Abstract
Cellular exposure to ionizing radiation leads to oxidizing events that alter atomic structure through direct interactions of radiation with target macromolecules or via products of water radiolysis. Further, the oxidative damage may spread from the targeted to neighboring, non-targeted bystander cells through redox-modulated intercellular communication mechanisms. To cope with the induced stress and the changes in the redox environment, organisms elicit transient responses at the molecular, cellular and tissue levels to counteract toxic effects of radiation. Metabolic pathways are induced during and shortly after the exposure. Depending on radiation dose, dose-rate and quality, these protective mechanisms may or may not be sufficient to cope with the stress. When the harmful effects exceed those of homeostatic biochemical processes, induced biological changes persist and may be propagated to progeny cells. Physiological levels of reactive oxygen and nitrogen species play critical roles in many cellular functions. In irradiated cells, levels of these reactive species may be increased due to perturbations in oxidative metabolism and chronic inflammatory responses, thereby contributing to the long-term effects of exposure to ionizing radiation on genomic stability. Here, in addition to immediate biological effects of water radiolysis on DNA damage, we also discuss the role of mitochondria in the delayed outcomes of ionization radiation. Defects in mitochondrial functions lead to accelerated aging and numerous pathological conditions. Different types of radiation vary in their linear energy transfer (LET) properties, and we discuss their effects on various aspects of mitochondrial physiology. These include short and long-term in vitro and in vivo effects on mitochondrial DNA, mitochondrial protein import and metabolic and antioxidant enzymes.
Keywords: Ionizing radiation, Reactive oxygen/nitrogen species, Oxidative metabolism, Mitochondria, Genomic instability, adaptive responses, bystander effects
1. Introduction
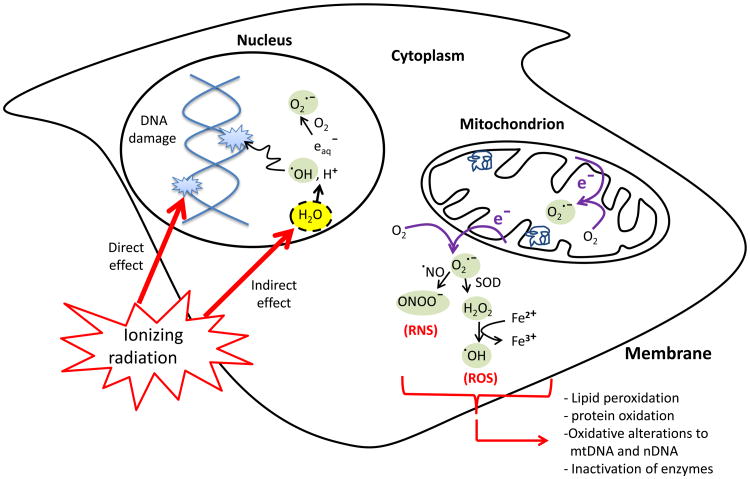
The absorption of ionizing radiation by living cells can directly disrupt atomic structures, producing chemical and biological changes. It can also act indirectly through radiolysis of water, thereby generating reactive chemical species that may damage nucleic acids, proteins and lipids [1] (Fig. 1). Together, the direct and indirect effects of radiation initiate a series of biochemical and molecular signaling events that may repair the damage or culminate in permanent physiological changes or cell death [2].
Figure 1.
The direct and indirect cellular effects of ionizing radiation on macromolecules. Absorption of ionizing radiation by living cells directly disrupts atomic structures, producing chemical and biological changes and indirectly through radiolysis of cellular water and generation of reactive chemical species by stimulation of oxidases and nitric oxide synthases. Ionizing radiation may also disrupt mitochondrial functions significantly contributing to persistent alterations in lipids, proteins, nuclear DNA (nDNA) and mitochondrial DNA (mtDNA).
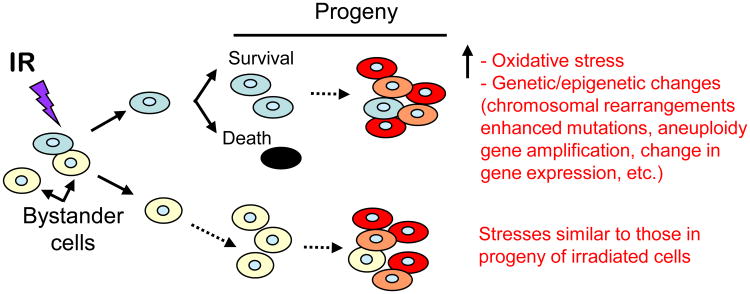
Interestingly, the early biochemical modifications, which occur during or shortly after the radiation exposure, were thought to be responsible for most of the effects of ionizing radiation in mammalian cells. However, oxidative changes may continue to arise for days and months after the initial exposure presumably because of continuous generation of reactive oxygen (ROS) and nitrogen (RNS) species [3]. Remarkably, these processes occur not only in the irradiated cells but also in their progeny [2; 4; 5]. Furthermore, radiation-induced oxidative stress may spread from targeted cells to non-targeted bystander cells through intercellular communication mechanisms [6; 7; 8; 9]. The progeny of these bystander cells also experience perturbations in oxidative metabolism and exhibit a wide range of oxidative damages, including protein carbonylation, lipid peroxidation, and enhanced rates of spontaneous gene mutations and neoplastic transformation [10; 11] (Fig. 3). The persistence of such stressful effects in progeny cells has profound implications for long-term health risks, including emergence of a second malignancy following radiotherapy treatments [12; 13; 14; 15]. Oxidative DNA damages in key genes such as the tumor suppressors P53 and RETINOBLASTOMA may be responsible for the induction of such malignancies [16; 17]. Increasing evidence also supports the role of chronic oxidative stress in the progression of degenerative diseases and radiation-induced late tissue injury [2; 18; 19]. Therefore, understanding the molecular and biochemical events that promote early and late oxidative stress in irradiated cells/tissues will be informative for counteracting adverse health effects of ionizing radiation.
Figure 3.
Ionizing radiation (IR) induces targeted and non-targeted (bystander) effects. Communication of stress-inducing molecules from cells exposed to IR propagates stressful effects, including oxidative stress, to the bystander cells and their progeny. The induced effects may be similar in nature to those observed in progeny of irradiated cells.
2. Primary effects of ionizing radiation
2.1. Water radiolysis and generation of reactive oxygen species
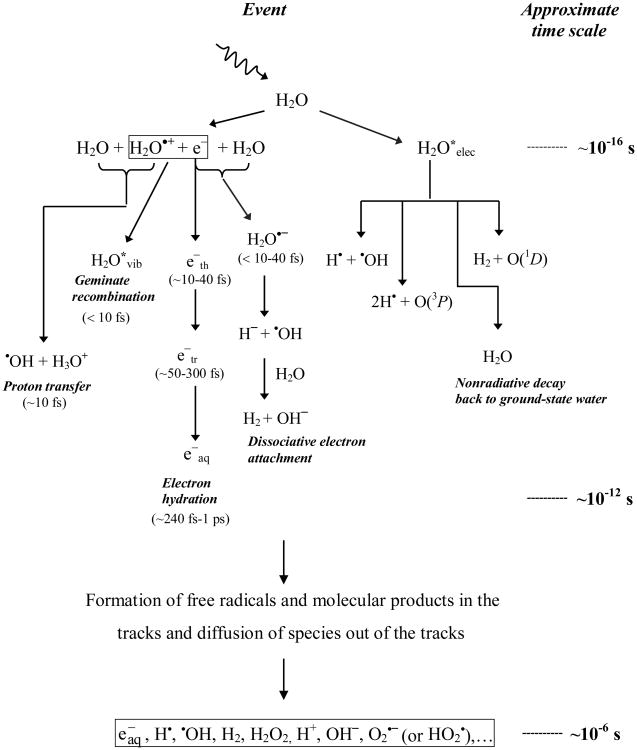
Water is the major (∼80%) constituent of cells. A thorough knowledge of water radiolysis is therefore critical for understanding radiobiological effects. The absorption of energetic radiations by water results in both excitations and ionizations leading to production of free radicals that in turn can attack other critical molecules (indirect effect) (Fig. 1). For brevity, the complex events that accompany the absorption of high-energy photons or the passage of fast charged particles can be divided into four, more or less clearly demarcated, consecutive, temporal stages [20]. During the first or “physical” stage, the energy deposition is caused by the incident radiation and secondary electrons are generated. The resulting species are extremely unstable and undergo fast reorganization in the second or “physicochemical” stage. These processes produce radical and molecular products of radiolysis that are distributed in a highly non-homogeneous track structure. Secondary electrons slow down to sub-excitation energies and following thermalization, they become trapped (e−tr) and hydrated (e−aq). The initial (∼10−12 s) spatial distribution of reactants is then directly used as the starting point for the so-called stage of “non-homogeneous chemistry”. During this third stage, the various chemically reactive species diffuse and react with one another or with the environment, until all intra-track reactions are complete (∼10−6 s). Finally, in a physiologic system, there follows a “biological” stage in which the cells respond to the damage resulting from the products formed in the preceding stages. During this stage (∼10−3 s or longer, depending very much upon the medium), the biological responses affecting the long-term consequences of radiation exposure are induced. Figure 2 illustrates the time scale of the stages of water radiolysis by sparsely ionizing types (e.g. cobalt-60 or cesium-137 γ rays). It also shows the time scale of chemical reactions leading to generation of specific radiolytic products.
Figure 2. Time scale of events in the radiolysis of water by low linear energy transfer radiations.
On a quantitative basis, the species produced in the radiolysis of pure deaerated water are e−aq, •OH, H•, H2, and H2O2 [21; 22] (Fig. 2). In the presence of oxygen, e−aq and H• atoms are rapidly converted to superoxide/perhydroxyl (O2•−/HO2•) radicals, where the O2•− radical exists in a pH-dependent equilibrium with its conjugate acid (pKa = 4.8) [23]. Thus, in an aerobic cellular environment at physiological pH, the major reactive species at homogeneity (∼10−6 s) include O2•−, •OH, and H2O2 (Fig. 2). H2 plays only a limited role in the radiolysis of aqueous solutions, and most of it escapes from solution.
In biological systems, organic radicals (R•) are also formed, most often by H-abstraction reactions (initiated by •OH radicals for example). These carbon-centered radicals usually react rapidly with O2 to give peroxyl radicals (RO2•), which are stronger oxidizing agents than their parent radicals [24]. The RO2• radicals can abstract H• from other molecules to form hydroperoxides (ROOH), a reaction known to be involved in lipid peroxidation. In this context, the progression of radiation damage in cells likely involves persistent lipid peroxidation reactions that are intertwined with protein inactivation [3; 25].
2.2. Generation of reactive nitrogen species
Ionizing radiation can also stimulate inducible nitric oxide synthase (NOS) activity in hit cells [26], thereby generating large amounts of nitric oxide (•NO). Although •NO is chemically inert toward most cellular constituents (except for heme), it reacts with O2•− to form the peroxynitrite anion (ONOO−) with a rate constant that is larger than that for the superoxide dismutase (SOD)-catalyzed dismutation of O2•−[27]. Like hydroxyl radicals, ONOO− is also highly reactive and capable of attacking a wide range of cellular targets, including lipids, thiols, proteins and DNA bases. This high reactivity of ONOO− implies low selectivity, confined reactivity with molecules in immediate vicinity, and inability to act as a cellular messenger. By contrast, the much lower reactivity of H2O2 and O2•− (or HO2•, depending on pH) allows them to diffuse a longer distance away from the originating site (the diffusion coefficients of H2O2, O2•−, and HO2• being 2.3 × 10−9, 1.75 × 10−9, and 2.3 × 10−9 m2 s−1, respectively [28]). In the presence of catalytic redox metal ions (principally iron and copper ions), these species lead to the production of •OH radicals via Fenton and Haber-Weiss chemistry [29], which can enhance damage [30].
In summary, the radiolysis of water and early activation of nitric oxide synthases is a major source of ROS/RNS in irradiated cells under ambient oxygen. Interestingly, the yield of these species is strongly modulated by different types of radiation. With increasing linear energy transfer (LET) of the irradiating particles, an increase in the yield of molecular products (such as H2O2) is accompanied by a corresponding decrease in the yield of radicals (such as •OH). In contrast, O2•− (or HO2•) is the most abundant radical species produced by radiations with high LET character [31; 32]. Evidently, the yield of these products and their concentrations along the tracks of irradiating particles has important consequences to the extent and nature of induced DNA damages [33; 34]. Reactive oxygen and nitrogen species can attack DNA resulting in several alterations, including DNA breaks, base damage, destruction of sugars, crosslinks and telomere dysfunction [35; 36; 37]. If unrepaired or mis-repaired, these damages may lead to mutations and promote neoplastic transformation or cell death [4].
2.3. Ionizing radiation track structure and the nature of induced biological effects
Ionizing radiation is classified as either electromagnetic or particulate. Whereas X and γ rays belong to electromagnetic radiation, energetic electrons, protons, neutrons, α particles and heavy charged particles are different forms of particulate radiation [1]. Many of the damaging effects of water radiolysis are due to the geometry of the physical energy deposition of the impacting radiation, referred to as the track structure or LET effects [38]. In irradiated cells, such energy deposition causes endogenous bursts of ROS in and around the radiation track as well as in the intercellular matrix. The track structure determines the relative potency of different types of radiation in causing biological effects [39; 40]. Following exposure to high LET radiations (e.g. α particles, high charge and high energy (HZE) particles), the yield of locally multiply damaged (LMDS) sites in DNA is greatly increased [4; 41; 42]. The clustering of lesions induced by ionizing radiation is thought to play a central role in long-term biological effects [43]. However, the concept that low doses/low fluences of ionizing radiation generate LMDS is not universally accepted [44].
Whereas ∼60 ROS per nanogram of tissue are generated within less than a microsecond from a hit caused by 137Cs γ rays, ∼2000 ROS are generated from a 3.2 MeV α particle traversal, which corresponds to a ROS concentration of ∼19 nM in the nucleus [45]. Such a nuclear ROS concentration can obviously cause extensive oxidative injury and modify normal biochemical reactions [46; 47]. As a result, different signaling cascades responding to these stress conditions are triggered. For example, adaptive responses encompassing DNA repair and antioxidation reactions may be triggered following exposures to low doses of low LET radiations (X and γ rays) [48; 49; 50]. The protective mechanisms may over-compensate, resulting in stimulatory responses that enhance the well-being of the organism long after the exposure [51; 52]. In contrast, basal and induced signaling cascades do not seem to completely alleviate the complex damages induced by low fluences of high LET radiations (e.g. α and HZE particles) [53]. Damaging effects endure and may spread to neighboring bystander cells [54] and persist in their progeny [11; 55] (Fig. 3). Since low-dose radiation-induced bystander effects and adaptive responses appear to involve ROS and RNS [2; 56], the track structure is crucial for dictating the size and precise location of the initial radiation-induced ROS bursts and their subsequent signaling or damaging effects [57; 58; 59; 60]. As discussed below, the track structure of the impacting radiation is also critical in determining the nature of long-term effects on oxidative metabolism [10; 11]. The bursts of ROS and RNS may affect directly or indirectly proteins/genes that participate in oxidative metabolism [26; 61]. The persistence of such perturbations in the normal oxidative metabolism is associated with chronic inflammatory responses [4; 62; 63]. The latter is a field of growing interest from radiotherapy [19] and radiation protection [18; 64] perspectives; it is the subject of intense investigations (reviewed in [4; 63; 65]). Levels of the inflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), white cell blood counts, and sialic acid levels were found to be increased in survivors of the A-bomb long after the event [64; 66].
Chronic inflammation is a dynamic and progressive process. When recruited to sites of inflammation, macrophages and neutrophils generate diffusible and reactive species, including ROS and RNS. These species can cause a large spectrum of oxidative DNA damage that can lead to mutations as well as DNA cross-links in neighboring cells (reviewed in [67; 68]). Further, the processing of closely spaced oxidative lesions (oxidatively-induced clustered clustered DNA lesions or OCDLs) can induce DNA double strand breaks, a serious type of DNA lesion that leads to cell death or long-term stressful effects in surviving cells [69; 70; 71]. Increasing evidence indicates that inflammatory cells in circulating blood of patients that received partial body irradiation may also induce DNA damage at sites that are distant from the irradiated target [4; 72], hence contributing to ‘out-of-field’or abscopal effects [5; 73; 74; 75]. Macrophages also secrete cytokines [76] that may perturb physiological functions in normal surrounding cells. Hence, the effects of localized energy deposition events in a cell may not be assessed independently of neighboring cells. Whereas, certain genetic and epigenetic changes in targeted and non-targeted cells may be observed shortly after exposure [5; 6; 54; 77], others require several generations to be expressed [10; 78; 79; 80]. Certain effects (e.g. microRNA expression) were detected as early as several hours after whole body irradiation of mice, and persisted for days, weeks, and even months after irradiation [79]. Together, the changes due to radiation-induced chronic inflammation and perturbations in oxidative metabolism can lead to genomic instability in targeted and non-targeted cells [81], causing serious health effects, including neurodegeneration, cardiovascular diseases and cancer [82; 83; 84].
2.4 Endogenous and radiation-induced DNA alterations
A strong emphasis has been on the effect of exogenous agents such as radiation on DNA damage. However, improvements in the sensitivity of analytic methods to measure oxidative damage [68] have revealed altered bases and nucleotides in the DNA of normal cells that have not been exposed to ionizing radiation or other mutagens [85]. The analyses have shown that endogenous biochemical processes greatly contribute to genome mutations. The ROS produced during normal cellular metabolic processes (mainly O2•− and H2O2) cause extensive depurinations and to lesser extent depyrimidinations. In addition, ROS can oxidize bases in DNA, such as the oxidation of deoxyguanosine (dG) to 8-hydroxyguanine (8-oxodG), with ∼ 100-500 of such lesion being formed per day in a human cell [86]. The rate of occurrence of these alterations has been closely linked to the rate of oxidative metabolism: higher oxygen consumption in different species correlated with an increased rate of base oxidation in DNA [87]. A failure to repair oxidized bases creates a risk of mutation during DNA replication. For example, 8-oxodG mispairs with deoxyadenosine (dA) rather than deoxycytosine (dC) resulting in a C-A point mutation.
In addition to base oxidation, depurination and depyrmidination, other spontaneous damages can also occur in DNA. The modification of cytosine to 5-methylcytosine (5mC) caused by reaction with S-adenosylmethionine, mainly in CpG doublets in the mammalian genome [88], creates a mutable site that generates thymine. The latter is a component of normal DNA, and the T:G basepair may escape detection and serves as template in a subsequent cycle of DNA replication, leading to a C- to T point mutation.
Several defenses act to restore DNA integrity. In response to DNA damage, cells activate cell cycle checkpoints that provide time for DNA repair machinery to mend the damage. Base excision repair (BER) recognizes and restores spontaneous base modifications, abasic sites and single strand breaks [89; 90]. Other modes of DNA repair, including nucleotide excision repair (NER), mismatch repair and double strand break repair restitute other types of DNA damage [91].
The spectrum of ROS generated during and shortly after irradiation is similar to that produced by metabolic processes. However, differences exist in microdistribution (single molecules and clusters of ROS produced by radiation vs. single molecules produced by endogenous processes), relative yield of specific products (mainly O2•− and H2O2 produced by endogenous processes vs. •OH in highest yield in irradiated cells) and timing of production (chronic release of endogenous ROS versus instantaneous production during irradiation) [92], As a result, damage from metabolic ROS is randomly distributed in the DNA, radiation-induced DNA damage frequently occurs in clusters [93]. Whereas ∼ 1/3 of DNA damage from ionizing radiation (low LET type) emanates from direct interaction of DNA with the irradiating particle and 2/3 due to indirect effects (Fig. 1), DNA damage from endogenous sources is due primarily to indirect effect.
Techniques such as the ‘comet assay’ [93; 94] and newer techniques (e.g. gas chromatography, high pressure liquid chromatography, immunoassays) [4; 68] can distinguish gross DNA damage produced by ionizing radiation and damage from oxidative attacks. For low doses of radiation, the total number of induced DNA alterations is probably small when compared with the total number of equivalent alterations from endogenous sources [95].
3. Reactive oxygen species and cellular homeostasis
Although excess ROS produced by ionizing radiation is toxic, intra-cellular ROS produced under physiologic conditions serve as essential signaling molecules that regulate numerous cellar processes [96; 97; 98]. Homeostatic cellular functions therefore require tight control of the redox environment [2; 99]. At low levels, ROS participate in signaling pathways that maintain normal cellular functions by regulating the expression of specific genes [100; 101; 102; 103], modulating ion channel activities [104], and mimicking or affecting intermediates (e.g. second messengers) in signal transduction [105]. NAD(P)H-oxidases, lipoxygenases, nitric oxide synthases, xanthine oxidase, microsomal cytochrome P-450, and mitochondrial electron transport chain are the major sources of cellular ROS [106]. The levels of these ROS greatly depend on the availability of cellular oxygen [30]. Most of the oxidants and their byproducts are metabolized by various enzymes and small molecule antioxidants [97]. Most likely, it is the ROS that escape antioxidant defense participate in homeostatic regulation of redox signaling [107]. Certain ROS (e.g. HO2•, H2O2) can permeate lipid bilayers [108] and traverse membranes [109], thus contributing to the propagation of signaling events among cells in a confluent cell culture.
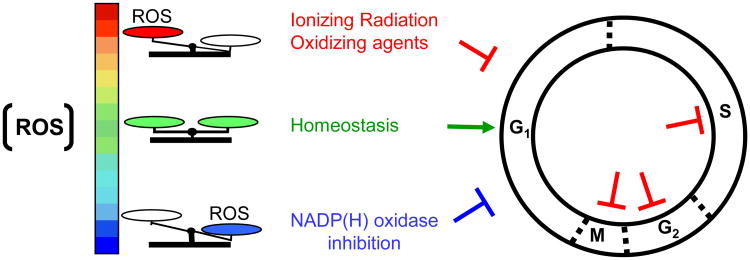
Although a major emphasis in the literature has been on physiological functions at homeostatic or higher than normal levels of ROS [110], lower ROS levels also result in important changes in cellular functions [111]. For example, inhibition of superoxide production by NAD(P)H-oxidases (but not nitric oxide generated by nitric oxide synthases) leads to strong arrest of cells in G1 phase of the growth cycle [112] (Fig. 4). The effect occurs in the absence of induced DNA damage and is dependent on the Ataxia Telangiectasia Mutated (ATM) gene, a critical player in the early detection and repair of ionizing radiation-induced DNA damage [113]. Importantly, the induced G1-delay is attenuated when cells treated with inhibitors of NAD(P)H-oxidases are simultaneously exposed to low dose-rate γ rays. These results suggest that ROS generated by γ rays substitute for effects of ROS generated by NAD(P)H-oxidases [112]. It is often asked whether the chemical species and/or genetic lesions induced by ionizing radiation and those induced by normal oxidative metabolism are distinct or similar [92]. Therefore, the ability of radiation-induced ROS to replace ROS generated by flavin-oxidases (e.g. NAD(P)H-oxidase) for restoration of normal cell cycle progression suggests that they are similar.
Figure 4.
Reactive oxygen species (ROS) and the regulation of cell proliferation. Higher or lower than normal levels of ROS can induce cell cycle delays in different phases of the cell cycle.
The findings as described above emphasize the multi-functionality of oxidases in modulating normal cellular responses and highlight a regulatory link between oxidative metabolic processes and cell-cycle functions [98]. In addition to mediating the anti-microbial activity of phagocytes [114], the multicomponent NAD(P)H oxidase affects signal transduction by growth factor receptors [115] and promotes proliferation in a variety of cell types [116]. In turn, mitogenic signaling elicited by cytokines or growth factors induces intracellular ROS production via activation of the PI3K pathway resulting in stimulation of Rac1, which up-regulates NAD(P)H-oxidase activity [117]. However, the exact molecular and biochemical events by which non-phagocytic NAD(P)H-oxidase (and/or other oxidases) mediate cellular proliferation under physiological oxidative conditions are not clear. Nevertheless, the levels and activity of cyclins that drive cellular proliferation (e.g. cyclin D) are redox sensitive [112; 118]. Therefore, the cell cycle arrest in G1 phase through modulation of the cellular redox environment offers a therapeutic potential to control tumor growth. Notably, many cancer cells show increased production of hydrogen peroxide and other oxidizing species, which are associated with cellular proliferation [119]. Hence, the approach of inhibiting basal oxidant production by flavin-oxidases could be an attractive mode of cancer therapy [111; 118; 120; 121; 122]. Importantly, most tumors harbor proliferating cells in different phases of the growth cycle. Blocking cells in G1 phase, by inhibiting ROS generation by flavin-containing oxidases, could result in depletion of tumor cells from the relatively radioresistant S-phase compartment [123], thereby enhancing radiosensitivity and therapeutic response. This approach may complement radiotherapy of cancer cells, which are normally not arrested in G1 phase after irradiation [124]. Aberrant cell proliferation associated with oxidative stress also contributes to other pathological conditions such as neurodegenerative and cardiovascular diseases and diabetes [125]. Reestablishing cell cycle regulation by manipulating the cellular redox environment may help ameliorate some of these disorders [98].
4. Mitochondria and delayed effects of ionizing radiation
Upon cellular exposure to ionizing radiation, ROS generating-oxidases may be activated, antioxidants modulated, and metabolic activity altered in response to the oxidative insult. Among the multitude of induced effects, ionizing radiation may disrupt mitochondrial functions because of several reasons. For example, mitochondria occupy a fairly substantial fraction of cell volume (4-25% depending on the cell) [126], which renders them a likely target of radiation traversal through the cell. More importantly, mitochondria consume about 90% of the body's oxygen and are the richest source of ROS [127; 128; 129; 130]. They divert about 1-5% of electrons from the respiratory chain to the formation of superoxide radicals by ubiquinone-dependent reduction [131]. Mitochondrial dysfunction in irradiated cells may thus significantly contribute to perturbation in oxidation-reduction reactions that determine the cellular redox environment. The mitochondrion serves as the “real power plant of the cell” through aerobic respiration [132]. It is the cell's principal source of energy that includes the tricarboxylic acid (TCA) cycle, electron transport chain (ETC), and oxidative phosphorylation.
The TCA cycle occurs in the mitochondrial matrix, and oxidative metabolism of carbohydrates, lipids, and amino acids integrate into the TCA cycle to produce NADH and FADH2. These essential intermediates are electron-rich donors that enter the ETC on the mitochondrial inner membrane for use in ATP production. The ETC is composed of 5 protein complexes (I-V) that perform a series of oxidation-reduction reactions in which O2 serves as the final electron acceptor and is reduced to H2O. Electron transfer is coupled with the ejection of H+ out of the matrix into the inter-membrane space, creating a proton gradient that drives the production of ATP through oxidative phosphorylation [133]. A consequence of this energy production is the generation of ROS byproducts.
The premature leakage of electrons (mainly from complexes I and III, and complex II to a minor extent) results in the reduction of O2 to create superoxide (O2•) [134; 135; 136]. Apart from this normal basal level of ROS production, radiation causes further leakage of electrons from the ETC, and therefore results in excess O2•− generation [97]. This occurs in addition to the ROS produced during water radiolysis. ROS production by mitochondria plays multiple roles in signaling cascades [137; 138; 139] and mediates apoptosis [140]. Excess ROS may cause mutations in mitochondrial DNA, and damage or alter the expression of proteins required for critical mitochondrial and cellular functions. Several endpoints have been tested to examine mitochondrial dysfunction in irradiated cells and their progeny. For example, early and late generation of radiation-induced mitochondrial ROS/RNS mediates changes in mitochondrial DNA copy number [141], mutations [142] and gene expression [143; 144], autophagy [145; 146], apoptosis [147; 148; 149], propagation of non-targeted responses [73; 150; 151; 152; 153; 154; 155], the induction of nuclear DNA damage [156], genomic instability [157], neoplastic transformation [158], and degenerative conditions [37; 63; 159] among other outcomes [2; 37]. We investigated effects of ionizing radiation on mitochondrial protein import, assembly of large protein complexes, protein oxidation and activity of metabolic and antioxidant enzymes [10; 11; 45; 48; 160; 161]. Significantly, the persistence of radiation effects that lead to mitochondrial dysfunction in progeny cells has serious consequences to health risks [2; 162].
4.1. Mitochondrial DNA
Human mitochondrial DNA (mtDNA) is a circular molecule; it is among the smallest known mtDNAs and contains 16,659 bases. A mammalian cell contains several copies of mtDNA that code for rRNA, tRNA and proteins (13 in humans). The close proximity of mtDNA to sites of ROS production renders it particularly susceptible to damage, which led Harman to propose that damage to mtDNA contributes to the aging process [163]. Various pathologies have been attributed to mutations in mtDNA; their severity depends on the nature of the mutation and on the proportion of mutant and wild-type mtDNA present in a particular cell type [164; 165; 166]. Tissues that have a high requirement for ATP are most affected by mutations in mtDNA [167], and express a variety of pathologies as well as age associated disease [168; 169].
Short- and long-term radiation-induced ROS/RNS could result in damage to mtDNA and/or nuclear DNA (nuDNA) coding for mitochondrial electron transport chain (ETC) subunits as well as the biochemical machinery necessary for their proper expression and assembly [170]. Interestingly, cells deficient in mitochondrial electron transport (rho(o) cells) do not experience radiation-induced ROS/RNS production [126]. Rho(o) cells are more resistant to radiation-induced cell killing than rho(+) cells; they show delayed G2 arrest and decreased ability to recover from the G2 checkpoint than rho(+) cells [171].
Recent evidence has indicated that base excision repair [172] and mismatch repair [173] may be induced in mitochondria during oxidative insult. However, excess oxidative stress appears to target the mitochondrial DNA polymerase γ (pol γ) activity required for replication and repair of mtDNA [174], thereby reducing the overall repair capacity [175; 176; 177]. In addition, cells from knockout mice for 8-oxoguanine DNA glycosylase (ogg1) spontaneously accumulate mtDNA damage [178]. Therefore, subsequent to radiation exposure, mtDNA might be preferentially damaged or lost due to oxidative stress with an ensuing decrease in respiratory chain activity and decrease of mitochondrial function. Mutations in mtDNA causing disruptions in the proper assembly and/or function of mitochondrial ETCs could lead to an increase in accessibility of reduced components of the ETCs to O2, which may result in an increase in prooxidant formation [2]. The net consequence being a condition of persistent metabolic oxidative stress that continues to cause de-novo oxidative damage to critical biological structures long after the radiation exposure. Radiation-induced mtDNA mutations may segregate in post-mitotic cells [179] and become heritable traits that contribute to genomic instability [180]. Changes in the structure and/or function of genes coding for mitochondrial ETC proteins in progeny cells can give rise to increases in mitochondria-derived oxidants that contribute to nuDNA damage [162].
The ‘common deletion’ in the mitochondrial genome is also induced following cellular exposure to ionizing radiation [181]. This deletion involves the loss of 4977 base pairs coding for genes that include subunits of the mitochondrial ATPase, NADH dehydrogenase complex I and cytochorome c oxidase. Once formed, the ‘common deletion’ becomes stable [182]. It has been proposed that the ‘common deletion’ leads to inefficient mitochondrial metabolism and thus increased ROS production [183]. Whereas irradiated normal cells accumulate significant levels of the 4977 base-deletion, tumor cells show only a modest induction [181]. Surprisingly, a relationship between radiation-induced deletions and sensitivity to cell killing by radiation was not observed in the same study among the various cell strains/lines tested [181].
The traffic of DNA from mitochondria to the nucleus has been demonstrated in Saccharomyces cerevisiae [184], and mtDNA sequences are found in at least 27 sites in human chromosomes [185] . It is therefore attractive to speculate that migration of mtDNA fragments to the nucleus following exposure to ionizing radiation may lead to their insertion in nuclear genome, particularly during repair of DNA double strand breaks [186], which would contribute to alterations in genomic DNA and the induction of genomic instability. Irradiation of mice stimulated the appearance of mtDNA fragments in the cytosolic fractions of the brain as early as 1 h after exposure to 5 Gy of 137CS γ rays [187]. By targeting regulatory sequences, insertion of mtDNA in nuDNA may result in permanent changes in gene expression [180]. Therefore, a persistent increase in mitochondrial oxidative stress following exposure to ionizing radiation may lead to a mutagenic phenomenon that could contribute to cancer and accelerated aging [188; 189]. Consistent with a role of oxidative stress in the translocation of mtDNA to the nucleus, experiments in yeast have shown that the migration rate increases during chronological aging [189], which suggests that a similar phenomenon may occur following exposure to moderate doses of radiation.
4.2. Effects of ionizing radiation on mitochondrial functions
4.2.1. Mitochondrial protein import
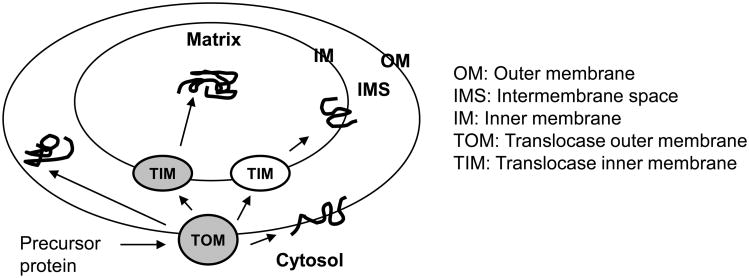
Although mitochondria contain their own DNA and complete systems for replication, transcription and translation, they synthesize only a few (13 in humans) proteins [190]. All other mitochondrial proteins are nuclear-encoded and are synthesized on cytoplasmic ribosomes. These proteins must be transported from cytosol to the correct mitochondrial sub-compartment – outer membrane, intermembrane space, inner membrane or matrix [191] (Fig. 5). Protein import into mitochondria is therefore a fundamental mechanism of mitochondrial biogenesis -maintenance and regeneration of mitochondria. Defects in mitochondrial protein import resulting from exposure to ionizing radiation may amplify the oxidative stress and lead to late health effects, including degenerative diseases and metabolic disorders [192; 193; 194; 195].
Figure 5. Schematic of protein import into mitochondria.
We have demonstrated that mitochondrial protein import could be used as a sensitive marker for early detection of long-term effects of ionizing radiation [161]. Specifically, irradiated normal human diploid cells show differential effects of high and low doses of γ rays on mitochondrial membrane potential (Δψ) and protein import [161]. Protein import into the mitochondrial matrix requires Δψ across the inner membrane, and our data show that ionizing radiation-induced defects in import are not solely due to changes in Δψ. These import studies were performed using the frataxin precursor protein as a model substrate, and it remains to be determined if radiation affects mitochondrial import of other proteins as well. For example, cellular exposure to ionizing radiation may cause damages to key components of the protein translocation machinery, and this in turn may result in a global defect in protein targeting/import.
We also found that exposure to ionizing radiation causes a decrease in the levels of some of the components of the protein import machinery in vivo. These observations were made with tissues of rats exposed to mean moderate doses (50 cGy) of high LET radiations (HZE particles). The effects persisted for months after the exposure, occurred in both the targeted and non-targeted tissues of irradiated animals, and correlated with greatly reduced mitochondrial protein import. These novel findings highlight the physiological relevance of radiation dose and quality (LET) with respect to long-term effects on mitochondria. The results also show that radiation dose-rate is an important factor modulating mitochondrial biogenesis. Compared to cultured cells or rodents exposed to acute radiation doses, protraction of delivery of the low LET radiation dose attenuated reduction in mitochondrial protein import. A reduction in import efficiency is normally indicative of stress conditions. However, it may also represent an adaptive response to minimize ROS-mediated metabolic changes. In fact, proteomic analyses of mitochondria from tissues of irradiated rodents [73] reveal both stressful effects (e.g. decrease in proteins involved in fatty acid elongation, a process required for energy storage and synthesis of lipids [196]), and protective responses (e.g. upregulation of Nrf2 signaling implicated in important antioxidant functions [197; 198]).
4.2.2. Metabolic enzymes: Aconitases
Iron-sulfur (Fe-S) clusters are ancient modular co-factors of proteins that are involved in many cellular processes, including enzymatic catalysis, electron transport, and regulation of gene expression [199]. Mammalian cells contain two aconitases that contain Fe-S clusters: the mitochondrial enzyme (ACO2) involved in the TCA cycle, and a cytosolic enzyme referred to as the iron regulatory protein-1 (IRP1).
The TCA cycle in the mitochondrial matrix is a central pathway of oxidative metabolism. This pathway is critical for the oxidation of acetyl-CoA and for the production of reducing equivalents that are used by the respiratory complexes to produce ATP. Aconitase (ACO2) is one of the eight enzymes that participate in the TCA cycle. It reversibly catalyzes the conversion of citrate to isocitrate. The cubane [4Fe-4S]2+ cluster in the active site of aconitase is essential for this catalytic activity, but it also renders the enzyme highly vulnerable to oxidative stress. The cytosolic IRP1 in its [4Fe-4S] cluster form exhibits aconitase activity. As in the case for the mitochondrial aconitase (ACO2), the [4Fe-4S] cluster of cytosolic IRP1 is also highly susceptible to oxidative damage.
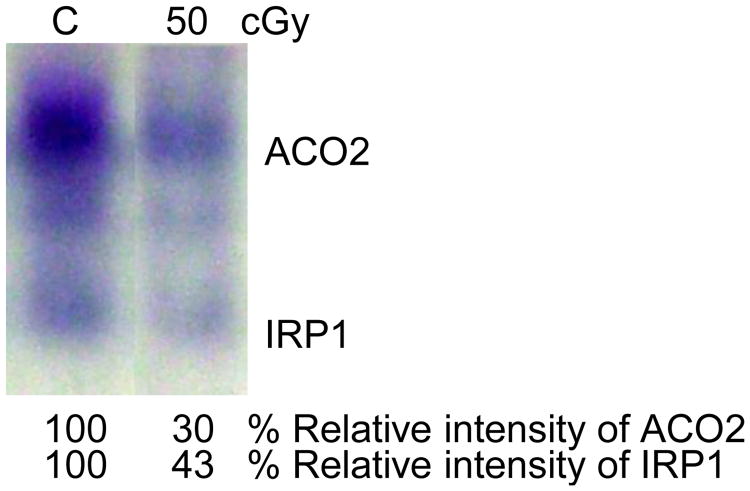
The data presented in Fig. 6 illustrate in-gel measurement of aconitase activity 24 h after exposure of Chinese hamster lung fibroblasts to a moderate dose of γ-rays (50 cGy). The assay differentiates between mitochondrial and cytoplasmic aconitase activities, and shows that both are decreased in irradiated cells (Fig. 6).
Figure 6. Native in-gel assay for the mitochondrial (ACO2) and cytoplasmic (IRP1) aconitase activities in control and γ-ray-exposed Chinese hamster lung fibroblasts.
We have also shown that ACO2 activity is decreased in tissues of irradiated mice or in cultured cells after exposure to doses as low as 1 cGy from sparsely ionizing 137Cs γ rays. The decreases were transient and the activity returned to basal levels within 48 to 120 h after irradiation. This is an important finding because such a low dose is often used for certain diagnostic procedures. In confluent cell cultures exposed to low fluences of densely ionizing radiations (e.g. HZE particles such as Fe or Ti ions found in deep space), significant decreases in ACO2 were detected in protein lysates even when only ∼1-5% of the cells in the culture were irradiated. These data suggest that oxidative stress in irradiated cells is propagated to neighboring bystander cells, and this notion has been confirmed in co-culture studies of HZE-particle-irradiated cells and bystander cells [10; 11]. Strikingly, the effect on aconitase activity in the progeny of bystander cells persisted and was detected after 20 population doublings [11]. These results strongly suggest that perturbations in oxidative metabolism persist long after the radiation exposure. The effect was associated with enhanced levels of micronuclei (a form of DNA damage that arises mainly from DNA double strand breaks [200]), protein carbonylation, lipid peroxidation and decreases in cloning efficiency [11].
One possibility is that the decreases in aconitase activity leads to reduced NADH supply for electron transport, thereby limiting the production of free radical species [201]. Thus, in the case of sparsely ionizing radiations (i.e. γ rays), the transient decreases in ACO2 activity in cells and tissues exposed to low doses represent an adaptive response to reduce further generation of ROS until the redox environment is restored to homeostatic levels. However, the continual reduction in aconitase activity in distant progeny of bystander cells that were in co-culture with cells exposed to densely ionizing radiations is indicative of persistent oxidative stress [10; 11]. These radiations include α particles emitted from radon gas and HZE particles encountered during deep space travel, and are likely to be associated with significant health risks [202; 203].
Measurement of aconitase activity may be used as another sensitive marker of oxidative stress in irradiated cells and tissues. It may be particularly informative of the level of stress induced in situations of mixed exposures to both sparsely and densely ionizing radiations. The oxidized form of aconitase (ACO2) appears to be selectively degraded by the Lon protease in mitochondria [204; 205]. Thus, studies of mitochondrial aconitase activity/protein levels and degradation of the aconitase protein by Lon protease may provide critical understanding of radiation-induced biochemical events in mitochondria.
4.2.3. Large protein complexes and oxidative damage to mitochondrial proteins
Proteins that participate in critical mitochondrial functions often exist in large complexes (e.g. respiratory complexes I-V). These complexes may be susceptible to oxidative stress from exposure to ionizing radiation. Consistent with this concept, dysfunction in mitochondrial complex II was shown to contribute to genomic instability in progeny of cells exposed to ionizing radiation [206]. In these cells, aberrant expression of subunit B protein of complex II led to increased steady-state levels of O2•− and H2O2.
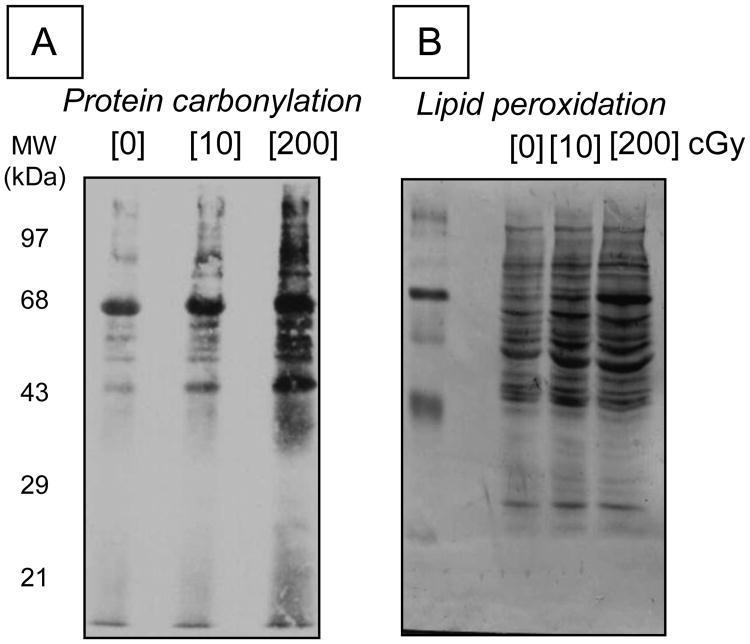
Radiation induced ROS may also result in oxidative damage to other proteins in mitochondria. Significant increases in protein carbonylation and also in levels of proteins with 4-hydroxynonenal adducts were observed in high LET-irradiated cells within hours after exposure [45]. 4-hydroxynonenal is a notoriously reactive molecule that covalently modifies proteins and DNA, thereby causing dysregulation of their function [207]. As shown in Fig. 7, these modifications were also detected in the distant progeny of bystander cells that were in co-culture with cells exposed to high LET radiations [11]. These findings are highly relevant to understanding of long-term health effects of radiation. Such a notion is consistent with our in vivo work showing extensive protein oxidation in targeted and non targeted tissues of mice and rats even 2 years after exposure to radiations with high LET character. These effects appear to be tissue specific and are associated with chronic inflammation, a dynamic and progressive process that begins with ROS and RNS generation and is closely associated with radiation-induced oxidative stress [19].
Figure 7.
Long-term effects of ionizing radiation on protein oxidation. Immunoblots showing protein carbonylation (panel A) and proteins with 4-hydroxynonenal (HNE) adducts (panel B) in progeny of bystander cells that were cultured for 5 h with 1 GeV/nucleon 56Fe-irradiated cells. Note that the radiation dose described in the figure refers to the absorbed dose by the irradiated cells.
Oxidative damage to proteins renders them prone to segregation and degradation. Further, carbonylation damage is unrepairable, which may impair the activity of key proteins essential for healthy survival [208]. It may disrupt protein structures with consequent loss of functions. It may also change the transport properties of lipid bilayers and the transmembrane potential of both plasma and nuclear membranes, and cause the accumulation of cytotoxic products [209]. Carbonylation of histones, the essential components of eukaryotic chromatin, has potentially severe consequences for the maintenance of genomic integrity [210]. It alters chromatin compactness with significant loss of DNA repair. Therefore, persistent oxidative damage in irradiated and non-targeted organs may be a factor in late tissue injury following radiotherapy [63; 106].
4.2.4. Modulation of antioxidants
Central to antioxidant defense are superoxide dismutases (SOD), glutathione, glutathione peroxidases, catalase as well as nutrient-derived antioxidant compounds such as vitamin E and selenium [211; 212]. The use of antioxidants in the treatment of radiation injury and other diseases associated with oxidative stress (e.g. Crohn's disease, rheumatoid arthritis, osteoarthritis) continues to be advocated [3; 106; 213]. For example, SOD has been shown to protect other enzymes, mitochondria, membranes, microsomes, DNA and normal mammalian cells [106]; its anti-inflammatory properties were discovered well before the protein was identified as an enzyme [214]. Indeed, the protective effects of antioxidants support the role of ROS in the progression of diseases [215]. However, the dose, timing and mode of delivery of antioxidants should be carefully evaluated. For example, the superoxide radical serve as both an initiator and a terminator of the free radical-mediated chain reaction that results in lipid peroxidation [216]. Thus, depending on dose, in some circumstances SOD may not alleviate radiation injury; in contrast, it may exacerbate the toxic effects.
Similar to mitochondrial protein import and aconitase activity described above, radiation also modulates antioxidant enzyme activity in a dose, dose-rate and LET-dependent manner. Low doses of low LET radiation (137Cs γ rays) delivered at low dose-rate up-regulate antioxidant defense (e.g. increase in the level of glutathione together with up-regulation of γ-glutamylcysteine synthetase expression [48; 217; 218]). By contrast, high LET radiation propagates oxidative stress in the irradiated cells and their neighboring bystanders [219; 220; 221]. In progeny of bystander cells, oxidative stress is associated with decreases in activity of MnSOD, CuZnSOD, catalase and glutathione peroxidase [11]. With relevance to radiation protection and radiotherapy, similar effects in progeny cells were not induced when bystander cells were co-cultured with low LET-irradiated cells [11].
5. Perspective
Reactive oxygen and nitrogen species have multiple roles that greatly depend on their concentrations. At normal physiologic levels, ROS/RNS participate in signal transduction functions that are essential for healthy survival [107]. However, at aberrant levels, they function as toxic agents and are associated with abnormal cell proliferation [215; 222]. Ionizing radiation is a strong inducer of ROS and RNS [35]. Depending on concentration, reactivity, spatial and temporal distribution, these species may mediate either adaptive/protective responses or genomic instability in progeny of irradiated cells and their neighboring bystanders [223; 224; 225; 226].
Against a background of ∼ 109 ROS/cell/day derived from metabolized oxygen [227], a few hundred ROS are generated from cellular exposure to low doses/low fluences of ionizing radiation mainly due to water radiolysis [95]. Yet, cells sense this increase and up-regulate signaling cascades to counteract the effects of additional reactive species. Metabolic protections are mobilized during and soon after the insult at molecular, cellular, tissue and organism levels [228]. In addition to up-regulation of DNA repair mechanisms, antioxidants scavenge excess ROS and proteases remove oxidized proteins. Further, cells/tissues may downregulate oxidative metabolism to lower the concentrations of reactive chemical species and may mobilize immune responses as well [11; 161; 229]. Whereas the induced mechanisms adequately protect against oxidative stress from low doses of low LET radiations delivered at low dose rates [48; 230; 231; 232; 233; 234; 235], they are inefficient at low fluences of high LET radiations. In fact, the latter radiations appear more effective at inducing long-term clastogenic/toxic effects [236; 237] than previously thought [238; 239]. The concentration of ROS/RNS in confined space along the track of densely ionizing radiations is an important factor that determines the damaging effects of high LET radiations [33; 47; 59; 240; 241]. Mounting evidence indicates that perturbations in oxidative metabolism may persist long after the decay of primary and secondary chemical species, and these conditions likely disrupt stability and activity of DNA repair proteins. Consequently, de novo DNA damages continue to occur [10; 242], thereby affecting gene expression. Therefore, understanding of the processes that lead to perturbations in oxidative metabolism would contribute to devising strategies to counteract oxidative stress (e.g. by administration of antioxidants, inhibitors of mitochondrial ATP-dependent proteases that target metabolic enzymes, etc.) to control the progression of cancer and other pathological states [243]. On the other hand, enhancing oxidative stress selectively in cancer cells to induce lethal effects is also desirable. Coupling radiotherapy with strategies to deplete cancer cells of antioxidants may enhance lethal effects in cancer cells. Further, enhancing the propagation of death-inducing events among irradiated cancer cells and between irradiated and bystander cancer cells through redox-modulated events would also contribute to tumor control [45; 75].
Although targets of ROS/RNS have been identified, many others remain to be explored [110]. For this purpose, proteomic analyses of covalent modifications (oxidative/nitrosative changes) in antioxidant enzymes and DNA repair proteins combined with studies of mitochondrial protein content [244] and functions may be informative. Revealing the association of these changes with radiation-induced protective or detrimental effects may identify regulated pathways in the context of metabolic changes. Such an approach may also uncover different mechanisms for early and late cellular responses to ionizing radiation. However care must be taken to precisely define the effects of dose, dose rate and radiation quality. For example, molecular and biochemical effects that underlie high dose radiations may not necessarily mediate low dose radiations. Further, when extrapolating results across species, the metabolic rate of the system being investigated may need to be integrated. Metabolic rates greatly vary, with that of humans being an order of magnitude lower than mice [87], which may be a factor in determining species-specific long-term effects. Likewise, basal metabolic activity in specific tissues is likely to be an important factor that determines radiation sensitivity. In sum, both genetic and metabolic susceptibility to ionizing radiation greatly affect the outcome of radiation exposure. At the organelle level, mitochondria being the major site of oxidative metabolism are almost certainly going to be affected. Whereas many studies have addressed the effects of ionizing radiation on biochemical changes in mitochondria, studies of the radiation-induced morphological changes of the organelle are lacking. This is particularly important since mitochondrial structure and bioenergetics may be tightly linked; mitochondrial size, shape and fusion/fission may significantly vary depending on cell type, the metabolic energy status, radiation dose and quality [244; 245; 246].
Acknowledgments
We are grateful to the members of our laboratories for their diligent input into the research. This work was supported by grants from the National Aeronautical Space Radiation Administration (Grant NNJ06HD91G), the US Department of Energy Low Dose Radiation Research Program (Grant FG02-02ER63447) and the National Institute of Health (Grant CA049062) to E.I.A., the Natural Sciences and Engineering Research Council of Canada to J.-P.J.-G, and the National Institute of Aging (Grant AG030504) and American Heart Association (Grant 09GRNT2260364) to D.P.
Footnotes
Conflict of interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 2.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 3.Petkau A. Role of superoxide dismutase in modification of radiation injury. Br J Cancer Suppl. 1987;8:87–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Tamminga J, Kovalchuk O. Role of DNA damage and epigenetic DNA methylation changes in radiation-induced genomic instability and bystander effects in germline in vivo. Current molecular pharmacology. 2011;4:115–125. doi: 10.2174/1874467211104020115. [DOI] [PubMed] [Google Scholar]
- 6.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 7.Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Current molecular pharmacology. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mothersill C, Seymour CB. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 9.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonanno M, de Toledo SM, Azzam EI. Increased frequency of spontaneous neoplastic transformation in progeny of bystander cells from cultures exposed to densely-ionizing radiation. PloS one. 2011;6 doi: 10.1371/journal.pone.0021540. art. no. e21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonanno M, De Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlatewith oxidative stress. Radiat Res. 2011;175:405–415. doi: 10.1667/RR2461.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucinotta FA, Chappell LJ. Non-targeted effects and the dose response for heavy ion tumor induction. Mutat Res. 2010;687:49–53. doi: 10.1016/j.mrfmmm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Hall EJ, Henry S. Kaplan Distinguished Scientist Award 2003. The crooked shall be made straight; dose-response relationships for carcinogenesis. Int J Radiat Biol. 2004;80:327–337. doi: 10.1080/09553000410001695895. [DOI] [PubMed] [Google Scholar]
- 14.Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol. 2009;91:4–15. doi: 10.1016/j.radonc.2008.12.016. discussion 11-13. [DOI] [PubMed] [Google Scholar]
- 15.BEIR-VII. Health Risks from Exposure to Low Levels of Ionizing Radiation. National Research Council of the National Academies; Washington, D.C: 2005. [PubMed] [Google Scholar]
- 16.Hendry JH. Genomic instability: potential contributions to tumour and normal tissue response, and second tumours, after radiotherapy. Radiother Oncol. 2001;59:117–126. doi: 10.1016/s0167-8140(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 17.Robles AI, Linke SP, Harris CC. The p53 network in lung carcinogenesis. Oncogene. 2002;21:6898–6907. doi: 10.1038/sj.onc.1205563. [DOI] [PubMed] [Google Scholar]
- 18.Schonfeld SJ, Bhatti P, Brown EE, Linet MS, Simon SL, Weinstock RM, Hutchinson AA, Stovall M, Preston DL, Alexander BH, Doody MM, Sigurdson AJ. Polymorphisms in oxidative stress and inflammation pathway genes, low-dose ionizing radiation, and the risk of breast cancer among US radiologic technologists. Cancer Causes Control. 2010;21:1857–1866. doi: 10.1007/s10552-010-9613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 20.Platzman R . The physical and chemical basis of mechanisms in radiation biology. Radiation Biology and Medicine. In: Claus W, editor. Selected Reviews in the Life Sciences. Addison-Wesley; Reading, MA: 1958. pp. 15–72. [Google Scholar]
- 21.Ferradini C, Jay-Gerin JP. Radiolysis of water and aqueous solutions: histrory and present state of the science. Can J Chem. 1999;77:1542–1575. [Google Scholar]
- 22.Spinks JWT, Wodds RJ. An Introduction to Radiation Chemistry. 3rd. Wiley; New York: 1990. [Google Scholar]
- 23.Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of HO2/O2- radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1041–1100. [Google Scholar]
- 24.Alfassi ZBE. Peroxyl Radicals. Wiley; Chichester: 1997. [Google Scholar]
- 25.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 27.Jay-Gerin JP, Ferradini C. Are there protective enzymatic pathways to regulate high local nitric oxide (•NO) concentrations in cells under stress conditions? Biochimie. 2000;82:161–166. doi: 10.1016/s0300-9084(00)00062-6. [DOI] [PubMed] [Google Scholar]
- 28.Frongillo Y, Goulet T, Fraser MJ, Cobut V, Patau JP, Jay-Gerin JP. Monte Carlo simulation of fast electron and proton tracks in liquid water. II. Nonhomogeneous chemistry. Radiat Phys Chem. 1998;51:245–254. [Google Scholar]
- 29.Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th. Oxford University Press; Oxford: 2007. [Google Scholar]
- 31.La Verne JA. Radiation chemical effects of heavy ions. In: Mozumder A, Hatano Y, editors. Charged Particle and Photon Interactions with Matter Chemical, Physicochemical, and Biological Consequences with Applications. Marcel Dekker; New York: 2004. pp. 403–429. [Google Scholar]
- 32.Meesungnoen J, Jay-Gerin JP. Radiation chemistry of liquid water with heavy ions: Monte Carlo simulation studies. In: K Y, M A, Hatano Y, editors. Charged Particle and Photon Interactions with Matter Recent Advances, Applications, and Interfaces. Taylor and Francis; Boca Raton, FL: 2011. pp. 355–400. [Google Scholar]
- 33.Goodhead DT. The initial physical damage produced by ionizing radiations. Int J Radiat Biol. 1989;56:623–634. doi: 10.1080/09553008914551841. [DOI] [PubMed] [Google Scholar]
- 34.Campa A, Ballarini F, Belli M, Cherubini R, Dini V, Esposito G, Friedland W, Gerardi S, Molinelli S, Ottolenghi A, Paretzke H, Simone G, Tabocchini MA. DNA DSB induced in human cells by charged particles and gamma rays: experimental results and theoretical approaches. Int J Radiat Biol. 2005;81:841–854. doi: 10.1080/09553000500530888. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill P, Wardman P. Radiation chemistry comes before radiation biology. Int J Radiat Biol. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- 36.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Molecular cancer therapeutics. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 38.Roth RA, Sharma SC, Katz R. Systematic evaluation of cellular radiosensitivity parameters. Phys Med Biol. 1976;21:491–503. doi: 10.1088/0031-9155/21/4/001. [DOI] [PubMed] [Google Scholar]
- 39.Ottolenghi A, Merzagora M, Paretzke HG. DNA complex lesions induced by protons and alpha-particles: track structure characteristics determining linear energy transfer and particle type dependence. Radiat Environ Biophys. 1997;36:97–103. doi: 10.1007/s004110050060. [DOI] [PubMed] [Google Scholar]
- 40.Goodhead DT. Energy deposition stochastics and track structure: what about the target? Radiat Prot Dosimetry. 2006;122:3–15. doi: 10.1093/rpd/ncl498. [DOI] [PubMed] [Google Scholar]
- 41.Ward JF. Biochemistry of DNA lesions. Radiat Res Suppl. 1985;8:S103–111. [PubMed] [Google Scholar]
- 42.Georgakilas AG. From chemistry of DNA damage to repair and biological significance. Comprehending the future. Mutat Res. 2011;711:1–2. doi: 10.1016/j.mrfmmm.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Gollapalle E, Wang R, Adetolu R, Tsao D, Francisco D, Sigounas G, Georgakilas AG. Detection of oxidative clustered DNA lesions in X-irradiated mouse skin tissues and human MCF-7 breast cancer cells. Radiat Res. 2007;167:207–216. doi: 10.1667/rr0659.1. [DOI] [PubMed] [Google Scholar]
- 44.Boucher D, Testard I, Averbeck D. Low levels of clustered oxidative DNA damage induced at low and high LET irradiation in mammalian cells. Radiat Environ Biophys. 2006;45:267–276. doi: 10.1007/s00411-006-0070-3. [DOI] [PubMed] [Google Scholar]
- 45.Autsavapromporn N, de Toledo SM, Little JB, Jay-Gerin JP, Harris AL, Azzam EI. The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose α-particle-irradiated human cells. Radiat Res. 2011;175:347–357. doi: 10.1667/RR2372.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc Natl Acad Sci U S A. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikjoo H, Goodhead DT, Charlton DE, Paretzke HG. Energy deposition in small cylindrical targets by monoenergetic electrons. Int J Radiat Biol. 1991;60:739–756. doi: 10.1080/09553009114552561. [DOI] [PubMed] [Google Scholar]
- 48.de Toledo SM, Asaad N, Venkatachalam P, Li L, Howell RW, Spitz DR, Azzam EI. Adaptive responses to low-dose/low-dose-rate γ rays in normal human fibroblasts: the role of growth architecture and oxidative metabolism. Radiat Res. 2006;166:849–857. doi: 10.1667/RR0640.1. [DOI] [PubMed] [Google Scholar]
- 49.Feinendegen LE, Pollycove M, Neumann RD. Whole-body responses to low-level radiation exposure: new concepts in mammalian radiobiology. Exp Hematol. 2007;35:37–46. doi: 10.1016/j.exphem.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106(1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomura T, Li XH, Ogata H, Sakai K, Kondo T, Takano Y, Magae J. Suppressive Effects of Continuous Low-Dose-Rate gamma Irradiation on Diabetic Nephropathy in Type II Diabetes Mellitus Model Mice. Radiat Res. 2011;176:356–365. doi: 10.1667/rr2559.1. [DOI] [PubMed] [Google Scholar]
- 52.Azzam EI. Exposure to low level environmental agents: The induction of hormesis. Mutat Res. 2011 doi: 10.1016/j.mrgentox.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. Transmission of chromosomal instability after plutonium α-particle irradiation [see comments] Nature. 1992;355:738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- 54.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 55.Ponnaiya B, Suzuki M, Tsuruoka C, Uchihori Y, Wei Y, Hei TK. Detection of chromosomal instability in bystander cells after Si490-ion irradiation. Radiat Res. 2011;176:280–290. doi: 10.1667/rr2428.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto H, Tomita M, Otsuka K, Hatashita M, Hamada N. Nitric oxide is a key molecule serving as a bridge between radiation-induced bystander and adaptive responses. Curr Mol Pharmacol. 2011;4 doi: 10.2174/1874467211104020126. [DOI] [PubMed] [Google Scholar]
- 57.Cucinotta FA, Katz R, Wilson JW. Radial distribution of electron spectra from high-energy ions. Radiat Environ Biophys. 1998;37:259–265. doi: 10.1007/s004110050127. [DOI] [PubMed] [Google Scholar]
- 58.Goodhead DT, Thacker J, Cox R. Effects of radiations of different qualities on cells: molecular mechanisms of damage and repair (Weiss Lecture) Int J Radiat Biol. 1993;63:543–556. doi: 10.1080/09553009314450721. [DOI] [PubMed] [Google Scholar]
- 59.Muroya Y, Plante I, Azzam EI, Meesungnoen J, Katsumura Y, Jay-Gerin JP. High-LET ion radiolysis of water: visualization of the formation and evolution of ion tracks and relevance to the radiation-induced bystander effect. Radiat Res. 2006;165:485–491. doi: 10.1667/rr3540.1. [DOI] [PubMed] [Google Scholar]
- 60.Plante I, Ponomarev A, Cucinotta FA. 3D visualisation of the stochastic patterns of the radial dose in nano-volumes by a Monte Carlo simulation of HZE ion track structure. Radiat Prot Dosimetry. 2011;143:156–161. doi: 10.1093/rpd/ncq526. [DOI] [PubMed] [Google Scholar]
- 61.Wu C, Parrott AM, Fu C, Liu T, Marino SM, Gladyshev VN, Jain MR, Baykal AT, Li Q, Oka S, Sadoshima J, Beuve A, Simmons WJ, Li H. Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal. 2011;15:2565–2604. doi: 10.1089/ars.2010.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res. 2008;10:215. doi: 10.1186/bcr2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 64.Hayashi T, Kusunoki Y, Hakoda M, Morishita Y, Kubo Y, Maki M, Kasagi F, Kodama K, Macphee DG, Kyoizumi S. Radiation dose-dependent increases in inflammatory response markers in A-bomb survivors. Int J Radiat Biol. 2003;79:129–136. [PubMed] [Google Scholar]
- 65.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83:819–825. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- 66.Neriishi K, Nakashima E, Delongchamp RR. Persistent subclinical inflammation among A-bomb survivors. Int J Radiat Biol. 2001;77:475–482. doi: 10.1080/09553000010024911. [DOI] [PubMed] [Google Scholar]
- 67.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Cadet J, Douki T, Ravanat JL. Measurement of oxidatively generated base damage in cellular DNA. Mutat Res. 2011;711:3–12. doi: 10.1016/j.mrfmmm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Redon CE, Dickey JS, Nakamura AJ, Kareva IG, Naf D, Nowsheen S, Kryston TB, Bonner WM, Georgakilas AG, Sedelnikova OA. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc Natl Acad Sci U S A. 2010;107:17992–17997. doi: 10.1073/pnas.1008260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hair JM, Terzoudi GI, Hatzi VI, Lehockey KA, Srivastava D, Wang W, Pantelias GE, Georgakilas AG. BRCA1 role in the mitigation of radiotoxicity and chromosomal instability through repair of clustered DNA lesions. Chem Biol Interact. 2010;188:350–358. doi: 10.1016/j.cbi.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 71.Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin OA, Redon CE, Nakamura AJ, Dickey JS, Georgakilas AG, Bonner WM. Systemic DNA damage related to cancer. Cancer Res. 2011;71:3437–3441. doi: 10.1158/0008-5472.CAN-10-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain MR, Li M, Chen W, Liu T, De Toledo SM, Pandey BN, Li H, Rabin BM, Azzam EI. In vivo space radiation-induced non-targeted responses: late effects on molecular signaling in mitochondria. Current molecular pharmacology. 2011;4:106–114. doi: 10.2174/1874467211104020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 75.Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I, Kohno S. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 77.Little JB. Failla Memorial Lecture. Changing views of cellular radiosensitivity. Radiat Res. 1994;140:299–311. [PubMed] [Google Scholar]
- 78.Aypar U, Morgan WF, Baulch JE. Radiation-induced genomic instability: are epigenetic mechanisms the missing link? Int J Radiat Biol. 2011;87:179–191. doi: 10.3109/09553002.2010.522686. [DOI] [PubMed] [Google Scholar]
- 79.Ilnytskyy Y, Kovalchuk O. Non-targeted radiation effects-an epigenetic connection. Mutat Res. 2011;714:113–125. doi: 10.1016/j.mrfmmm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Dubrova YE, Jeffreys AJ, Malashenko AM. Mouse minisatellite mutations induced by ionizing radiation. Nat Genet. 1993;5:92–94. doi: 10.1038/ng0993-92. [DOI] [PubMed] [Google Scholar]
- 81.Lorimore SA, Mukherjee D, Robinson JI, Chrystal JA, Wright EG. Long-lived inflammatory signaling in irradiated bone marrow is genome dependent. Cancer Res. 2011;71:6485–6491. doi: 10.1158/0008-5472.CAN-11-1926. [DOI] [PubMed] [Google Scholar]
- 82.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 83.Morgan WF, Day JP, Kaplan MI, McGhee EM, Limoli CL. Genomic instability induced by ionizing radiation. Radiat Res. 1996;146:247–258. [PubMed] [Google Scholar]
- 84.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinberg RA. The biology of cancer, Garland Science. Taylor & Francis Group, LLC; New York: 2007. [Google Scholar]
- 86.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 87.Ames BN. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7:121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- 88.Holliday R, Grigg GW. DNA methylation and mutation. Mutat Res. 1993;285:61–67. doi: 10.1016/0027-5107(93)90052-h. [DOI] [PubMed] [Google Scholar]
- 89.Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 90.Wilson DM, 3rd, Kim D, Berquist BR, Sigurdson AJ. Variation in base excision repair capacity. Mutat Res. 2011;711:100–112. doi: 10.1016/j.mrfmmm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friedberg EC, McDaniel LD, Schultz RA. The role of endogenous and exogenous DNA damage and mutagenesis. Curr Opin Genet Dev. 2004;14:5–10. doi: 10.1016/j.gde.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Feinendegen LE, Neumann RD, editors. Workshop Report: Cellular Responses to Low Doses of Ionizing Radiation. United States Department of Energy and the National Institutes of Health; Bethesda: 1999. [Google Scholar]
- 93.Georgakilas AG, Holt SM, Hair JM, Loftin CW. Measurement of oxidatively-induced clustered DNA lesions using a novel adaptation of single cell gel electrophoresis (comet assay) Curr Protoc Cell Biol. 2010;Chapter 6:Unit 6 11. doi: 10.1002/0471143030.cb0611s49. [DOI] [PubMed] [Google Scholar]
- 94.Olive PL. Impact of the comet assay in radiobiology. Mutat Res. 2009;681:13–23. doi: 10.1016/j.mrrev.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol. 2002;21:85–90. doi: 10.1191/0960327102ht216oa. [DOI] [PubMed] [Google Scholar]
- 96.Burdon RH, Gill V, Rice-Evans C. Cell proliferation and oxidative stress. Free Radic Res Commun. 1989;7:149–159. doi: 10.3109/10715768909087937. [DOI] [PubMed] [Google Scholar]
- 97.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 98.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 100.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 101.Herrlich P, Bohmer FD. Redox regulation of signal transduction in mammalian cells. Biochem Pharmacol. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 102.Meplan C, Richard MJ, Hainaut P. Redox signalling and transition metals in the control of the p53 pathway. Biochem Pharmacol. 2000;59:25–33. doi: 10.1016/s0006-2952(99)00297-x. [DOI] [PubMed] [Google Scholar]
- 103.Price BD, Calderwood SK. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 1992;52:3814–3817. [PubMed] [Google Scholar]
- 104.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 105.Schulze-Osthoff K, Bauer M, Vogt M, Wesselborg S, Baeuerle PA. Reactive Oxygen Intermediates as Primary Signals and Second Mesengers in the Activation of transcription Factors. In: Forman HJ, Cadenas E, editors. Oxidative Stress and Signal Transduction. Chapman & Hall; New York: 1997. pp. 239–259. [Google Scholar]
- 106.Petkau A. Scientific basis for the clinical use of superoxide dismutase. Cancer Treat Rev. 1986;13:17–44. doi: 10.1016/0305-7372(86)90012-5. [DOI] [PubMed] [Google Scholar]
- 107.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 108.Rumyantseva GV, Weiner LM, Molin YN, Budker VG. Permeation of liposome membrane by superoxide radical. FEBS Lett. 1979;108:477–480. doi: 10.1016/0014-5793(79)80592-x. [DOI] [PubMed] [Google Scholar]
- 109.Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- 110.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 111.Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H, Goswami PC. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 2003;63:2109–2117. [PubMed] [Google Scholar]
- 112.Venkatachalam P, de Toledo SM, Pandey BN, Tephly LA, Carter AB, Little JB, Spitz DR, Azzam EI. Regulation of normal cell cycle progression by flavin-containing oxidases. Oncogene. 2008;27:20–31. doi: 10.1038/sj.onc.1210634. [DOI] [PubMed] [Google Scholar]
- 113.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 114.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 115.Ammendola R, Ruocchio MR, Chirico G, Russo L, De Felice C, Esposito F, Russo T, Cimino F. Inhibition of NADH/NADPH oxidase affects signal transduction by growth factor receptors in normal fibroblasts. Arch Biochem Biophys. 2002;397:253–257. doi: 10.1006/abbi.2001.2641. [DOI] [PubMed] [Google Scholar]
- 116.Irani K, Goldschmidt-Clermont PJ. Ras, superoxide and signal transduction. Biochem Pharmacol. 1998;55:1339–1346. doi: 10.1016/s0006-2952(97)00616-3. [DOI] [PubMed] [Google Scholar]
- 117.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 118.Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE, Oberley LW, Goswami PC. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007;67:6392–6399. doi: 10.1158/0008-5472.CAN-07-0225. [DOI] [PubMed] [Google Scholar]
- 119.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 120.Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- 121.Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Fujita Y, Ali N, Ishizawa K, Yamauchi A, Tamaki T. Antioxidant effects of stereoisomers of N-acetylcysteine (NAC), L-NAC and D-NAC, on angiotensin II-stimulated MAP kinase activation and vascular smooth muscle cell proliferation. J Pharmacol Sci. 2004;95:483–486. doi: 10.1254/jphs.sc0040061. [DOI] [PubMed] [Google Scholar]
- 122.Venkatachalam P, de Toledo SM, Azzam EI. Flavin-containing oxidases regulate progression from G1 to S phase of the cell cycle in normal human fibroblasts. Radiat Phys Chem. 2004;72:315–321. [Google Scholar]
- 123.Terasima R, Tolmach LJ. X-ray sensitivity and DNA synthesis in synchronous populations of Hela cells. Science. 1963;140:490–492. doi: 10.1126/science.140.3566.490. [DOI] [PubMed] [Google Scholar]
- 124.Nagasawa H, Li CY, Maki CG, Imrich AC, Little JB. Relationship between radiation-induced G1 phase arrest and p53 function in human tumor cells. Cancer Res. 1995;55:1842–1846. [PubMed] [Google Scholar]
- 125.Kar S, Subbaram S, Carrico PM, Melendez JA. Redox-control of matrix metalloproteinase-1: a critical link between free radicals, matrix remodeling and degenerative disease. Respir Physiol Neurobiol. 2010;174:299–306. doi: 10.1016/j.resp.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 127.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 129.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 130.Los M, Schenk H, Hexel K, Baeuerle PA, Droge W, Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. Embo J. 1995;14:3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boveris A, Cadenas E, Stoppani AO. Role of ubiquinone in the mitochond rial generation of hydrogen peroxide. Biochem J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Claude A. The coming of age of the cell. Science. 1975;189:433–435. doi: 10.1126/science.1098146. [DOI] [PubMed] [Google Scholar]
- 133.Champe PC, Harvey RA, Ferrier DR. Biochemistry. 4th. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 134.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 135.Nohl H, Jordan W. The mitochondrial site of superoxide formation. Biochem Biophys Res Commun. 1986;138:533–539. doi: 10.1016/s0006-291x(86)80529-0. [DOI] [PubMed] [Google Scholar]
- 136.Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- 137.Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J. Redox regulation of TNF signaling. Biofactors. 1999;10:145–156. doi: 10.1002/biof.5520100210. [DOI] [PubMed] [Google Scholar]
- 138.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 139.Werner E, Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J Cell Biol. 2002;158:357–368. doi: 10.1083/jcb.200111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sidoti-de Fraisse C, Rincheval V, Risler Y, Mignotte B, Vayssiere JL. TNF-alpha activates at least two apoptotic signaling cascades. Oncogene. 1998;17:1639–1651. doi: 10.1038/sj.onc.1202094. [DOI] [PubMed] [Google Scholar]
- 141.Malakhova L, Bezlepkin VG, Antipova V, Ushakova T, Fomenko L, Sirota N, Gaziev AI. The increase in mitochondrial DNA copy number in the tissues of gamma-irradiated mice. Cell Mol Biol Lett. 2005;10:721–732. [PubMed] [Google Scholar]
- 142.Antipova VN, Malakhova LV, Bezlepkin VG. Detection of large deletions of mitochondrial DNA in tissues of mice exposed to X-rays. Biofizika. 2011;56:439–445. [PubMed] [Google Scholar]
- 143.Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ. 2003;10:323–334. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chaudhry MA, Omaruddin RA. Mitochondrial Gene Expression in Directly Irradiated and Nonirradiated Bystander Cells. Cancer Biother Radiopharm. 2011 doi: 10.1089/cbr.2010.0940. [DOI] [PubMed] [Google Scholar]
- 145.Chiu HW, Lin W, Ho SY, Wang YJ. Synergistic effects of arsenic trioxide and radiation in osteosarcoma cells through the induction of both autophagy and apoptosis. Radiat Res. 2011;175:547–560. doi: 10.1667/RR2380.1. [DOI] [PubMed] [Google Scholar]
- 146.Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T, Brodie C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125:717–722. doi: 10.1002/ijc.24402. [DOI] [PubMed] [Google Scholar]
- 147.Cuisnier O, Serduc R, Lavieille JP, Longuet M, Reyt E, Riva C. Chronic hypoxia protects against gamma-irradiation-induced apoptosis by inducing bcl-2 up-regulation and inhibiting mitochondrial translocation and conformational change of bax protein. Int J Oncol. 2003;23:1033–1041. [PubMed] [Google Scholar]
- 148.Ogura A, Oowada S, Kon Y, Hirayama A, Yasui H, Meike S, Kobayashi S, Kuwabara M, Inanami O. Redox regulation in radiation-induced cytochrome c r elease from mitochondria of human lung carcinoma A549 cells. Cancer Lett. 2009;277:64–71. doi: 10.1016/j.canlet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 149.Cherbonnel-Lasserre C, Gauny S, Kronenberg A. Suppression of apoptosis by Bcl-2 or Bcl-xL promotes susceptibility to mutagenesis. Oncogene. 1996;13:1489–1497. [PubMed] [Google Scholar]
- 150.Chen S, Zhao Y, Zhao G, Han W, Bao L, Yu KN, Wu L. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutat Res. 2009;666:68–73. doi: 10.1016/j.mrfmmm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 151.Maguire P, Mothersill C, Seymour C, Lyng FM. Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human keratinocytes. Radiat Res. 2005;163:384–390. doi: 10.1667/rr3325. [DOI] [PubMed] [Google Scholar]
- 152.Rajendran S, Harrison SH, Thomas RA, Tucker JD. The role of mitochondria in the radiation-induced bystander effect in human lymphoblastoid cells. Radiat Res. 2011;175:159–171. doi: 10.1667/rr2296.1. [DOI] [PubMed] [Google Scholar]
- 153.Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67:5872–5879. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects. Cancer Res. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He M, Zhao M, Shen B, Prise KM, Shao C. Radiation-induced intercellular signaling mediated by cytochrome-c via a p53-dependent pathway in hepatoma cells. Oncogene. 2011;30:1947–1955. doi: 10.1038/onc.2010.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Choi KM, Kang CM, Cho ES, Kang SM, Lee SB, Um HD. Ionizing radiation-induced micronucleus formation is mediated by reactive oxygen species that are produced in a manner dependent on mitochondria, Nox1, and JNK. Oncol Rep. 2007;17:1183–1188. [PubMed] [Google Scholar]
- 157.Limoli CL, Giedzinski E, Morgan WF, Swarts SG, Jones GD, Hyun W. Persistent oxidative stress in chromosomally unstable cells. Cancer Res. 2003;63:3107–3111. [PubMed] [Google Scholar]
- 158.Du C, Gao Z, Venkatesha VA, Kalen AL, Chaudhuri L, Spitz DR, Cullen JJ, Oberley LW, Goswami PC. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer biology & therapy. 2009;8:1962–1971. doi: 10.4161/cbt.8.20.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Autsavapromporn N, de Toledo SM, Buonanno M, Jay-Gerin JP, Harris AL, Azzam EI. Intercellular communication amplifies stressful effects in high-charge, high-energy (HZE) particle-irradiated human cells. J Radiat Res (Tokyo) 2011;52:408–414. doi: 10.1269/jrr.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pandey BN, Gordon DA, Pain D, Azzam EI. Normal human fibroblasts exposed to high or low dose ionizing radiation: differential effects on mitochondrial protein import and membrane potential. Antioxid Redox Signal. 2006;8:1253–1261. doi: 10.1089/ars.2006.8.1253. [DOI] [PubMed] [Google Scholar]
- 162.Kim GJ, Fiskum GM, Morgan WF. A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res. 2006;66:10377–10383. doi: 10.1158/0008-5472.CAN-05-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 164.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 165.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, 2nd, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 166.Wallace DC, Zheng XX, Lott MT, Shoffner JM, Hodge JA, Kelley RI, Epstein CM, Hopkins LC. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988;55:601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- 167.DiMauro S, Hirano M. Pathogenesis and treatment of mitochondrial disorders. Adv Exp Med Biol. 2009;652:139–170. doi: 10.1007/978-90-481-2813-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 169.Lu CY, Lee HC, Fahn HJ, Wei YH. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat Res. 1999;423:11–21. doi: 10.1016/s0027-5107(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 170.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 6th. W. H. Freeman and Company; New York, NY: 2006. [Google Scholar]
- 171.Kawamura S, Takada D, Watanabe K, Hayashi J, Hayakawa K, Akashi M. Role of mitochondrial DNA in cells exposed to irradiation: generation of reactive oxygen species (ROS) is required for G2 checkpoint upon irradiation. Journal of Health Science. 2005;51:385–393. [Google Scholar]
- 172.Grishko VI, Rachek LI, Spitz DR, Wilson GL, LeDoux SP. Contribution of mitochondrial DNA repair to cell resistance from oxidative stress. J Biol Chem. 2005;280:8901–8905. doi: 10.1074/jbc.M413022200. [DOI] [PubMed] [Google Scholar]
- 173.Mason PA, Matheson EC, Hall AG, Lightowlers RN. Mismatch repair activity in mammalian mitochondria. Nucleic Acids Res. 2003;31:1052–1058. doi: 10.1093/nar/gkg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Graziewicz MA, Day BJ, Copeland WC. The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic Acids Res. 2002;30:2817–2824. doi: 10.1093/nar/gkf392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zastawny TH, Dabrowska M, Jaskolski T, Klimarczyk M, Kulinski L, Koszela A, Szczesniewicz M, Sliwinska M, Witkowski P, Olinski R. Comparison of oxidative base damage in mitochondrial and nuclear DNA. Free Radic Biol Med. 1998;24:722–725. doi: 10.1016/s0891-5849(97)00331-6. [DOI] [PubMed] [Google Scholar]
- 176.Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 177.Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjoras M, Eide L. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci. 2011;31:9746–9751. doi: 10.1523/JNEUROSCI.0852-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bogenhagen D, Clayton DA. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell. 1977;11:719–727. doi: 10.1016/0092-8674(77)90286-0. [DOI] [PubMed] [Google Scholar]
- 180.Gaziev AI, Shaikhaev GO. Ionizing radiation can activate the insertion of mitochondrial DNA fragments in the nuclear genome. Radiatsionnaia biologiia, radioecologiia / Rossiiskaia akademiia nauk. 2007;47:673–683. [PubMed] [Google Scholar]
- 181.Prithivirajsingh S, Story MD, Bergh SA, Geara FB, Ang KK, Ismail SM, Stevens CW, Buchholz TA, Brock WA. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004;571:227–232. doi: 10.1016/j.febslet.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 182.Chen B, Zhong Y, Peng W, Sun Y, Hu YJ, Yang Y, Kong WJ. Increased mitochondrial DNA damage and decreased base excision repair in the auditory cortex of D-galactose-induced aging rats. Mol Biol Rep. 2011;38:3635–3642. doi: 10.1007/s11033-010-0476-5. [DOI] [PubMed] [Google Scholar]
- 183.Biskup S, Moore DJ. Detrimental deletions: mitochondria, aging and Parkinson's disease. Bioessays. 2006;28:963–967. doi: 10.1002/bies.20471. [DOI] [PubMed] [Google Scholar]
- 184.Thorsness PE, Fox TD. Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature. 1990;346:376–379. doi: 10.1038/346376a0. [DOI] [PubMed] [Google Scholar]
- 185.Ricchetti M, Tekaia F, Dujon B. Continued colonization of the human genome by mitochondrial DNA. PLoS Biol. 2004;2:E273. doi: 10.1371/journal.pbio.0020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ricchetti M, Fairhead C, Dujon B. Mitochondrial DNA repairs double-strand breaks in yeast chromosomes. Nature. 1999;402:96–100. doi: 10.1038/47076. [DOI] [PubMed] [Google Scholar]
- 187.Patrushev M, Kasymov V, Patrusheva V, Ushakova T, Gogvadze V, Gaziev AI. Release of mitochondrial DNA fragments from brain mitochondria of irradiated mice. Mitochondrion. 2006;6:43–47. doi: 10.1016/j.mito.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 188.Shay JW, Werbin H. New evidence for the insertion of mitochondrial DNA into the human genome: significance for cancer and aging. Mutat Res. 1992;275:227–235. doi: 10.1016/0921-8734(92)90026-l. [DOI] [PubMed] [Google Scholar]
- 189.Cheng X, Ivessa AS. The migration of mitochondrial DNA fragments to the nucleus affects the chronological aging process of Saccharomyces cerevisiae. Aging Cell. 2010;9:919–923. doi: 10.1111/j.1474-9726.2010.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Scheffler IE. Mitochondria. 2nd. Wiley-Liss; Hoboken, NJ: 2008. [Google Scholar]
- 191.Pain D, Murakami H, Blobel G. Identification of a receptor for protein import into mitochondria. Nature. 1990;347:444–449. doi: 10.1038/347444a0. [DOI] [PubMed] [Google Scholar]
- 192.Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, Snyder FF, Bridge PJ, Bernier FP. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J Med Genet. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huckriede A, Heikema A, Sjollema K, Briones P, Agsteribbe E. Morphology of the mitochondria in heat shock protein 60 deficient fibroblasts from mitochondrial myopathy patients. Effects of stress conditions. Virchows Arch. 1995;427:159–165. doi: 10.1007/BF00196521. [DOI] [PubMed] [Google Scholar]
- 194.Rothbauer U, Hofmann S, Muhlenbein N, Paschen SA, Gerbitz KD, Neupert W, Brunner M, Bauer MF. Role of the deafness dystonia peptide 1 (DDP1) in import of human Tim23 into the inner membrane of mitochondria. J Biol Chem. 2001;276:37327–37334. doi: 10.1074/jbc.M105313200. [DOI] [PubMed] [Google Scholar]
- 195.Takakubo F, Cartwright P, Hoogenraad N, Thorburn DR, Collins F, Lithgow T, Dahl HH. An amino acid substitution in the pyruvate dehydrogenase E1 alpha gene, affecting mitochondrial import of the precursor protein. Am J Hum Genet. 1995;57:772–780. [PMC free article] [PubMed] [Google Scholar]
- 196.Wang C, Rajput S, Watabe K, Liao DF, Cao D. Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Frontiers in bioscience (Scholar edition) 2:515–526. doi: 10.2741/s82. [DOI] [PubMed] [Google Scholar]
- 197.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 199.Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nature reviews. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- 200.Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 201.Bulteau AL, Ikeda-Saito M, Szweda LI. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42:14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 202.Academies NRCotN., editor. Managing Space Radiation Risk in the New Era of Space Exploration. The National Academies Press; Washington, D.C: 2008. [Google Scholar]
- 203.BEIR-IV, Health risks of radon and other internally deposited alpha-emitters (BEIR IV) National Academy Press; Washington, D.C: 1988. [PubMed] [Google Scholar]
- 204.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 205.Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science. 1994;264:891. doi: 10.1126/science.8178144. [DOI] [PubMed] [Google Scholar]
- 206.Dayal D, Martin SM, Owens KM, Aykin-Burns N, Zhu Y, Boominathan A, Pain D, Limoli CL, Goswami PC, Domann FE, Spitz DR. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–745. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc. 2006;128:1879–1885. doi: 10.1021/ja057358l. [DOI] [PubMed] [Google Scholar]
- 208.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. Embo J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ziegler C, Wessels JM. Investigation of lipid peroxidation in liposomes induced by heavy ion irradiation. Radiat Environ Biophys. 1998;37:95–100. doi: 10.1007/s004110050100. [DOI] [PubMed] [Google Scholar]
- 210.Wondrak GT, Cervantes-Laurean D, Jacobson EL, Jacobson MK. Histone carbon ylation in vivo and in vitro. Biochem. 2000;J 351 Pt 3:769–777. [PMC free article] [PubMed] [Google Scholar]
- 211.Lu SC. Regulation of glutathione synthesis. Curr Top Cell Regul. 2000;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]
- 212.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 213.Epperly MW, Wang H, Jones JA, Dixon T, Montesinos CA, Greenberger JS. Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res. 2011;175:759–765. doi: 10.1667/RR2398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 215.Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L, Spitz DR, Oberley LW. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic Biol Med. 2006;41:226–237. doi: 10.1016/j.freeradbiomed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 216.McCord JM. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Response. 2008;6:223–238. doi: 10.2203/dose-response.08-012.McCord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kojima S, Ishida H, Takahashi M, Yamaoka K. Elevation of glutathione induced by low-dose gamma rays and its involvement in increased natural killer activity. Radiat Res. 2002;157:275–280. doi: 10.1667/0033-7587(2002)157[0275:eogibl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 218.Bravard A, Luccioni C, Moustacchi E, Rigaud O. Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int J Radiat Biol. 1999;75:639–645. doi: 10.1080/095530099140285. [DOI] [PubMed] [Google Scholar]
- 219.Azzam EI, de Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 220.Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 221.Narayanan PK, LaRue KEA, Goodwin EH, Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiat Res. 1999;152:57–63. [PubMed] [Google Scholar]
- 222.Oberley LW, Oberley TD. Role of antioxidant enzymes in cell immortalization and transformation. Mol Cell Biochem. 1988;84:147–153. doi: 10.1007/BF00421049. [DOI] [PubMed] [Google Scholar]
- 223.Azzam EI, Little JB. The radiation-induced bystander effect: evidence and significance. Hum Exp Toxicol. 2004;23:61–65. doi: 10.1191/0960327104ht418oa. [DOI] [PubMed] [Google Scholar]
- 224.de Toledo SM, Azzam EI. Adaptive and bystander responses in human and rodent cell cultures exposed to low level ionizing radiation: the impact of linear energy transfer. Dose Response. 2006;4:291–301. doi: 10.2203/dose-response.06-103.deToledo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brooks AL. Paradigm shifts in radiation biology: their impact on intervention for radiation-induced disease. Radiat Res. 2005;164:454–461. doi: 10.1667/rr3324.1. [DOI] [PubMed] [Google Scholar]
- 226.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 227.Lindahl T. DNA repair enzymes acting on spontaneous lesions in DNA. In: Nichols WW, Murphy DG, editors. DNA Repair Processes. Symposia Specialists; Miami: 1977. pp. 225–240. [Google Scholar]
- 228.Feinendegen LE, Brooks AL, Morgan WF. Biological consequences and health risks of low-level exposure to ionizing radiation: commentary on the workshop. Health Phys. 2011;100:247–259. doi: 10.1097/HP.0b013e31820a83ae. [DOI] [PubMed] [Google Scholar]
- 229.Ina M, Sakai K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: analysis of immune cell populations and surface molecules. Int J Radiat Biol. 2005;81:721–729. doi: 10.1080/09553000500519808. [DOI] [PubMed] [Google Scholar]
- 230.Azzam EI, de Toledo SM, Raaphorst GP, Mitchel RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- 231.Azzam EI, Raaphorst GP, Mitchel RE. Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T1/2 mouse embryo cells. Radiat Res. 1994;138:S28–31. [PubMed] [Google Scholar]
- 232.Mitchel RE, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- 233.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 234.Redpath JL, Antoniono RJ. Induction of an adaptive response against spontaneous neoplastic transformation in vitro by low-dose gamma radiation. Radiat Res. 1998;149:517–520. [PubMed] [Google Scholar]
- 235.Le XC, Xing JZ, Lee J, Leadon SA, Weinfeld M. Inducible repair of thymine glycol detected by an ultrasensitive assay for DNA damage. Science. 1998;280:1066–1069. doi: 10.1126/science.280.5366.1066. [DOI] [PubMed] [Google Scholar]
- 236.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brenner DJ, Elliston CD. The potential impact of bystander effects on radiation risks in a Mars mission. Radiat Res. 2001;156:612–617. doi: 10.1667/0033-7587(2001)156[0612:tpiobe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 238.Barendsen GW. Differences between biological effects of high LET and low LET radiations in relation to their application in radiotherapy. Radiol Clin (Basel) 1977;46:380–389. [PubMed] [Google Scholar]
- 239.Brooks AL. Chromosome damage in liver cells from low dose rate alpha, beta, and gamma irradiation: Derivation of RBE. Science. 1975;190:1090–1092. doi: 10.1126/science.1188384. [DOI] [PubMed] [Google Scholar]
- 240.Cucinotta FA, Wu H, Shavers MR, George K. Radiation dosimetry and biophysical models of space radiation effects. Gravit Space Biol Bull. 2003;16:11–18. [PubMed] [Google Scholar]
- 241.Meesungnoen J, Benrahmoune M, Filali-Mouhim A, Mankhetkorn S, Jay-Gerin JP. Monte Carlo calculation of the primary radical and molecular yields of liquid water radiolysis in the linear energy transfer range 0.3-6.5 keV/um: application to 137Cs gamma rays. Radiat Res. 2001;155:269–278. doi: 10.1667/0033-7587(2001)155[0269:mccotp]2.0.co;2. Erratum Published in Radiat Res 155 (2001) 2873. [DOI] [PubMed] [Google Scholar]
- 242.Poulose SM, Bielinski DF, Carrihill-Knoll K, Rabin BM, Shukitt-Hale B. Exposure to 16O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat Res. 2011;176:761–769. doi: 10.1667/rr2605.1. [DOI] [PubMed] [Google Scholar]
- 243.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 244.Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brocard JB, Rintoul GL, Reynolds IJ. New perspectives on mitochondrial morphology in cell function. Biol Cell. 2003;95:239–242. doi: 10.1016/s0248-4900(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 246.Griparic L, van der Bliek AM. The many shapes of mitochondrial membranes. Traffic. 2001;2:235–244. doi: 10.1034/j.1600-0854.2001.1r008.x. [DOI] [PubMed] [Google Scholar]