Abstract
Object
To evaluate the utility of a temporally-extended Signal Space Separation algorithm (tSSS) for patients with vagal nerve stimulator (VNS)
Methods
We evaluated median nerve somatosensory evoked responses (SER) of magnetoencephalography (MEG) in 27 VNS patients (48 sides) with/without tSSS processing. We classified SER dipoles as ‘acceptable’ if: A) the location of the dipole was in the expected location in the central sulcus, and B) the Goodness Of Fit value (GOF) was greater than 80%. We evaluated 1) the number of sides which produced acceptable dipoles in each dataset (i.e. with/without tSSS processing), and in cases where the both data produced reliable dipoles, 2) compared their GOFs and the 95% Confidence Volumes (CV) (mm3). Statistical differences in the GOF and CV between with/without tSSS conditions were determined by paired t test.
Results
Only 11 (23%) responses had reliable dipoles without tSSS processing, while all 48 (100%) had acceptable dipoles under tSSS processing. Additionally, the latter group had significantly higher GOF (increased by 7% on average) and lower CV (mean decrease of 200 mm3) than the former (p < 0.01).
Conclusion
Processing with tSSS quantitatively improves dipole fitting of known sources in VNS patients.
Significance
This algorithm permits satisfactory MEG testing in the relatively commonly encountered epilepsy patient with VNS.
Keywords: a temporally-extended Signal Space Separation algorithm, vagal nerve stimulator, magnetoencephalography
INTRODUCTION
Vagal Nerve Stimulation, an option for treatment of epilepsy often used in intractable cases, has shown benefit in both focal and generalized epilepsy (Ben-Menachem et al., 1994). Vagal nerve stimulators (VNS) are implanted in the chest, and the stimulation electrodes are threaded up into the neck, where they are wrapped around the vagus nerve.
Magnetoencephalography (MEG) is a noninvasive technique, which is most commonly used to estimate the sources inside the brain from extracranial recordings of magnetic fields. The MEG signal is, however, easily obscured by magnetic noise produced by electrical devices, particularly VNS (Tanaka et al., 2009). Even when the VNS stimulator is switched off, the magnetic noise produced by movement of the metallic components of the VNS affect the MEG recording. Because of this known magnetic artifact, many centers do not permit epilepsy patient with VNS to undergo MEG testing (Donahue et al., 2007). Despite the likelihood of obtaining clinically significant information, these centers have apparently been unable to obtain satisfactory diagnostic results from their MEG recordings.
Recent advances in signal processing have improved our ability to differentiate and remove noise from brain signal. In particular, the temporally-extended signal space separation (SSS) algorithm (tSSS) is much better at removing noise that comes from close to the sensor array than the conventionally available SSS algorithms (Taulu and Simola, 2006). The advantages of tSSS are especially beneficial in VNS patients, because the close proximity to the sensor array of the vagus nerve stimulating electrode in the left neck generates relatively high amplitude magnetic noise (Song et al., 2009).
The usefulness of tSSS in cases with VNS has thus far been reported in only a small number of patients. Tanaka et al. reported on two epilepsy cases with VNS in whom tSSS successfully eliminated magnetic noise, enabling spike dipole localization that was concordant with either the intracranial electrode finding or clinical profile (Tanaka et al., 2009). Song et al. showed that spontaneous recordings of spike activity from 4 cases with VNS, as well as auditory and somatosensory evoked magnetic responses (AER, SER) were successful only after applying tSSS (Song et al., 2009). The most recent study, reported by Carette et al., demonstrated the contribution of this algorithm to patient management by enabling the delineation of the epileptogenic zone in 6 of 10 epilepsy patients with VNS (Carrette et al., 2011).
However, there is thus far no study with relatively large numbers of patients that provides direct evidence of the usefulness of tSSS in MEG recording in cases with VNS. At our institution,Jin et al. (2008) investigated the benefit of tSSS on spontaneous MEG, and demonstrated that acceptable dipole localization was possible without tSSS in only 3 out of 12 consecutive patients who had clusters of interictal sources. The 9 patients requiring tSSS processing to obtain adequate results included all (4) of the patients with VNS. In this new study reported here, we evaluated SER in 27 cases (48 sides) with VNS by comparing both data with/without tSSS processing, in an effort to determine how tSSS contributed to improvement of signal quality in VNS cases.
METHODS
Patients
Of 300 patients who underwent MEG recording for presurgical evaluation in our laboratory from 2008 to 2011, we evaluated median nerve SERs in 48 sides of 27 intractable epilepsy patients who had vagal nerve stimulators with the stimulating wire attached to the left vagal nerve. In 21 patients, we carried out the ordinary bilateral evaluation, while in 6 only unilateral evaluations were done for various technical reasons (e.g. I Vs, spasticity, etc). Table 1 shows the clinical profiles of these 27 cases, including epilepsy classification, etiology, and MRI findings. Eleven of the 27 cases had an etiological diagnosis, and ten cases had an MRI-visible abnormality. The study was approved by the Institutional Review Board of the Cleveland Clinic Foundation. In all cases, the VNS was turned off prior to MEG SER recording, then turned back on upon departure from the MEG lab.
Table 1.
Clinical profiles of the 27 cases.
| Case | Sex | Sz onset (age) |
Epilepsy Classification | Etiology | MRI |
|---|---|---|---|---|---|
| 1 | F | 17 | R Hemispheric | MCD | Cystic encephalomalacia (R Frontal) |
| 2 | M | 8 | L Perisylvian | Unknown | Normal |
| 3 | F | 11 | L or R Hemispheric | Unknown | Normal |
| 4 | M | 2 | L Fronto-Central | Unknown | Normal |
| 5 | F | 21 | L Temporal | Unknown | Normal |
| 6 | M | 6mo | L Hemispheric | Unknown | Normal |
| 7 | M | 4 | R Frontal | MCD, probable | T2 high (R posterior cingulate ) |
| 8 | F | 49 | L Temporal | Unknown | Normal |
| 9 | F | 3 | R Occipital | MCD | Abnormal sulcation (R Occipital) |
| 10 | F | 7 | L Hemispheric | MCD,Probable | L hemicranial atrophy |
| 11 | M | 14 | R Parietal | Neoplasm | Scar related to DNET resection (R Parietal) |
| 12 | F | 5 | L Hemispheric | Unknown | Abnormal sulcation (L Frontal) |
| 13 | M | 8 | Multiregional | Tuberous sclerosis | Sub-ependymal nodule (L ventricle) |
| 14 | M | 14 | R Fronto-Temporal | Unknown | Normal |
| 15 | M | 14 | R Hemispheric | Unknown | Normal |
| 16 | F | 4 | R Hemispheric | MCD, probable | Malformation (R hemisphere) |
| 17 | F | 4mo | Generalized | Unknown | Normal |
| 18 | M | 3 | R Temporal | Unknown | Normal |
| 19 | F | 11 | L Hemispheric | Unknown | Normal |
| 20 | F | 17 | L & R Temporal | Unknown | Normal |
| 21 | F | 8 | Multiregional | Viral encephalitis | Normal |
| 22 | F | 9 | L & R Parieto-Occipital | Unknown | Normal |
| 23 | F | 16 | L Temporal | Viral encephalitis | Normal |
| 24 | M | 8 | R Hemispheric | Unknown | Normal |
| 25 | M | 4 | L Fronto-Temporal | MCD | Polymicrogyria (R>L) |
| 26 | F | 50 | Frontal | Trauma | Normal |
| 27 | F | 51 | Bi Fronto-Temporal | Unknown | L thalamic infarction |
Abbreviations: MCD, Malformation of cortical development; DNET, dysembryoplastic neuroepithelial tumors
MEG recording and analysis
SER were measured in a magnetically shielded room using a whole-head MEG system with 204 spatial-type gradiometers (Elekta Ltd, Helsinki, Finland). Because it interferes with artifact detection and trial rejection, continuous head position monitoring was not employed during these short SER recordings. In addition, the vendor’s closed-loop real-time noise cancellation (“MaxShield”, Elekta Neuromag, Helsinki, Finland) was not used in this study either. The median nerves were stimulated unilaterally using surface electrodes by monophasic square-wave pulses of 2 ms duration at 2.6 Hz. SER signals were acquired continuously at a sampling rate of 1000 Hz and bandpass filtered between 0.1 and 333 Hz. The acquired MEG data was analyzed after subsequent digital filtering between 5 and 100 Hz. The tSSS processing was carried out by a commercial software package provided by the MEG vendor, “MaxFilter” (Elekta Neuromag, 2004). Recorded responses were averaged from the raw data off-line in two batches: 1) with no additional processing, and 2) after tSSS processing (Song et al., 2009). For the tSSS processing, a 4-second time window and a subspace correlation limit of 0.9 were applied. Artifact-rejection thresholds were set at the lowest of 3000, 5000, or 10000 fT/cm that produced about 200 acceptable trials; in most cases a threshold of 3000 fT/cm was used.
All SERs were analyzed under two conditions: 1) with tSSS processing and 2) without tSSS processing but with standard signal space projection (SSP, Tesche et al. 1995). I.e. each recording was analyzed twice independently, and we then compared the results of the two conditions. The initial baseline noise level of the MEG signal was chosen from the prestimulus period, over the range from -50 to -5 msec. Prominent peaks were visually identified approximately 20 ms after median nerve stimulation; in the very rare cases with weak response, we instead used the second peak of the response (Allison et al., 1989). Equivalent current dipole sources were estimated individually using a single dipole source model (Hämäläinen et al., 1993) with the head modeled as a sphere for the forward calculation of the measured magnetic fields (Hämäläinen et al., 1993). SERs were identified over the contralateral primary somatosenory cortex by selecting 48 gradiometer sensors around the locus of the maximum response (Airaksinen et al., 2011). The estimated dipoles were superimposed onto the patient’s MR images using the vendor’s coregistration procedure.
‘Acceptable’ dipoles were defined based on the following criteria: 1) the dipole is estimated on the central sulcus of the hemisphere contralateral to stimulation, and 2) its goodness of fit value (GOF) is over 80% (Airaksinen et al., 2011). The results were evaluated in two ways:
Study 1: We measured the number of responses (sides) which showed an acceptable dipole in each data set with/without tSSS processing. In order to eliminate the possibility that the left-hemispheric responses (i.e. the VNS side) were poorer in the non-tSSS processed data simply due to their proximity to the VNS, we looked at the percentage of cases in which either or both hemispheres had acceptable results.
Study 2: For SERs where the dipoles were deemed acceptable in study 1 under both the with and without tSSS conditions, the quality of the dipoles was compared.
Statistical analysis
We compared the value of GOF and the 95% confidence limit of the volume (CV) (mm3) to quantitatively evaluate the performance of the tSSS algorithm. Statistical differences in the GOF and CV under the with/without tSSS conditions were tested by paired t test, and. p values less than 0.05 were considered significant.
RESULTS
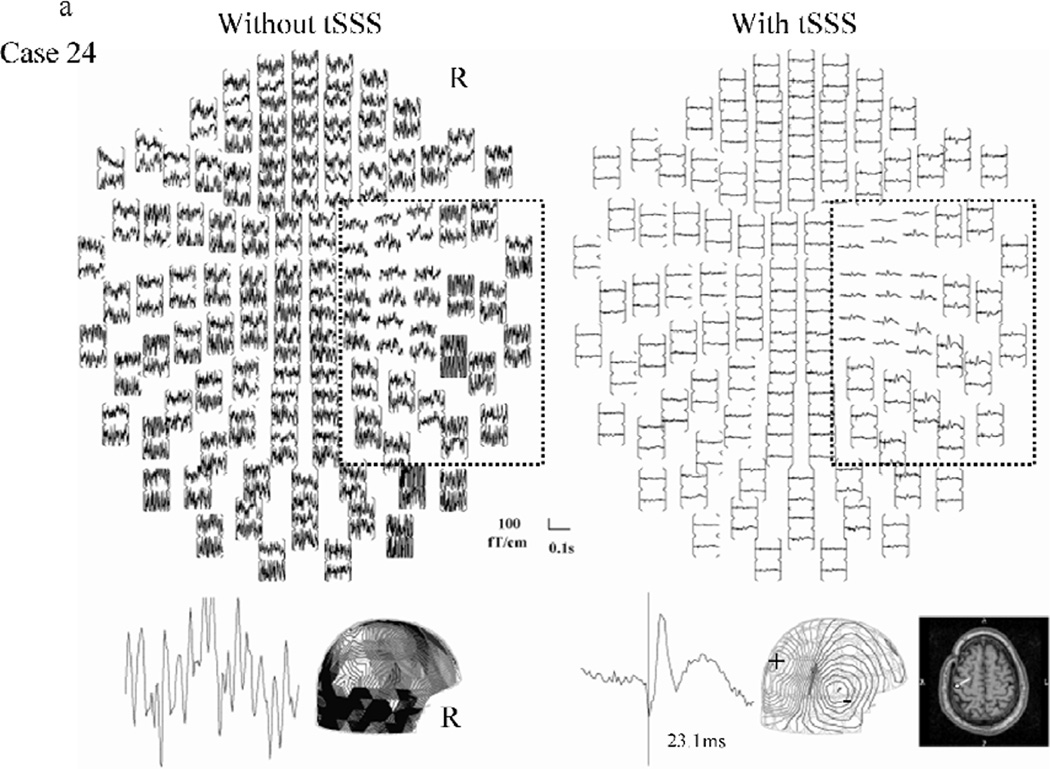
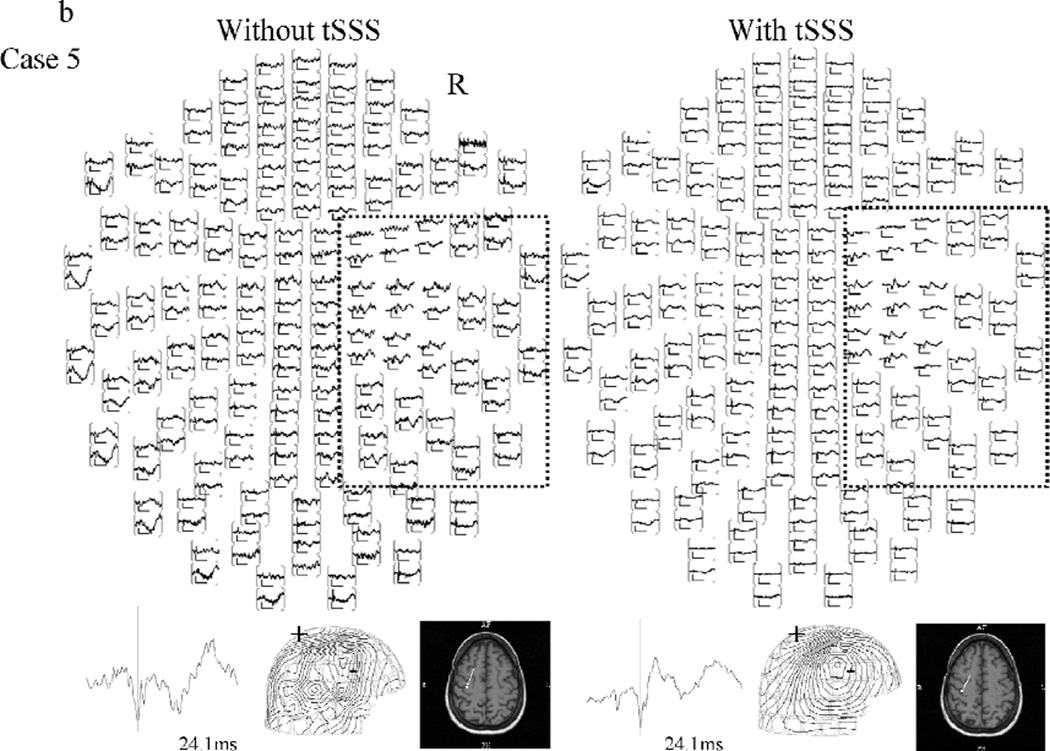
Figure 1 shows representative SERs to left median nerve stimulation (right hemispheric response) from two patients, with/without tSSS processing. Figure 1a, from case 24, illustrates a dramatic reduction in noise. The upper left panel of fig 1a shows that the data was initially overwhelmed by magnetic noise, making source analysis impossible. In figure 1b, taken from case 5, the visual reduction of noise is modest, but the dipole quality is improved with tSSS. There is little difference in the appearance of figure 1b’s left hand and right hand full-view waveforms; the right hemispheric response and the associated dipole localization on the central sulcus can be seen in both. However, the high amplitude, low frequency artifact can be clearly seen in the posterior quadrant channels of the left panel. The contour map of the response after tSSS showed a clearer pattern than that without tSSS, as seen in 1b, lower right panel, The type of analysis illustrated in Figure 1 was performed for all 48 sides in all 27 patients. In one case (25) the second peak of the right hemispheric SER response (left side stimulation) was analyzed because the first peak of the response did not show a clear field pattern.
Figure 1.
Representative right hemisphere somatosensory evoked response to median nerve stimulation without (left) and with (right) tSSS processing. Examples from cases 24 (a: high noise) and 5 (b: lower noise) are shown.
Top view image of evoked responses (upper) over all 204 sensors, representative waveform (lower left), contour map and, if available, its dipole superimposed on the patient MRI (lower right) are shown. The area outlined on the top view image indicates the region of interest covering the right central sulcus where the response is expected to come from. On the contour map, arrows indicate the estimated dipole. The (+) indicates magnetic field efflux, and the (-) indicates magnetic field influx relative to the scalp surface.
Notice that in the left-hand image of 1a without tSSS, noise overwhelms most of the signal and no valid response could be obtained. On the right-hand side, the application of tSSS eliminated the magnetic noise and produced a clear response. The dipole source was successfully estimated on the posterior bank of the central sulcus. In fig 1b, both of the images without (left-hand) and with (right-hand) tSSS showed a response in the right hemisphere, demonstrating that even when tSSS is not critical, no corruption of the signal occurs. In this case, the dipole sources are estimated on central sulcus in either circumstance, but the contour map of the response with tSSS showed a clearer pattern than that without tSSS.
The two examples in this figure demonstrate that the tSSS algorithm not only reduces noise so that otherwise undetectable SERs can be identified, but also improves dipole fitting quantitatively.
When comparing the number of patients who showed reliable dipole estimation under the two conditions, we found that only 11 (23%) responses had acceptable dipoles without tSSS processing (such as the example in figure 1b), while all 48 (100%) had acceptable dipoles after the application of tSSS (Table 2).
Table 2.
MEG results of the 27 cases.
| SER of Left MN* |
SER of Right MN* |
||||
|---|---|---|---|---|---|
| Case | Age of MEG (y.o.) | without tSSS |
with tSSS |
without tSSS |
with tSSS |
| 1 | 34 | A | A | A | A |
| 2 | 20 | A | A | A | A |
| 3 | 63 | A | A | A | A |
| 4 | 16 | - | - | A | A |
| 5 | 45 | A | A | na | A |
| 6 | 15 | A | A | na | A |
| 7 | 19 | na | A | A | A |
| 8 | 49 | A | A | na | A |
| 9 | 33 | na | A | na | A |
| 10 | 10 | na | A | na | A |
| 11 | 30 | na | A | na | A |
| 12 | 27 | na | A | na | A |
| 13 | 11 | na | A | na | A |
| 14 | 47 | na | A | na | A |
| 15 | 22 | na | A | na | A |
| 16 | 17 | na | A | na | A |
| 17 | 19 | na | A | na | A |
| 18 | 26 | na | A | na | A |
| 19 | 14 | na | A | na | A |
| 20 | 43 | - | - | na | A |
| 21 | 13 | - | - | na | A |
| 22 | 25 | - | - | na | A |
| 23 | 27 | - | - | na | A |
| 24 | 44 | na | A | - | - |
| 25 | 61 | na | A | na | A |
| 26 | 50 | na | A | na | A |
| 27 | 51 | na | A | na | A |
| # of sides | 22 | 26 | |||
| # of acceptable | 6 | 22 | 5 | 26 | |
| (vs not acceptable) | (16) | (0) | (21) | (0) | |
Studies shaded in gray were those eligible to be carried over to study 2 (see table 3).
Abbreviations: A, acceptable; MN, median nerve stimulation; na, not acceptable; SER, somatosensory evoked responses; tSSS, temporally-extended signal space separation algorithm
When analysis was performed without tSSS on the 21 patients who had bilateral SER, there were four patients (19%) whose results were different for the two sides (3 had acceptable response in the right hemisphere only and one in the left hemisphere only). However, the majority (17) demonstrated no left-right difference (3 had acceptable responses bilaterally, 14 had no acceptable response bilaterally)
There were 11 hemispheric responses where we could compare the dipole estimation parameters under both conditions. The condition with tSSS had significantly higher GOF and lower CV than without tSSS (p < 0.01) as shown in Table 3.
Table 3.
Comparison of dipole estimation parameters under with/without tSSS (temporally-extended signal space separation algorithm) conditions.
| Goodness of fit (%) |
Confidence volume (mm3) |
||||
|---|---|---|---|---|---|
| Case | side | Without tSSS |
with tSSS | without tSSS |
with tSSS |
| 1 | L | 90.6 | 90.7 | 169.0 | 85.3 |
| R | 83.5 | 96.2 | 255.3 | 13.2 | |
| 2 | L | 90.4 | 96.1 | 200.0 | 55.7 |
| R | 93.0 | 98.3 | 46.0 | 19.2 | |
| 3 | L | 92.2 | 95.1 | 412.9 | 59.9 |
| R | 80.6 | 86.7 | 510.0 | 141.6 | |
| 4 | R | 82.9 | 94.8 | 495.7 | 312.3 |
| 5 | L | 80.5 | 93.8 | 453.5 | 206.3 |
| 6 | L | 92.9 | 98.9 | 20.2 | 4.3 |
| 7 | R | 80.5 | 86.7 | 440.4 | 62.4 |
| 8 | L | 92.6 | 97.3 | 170.8 | 11.3 |
| mean | 87.2 | 94.1 | 288.5 | 88.3 | |
| SD | 5.5 | 4.3 | 180.5 | 96.4 | |
| p value | p<0.01 | p<0.01 | |||
DISCUSSION
This is the first MEG study to test the usefulness of the tSSS algorithm in a relatively large number of epilepsy patients with VNS. We chose to test this algorithm’s effect on a signal with a known morphology and location, i.e. the averaged SER to median nerve stimulation. The present results are in accordance with previous small case series showing the possible utility of the algorithm for noise reduction due to VNS in spontaneous recordings (Tanaka et al, 2009; Song et al., 2009), and by application to SERs provides stronger evidence and statistical significance. This result should allow and encourage MEG evaluation of patients with VNS. VNS patients are a population in whom MEG recordings were previously difficult, and they belong to a class of refractory epilepsy patients who, after careful localization with MEG, can be considered for epilepsy surgery.
Of course magnetic interference from implanted devices is not the sole obstacle to MEG recordings of adequate fidelity. The presence or absence of an evoked response can be affected by various factors, including the customary dependence on the amplitude and number of stimuli. Our stimulation methodology employed standard settings for median nerve stimulation (Iwasaki et al, 2001; Burgess et al, 2011). The fact that for all of the SERs processed by the tSSS algorithm the responses generated an ‘acceptable’ dipole suggests that the stimulation settings were appropriate. In one dataset (right hemispheric response in case 25) we were forced to perform dipole estimation on the second component because the first component did not show a clear field pattern. Unilateral MEG somatosensory evoked responses are not that uncommon. Ishitobi et al. reported that in unilateral polymicrogyria (Ishitobi et al, 2005) the response of the affected side can show an abnormal response or an inappropriate location of the SEF dipole. We speculate that in case 25 perhaps our inability to fit the cortical source in response to left median nerve stimulation at the first peak may relate to the underlying cortical abnormality.
It has long been recognized that many MEG recordings, not only for evoked responses but also for detection of interictal spikes, and especially in epileptic patients with VNS devices, are contaminated by large amounts of noise (Tanaka et al, 2009; Song et al., 2009, Carrette et al, 2011). Nevertheless, 23 % of the sides in our study had acceptable dipoles --- even without the application of tSSS. The variability of noise contamination could be related to the smaller size of other studies, which may by chance have included mostly magnetically-noisy cases. One might presume that the responses from the left hemisphere, i.e. the side of VNS implantation, would be much more affected by magnetic noise because of the closer proximity of the cortical source to the implanted device. However, as shown in our study, a substantial majority of the cases (over 80%) showed no difference between sides. This result suggests that although VNS does produce significant magnetic noise, the interference would be widespread and the effect is generally not much worse on the implanted side.
Beyond the known variability in evoked responses, the cause of additional variability in signal to noise ratio between cases in this study cannot be entirely known. Since continuous head position monitoring was not employed, some head movement during recording may have decreased the consistency of the responses. Although the “empty room” noise measurements just before each recording showed consistently low average levels (approximately 2.5 – 2.8 fT), the level of external environmental noise during recordings may also have varied.
It is important to note that the tSSS algorithm not only reduces noise so that otherwise undetectable SERs can be identified, but also improves dipole fitting quantitatively. This contribution of the algorithm to improvement in dipole quality in SER has not been clear in previous papers. Part of the reason, again probably related to study size, is that very few of the previously reported cases showed clear responses without the application of tSSS, precluding a direct quality comparison between the dipole fits with and without tSSS. In conclusion, the tSSS algorithm permits satisfactory MEG testing in the relatively commonly encountered epilepsy patient with an implanted VNS.
Highlights.
-
◦
This is the first MEG study to test the usefulness of the temporally-extended Signal Space Separation algorithm (tSSS) algorithm in a relatively large number of epilepsy patients with vagal nerve stimulator (VNS).
-
◦
The tSSS algorithm not only reduces noise so that otherwise undetectable somatosensory evoked responses (SERs) can be identified, but also improves dipole fitting quantitatively.
-
◦
The algorithm permits satisfactory MEG testing in the relatively commonly encountered epilepsy patient with an implanted VNS.
ACKNOWLEDGMENT
This work was supported in part by the National Institutes of Health under grants DP2-OD006469, R01-EB009048, R01-NS074980, and by the Epilepsy Center of the Cleveland Clinic Neurological Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
No financial interest is involved in the publication of this manuscript.
REFERENCES
- Airaksinen K, Mäkelä JP, Taulu S, Ahonen A, Nurminen J, Schnitzler A, et al. Effects of DBS on auditory and somatosensory processing in Parkinson's disease. Hum Brain Mapp. 2011;32:1091–1099. doi: 10.1002/hbm.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol. 1989;62:694–710. doi: 10.1152/jn.1989.62.3.694. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–626. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Burgess RC, Funke ME, Bowyer SM, Lewine JD, Kirsch HE, Bagic AI. American Clinical Magnetoencephalography Society Clinical Practice Guideline 2: Presurgical Functional Brain Mapping Using Magnetic Evoked Fields. J Clin Neurophysiol. 2011;28:355–361. doi: 10.1097/WNP.0b013e3182272ffe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrette E, De Tiège X, Op De Beeck M, De Herdt V, Meurs A, Legros B, et al. Magnetoencephalography in epilepsy patients carrying a vagus nerve stimulator. Epilepsy Res. 2011;93:44–52. doi: 10.1016/j.eplepsyres.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Donahue D, Sanchez R, Hernandez A, Malik S, Black CT, Honeycutt J. Preservation of a subcutaneous pocket for vagus nerve stimulation pulse generator during magnetoencephalography. Technical note. J Neurosurg. 2007;107(6 Suppl):519–520. doi: 10.3171/PED-07/12/519. [DOI] [PubMed] [Google Scholar]
- Elekta Neuromag. Source Modeling Software User’s Guide. 2004. [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography–theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–497. [Google Scholar]
- Ishitobi M, Nakasato N, Yoshimoto T, Iinuma K. Abnormal primary somatosensory function in unilateral polymicrogyria: an MEG study. Brain Dev. 2005;27:22–29. doi: 10.1016/j.braindev.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Nakasato N, Kanno A, Hatanaka K, Nagamatsu K, Nagamine Y, et al. Somatosensory evoked fields in comatose survivors after severe traumatic brain injury. Clin Neurophysiol. 2001;112:205–211. doi: 10.1016/s1388-2457(00)00506-x. [DOI] [PubMed] [Google Scholar]
- Jin K, Burgess RC, Alexopoulos AV, Mosher JC. Clinical application of spatiotemporal signal space separation ( tSSS ) method for neuromagnetic recordings of epilepsy patients. Epilepsia. 2008;49(Suppl 7):199–200. [Google Scholar]
- Song T, Cui L, Gaa K, Feffer L, Taulu S, Lee RR, et al. Signal space separation algorithm and its application on suppressing artifacts caused by vagus nerve stimulation for magnetoencephalography recordings. J Clin Neurophysiol. 2009;26:392–400. doi: 10.1097/WNP.0b013e3181c29896. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Thiele EA, Madsen JR, Bourgeois BF, Stufflebeam SM. Magnetoencephalographic analysis in patients with vagus nerve stimulator. Pediatr Neurol. 2009;41:383–387. doi: 10.1016/j.pediatrneurol.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Uusitalo MA, Ilmoniemi RJ, Huotilainen M, Kajola M, Salonen O. Signalspace projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol. 1995;95:189–200. doi: 10.1016/0013-4694(95)00064-6. [DOI] [PubMed] [Google Scholar]