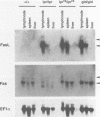
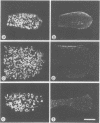
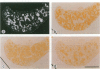
Abstract
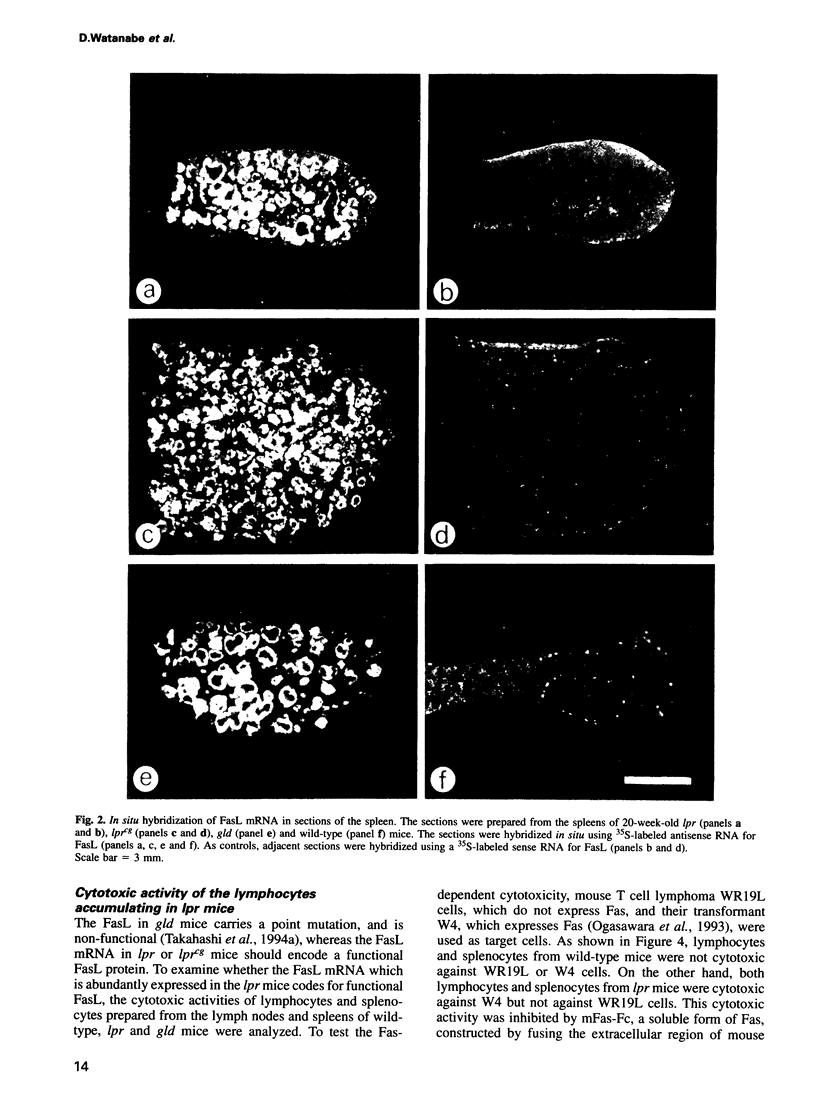
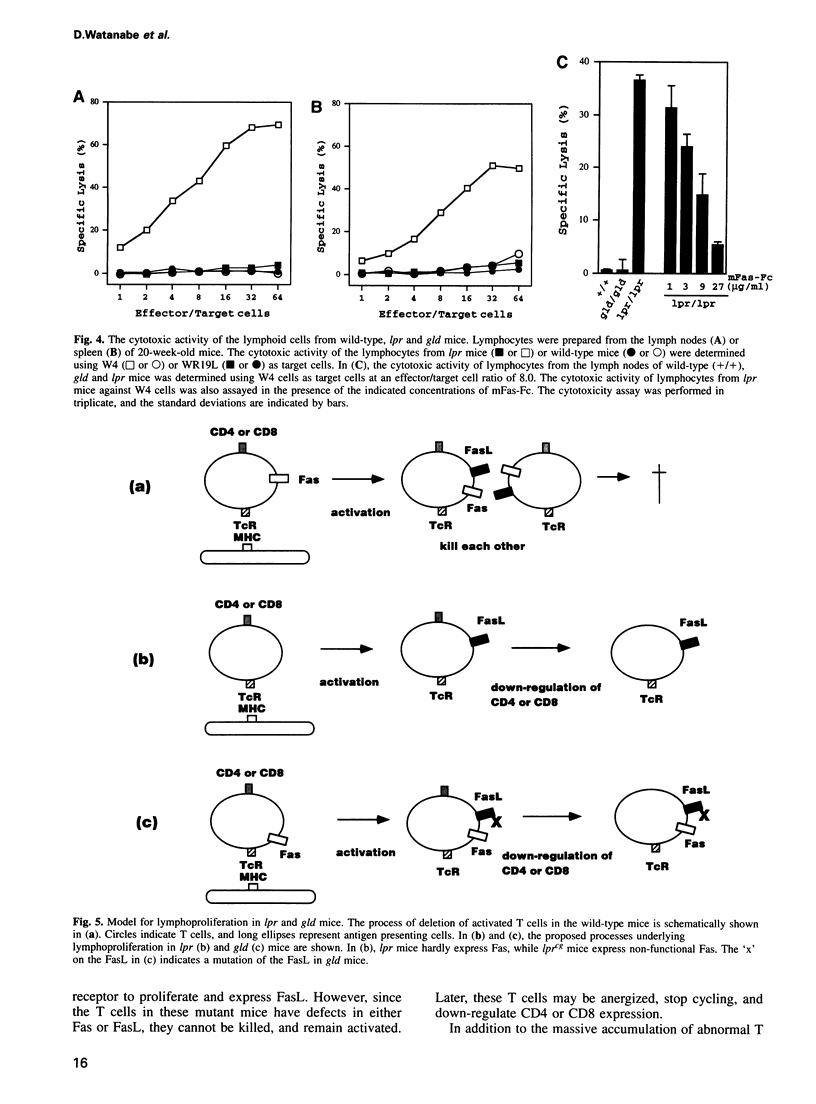
Mice homozygous for lpr (lymphoproliferation) or gld (generalized lymphoproliferative disease) develop lymphadenopathy and splenomegaly and suffer from autoimmune disease. The lpr mice have a defect in a cell-surface receptor, Fas, that mediates apoptosis, while gld mice have a mutation in the Fas ligand (FasL). Northern hybridization with the FasL cDNA as probe indicated that the cells accumulating in lpr and gld mice abundantly express the FasL mRNA without stimulation. By means of in situ hybridization and immunohistochemistry, we identified the cells expressing the FasL mRNA as CD4-CD8- double negative T cells. The T cells from lpr mice were specifically cytotoxic against Fas-expressing cells. Since FasL is normally expressed in activated mature T cells these results indicate that the double negative T cells accumulating in lpr and gld mice are activated once, and support the notion that the Fas/FasL system is involved in activation-induced suicide of T cells. Furthermore, the graft-versus host disease caused by transfer of lpr bone marrow to wild-type mice can be explained by the constitutive expression of the FasL in lpr-derived T cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D., Marshall J. D., Roths J. B., Sidman C. L. Differences defined by bone marrow transplantation suggest that lpr and gld are mutations of genes encoding an interacting pair of molecules. J Exp Med. 1990 Nov 1;172(5):1367–1375. doi: 10.1084/jem.172.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A. Abnormal antigen receptor-initiated signal transduction in lpr T lymphocytes. Semin Immunol. 1994 Feb;6(1):9–17. doi: 10.1006/smim.1994.1003. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Drappa J., Brot N., Elkon K. B. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain, MRL lpr/lpr. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R., Wang J. K., Bossu P., Papas K., Sidman C. L., Abbas A. K., Marshak-Rothstein A. Functional distinctions between MRL-lpr and MRL-gld lymphocytes. Normal cells reverse the gld but not lpr immunoregulatory defect. J Immunol. 1994 Feb 15;152(4):1557–1568. [PubMed] [Google Scholar]
- Fraziano M., Montesano C., Sammarco I., di Cesare S., Caroleo M. C., Mattei M., Cannata S., Poccia F., Bellavia A., Salerno A. Induction of graft versus host disease in SCID mice by MRL/lpr cell transfer. Clin Immunol Immunopathol. 1994 Jun;71(3):265–272. doi: 10.1006/clin.1994.1085. [DOI] [PubMed] [Google Scholar]
- Giese T., Davidson W. F. Evidence for early onset, polyclonal activation of T cell subsets in mice homozygous for lpr. J Immunol. 1992 Nov 1;149(9):3097–3106. [PubMed] [Google Scholar]
- Hashimoto H., Ishihara T., Shigemoto R., Mori K., Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993 Aug;11(2):333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Katagiri T., Ting J. P., Dy R., Prokop C., Cohen P., Earp H. S. Tyrosine phosphorylation of a c-Src-like protein is increased in membranes of CD4- CD8- T lymphocytes from lpr/lpr mice. Mol Cell Biol. 1989 Nov;9(11):4914–4922. doi: 10.1128/mcb.9.11.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Urakawa K., Yamanashi Y., Semba K., Takahashi T., Toyoshima K., Yamamoto T., Kano K. Overexpression of src family gene for tyrosine-kinase p59fyn in CD4-CD8- T cells of mice with a lymphoproliferative disorder. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10064–10068. doi: 10.1073/pnas.86.24.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klas C., Debatin K. M., Jonker R. R., Krammer P. H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993 Jun;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Hirano T., Kakinuma M., Uede T. Transcriptional repression and differential splicing of Fas mRNA by early transposon (ETn) insertion in autoimmune lpr mice. Biochem Biophys Res Commun. 1993 Mar 15;191(2):617–624. doi: 10.1006/bbrc.1993.1262. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Steinberg A. D., Klinman D. M., Smith H. R., Mushinski J. F. Autoimmunity and increased c-myb transcription. Science. 1984 Nov 30;226(4678):1087–1089. doi: 10.1126/science.6494925. [DOI] [PubMed] [Google Scholar]
- Muraoka S., Miller R. G. The autoimmune mouse MRL/Mp-lpr/lpr contains cells with spontaneous cytotoxic activity against target cells bearing self-determinants. Cell Immunol. 1988 Apr 15;113(1):20–32. doi: 10.1016/0008-8749(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Negative selection of lymphocytes. Cell. 1994 Jan 28;76(2):229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 Aug 26;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Yonehara S., Crump W. L., 3rd, Grimm E. A. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992 Mar;140(1):197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., Rush B., Weaver C., Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., Wang R. Autoimmune gld mutation uncouples suicide and cytokine/proliferation pathways in activated, mature T cells. Eur J Immunol. 1993 Sep;23(9):2379–2382. doi: 10.1002/eji.1830230951. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Davidson W. F., Morse H. C., 3rd, Klausner R. D. Abnormal tyrosine phosphorylation on T-cell receptor in lymphoproliferative disorders. Nature. 1986 Dec 18;324(6098):674–676. doi: 10.1038/324674a0. [DOI] [PubMed] [Google Scholar]
- Sobel E. S., Katagiri T., Katagiri K., Morris S. C., Cohen P. L., Eisenberg R. A. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of murine systemic autoimmunity. J Exp Med. 1991 Jun 1;173(6):1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994 Mar 1;179(3):873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Inazawa J., Abe T., Suda T., Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994 Oct;6(10):1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Balderas R. S., Gozes Y., Aguado M. T., Hang L. M., Morrow P. R., Dixon F. J. Association of lpr gene with graft-vs.-host disease-like syndrome. J Exp Med. 1985 Jul 1;162(1):1–18. doi: 10.1084/jem.162.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Uetsuki T., Naito A., Nagata S., Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. J Biol Chem. 1989 Apr 5;264(10):5791–5798. [PubMed] [Google Scholar]
- Vignaux F., Golstein P. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur J Immunol. 1994 Apr;24(4):923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- Wang W., Kobayashi S., Mori K., Harada H., Miyake K., Uede T. Spontaneous and antibody directed cytotoxicity of double-negative T cells from autoimmune mice. Int Immunol. 1993 Apr;5(4):361–369. doi: 10.1093/intimm/5.4.361. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhou T., He J., Mountz J. D. Autoimmune disease in mice due to integration of an endogenous retrovirus in an apoptosis gene. J Exp Med. 1993 Aug 1;178(2):461–468. doi: 10.1084/jem.178.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994 Jan 28;76(2):219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]