmium tetroxide with special reference to membranes and the extracelluar space. J. Ultrastruct. Res. 1965;12:160–186. doi: 10.1016/s0022-5320(65)80014-4. [DOI] [PubMed] [Google Scholar]
- 53.Suter U., Snipes G.J., Schoener-Scott R., Welcher A.A., Pareek S., Lupski J.R., Murphy R.A., Shooter E.M., Patel P.I. Regulation of tissue-specific expression of alternative peripheral myelin protein-22 (PMP22) gene transcripts by two promoters. J. Biol. Chem. 1994;269:25795–25808. [PubMed] [Google Scholar]
- 54.van de Wetering R.A., Gabreëls-Festen A.A., Kremer H., Kalscheuer V.M., Gabreëls F.J., Mariman E.C. Regulation and expression of the murine PMP22 gene. Mamm. Genome. 1999;10:419–422. doi: 10.1007/s003359901015. [DOI] [PubMed] [Google Scholar]
- 55.Ohsawa Y., Murakami T., Miyazaki Y., Shirabe T., Sunada Y. Peripheral myelin protein 22 is expressed in human central nervous system. J. Neurol. Sci. 2006;247:11–15. doi: 10.1016/j.jns.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 57.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J.G., Hudson L.D. Novel member of the zinc finger superfamily: A C2-HC finger that recognizes a glia-specific gene. Mol. Cell. Biol. 1992;12:5632–5639. doi: 10.1128/mcb.12.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang E., Cambi F. MicroRNA expression in mouse oligodendrocytes and regulation of proteolipid protein gene expression. J. Neurosci. Res. 2012;90:1701–1712. doi: 10.1002/jnr.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zolova O.E., Wight P.A. YY1 negatively regulates mouse myelin proteolipid protein (Plp1) gene expression in oligodendroglial cells. ASN Neuro. 2011;3:3. doi: 10.1042/AN20110021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nave K.A., Lemke G. Induction of the myelin proteolipid protein (PLP) gene in C6 glioblastoma cells: functional analysis of the PLP promotor. J. Neurosci. 1991;11:3060–3069. doi: 10.1523/JNEUROSCI.11-10-03060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janz R., Stoffel W. Characterization of a brain-specific Sp1-like activity interacting with an unusual binding site within the myelin proteolipid protein promoter. Biol. Chem. Hoppe Seyler. 1993;374:507–517. doi: 10.1515/bchm3.1993.374.7-12.507. [DOI] [PubMed] [Google Scholar]
- 63.Berndt J.A., Kim J.G., Tosic M., Kim C., Hudson L.D. The transcriptional regulator Yin Yang 1 activates the myelin PLP gene. J. Neurochem. 2001;77:935–942. doi: 10.1046/j.1471-4159.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 64.Dobretsova A., Johnson J.W., Jones R.C., Edmondson R.D., Wight P.A. Proteomic analysis of nuclear factors binding to an intronic enhancer in the myelin proteolipid protein gene. J. Neurochem. 2008;105:1979–1995. doi: 10.1111/j.1471-4159.2008.05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuason M.C., Rastikerdar A., Kuhlmann T., Goujet-Zalc C., Zalc B., Dib S., Friedman H., Peterson A. Separate proteolipid protein/DM20 enhancers serve different lineages and stages of development. J. Neurosci. 2008;28:6895–6903. doi: 10.1523/JNEUROSCI.4579-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung-Testas I., Do Thi A., Koenig H., Désarnaud F., Shazand K., Schumacher M., Baulieu E.E. Progesterone as a neurosteroid: synthesis and actions in rat glial cells. J. Steroid Biochem. Mol. Biol. 1999;69:97–107. doi: 10.1016/s0960-0760(98)00149-6. [DOI] [PubMed] [Google Scholar]
- 67.Blaustein J.D. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann. N Y Acad. Sci. 2003;1007:238–250. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 68.Baulieu E.E. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 69.Schumacher M., Guennoun R., Robert F., Carelli C., Gago N., Ghoumari A., Gonzalez Deniselle M.C., Gonzalez S.L., Ibanez C., Labombarda F. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm. IGF Res. 2004;14(Suppl A):S18–S33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 70.MacLusky N.J., McEwen B.S. Progestin receptors in the developing rat brain and pituitary. Brain Res. 1980;189:262–268. doi: 10.1016/0006-8993(80)90026-8. [DOI] [PubMed] [Google Scholar]
- 71.Lauber A.H., Romano G.J., Pfaff D.W. Gene expression for estrogen and progesterone receptor mRNAs in rat brain and possible relations to sexually dimorphic functions. J. Steroid Biochem. Mol. Biol. 1991;40:53–62. doi: 10.1016/0960-0760(91)90167-4. [DOI] [PubMed] [Google Scholar]
- 72.Hagihara K., Hirata S., Osada T., Hirai M., Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res. Mol. Brain Res. 1992;14:239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- 73.Beato M., Chalepakis G., Schauer M., Slater E.P. DNA regulatory elements for steroid hormones. J. Steroid Biochem. 1989;32:737–747. doi: 10.1016/0022-4731(89)90521-9. [DOI] [PubMed] [Google Scholar]
- 74.Beato M., Klug J. Steroid hormone receptors: an update. Hum. Reprod. Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 75.Sandelin A., Wasserman W.W. Prediction of nuclear hormone receptor response elements. Mol. Endocrinol. 2005;19:595–606. doi: 10.1210/me.2004-0101. [DOI] [PubMed] [Google Scholar]
- 76.Martini L., Magnaghi V., Melcangi R.C. Actions of progesterone and its 5alpha-reduced metabolites on the major proteins of the myelin of the peripheral nervous system. Steroids. 2003;68:825–829. doi: 10.1016/s0039-128x(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 77.Schumacher M., Guennoun R., Mercier G., Désarnaud F., Lacor P., Bénavides J., Ferzaz B., Robert F., Baulieu E.E. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res. Brain Res. Rev. 2001;37:343–359. doi: 10.1016/s0165-0173(01)00139-4. [DOI] [PubMed] [Google Scholar]
- 78.Afhüppe W., Beekman J.M., Otto C., Korr D., Hoffmann J., Fuhrmann U., Möller C. In vitro characterization of ZK 230211—A type III progesterone receptor antagonist with enhanced antiproliferative properties. J. Steroid Biochem. Mol. Biol. 2010;119:45–55. doi: 10.1016/j.jsbmb.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Jonat W., Bachelot T., Ruhstaller T., Kuss I., Reimann U., Robertson J.F. Randomized phase II study of lonaprisan as second-line therapy for progesterone receptor-positive breast cancer. Ann. Oncol. 2013;24:2543–2548. doi: 10.1093/annonc/mdt216. [DOI] [PubMed] [Google Scholar]
- 80.Ip C.W., Kroner A., Groh J., Huber M., Klein D., Spahn I., Diem R., Williams S.K., Nave K.A., Edgar J.M., Martini R. Neuroinflammation by cytotoxic T-lymphocytes impairs retrograde axonal transport in an oligodendrocyte mutant mouse. PLoS ONE. 2012;7:e42554. doi: 10.1371/journal.pone.0042554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tait A.S., Butts C.L., Sternberg E.M. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J. Leukoc. Biol. 2008;84:924–931. doi: 10.1189/jlb.0208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Renault T.T., Manon S. Bax: Addressed to kill. Biochimie. 2011;93:1379–1391. doi: 10.1016/j.biochi.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 83.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 84.Lüscher B., Vervoorts J. Regulation of gene transcription by the oncoprotein MYC. Gene. 2012;494:145–160. doi: 10.1016/j.gene.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 85.Sereda M., Griffiths I., Pühlhofer A., Stewart H., Rossner M.J., Zimmerman F., Magyar J.P., Schneider A., Hund E., Meinck H.M. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron. 1996;16:1049–1060. doi: 10.1016/s0896-6273(00)80128-2. [DOI] [PubMed] [Google Scholar]
- 86.Fledrich R., Stassart R.M., Sereda M.W. Murine therapeutic models for Charcot-Marie-Tooth (CMT) disease. Br. Med. Bull. 2012;102:89–113. doi: 10.1093/bmb/lds010. [DOI] [PubMed] [Google Scholar]
- 87.Schenone A., Nobbio L., Monti Bragadin M., Ursino G., Grandis M. Inherited neuropathies. Curr. Treat. Options Neurol. 2011;13:160–179. doi: 10.1007/s11940-011-0115-z. [DOI] [PubMed] [Google Scholar]
- 88.Meyer zu Horste G., Prukop T., Liebetanz D., Mobius W., Nave K.A., Sereda M.W. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann. Neurol. 2007;61:61–72. doi: 10.1002/ana.21026. [DOI] [PubMed] [Google Scholar]
- 89.Nave K.A., Sereda M.W., Ehrenreich H. Mechanisms of disease: inherited demyelinating neuropathies—from basic to clinical research. Nat. Clin. Pract. Neurol. 2007;3:453–464. doi: 10.1038/ncpneuro0583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


