Abstract
Recently we described an ischemic preconditioning induced by repetitive coronary stenosis, which is induced by 6 episodes of non-lethal ischemia over 3 days, and which also resembles the hibernating myocardium phenotype. When compared with traditional second window of ischemic preconditioning using DNA microarrays, many genes which differed in the repetitive coronary stenosis appeared targeted to metabolism. Accordingly, the goal of this study was to provide a more in depth analysis of changes in metabolism in the different models of delayed preconditioning, i.e., second window and repetitive coronary stenosis. This was accomplished using a metabolomic approach based on liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) techniques. Myocardial samples from the ischemic section of porcine hearts subjected to both models of late preconditioning were compared against sham controls. Interestingly, although both models involve delayed preconditioning, their metabolic signatures were radically different; of the total number of metabolites that changed in both models (135 metabolites) only 7 changed in both models, and significantly more, p<0.01, were altered in the repetitive coronary stenosis (40%) than in the second window (8.1%). The most significant changes observed were in energy metabolism, e.g., phosphocreatine was increased 4 fold and creatine kinase activity increased by 27.2%, a pattern opposite from heart failure, suggesting that the repetitive coronary stenosis and potentially hibernating myocardium have enhanced stress resistance capabilities. The improved energy metabolism could also be a key mechanism contributing to the cardioprotection observed in the repetitive coronary stenosis and in hibernating myocardium.
Keywords: metabolomics, delayed cardiac ischemic preconditioning
1. Introduction
Delayed ischemic preconditioning; the second window of ischemic preconditioning, described 19 years ago, involves one or several ischemic episodes on one day leading to ischemic protection 1-2 days later [1, 2]. More recently another model of delayed preconditioning induced by 6 episodes of 90 minutes of coronary stenosis every 12 hours for 3 days, was described and referred to as repetitive coronary stenosis [3, 4]. In both models coronary blood flow is normal as is global left ventricular (LV) function, however in the repetitive coronary stenosis, contractile function in the previously ischemic zone remains depressed due to myocardial stunning [5]. The repetitive coronary stenosis resembles hibernating myocardium in many ways, including an adaptative decrease in myocardial oxygen consumption and contractile power [3, 6]. Repetitive coronary stenosis induces as robust cardioprotection as the second window in terms of infarct size reduction, but their underlying molecular mechanisms are substantially different [4, 7].
When these two models of delayed preconditioning were compared using cDNA microarray techniques, it was apparent that many of the genes that changed, particularly in repetitive coronary stenosis, were related to myocardial energy metabolism [4, 7]. However, despite the potential importance of this topic and mechanisms for mediating cardiac protection, relatively little has been investigated in delayed preconditioning, with almost no prior studies examining how preconditioning can alter baseline metabolism, even prior to an ischemic intervention. In order to provide an in depth analysis of changes in metabolism in these models of delayed preconditioning, we employed a comprehensive metabolomic approach, which is based on technology that utilizes liquid chromatography–tandem mass spectrometry (LC/MS/MS2) and gas chromatography–mass spectrometry (GC/MS) platforms; allowing for an untargeted assessment of the concentration of small compounds as metabolites through a wide range of biochemical pathways. Of specific interest was to determine novel mechanisms that could protect preconditioned hearts from superimposed stress, including myocardial ischemia. We compared a well-established chronically instrumented swine model of the second window of ischemic preconditioning [8] with a more novel model of delayed ischemic preconditioning induced by repetitive coronary stenosis [3, 4, 7]; both models induce marked cardioprotection following a more prolonged ischemic insult which results in myocardial infarction [4, 7].
Myocardial samples were obtained in the preconditioned state in order to assess the myocardial metabolomic changes that are associated with the two forms of delayed ischemic preconditioning.
2. Materials and methods
2.1. Animal models
All protocols concerning animal use were approved by the Institutional Animal Care and Use Committee at the New Jersey Medical School. All of the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
Thirty female 3.1 month old Yorkshire pigs, weighing 26.9±0.9 kg, were randomly distributed into three experimental groups; sham operated (n=10); second window (n=10); and repetitive coronary stenosis (n=10). The myocardial samples were derived from pigs studied in prior publications using the repetitive coronary stenosis model [4, 7].
The second window of ischemic preconditioning and repetitive coronary stenosis were induced in conscious, chronically instrumented pigs after full recovery from instrumentation, as previously described in detail (n=10/group) [4, 7]. Briefly, the second window was induced by a preconditioning stimulus consisting of two episodes of total left anterior descending (LAD) coronary artery occlusion for 10 min and 10 min of coronary artery reperfusion (CAR), followed by 24 hours of CAR. Repetitive coronary artery stenosis (CAS) was induced by inflating the proximal LAD occluder to reduce coronary blood flow approximately 40% from baseline for 90 minutes each 12 hours, 6 times in total (Figure S1).
The animals for the sham group (1 hour after surgery) (n=10), the second window group (24 hours after the preconditioning stimulus), and the repetitive coronary stenosis group (1 hour after the sixth CAS), were euthanized with a mixture of pentobarbital 390 mg/ml + phenytoin 50 mg/ml in a dose of 1 ml each 10 pounds (4.5 kg) and a section of the anterior LV wall from the preconditioned zone and a similar level from the sham group was excised and quickly frozen in liquid nitrogen. In these models of ischemic preconditioning, necrosis is negligible without a superimposed sustained coronary artery occlusion [3, 9, 10].
2.2. Metabolomics measurements
The sample preparation process was carried out using the automated MicroLab STAR® system from the Hamilton Company. Recovery standards were added prior to the first step in the extraction process for quality control purposes. Sample preparation was conducted using a proprietary series of organic and aqueous extractions to remove the protein fraction while allowing maximum recovery of small molecules. The resulting extract was divided into two fractions; one for analysis by liquid chromatography (LC) and one for analysis by gas chromatography (GC). Samples were placed briefly on a TurboVap® (Zymark) to remove the organic solvent. Each sample was then frozen and dried under vacuum.
The analytical platform incorporates two separate ultrahigh-performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS2) injections and one gas chromatography/mass spectrometry (GC/MS) injection per sample. A total of 287 metabolites were measured, spanning several relevant classes. The detection of the entire panel was carried out with 24 minutes of instrument analysis time (two injections at 12 minutes each), while maintaining low median process variability (<12% across all compounds). The resulting MS/MS2 data were searched against a standard library that included retention time, molecular mass to charge ratio (m/z), preferred adducts and in-source fragments, as well as their associated MS/MS2 spectra for all molecules in the library. The library allowed for the identification of the experimentally detected molecules on the basis of a multiparameter match without the need for additional analyses. For the tissue analysis, an equivalent amount of sample from each tissue was loaded on the instruments after adjusting the extraction volumes to the weights of the tissues; therefore, no other normalization process was needed.
For the purpose of bioinformatics analysis, the metabolomic data were classified into clusters of related pathways that share a common metabolite called super-pathways, furthermore these clusters are subdivided by more specific pathways, defined as sub-pathways. For this specific metabolomic analysis, the results were divided into the following seven super-pathways: amino acids, peptides, carbohydrates, energy, lipids, nucleotides, cofactor and vitamins (Table S1).
2.3. Western blotting analysis
Protein expression of creatine kinase was assessed by western blot using commercial anti-creatine kinase (CK) MM and CK-MT antibodies (Abcam) as previously described [11].
2.4. Enzymatic Activity
Total CK was measured using EnzyChrom™ Creatine Kinase Assay Kit (BioAssay Systems). From whole cardiac tissue lysate, 50 ng of protein was analyzed following the instructions of the manufacturer and the enzymatic activity was represented as units per liter (U/L).
2.5. Statistical analysis
ANOVA with Bonferroni post hoc analysis was used to compare among all three experimental groups (Figures 4-6). χ2 two tails was used to compare proportions. A principal component analysis (PCA) to cluster samples based on variance was performed. The data are presented as fold change (FC) with respect to the sham group. For supplemental table 1 (Table S1), Welch's two-sample t-test was used to identify biochemicals that differed significantly between experimental groups.
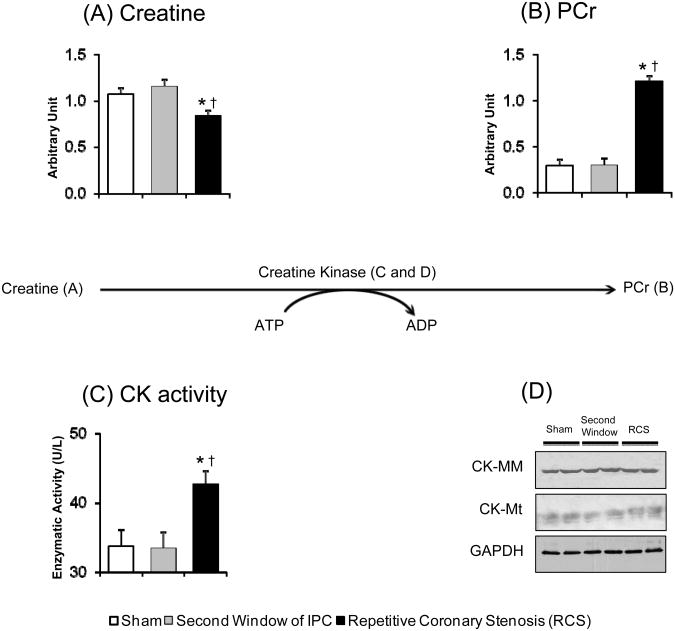
Figure 4.
PCr synthesis pathway. Bar graph shows the concentration of (A) creatine, (B) PCr, (C) the total CK activity and (D) western blotting for CK-MM and CK-MT isoforms. ANOVA and Bonferroni post hoc test: * p<0.05 different from sham, † p<0.05 different from second window of ischemic preconditioning. N=10 pigs per group.
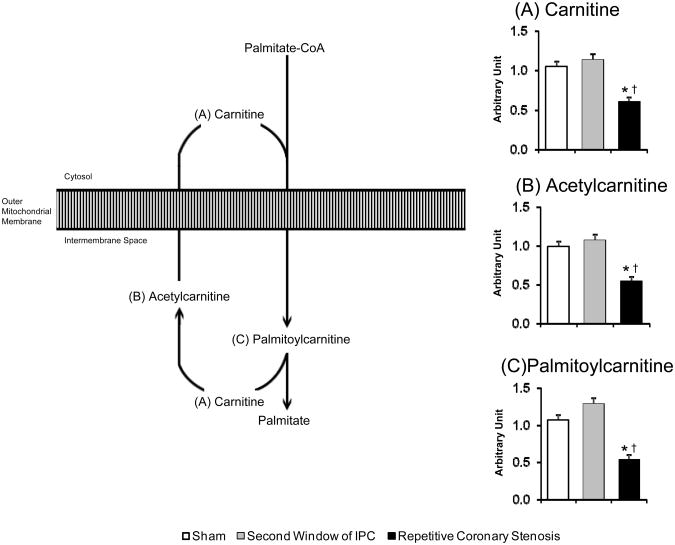
Figure 6.
Mitochondrial fatty acid uptake. Bar graph shows the concentration of (A) carnitine, (B) acetylcarnitine, (C) palmitoylcarnitine. ANOVA and Bonferroni post hoc test: * p<0.05 different from sham, † p<0.05 different from second window of ischemic preconditioning. N=10 pigs per group.
3. Results
3.1. Global metabolomic profile
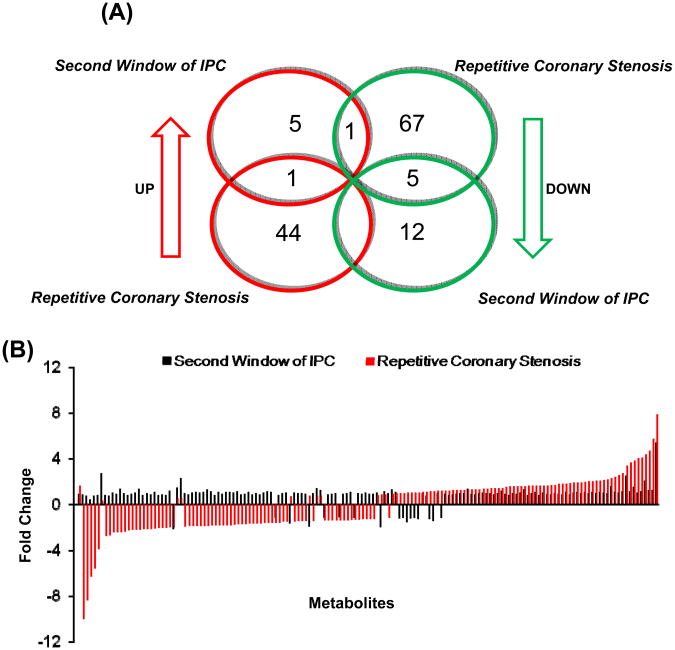
There were major differences in the changes in metabolites in the second window of ischemic preconditioning versus the repetitive coronary stenosis model. Interestingly, although both are models of delayed preconditioning, the total number of metabolites that changed in both models (135 metabolites) only 7 changed in both models. Of these 7, only 1 increased in both models (ophthalmate) whereas 5 decreased in both models (malate, phosphate, methyl palmitate, inosine and vitamin C) and the one that went up in the second window and down with repetitive coronary stenosis was adenosine 5′-diphosphate (Figure 1A and Table S1). More importantly, significantly more metabolites rose with repetitive coronary stenosis (45 metabolites or 15.6%) compared with second window (7 metabolites or 2.4%) p<0.05, and significantly more metabolites fell with repetitive coronary stenosis (73 metabolites or 25.4%) than in the second window (17 metabolites or 5.9%) p<0.05. Furthermore, not only was the frequency of the changes more with repetitive coronary stenosis than in second window (41.1% versus 8.3%; p<0.05), but also the magnitude was greater for those metabolites that increased or decreased with repetitive coronary stenosis (Figure 1B and Table S1). All the metabolites that changed are noted in Table S1.
Figure 1.
(A) Venn diagram shows the number of significantly increased or decreased metabolites in second and sham group. (B) The graph at the bottom shows metabolite fold change over sham group, in both second window of ischemic preconditioning and repetitive coronary stenosis.
3.2. Principal Component Analysis
The PCA clusters variables based on variance. The first principal component accounts for the highest variance and the additional principal components account for lower variance.
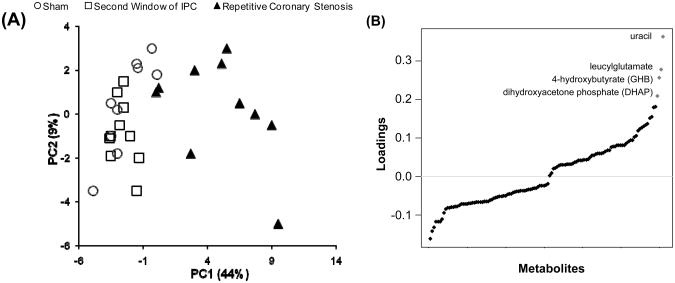
In the present study the first principal component contained 44.4% of the total variance and the second principal component contains metabolites with 9% of the total variance (Figure 2A). Moreover, the PCA showed that there is not a clear separation between the second window and sham groups, indicating that they are similar in terms of variance; however the repetitive coronary stenosis model showed a clear separation from the other groups, indicating that this preconditioning model is different not only from sham, but also from the second window model in terms of variance in metabolites (Figure 2A).
Figure 2.
(A) Score plot of the top two principal components (PCs) from the PCA using 122 metabolites, which showed significant changes among three groups (p<0.05, ANOVA). As indicated, PC1 and PC2 account for 44% and 9% of the total variation, respectively. Sample groups are shown in different symbols. It appears that PC1 can separate repetitive coronary stenosis samples from sham and second window samples. (B) Loading plot for PC1. The metabolites were sorted on the x-axis by their contribution (loading) to PC1. Red dots are metabolites whose value is > 0.2.
Those metabolites that contribute more to the total variance within principal component 1 are: uracil, leucylglutamate, 4-hydroxybutyrate (GHB) and dihydroxyacetone phosphate (DHAP) (Figure 2B).
3.3. Super- and sub-pathways
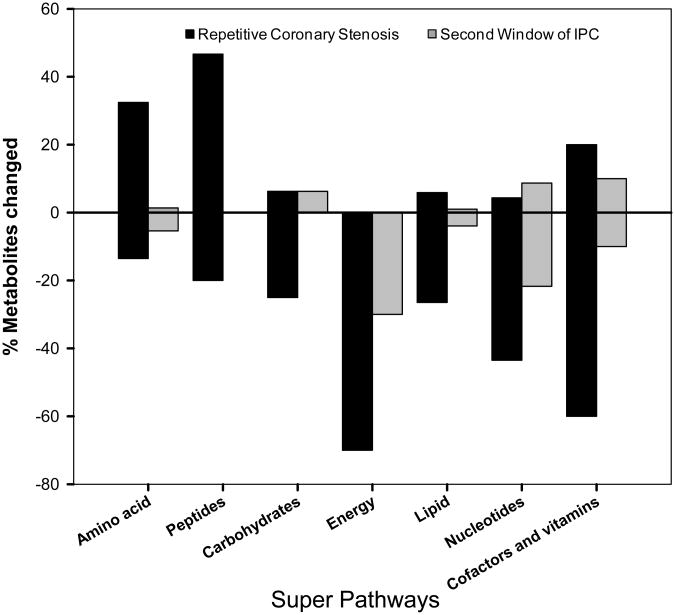
Seven super-pathways were examined, i.e., amino acid, peptides, carbohydrate, energy, lipid, nucleotides and, cofactor and vitamins (Figure 3).
Figure 3.
Bar graph shows the percentage of metabolites significantly changed (up or down) in the super-pathways for amino acid, peptides, carbohydrate, energy, lipid, nucleotides and, cofactor and vitamins. N=10 pigs per group.
In the amino acid super-pathway, like the peptide super-pathway, these metabolites were increased with repetitive coronary stenosis but not in second window, suggesting that the repetitive coronary stenosis induces proteolysis. In the carbohydrate super-pathway, the metabolites were decreased with repetitive coronary stenosis, but not in the second window of preconditioning. A greater decrease was seen in the metabolites related to the energy super-pathway, indicating a decrease in energy transfer with repetitive coronary stenosis. In the lipid super-pathway, the metabolites were decreased more than in the second window suggesting an enhancement in the breakdown of complex lipids with repetitive coronary stenosis. In the nucleotide, cofactor and vitamin super-pathways, the metabolites were significantly decreased with repetitive coronary stenosis, and to a much lesser extent in the second window. In summary, these points reinforce the overall data demonstrating that a greater number of metabolites changed with repetitive coronary stenosis than in the second window, suggesting enhanced metabolic rearrangement (Figure 3).
Within the 7 super-pathways, three sub-pathways were involved in energy transfer in the cardiomyocyte, i.e. creatine metabolism, Krebs cycle and carnitine metabolism which showed changes in more than 60% of the measured metabolites in the repetitive coronary stenosis model, as detailed below.
3.4. Creatine metabolism
Within the creatine metabolic pathway, again there was greater activity with repetitive coronary stenosis compared to the second window. Creatine concentration was significantly decreased, p<0.05 (Figure 4A), while phosphocreatine (PCr), was increased, p<0.05 (Figure 4B). The total CK activity was increased (Figure 4C); with no change in the protein concentration measured by western blot (Figure 4D). Thus, these were major changes present only with repetitive coronary stenosis which suggests improvement in the energetic status of the heart.
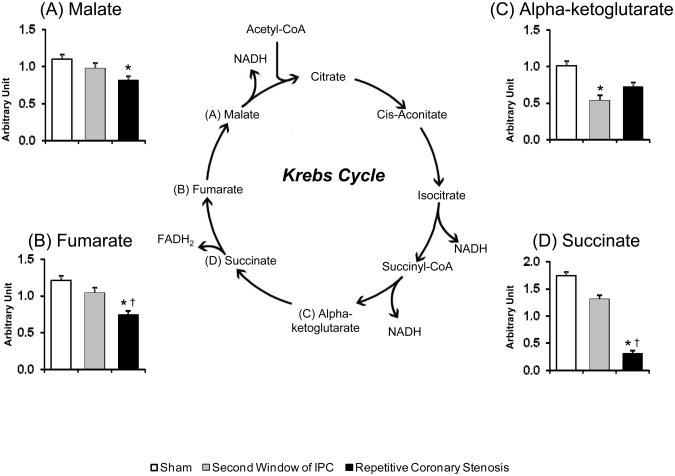
3.5. Krebs cycle
In the energy super-pathway, the repetitive coronary stenosis model showed greater decreases than in the second window in the concentration of metabolites within the Krebs cycle, e.g., malate, succinate and fumarate (p<0.05) (Figure 5A, 5B and 5D). Second window showed a decrease only in alpha-ketoglutarate (Figure 5C).
Figure 5.
Krebs cycle. Bar graph shows the concentration of (A) malate, (B) fumarate and (C) alpha-ketoglutarate and (D) succinate. ANOVA and Bonferroni post hoc test: * p<0.05 different from sham, † p<0.05 different from second window of ischemic preconditioning. N=10 pigs per group.
3.6. Carnitine metabolism
In the lipid metabolism super-pathway, specifically in the carnitine sub pathway, again repetitive coronary stenosis showed a greater change, as reflected by the decrease in the concentrations of carnitine (Figure 6A), acetylcarnitine (Figure 6B) and palmitoylcarnitine (Figure 6C), suggesting that the repetitive coronary stenosis produced a deficiency in mitochondrial fatty acid uptake, due to a decrease in carnitine and carnitine esters that are required for mitochondrial lipid uptake and oxidation.
4. Discussion
There are several models of delayed preconditioning; the second window, described 19 years ago, utilizes similar preconditioning stimuli as in the first window [12], but the ischemic protection reappeared 1-2 days later [1, 2]. Another model was described where the preconditioning was induced by microembolization and the protection was present 6 hours after the stimulus [13, 14]. More recently, our laboratory described another model of delayed preconditioning induced by 6 episodes of 90 min coronary stenosis delivered 12 hours apart, which we refer to as repetitive coronary stenosis [3]. The cardioprotection in the repetitive coronary stenosis model is equivalent to that in the second window [7], but the molecular mechanisms mediating the two are radically different. For example, nitric oxide mediates the second window, but not the repetitive coronary stenosis model [7, 15]. More importantly, the repetitive coronary stenosis model more closely resembles the clinical situation in patients with recurrent ischemic episodes.
Despite the critical link between myocardial metabolism and cardiomyocyte death under conditions of limited coronary blood supply, relatively little has been studied on this topic related to ischemic preconditioning. Except for the demonstration of altered fatty acid metabolism in the second window [8], relatively little else has been found. The data from the current study confirm surprisingly few of the changes in metabolism in the second window of preconditioning, but demonstrate profound alterations in the myocardial metabolomic profile in the repetitive coronary stenosis model. Significantly more metabolites rose with repetitive coronary stenosis (45 metabolites) compared with second window (7 metabolites) and significantly more metabolites fell in the repetitive coronary stenosis model (73 metabolites) than in the second window (17 metabolites). Thus, application of an unbiased metabolomics approach was successful at unveiling novel changes to the myocardial metabolome induced by the repetitive coronary stenosis model.
A major impetus for the current investigation was derived from a prior study using cDNA microarray techniques, which identified a marked disparity in gene regulation induced by the second window of ischemic preconditioning and repetitive coronary stenosis and the finding that many of the altered genes in the repetitive coronary stenosis model related to metabolism [7]. This difference was supported in the current study using a metabolomic approach which verified that repetitive coronary stenosis induced significantly greater effects on metabolism than the second window although both models involve delayed preconditioning. Of the total number of metabolites that changed in both windows (135 metabolites) only 7 changed in both models and significantly more metabolites were upregulated and downregulated in the repetitive coronary stenosis model compared with the second window. Further, the second window showed little difference from sham-operated animals, but the repetitive coronary stenosis model was markedly different from sham. Thus, altogether this supports the concept that the mechanisms mediating the repetitive coronary stenosis model is distinct from those mediating classic second window of ischemic preconditioning.
Changes in amino acids, peptides, carbohydrates, energy, lipid, nucleotide and cofactor super-pathways were observed with repetitive coronary stenosis. The repetitive coronary stenosis model is closely linked to hibernating myocardium [16-19], which also has been shown to have features from short-term and chronic hibernating myocardium [20-22], such as altered regulation of metabolism, e.g., increase in intracellular glycogen deposits, enhancement in glucose uptake and decreases in phosphorylative oxidation, and particularly, changes related to energy status [23-26]. In the repetitive coronary stenosis model there was a significant reduction in metabolites related to the energy super-pathway that involve intermediates from the Krebs cycle and oxidative phosphorylation. These changes suggest a decrease in the capacity for mitochondrial ATP production in the repetitive coronary stenosis model, similar to that observed in hibernating myocardium [23, 25, 26], which would act to protect the myocardium under conditions of reduced blood flow. Important features of the changes in metabolites in the current study related to changes in the Krebs cycle and creatine metabolism, all crucial ingredients for energy metabolism. On the other hand, PCr and CK activity, central to high-energy phosphates in the cardiomyocyte and the transfer of energy from mitochondria to essential cytosolic ATPase, were significantly increased. This pattern is opposite to that which is observed in failing hearts [27, 28], indicating energy reserve in the repetitive coronary stenosis model can be utilized in situations of ischemic stress or increased contractile demand. Previous studies of the repetitive coronary stenosis model and short term hibernating myocardium have shown upregulation of glucose uptake, increases in anaerobic metabolism and optimization of energy utilization [3, 21, 22, 26, 29, 30]. This plus the increase in PCr and total CK activity are likely important for protection of myocardium subjected to ischemia [31-34]. Support from this concept comes from several clinical trials showing beneficial effects of exogenous PCr on the ischemic heart [31-34]. Furthermore, in the current study metabolites involved in the peptide and amino acid super-pathways were observed with repetitive coronary stenosis, suggesting enhanced proteolysis, consistent with chronic myocardial hibernation [20, 21, 35].
The metabolic pattern is the sum of a complex interaction among genome, proteome and environment. Whereas the untargeted assessment of small metabolites in metabolomic studies permits a snapshot of the broad sweep of changes in different metabolic pathways simultaneously, the weakness of this method is that the concentration of metabolites depends on the kinetics of the interaction among the reactants and the measured metabolites, which may not be in a steady state [25]. Nonetheless, the metabolomic approach provides a broad insight into changes in metabolism in any altered state, such as the heart under conditions of preconditioning, which was the focus of the current investigation. Furthermore, although we cannot be certain that the changes are in the cardiomyocytes, keep in mind that cardiomyocytes account about 67-80% of the myocardial volume and 90% of the cardiac mass [36], therefore, we believe that most of the metabolites that we present in this manuscript reflect what is happening in cardiomyocytes. Note that in numerous prior papers in cardiac metabolism [37-39], it is assumed that the changes observed reflect myocyte metabolism.
One may question why the results in the repetitive coronary stenosis model differ from those in the second window: is it the number of preconditioning stimuli? Is it the duration of the preconditioning stimuli? Is it the duration of the preconditioning stimuli, 3 days versus minutes or hours? Is it the persistence of stunned myocardium [3] in the preconditioned hearts following repetitive coronary stenosis? All of these factors may be involved. What is important is that the repetitive coronary stenosis model differs from the more widely studied classical models of preconditioning in that it more closely mimics the clinical situation and that this intervention induces powerful cardioprotection along with profound metabolic changes in the myocardium, which are not observed in other models of preconditioning.
5. Conclusion
Metabolomic analysis demonstrated remarkably few changes with the second window of ischemic preconditioning compared to normal myocardium, but revealed dramatic alteration with repetitive coronary stenosis. This metabolic rearrangement is important for understanding the different mechanisms of cardioprotection in the repetitive coronary stenosis versus second window of ischemic preconditioning, but also to short term and chronic hibernating myocardium, which are closely linked to repetitive coronary stenosis and consequent chronic stunning. The results of the current investigation should provide for further emphasis on how changes in metabolism are critical for ischemic preconditioning, which has previously been largely overlooked in studies of the second window of ischemic preconditioning.
Supplementary Material
Supplemental Figure 1 (Figure S1): All 3 experimental groups are schematically summarized including sham pigs, second window of ischemic preconditioning and repetitive coronary stenosis.
Supplemental Table 1 (Table S1): The values next to each metabolite represent the ratio of the average amount of metabolites in the two groups. Green or red color, indicates significant difference (p<0.05) between the groups using Welch's two-sample t-test.
Acknowledgments
Funding: This study was supported by funding from National Institutes of Health (P01HL069020, RO1HL093481, RO1HL106511, P01AG027211, R01HL102472, R01HL033107, T32HL069752, R01HL095888, R01HL091781, RO1HL093415).
Abbreviation
- CAR
Coronary artery reperfusion
- CAS
Coronary artery stenosis
- CK
Creatine kinase
- GC
Gas chromatography
- IPC
Ischemic preconditioning
- LAD
Left anterior descending
- LC
Liquid chromatography
- LV
Left ventricle
- PCA
Principal component analysis
- PCr
Phosphocreatine
- RCS
Repetitive coronary stenosis
- SWOP
Second window of ischemic preconditioning
Footnotes
Disclosures: No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circulation research. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 2.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, et al. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circulation research. 2003;92:1233–9. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 4.Shen YT, Depre C, Yan L, Park JY, Tian B, Jain K, et al. Repetitive ischemia by coronary stenosis induces a novel window of ischemic preconditioning. Circulation. 2008;118:1961–9. doi: 10.1161/CIRCULATIONAHA.108.788240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. The Journal of clinical investigation. 1975;56:978–85. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, et al. Program of cell survival underlying human and experimental hibernating myocardium. Circulation research. 2004;95:433–40. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 7.Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, et al. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. American journal of physiology Heart and circulatory physiology. 2010;299:H752–62. doi: 10.1152/ajpheart.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudej RK, Fasano M, Zhao X, Lopaschuk GD, Fischer SK, Vatner DE, et al. Second window of preconditioning normalizes palmitate use for oxidation and improves function during low-flow ischaemia. Cardiovascular research. 2011;92:394–400. doi: 10.1093/cvr/cvr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–9. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 10.Geft IL, Fishbein MC, Ninomiya K, Hashida J, Chaux E, Yano J, et al. Intermittent brief periods of ischemia have a cumulative effect and may cause myocardial necrosis. Circulation. 1982;66:1150–3. doi: 10.1161/01.cir.66.6.1150. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Park J, Ho D, Gao S, Yan L, Ge H, et al. Cardiomyocyte overexpression of the alpha1A-adrenergic receptor in the rat phenocopies second but not first window preconditioning. American journal of physiology Heart and circulatory physiology. 2012;302:H1614–24. doi: 10.1152/ajpheart.01072.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 13.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, et al. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circulation research. 2007;100:140–6. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 14.Heusch P, Skyschally A, Leineweber K, Haude M, Erbel R, Heusch G. The interaction of coronary microembolization and ischemic preconditioning: A third window of cardioprotection through TNF-alpha. Arch Med Sci. 2007;3:83–92. [Google Scholar]
- 15.Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, et al. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circulation research. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 16.Shen YT, Kudej RK, Bishop SP, Vatner SF. Inotropic reserve and histological appearance of hibernating myocardium in conscious pigs with ameroid-induced coronary stenosis. Basic research in cardiology. 1996;91:479–85. doi: 10.1007/BF00788729. [DOI] [PubMed] [Google Scholar]
- 17.Shen YT, Vatner SF. Mechanism of impaired myocardial function during progressive coronary stenosis in conscious pigs. Hibernation versus stunning? Circulation research. 1995;76:479–88. doi: 10.1161/01.res.76.3.479. [DOI] [PubMed] [Google Scholar]
- 18.Camici PG, Wijns W, Borgers M, De Silva R, Ferrari R, Knuuti J, et al. Pathophysiological mechanisms of chronic reversible left ventricular dysfunction due to coronary artery disease (hibernating myocardium) Circulation. 1997;96:3205–14. doi: 10.1161/01.cir.96.9.3205. [DOI] [PubMed] [Google Scholar]
- 19.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. The New England journal of medicine. 1998;339:173–81. doi: 10.1056/NEJM199807163390307. [DOI] [PubMed] [Google Scholar]
- 20.Heusch G. Hibernating myocardium. Physiological reviews. 1998;78:1055–85. doi: 10.1152/physrev.1998.78.4.1055. [DOI] [PubMed] [Google Scholar]
- 21.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. American journal of physiology Heart and circulatory physiology. 2005;288:H984–99. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 22.Martin C, Schulz R, Rose J, Heusch G. Inorganic phosphate content and free energy change of ATP hydrolysis in regional short-term hibernating myocardium. Cardiovascular research. 1998;39:318–26. doi: 10.1016/s0008-6363(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 23.Elsasser A, Muller KD, Skwara W, Bode C, Kubler W, Vogt AM. Severe energy deprivation of human hibernating myocardium as possible common pathomechanism of contractile dysfunction, structural degeneration and cell death. Journal of the American College of Cardiology. 2002;39:1189–98. doi: 10.1016/s0735-1097(02)01735-7. [DOI] [PubMed] [Google Scholar]
- 24.Jameel MN, Li Q, Mansoor A, Xiong Q, Swingen C, Zhang J. Long-term preservation of myocardial energetic in chronic hibernating myocardium. American journal of physiology Heart and circulatory physiology. 2011;300:H836–44. doi: 10.1152/ajpheart.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Q, Suzuki G, Young RF, Page BJ, Fallavollita JA, Canty JM., Jr Reductions in mitochondrial O(2) consumption and preservation of high-energy phosphate levels after simulated ischemia in chronic hibernating myocardium. American journal of physiology Heart and circulatory physiology. 2009;297:H223–32. doi: 10.1152/ajpheart.00992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFalls EO, Kelly RF, Hu Q, Mansoor A, Lee J, Kuskowski M, et al. The energetic state within hibernating myocardium is normal during dobutamine despite inhibition of ATP-dependent potassium channel opening with glibenclamide. American journal of physiology Heart and circulatory physiology. 2007;293:H2945–51. doi: 10.1152/ajpheart.00012.2007. [DOI] [PubMed] [Google Scholar]
- 27.Ingwall JS. On the control of metabolic remodeling in mitochondria of the failing heart. Circulation Heart failure. 2009;2:275–7. doi: 10.1161/CIRCHEARTFAILURE.109.885301. [DOI] [PubMed] [Google Scholar]
- 28.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circulation research. 2004;95:135–45. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 29.McFalls EO, Murad B, Liow JS, Gannon MC, Haspel HC, Lange A, et al. Glucose uptake and glycogen levels are increased in pig heart after repetitive ischemia. American journal of physiology Heart and circulatory physiology. 2002;282:H205–11. doi: 10.1152/ajpheart.2002.282.1.H205. [DOI] [PubMed] [Google Scholar]
- 30.Wiggers H, Noreng M, Paulsen PK, Bottcher M, Egeblad H, Nielsen TT, et al. Energy stores and metabolites in chronic reversibly and irreversibly dysfunctional myocardium in humans. Journal of the American College of Cardiology. 2001;37:100–8. doi: 10.1016/s0735-1097(00)01059-7. [DOI] [PubMed] [Google Scholar]
- 31.Stoica SC. High-energy phosphates and the human donor heart. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2004;23:S244–6. doi: 10.1016/j.healun.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Sharov VG, Afonskaya NI, Ruda MY, Cherpachenko NM, Pozin E, Markosyan RA, et al. Protection of ischemic myocardium by exogenous phosphocreatine (neoton): pharmacokinetics of phosphocreatine, reduction of infarct size, stabilization of sarcolemma of ischemic cardiomyocytes, and antithrombotic action. Biochemical medicine and metabolic biology. 1986;35:101–14. doi: 10.1016/0885-4505(86)90064-2. [DOI] [PubMed] [Google Scholar]
- 33.Cisowski M, Bochenek A, Kucewicz E, Wnuk-Wojnar AM, Morawski W, Skalski J, et al. The use of exogenous creatine phosphate for myocardial protection in patients undergoing coronary artery bypass surgery. The Journal of cardiovascular surgery. 1996;37:75–80. [PubMed] [Google Scholar]
- 34.Prabhakar G, Vona-Davis L, Murray D, Lakhani P, Murray G. Phosphocreatine restores high-energy phosphates in ischemic myocardium: implication for off-pump cardiac revascularization. Journal of the American College of Surgeons. 2003;197:786–91. doi: 10.1016/j.jamcollsurg.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Elsasser A, Schaper J. Hibernating myocardium: adaptation or degeneration? Basic research in cardiology. 1995;90:47–8. doi: 10.1007/BF00795119. [DOI] [PubMed] [Google Scholar]
- 36.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 37.Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI, et al. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. Journal of molecular and cellular cardiology. 2009;46:268–77. doi: 10.1016/j.yjmcc.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Souza AI, Cardin S, Wait R, Chung YL, Vijayakumar M, Maguy A, et al. Proteomic and metabolomic analysis of atrial profibrillatory remodelling in congestive heart failure. Journal of molecular and cellular cardiology. 2010;49:851–63. doi: 10.1016/j.yjmcc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Pinz I, Tian R, Belke D, Swanson E, Dillmann W, Ingwall JS. Compromised myocardial energetics in hypertrophied mouse hearts diminish the beneficial effect of overexpressing SERCA2a. The Journal of biological chemistry. 2011;286:10163–8. doi: 10.1074/jbc.M110.210757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 (Figure S1): All 3 experimental groups are schematically summarized including sham pigs, second window of ischemic preconditioning and repetitive coronary stenosis.
Supplemental Table 1 (Table S1): The values next to each metabolite represent the ratio of the average amount of metabolites in the two groups. Green or red color, indicates significant difference (p<0.05) between the groups using Welch's two-sample t-test.