Abstract
Background
For reasons that remain unclear, whether type 5 AC (AC5), one of two major AC isoforms in heart, is protective or deleterious in response to cardiac stress is controversial. To reconcile this controversy we examined the cardiomyopathy induced by chronic isoproterenol (ISO) in AC5 transgenic (Tg) mice and the signaling mechanisms involved.
Methods and Results
Chronic ISO increased oxidative stress and induced more severe cardiomyopathy in AC5 Tg, as left ventricular (LV) ejection fraction fell 1.9 fold more than wild type (WT), along with greater LV dilation and increased fibrosis, apoptosis and hypertrophy. Oxidative stress induced by chronic ISO, detected by 8-OhDG was 15% greater, p=0.007, in AC5 Tg hearts, while protein expression of MnSOD was reduced by 38%, indicating that the susceptibility of AC5 Tg to cardiomyopathy may be due to decreased MnSOD expression. Consistent with this, susceptibility of the AC5 Tg to cardiomyopathy was suppressed by overexpression of MnSOD, whereas protection afforded by the AC5 KO was lost in AC5 KO×MnSOD+/− mice. Elevation of MnSOD was eliminated by both sirtuin and MEK inhibitors, suggesting both the SIRT1/FoxO3a and MEK/ERK pathway are involved in MnSOD regulation by AC5.
Conclusion
Overexpression of AC5 exacerbates the cardiomyopathy induced by chronic catecholamine stress by altering regulation of SIRT1/FoxO3a, MEK/ERK and MnSOD, resulting in oxidative stress intolerance, thereby shedding light on new approaches for treatment of heart failure.
Keywords: Adenylyl cyclase, Adrenergic, Cardiomyopathy, Oxidative Stress
Introduction
Adenylyl cyclase (AC) is a key regulator of health and longevity in organisms ranging from yeast to mammals.1–5 In the heart AC is a critical link in sympathetic control and beta adrenergic receptor (beta-AR) signaling and therefore plays a fundamental role in mediating not only baseline cardiac function, but also the cardiac response to stress, e.g., in the pathogenesis of heart failure. Type 5 AC (AC5) is one of two major isoforms in heart, the other being type 6 AC (AC6). For reasons that remain unclear, whether AC5 is protective or deleterious in response to cardiac stress is controversial, particularly with respect to the signaling mechanisms involved, and whether these mechanisms are shared by AC6. It is generally accepted that cardiac-specific AC5 overexpressed (AC5 Tg) mice exhibit enhanced cardiac performance,6 which follows from the role of AC, which generates cyclic AMP upon beta-AR stimulation resulting in increased cardiac contractility and heart rate. However, the extent to which altered AC5 regulation is protective with chronic stress remains controversial. Prior studies examined whether overexpression or disruption of AC5 in the heart could affect the progression of cardiomyopathy induced by overexpression of Galphaq and beta1-AR. This was accomplished by mating overexpressed Galphaq and beta1-AR with AC5 Tg or AC5 knockout (KO) mice. These studies found that AC5 Tg rescued Galphaq cardiomyopathy,6 but not beta1-AR cardiomyopathy,7 and AC5 KO mice failed to rescue cardiomyopathy in Galphaq mice.8 In addition, AC5 KO mice rescued cardiomyopathies from chronic pressure overload,9 chronic catecholamine stress,10 and aging.1
Since beta-AR signaling, of which AC is central, plays a key role in the pathogenesis of heart failure and since beta-AR blockade therapy is widely used in patients with heart failure, but that therapy is still far from perfect, it becomes critical to reconcile the controversy and understand the role of AC in the heart in the development of cardiomyopathy and heart failure, which would eventually be of clinical importance. Accordingly, this was the overall goal of the current investigation. We first examined the extent to which manganese superoxide dismutase (MnSOD) regulation and oxidative stress were altered in AC5 Tg at baseline and in response to chronic beta-AR stimulation, since it is known that beta-AR stimulation increases oxidative stress,11, 12 and that MnSOD is upregulated in AC5 KO mice.1 The results of the experiments with bigenic mice (AC5 Tg × MnSOD Tg and AC5 KO × MnSOD+/−) led us to elucidate the signaling mechanisms linking AC5, MnSOD and oxidative stress, and the involvement of the SIRT1/FoxO3a pathway. The SIRT1/FoxO3a pathway was selected to investigate, because MnSOD is upregulated in the AC5 KO mouse, which lives longer than wild type (WT)1 and FoxO3a is the transcriptional factor most closely related to the anti-oxidative protective effects associated with longevity, as shown in several models: C.elegans,13, 14 rats15 and human quiescent cells.16 The final goal was to investigate whether this pathway is regulated specifically by AC5, or whether it is common to all AC signaling in the heart, which would mean that these mechanisms were shared by the other major cardiac AC isoform, AC6.
Methods
Mouse Models
Generation of AC5 Tg mice was described previously.17 AC5 KO × MnSOD+/− mice were generated by crossing AC5 KO mice with MnSOD heterozygous mice. AC5 Tg × MnSOD Tg were generated by crossing AC5 Tg mice with MnSOD Tg mice (From Jackson Laboratory, Stock ID: 009438). To produce catecholamine cardiomyopathy, ISO was delivered to 3–5 month old Tg mice, bigenic mice and corresponding control littermates for 7 days at a dose of 60 mg/kg/day with a miniosmotic pump (ALZET model 2001, DURECT Corp, Cupertino, California) as described.10 The severity of the cardiomyopathy was assessed by echocardiographic measurements of LV ejection fraction and LV end diastolic and end systolic diameter and histopatholical measurements of myocardial fibrosis, apoptosis and myocyte cross sectional area. For the Tempol treatment group, 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl (Tempol, Sigma) was administered to AC5 Tg mice by dissolving it in drinking water at a concentration of 1mmol/L for 1 month prior to chronic ISO infusion to block oxidative stress. Animals used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Eighth Edition 2011). This study was approved by the Animal Care and Use Committee at New Jersey Medical School.
Experimental procedures
All techniques are described in more detail in Supplemental materials with references to previous work with these techniques. Experimental procedures included: adenoviral construction (Figure S2), physiological studies,10 primary culture of neonatal rat ventricular myocytes,18 AC assay,10 immunoprecipitation, western blotting,1 quantitative RT-PCR,18 8-hydroxy-2’-deoxyguanosine (8-OHdG) ELISA assay, chemiluminescent assay for superoxide production,19 subcellular fractionation, luciferase activity, Chromatin Immunoprecipitation (ChIP) assay15 and histological analyses (apoptosis, fibrosis and cell size).20
Statistical analysis
Normally distributed data were presented as mean±SEM. Otherwise, data were summarized using the Median and range. When the data were normally distributed, we used Student’s unpaired t-test to compare two independent groups; otherwise, the difference was tested using the Mann-Whitney U test. For a comparison of three or more groups, one-way ANOVA was used if the sample population was normally distributed and within-group variances were approximately equal. The Student-Newman-Keuls test was used for post-hoc analysis. For data that did not meet the ANOVA assumptions, the Kruskal-Wallis test was applied and post hoc testing was carried out using the Mann-Whitney U test with Bonferroni correction. The Bonferroni correction factor is 3 for Figures 1, 5F and 5G. GraphPad-Prism 5.0 (GraphPad-Software, San Diego,CA), SPSS 20.0 (SPSS Inc, Chicago, IL) and SAS 9.3 (SAS, Research Triangle, NC) were used to perform the statistical analyses. P-values less than 0.05 defined statistical significance.
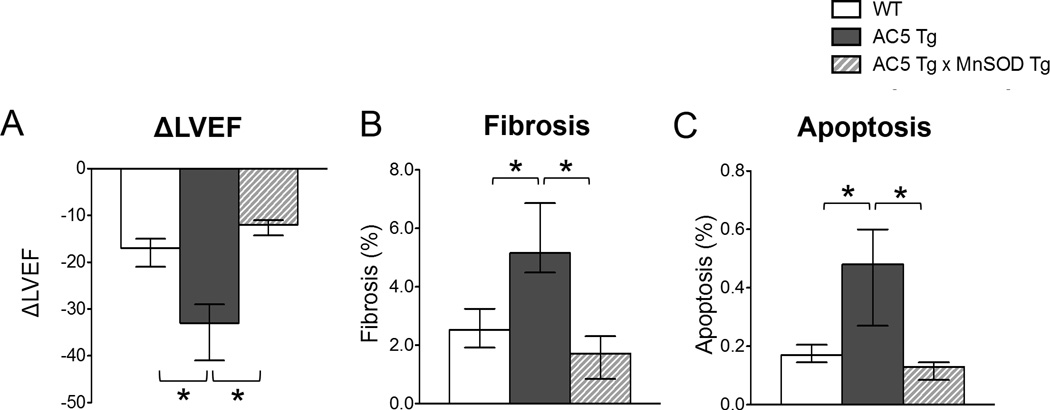
Figure 1.
Chronic ISO cardiomyopathy in AC5 Tg, compared with WT, was rescued by mating the AC5 Tg mice with MnSOD Tg (AC5 Tg × MnSOD Tg) mice. The asterix indicates a p<0.05 difference. A, Delta LV ejection fraction (EF) after ISO. AC5 Tg mice fell significantly more than that of WT mice, p<0.05. In the bigenic mice the fall in LVEF was significantly less, n=6–7/group. B, More fibrosis, p<0.05, was detected in AC5 Tg mice after ISO than in the other two groups after ISO n=5/group. C, Myocyte apoptosis, calculated by the percentage of total myocyte nuclei, also increased more in AC5 Tg than the other two groups. The data in panel A–C did not have a normal distribution and the appropriate statistical tests were used (see statistical analysis section). *p<0.05
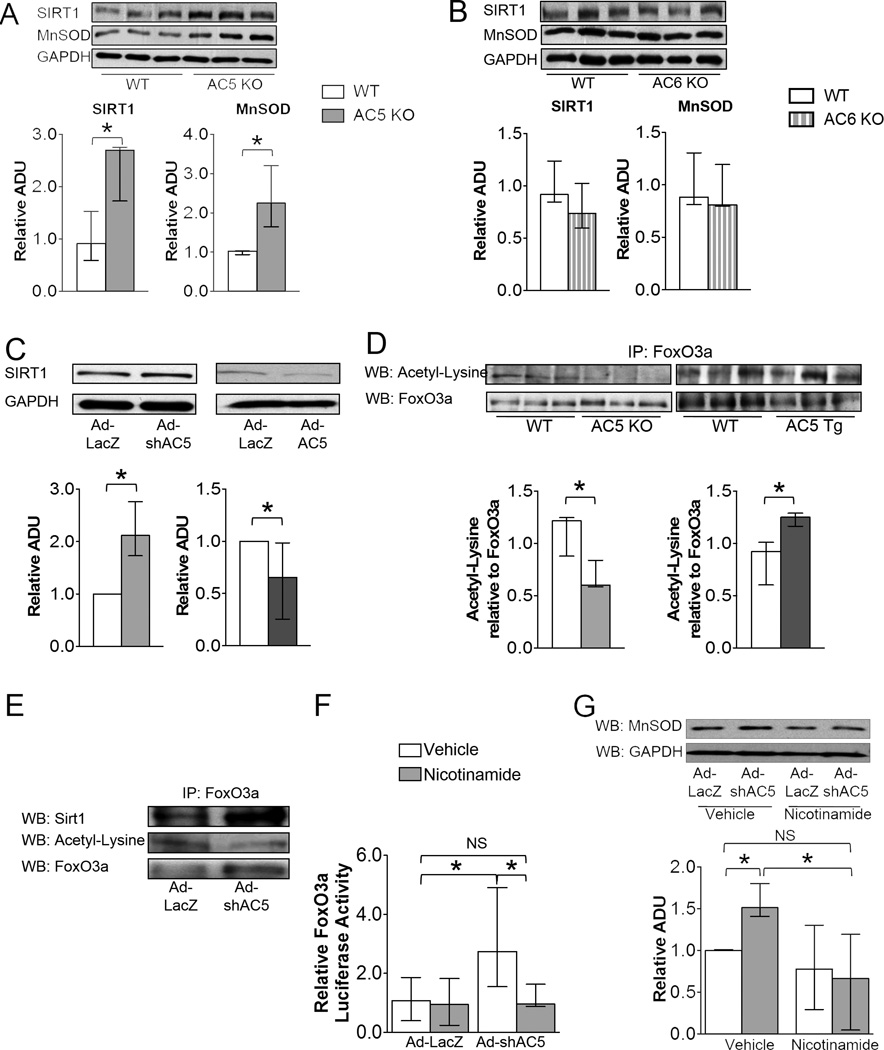
Figure 5.
AC5 regulated FoxO3a transcriptional activity through SIRT1. A, SIRT1 and MnSOD expression levels increased in AC5 KO heart, n=3/group, but not in AC6 KO hearts, n=3/group (B). C, SIRT1 expression levels increased in AC5 KD cardiomyocytes (left panel) (n=4) and decreased in AC5 OE cardiomyocytes (right panel) (n=5). D, Acetyl-lysine in FoxO3a was down-regulated in AC5 KO mice heart (left panel), but up-regulated in AC5 Tg mouse heart (right panel), n=3/group. E, Co-IP confirmed that SIRT1 de-acetylated FoxO3a by directly binding to FoxO3a in AC5 KD cardiomyocytes. F, FoxO3a transcriptional activity was inhibited in AC5 KD myocytes treated with nicotinamide, a Sirtuin inhibitor, n=9/group. The FoxO3a transcriptional activity of AC5 KD myocytes treated with nicotinamide showed no significant difference compared with cardiomyocytes infected with ad-LacZ, NS=non-significant. G, MnSOD expression level was inhibited in AC5 KD cardiomyocytes when the cells were treated with nicotinamide. n=6. The data in panels A – G did not have a normal distribution. The appropriate statistical tests are noted in the statistical analysis section. *p<0.05
Results
AC5 Tg Mouse Model and Cardiomyopathy Induced by Chronic Isoproterenol (ISO)
AC5 protein expression, assessed by western blot analysis, was increased 26-fold in AC5 Tg (Figure S1A). Basal AC activity was increased 13-fold in AC5 Tg mice hearts compared to WT, and was increased 10-fold with forskolin compared to WT (Figure S1B). The AC5 Tg exhibited increased left ventricular ejection fraction (LVEF), p=0.0009, without ISO (WT=73(67–74)%; AC5 Tg=78(75–81)%) and heart rate was not significantly different, p=0.3176, (WT=337(325–465) bpm; AC5 Tg= 442(355–500)bpm). The increase in LVEF in response to an ISO challenge was similar in AC5 Tg and WT mice(Figure S1C).
Chronic ISO infusion induced more severe cardiomyopathy in AC5 Tg compared with WT, i.e., LVEF was lower, p=0.0058, in AC5 Tg (45(30–49)%) compared to WT (54(47–58)%). Actually the decline in LVEF was even more significant, since that takes into account the different baseline levels where LVEF was higher in AC5 Tg and fell to a lower level, p=0.0021 (Figure 1A). In addition, the LV dilated more in AC5 Tg mice than WT (Table S1). Similarly, chronic ISO induced more fibrosis (2.0-fold) and more myocyte apoptosis (2.8-fold) in AC5 Tg mice compared with WT (Figure 1B and 1C). There was also more LV hypertrophy, as measured by myocyte cross sectional area, but the increase (1.2-fold) was not as great as with fibrosis and apoptosis.
Overexpression of AC5 Increased Oxidative Stress
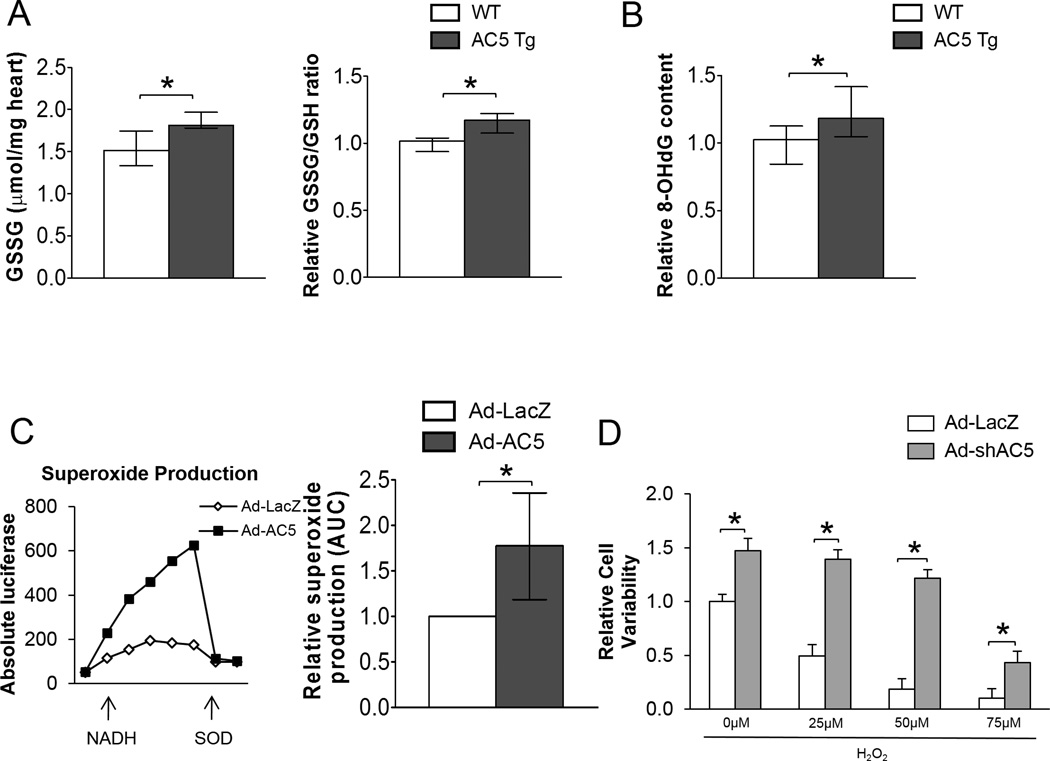
After chronic ISO stimulation, AC5 Tg mice exhibited 19% more GSSG content, an indicator of oxidative stress, than WT littermates (Figure 2A). Consistent with this, AC5 Tg mice had 15% more oxidative stress-induced DNA damage compared with WT mice after chronic ISO stimulation detected by 8-OHdG ELISA (Figure 2B). In AC5 overexpressed neonatal myocytes, superoxide production was approximately 2-fold more than in the control group (Figure 2C). AC5 knockdown (KD) myocytes increased cell survival with H2O2 treatment (Figure 2D). MnSOD is part of a mechanism that might be responsible for the opposite response of AC5 overexpressed (OE) and AC5 KD towards oxidative stress, since MnSOD is up-regulated in AC5 KO mice.
Figure 2.
Oxidative stress was detected in AC5 Tg mice. A, Higher GSSG content (left panel), as well as GSSG/GSH ratio (right panel) were detected in AC5 Tg heart after chronic ISO stimulation. n=5/group. B, The elevation of oxidative stress in AC5 Tg heart after chronic ISO stimulation was further confirmed by 8-OHdG (a marker of oxidative DNA damage) ELISA assay. n=7/group. C, Ad-AC5 infected myocytes released approximately 2-fold more superoxide radicals than control myocytes. n=4. D, Knocking down AC5 improved cell survival in myocytes treated with various concentrations of H2O2․ n=8. The data in panels A, B and C did not have a normal distribution and the appropriate statistical tests were used (see statistical analysis section). The data in panel D had a normal distribution and were presented as mean±sem. *p<0.05 by 1-way ANOVA with Student-Newman-Keuls post-hoc analysis. *p<0.05
AC5 Down-Regulates MnSOD
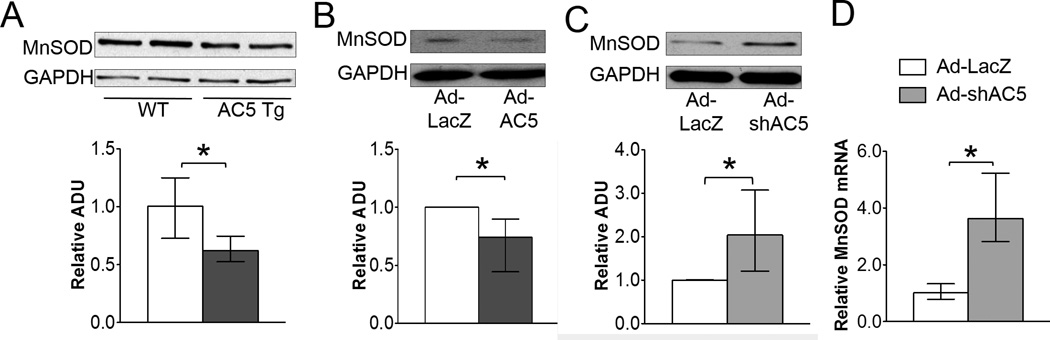
By western blotting, the protein expression of MnSOD was reduced 38% in AC5 Tg mice compared with WT (Figure 3A). On the cellular level, 26% less MnSOD was detected in AC5 OE myocytes and MnSOD protein was increased a 2-fold and mRNA a 3.6-fold in AC5 KD myocytes (Figure 3B, 3C and 3D). The data demonstrated that AC5 regulated the protein and mRNA expression level of MnSOD, which altered MnSOD function.
Figure 3.
AC5 regulated MnSOD expression in protein and mRNA levels. Panels A and B show the downregulation of MnSOD in AC5 Tg mice hearts (n=6/group) and Ad-AC5 infected myocytes (n=4). C, MnSOD was up-regulated in Ad-shAC5 infected myocytes, n=4. D, mRNA also increased in AC5 knock down myocytes, n=9/group. The data in panels A – D did not have a normal distribution and the appropriate statistical tests were used (see statistical analysis section). *p<0.05
MnSOD Overexpression Ameliorated Chronic ISO Cardiomyopathy in AC5 Tg
We increased MnSOD in AC5 Tg using a bigenic (AC5 Tg × MnSOD Tg) mouse. The cardiac specific MnSOD Tg mice had a 20-fold increase in SOD activity in the heart.21 Baseline LVEF was similar in AC5 Tg × MnSOD Tg mice (85(84–89)%) and AC5 Tg mice (78(75–81)%). After chronic ISO, the LVEF of bigenic mice decreased significantly less (Figure 1A, Table S1), p=0.0033, to 74(66–77)%, than in AC5 Tg (45(30–49)%) mice (Figure 1A, Table S1). The increases in LVEDD and LVESD were also no longer greater than observed in WT (Table S1), and the increases in fibrosis and apoptosis, observed in AC5 Tg on chronic ISO, were no longer observed in the bigenic mice (Figure 1B and 1C). Similarly, Tempol, which also protects against oxidative stress, rescued the adverse effects of the AC5 Tg after chronic ISO stimulation, i.e., the LVEF in AC5 Tg with ISO and Tempol (63(43–69)%) was higher than with ISO in AC5 Tg without Tempol (45(30–49)%). Thus, these data demonstrated that down-regulation of MnSOD in AC5 Tg mice is a key mechanism mediating the exacerbated cardiomyopathy induced by chronic ISO.
Down-regulation of MnSOD Eliminated Protective Effects of AC5 KO under Chronic Catecholamine Stress
To investigate whether MnSOD was important for the protective effects of AC5 KO mice, we crossed the AC5 KO mice with MnSOD heterozygous KO mice. Previously, we reported that the AC5 KO mice were protected against catecholamine stress.10 This was confirmed in the present study in a small cohort, where the fall in LVEF was less in the AC5 KO than WT with chronic ISO. This protection was lost in the bigenic mice, where LVEF after chronic ISO was decreased to 50(43–60)% (n=6), which was almost identical to the LVEF in the WT mice (53(39–62)%, n=11) (Table S1). Fibrosis, an indicator of the cardiomyopathy with chronic ISO was increased similarly in WT (2.69(1.64–3.84)%) and AC5 KO × MnSOD+/− mice (2.92(2.32–3.85)%) compared with AC5 KO mice with chronic ISO (1.13(0.81–1.37)%).
AC5 Regulated MnSOD Transcriptionally through the SIRT1/FoxO3a Pathway
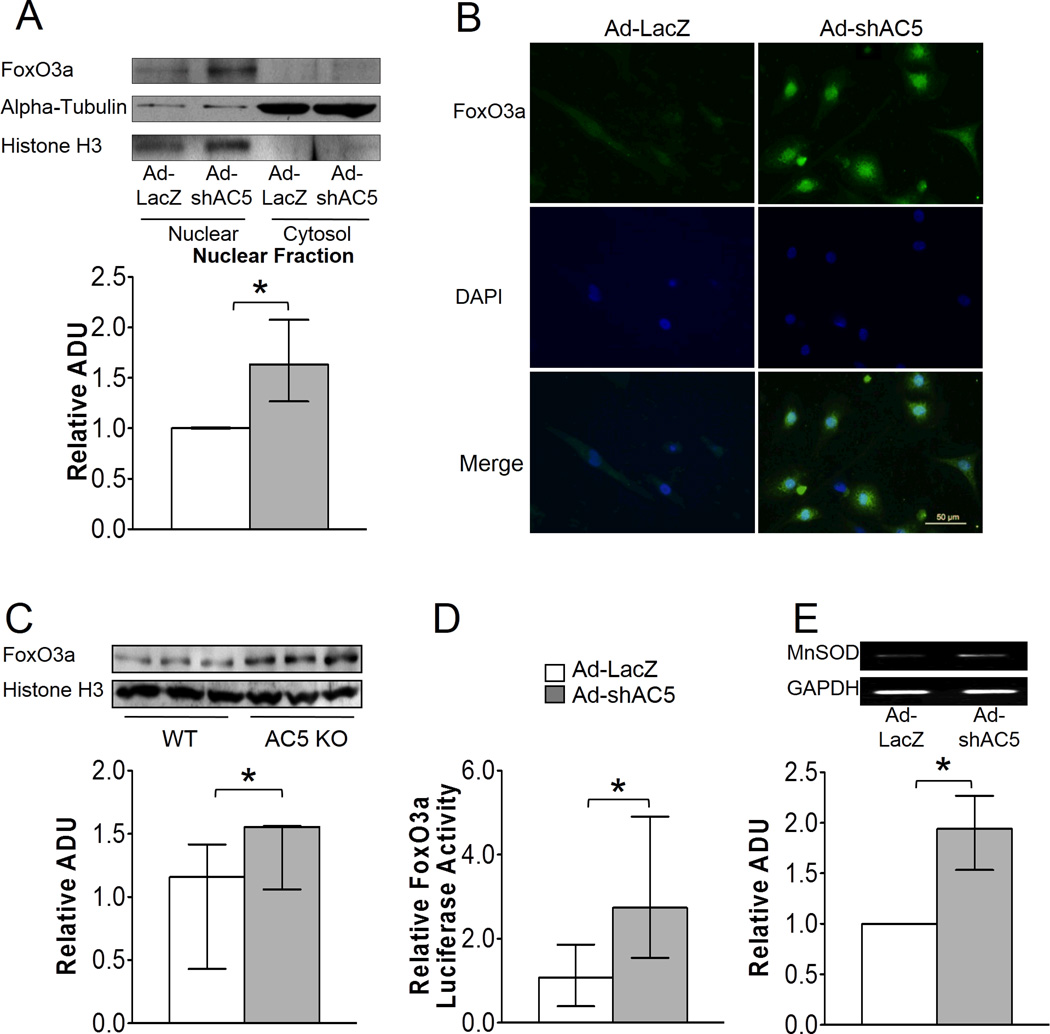
Since down-regulation of MnSOD is the key mechanism mediating the enhanced cardiomyopathy of AC5 Tg mice, we investigated the molecular pathway responsible. The mRNA level of MnSOD in AC5 KD myocytes was 3.6-fold higher than those infected by a control adenovirus, Ad-LacZ (Figure 3D), which suggested that transcriptional factors may be involved in the regulation of MnSOD, with FoxO3a a likely target, as noted earlier. To determine whether FoxO3a directly regulated MnSOD, we first examined the localization of FoxO3a in the AC5 KD neonatal myocytes. Immunostaining and western blotting detected more FoxO3a in the nucleus of AC5 KD myocytes compared with the control group (Figure 4A and 4B). Similarly in tissue, more FoxO3a was detected in the nucleus of AC5 KO mouse heart compared with WT (Figure 4C).
Figure 4.
AC5 regulated MnSOD transcriptionally through FoxO3a. A and B, Both immunostaining (40×) and western blotting confirm the nuclear localization of FoxO3a in AC5 KD myocytes, n=4. The data are normalized to the intensity of histone H3. C, More FoxO3a expression was detected in the nuclear fraction of AC5 KO heart, n=3/group. D, Myocytes infected with Ad-shAC5 showed significantly higher transcriptional activity compared with Ad-LacZ infected cells, n=9/group. E, 2 fold more FoxO3a binding to the MnSOD promoter in AC5 KD cells compared with LacZ control cells, n=3. The data in panels A – E did not have a normal distribution and the appropriate statistical tests were used (see statistical analysis section), *p<0.05
Next, we tested the transcriptional activity of FoxO3a using a luciferase assay by transfecting a FoxO3a luciferase vector, with a promoter containing 3 repeats of the forkhead response element (FRE), into neonatal myocytes.22 When AC5 was knocked down in myocytes, a 2.5-fold increase in luciferase activity was observed (Figure 4D). To examine whether FoxO3a increased the native MnSOD gene directly, we performed the ChIP assay on native MnSOD in the H9C2 rat cardiac myoblast cell line infected with Ad-LacZ and Ad-shAC5, respectively. We found 1.9-fold more FoxO3a binding to the specific FoxO-binding element within the MnSOD promoter region (Figure 4E) in AC5 KD myocytes. The data demonstrated that knock down of AC5 causes FoxO3a to localize to the nucleus and associate with the MnSOD promoter.
FoxO is known to be regulated by acetylation, a modification removed by the NAD+-responsive, metabolic sensor SIRT1.23 To test whether AC5 regulated SIRT1 directly; protein expression of SIRT1 was detected in AC5 KO mice hearts. SIRT1 was significantly up-regulated in AC5 KO mice hearts (Figure 5A). Consistent with the adult heart data, SIRT1 was up-regulated in AC5 KD myocytes and down-regulated in AC5 OE neonatal myocytes (Figure 5C).
To test whether this pathway was unique to AC5, we also examined AC6 KO, the other major cardiac AC isoform. In AC6 KO, SIRT1 was not up-regulated (Figure 5B). Paralleling SIRT1 expression, MnSOD was not up-regulated in the AC6 KO hearts. Thus, the up-regulation of SIRT1 and MnSOD expression was not due to a general response to AC activity.
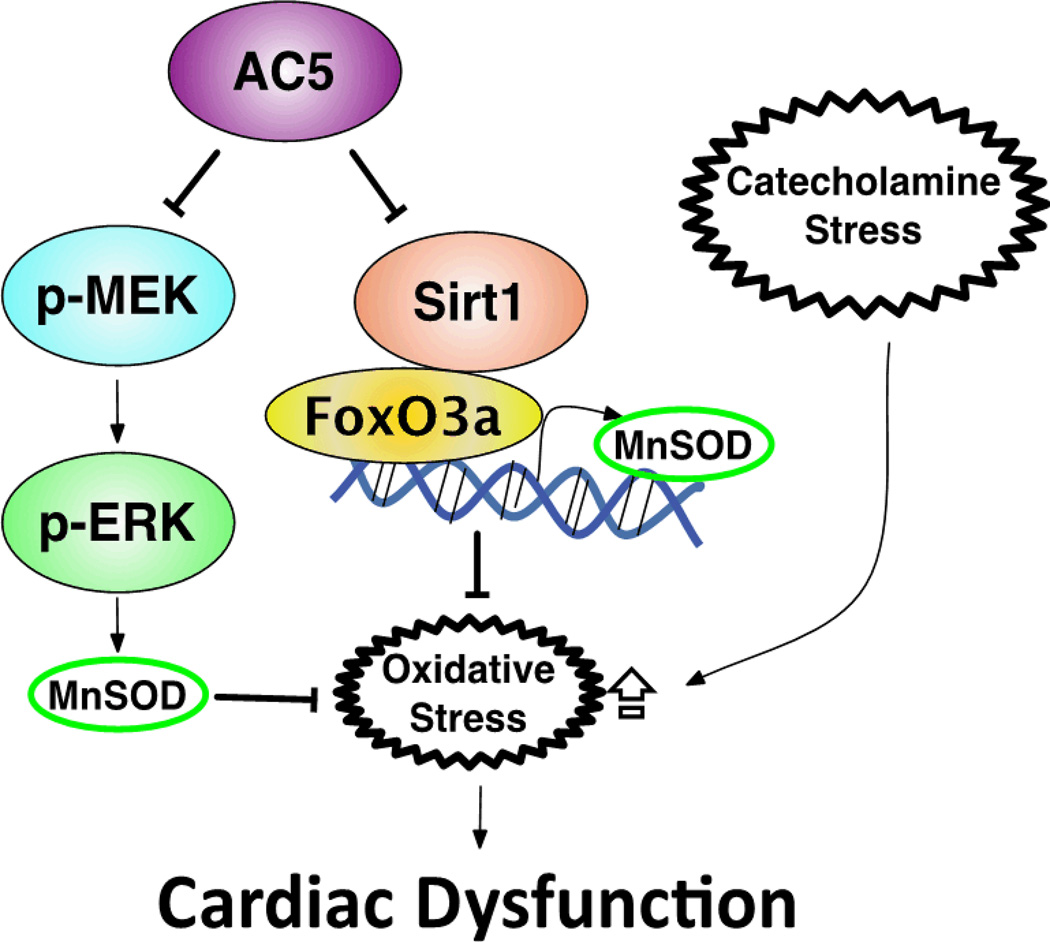
Given the apparent link between SIRT1 and FoxO3a, we found that the level of acetyl-FoxO3a in the heart of AC5 KO and AC5 Tg mice correlated inversely with the expression of SIRT1 (Figure 5D). In AC5 KD myocytes, SIRT1 was shown by co-immunoprecipitation (IP) to bind directly to FoxO3a (Figure 5E). These data suggest that FoxO3a is regulated by deacetylation in cardiac tissue and this is controlled by AC5 activity. To further investigate the role of SIRT1, we treated AC5 KD and the control group with nicotinamide, a sirtuin inhibitor, and then tested the transcriptional activity of FoxO3a in the control group. Nicotinamide did not change the activity in the control group, but reduced the transcriptional activity of FoxO3a to almost the same level in AC5 KD group as in the control group (Figure 5F). To investigate if the elevation of luciferase activity of FoxO3a contributed to MnSOD expression, we determined MnSOD protein levels in AC5 KD myocytes. The MnSOD level in nicotinamide treated AC5 KD myocytes was down-regulated to a similar level as the control group (Figure 5G). These data indicate that the increased transcriptional activity of FoxO3a and MnSOD expression in AC5 KD myocytes is due to increased SIRT1 activity. In summary, AC5 inhibited SIRT1 activity, which consequently decreased the interaction between SIRT1 and FoxO3a, thus decreasing MnSOD expression, resulting in less tolerance towards stress (Figure 6).
Figure 6.
Signaling diagram for AC5 regulation of MnSOD through the SIRT1/FoxO3a and the MEK/ERK pathway. Imbalance between reactive oxygen species production and the intracellular anti-oxidant system results in the intolerance of AC5 Tg to stress.
MnSOD also Regulated by MEK/ERK Signaling
We previously reported that both MEK/ERK/MnSOD and Akt pathways were involved in the protective mechanism of AC5 KO.1, 10 The elevation of MnSOD in myocytes infected with AC5 KD adenovirus was eliminated by both the MEK inhibitor (PD98059)(Figure S3B) and sirtuin inhibitor (nicotinamide) (Figure 5G), suggesting both the MEK/ERK and SIRT1/FoxO3a pathway are involved in MnSOD regulation. Furthermore, we examined the activity of Akt (represented as p-Akt/Akt) in AC5 Tg mice after chronic ISO. In contrast to AC5 KO mice, the activity of Akt was lower in AC5 Tg than in WT mice (Figure S4), indicating that the AC5 Tg failed to activate this protective mechanism induced by chronic ISO stress. Different from MnSOD basal regulation by AC5 (Figure S3A), Akt is involved in mediating the cardiac effects after chronic ISO stimulation.
To demonstrate that MnSOD is the only anti-oxidant gene targeted by the AC5 regulated Sirt1/FoxO3a pathway, we examined the expression of catalase, another downstream target of FoxO3a,24, 25 and found no differences in the hearts among WT, AC5 KO and AC5 Tg mice (Figure S5).
Discussion
Since the conclusions from previous studies examining the extent to which cardiac overexpression or deletion of AC5 affects the development of cardiomyopathy have been controversial6, 7, 17, 26 and no prior study examined the effects of cardiac overexpression of AC5 on the development of cardiomyopathy, the goal of the present investigation was to do just that, using the model of chronic catecholamine stress. The results indicate clearly that cardiac over-expression of AC5 increases the severity of the cardiomyopathy induced by chronic catecholamine stress, resulting in more severely compromised LV function and increased LV dilation, cardiac fibrosis and apoptosis. It is well recognized that catecholamines increase oxidative stress, which in turn, induces necrosis and results in cardiac fibrosis,27–30 which is an important mechanism mediating the decrease in function observed, not only in the cardiomyopathy induced by chronic ISO, but also in all cardiomyopathies. Although the most common cause of necrosis is myocardial ischemia, it can also result from an imbalance between myocardial oxygen supply and demand, particularly in the subendocardium, and there is also non-ischemic necrosis,27 all of which leads to a reduction in contractile units in the heart and increased fibrosis, which interferes with cardiac contraction. ISO also induces necrosis in myocytes in culture, independent of myocardial blood supply.27, 31, 32
There is another reason for controversial results in literature, i.e., it is not always possible to extrapolate linearly between in vivo and in vitro work and between Tg and KO models. A major strength of this investigation was the use of both Tg and KO models for AC5, which alleviated the criticisms that the high level of overexpression of AC5 in the Tg model, overwhelmed other mechanisms. In addition, finding reciprocal data in the KO and Tg model strengthens the conclusions.
Since MnSOD protects against oxidative stress in AC5 KO mice1 and since oxidative stress has been implicated in catecholamine induced cardiomyopathy,11, 27, 33 our hypothesis was that the adverse effects of AC5 overexpression in the heart are mediated by enhanced oxidative stress, primarily through an MnSOD mechanism. Confirming this hypothesis we found a 36% decrease of MnSOD expression in AC5 Tg mice and greater oxidative stress induced DNA damage. To further confirm our hypothesis that the reduced MnSOD was responsible for the enhanced oxidative stress, we restored MnSOD to the AC5 Tg mice by mating them with MnSOD Tg mice. The bigenic mice no longer responded to chronic ISO with more severe cardiomyopathy. To further confirm our hypothesis, we also examined whether reducing MnSOD eliminated the protection afforded to AC5 KO mice. Accordingly, we also mated MnSOD+/− mice with AC5 KO mice and then subjected the bigenic mice to chronic ISO stimulation. The bigenic mice were no longer protected from chronic catecholamine stress. Thus, the level of expression of MnSOD was responsible for the difference in responses to chronic catecholamine stimulation from WT in AC5 Tg and AC5 KO mice, and the opposite responses in AC5 Tg and AC5 KO mice. It was important to use the KO model in parallel with the AC5 Tg model, to avoid complicating influences derived from increasing gene expression to a high level. More importantly, the elimination of the protective effect in AC5 KO × MnSOD+/− mice is direct evidence that indicates the importance of MnSOD in the AC5 regulatory pathway. Other studies have found that oxidative stress is an important mechanism mediating several different cardiomyopathies,28–30, 33, 34 and Dai et al., showed the importance of mitochondrial oxidative stress.35 However, the signaling pathways have not been elucidated.
In this connection, it was previously shown that impaired mitochondrial function in cardiac myocytes from Sod2+/− mouse hearts is associated with a reduction in MnSOD activity36, which might suggest that the same phenomenon might occur in the AC5 Tg, where MnSOD activity is reduced, and that this may reduce cardiac function. However, we observed increased LV function at baseline in AC5 Tg and impaired function with chronic ISO. This apparent conundrum can be explained by the complex interaction of mechanisms controlling cardiac function in normal animals in vivo and more so in Tg animals. Since AC5 is a direct downstream target of the beta-AR, the increased basal LV function is a result of amplifying signals from the beta-AR, and not due to reduced MnSOD expression. Chronic ISO stress in AC5 Tg leads to the accumulation of oxidative stress, and an imbalance between oxygen supply and demand in the heart, leading to necrosis, apoptosis and fibrosis, which consequently reduced LV function and resulted in cardiomyopathy.
We then investigated the signaling pathway by which AC5 regulated MnSOD expression. In AC5 KD myocytes, we detected a significant increase in MnSOD mRNA, which suggests that AC5 also regulated MnSOD in a transcriptional manner. As noted earlier, the experiments demonstrating the role of MnSOD in mediating the enhanced cardiomyopathy with chronic ISO stress in AC5 Tg led us to investigate the SIRT1/FoxO3a pathway, in view of its protective role against oxidative stress associated with aging in C. elegans,13, 14 rats15 and human quiescent cells.16 In our study, we demonstrate for the first time the importance of AC5, an up-stream gene of the SIRT1/FoxO3a complex regulating MnSOD in cardiomyopathy. We also found activation of SIRT1 and FoxO3a in AC5 KD myocytes, whereas inhibition was detected in AC5 OE myocytes, suggesting that AC5 inhibits SIRT1 and FoxO3a activity, resulting in an impaired anti-oxidant system and induced cell death, suggesting that overexpression of SIRT1 and/or nicotinamide mononucleotide (NMN) or nicotinamide riboside treatment37 should be able to counteract the adverse effects of increased AC5 in the setting of chronic catecholamine cardiomyopathy. These conclusions are based in part on the acetylation experiments, which have the limitation of using the immunoprecipitation technique.38 A future direction will be to utilize a specific antibody for acetyl FoxO3a when it is available, which would permit a more definitive conclusion.
FoxO3a is known to regulate MnSOD transcriptionally, protecting cells from cellular oxidative stress.16 FoxO activity is regulated, in turn, by SIRT1, which also exerts favorable effects on oxidative stress resistance in cardiac myocytes.23 An interaction between the cyclic AMP/PKA pathway and Sir2 (an ortholog of SIRT1) in yeast has been reported.39 Recently a few studies indicated cyclic AMP/PKA dependent pathways of SIRT1 activation,40,41 which seem to be at variance with our findings. However, there are at least 4 important differences between these studies and ours. These studies were conducted in cancer, skeletal muscle, or hepatic cells, whereas we examined cardiomyocytes. Secondly, our findings are related only to AC5; it is conceivable that other AC isoforms, even AC6, the other major isoform in the heart could induce different regulation. Indeed, this is what we observed. Since in the studies by Noriega and Gerhart-Hines, forskolin was used to stimulate AC, which will activate all AC isoforms, this may have influenced AC6, which regulates AC activity to a greater effect than AC5 in the heart. Fourthly, our study was conducted under conditions of chronic activation or inhibition of the cyclic AMP/PKA pathway, e.g., in AC5 Tg and KO, and measured the change in protein expression of SIRT1, whereas the prior studies utilized more acute activation of cyclic AMP/PKA with forskolin, which activated SIRT1, either through the induction of its transcription or through SIRT1 phosphorylation. Finally, the results of our investigation relating AC5 to the SIRT1/FoxO pathway is consistent with other studies showing that SIRT1/FoxO protects the heart against oxidative stress, and that MnSOD plays an important role.42, 43 It has been shown that overexpression of SIRT1 no longer promotes cell survival when MnSOD was eliminated42 and nuclear translocation of FoxO induced transcriptional up-regulation of MnSOD, which protected the heart from myocardial infarction.43 In addition, we found translocation and activation of FoxO3a by SIRT1, consistent with a report in C. elegans.23 Since FoxO3a regulates several molecules involved in the cell cycle and oxidative stress, e.g., catalase24, 25 it is possible that MnSOD is not uniquely targeted. However, we found that catalase expression was not different in hearts from WT, AC5 Tg and AC5 KO (Figure S5), supporting the concept that MnSOD is uniquely regulated by FoxO3a with relation to AC5. Deacetylation of FoxO3a should activate the transcriptional activity as described previously.23 As shown in Figure 5F and 5G, inhibition of SIRT1 activity reduced the FoxO3a transcriptional activity and MnSOD expression in AC5 KD myocytes. Since MnSOD is one of the important down-stream targets of FoxO3a, it is expected that the binding affinity to the MnSOD promoter increases as FoxO3a is activated.
It is important that we found the signaling mechanisms differed in the two major cardiac AC isoforms, AC5 and AC6. There have been several instances of AC isoform differences within organs. AC1, AC5, and AC8 all are major isoforms in brain, AC1 and AC8 play an important role in memory and learning,44, 45 but not AC5. Similarly, AC1 KO and AC5 KO mice are resistant to pain stress,46, 47 but not AC8. Also there are apparent differences in AC5 and AC6 regulation in the heart, showing that the AC5 KO is protected against stress,9, 10 but not AC6 KO.48
We previously found that the MEK/ERK pathway regulated MnSOD in the AC5 KO.1 In the current investigation we found that MnSOD was upregulated similarly in AC5 KO mice with vehicle and with ISO (Figure S3A) and that both a MEK inhibitor and a sirtuin inhibitor blocked the elevation of MnSOD in AC5 KD myocytes (Figure S3B, 5G), suggesting that both of these pathways are involved in the regulation of MnSOD by AC5.
In summary, this study examined for the first time the effects of overexpression of AC5 on the response to cardiac stress. In contrast to conflicting results from prior studies in AC5 Tg,6, 7 the results of the current investigation indicate clearly that overexpression of AC5 is deleterious in response to cardiac stress. We also demonstrated a new pathway for cardiac dysfunction mediated by AC5; cardiac overexpression of AC5 exacerbates the cardiomyopathy induced by chronic catecholamine stress through a mechanism inhibiting SIRT1 and FoxO3a, which decreases MnSOD transcription. The impaired antioxidant system elevates the intercellular oxidative stress level with chronic ISO stimulation, inducing more cell death and resulting in augmented cardiac dysfunction.
Supplementary Material
Acknowledgements
We appreciated the generous gifts of the MnSOD+/− mouse model by Dr. Ting-Ting Huang. We thank Dr. Chunbo Wang and Dr. Yimin Tian for technical support of the immunohistology analyses.
Funding Sources
This study was supported by funding from National Institutes of Health (5R01HL093481, 1R01HL106511, 5P01AG027211, 1R01HL102472, 5R01HL033107, 5T32HL069752, 5R01HL095888, 5P01HL069020, 5R01HL091781, 5R01HL093415, 5R01AG028730, 1R01AG019719). Dr. David A. Sinclair is supported by the Glenn Foundation for Medical Research.
Footnotes
Disclosures
Dr. David A. Sinclair is a consultant to Sirtris, a GSK company working to develop sirtuin-targeted medicines.
References
- 1.Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Hines LM, Tabakoff B. Platelet adenylyl cyclase activity: A biological marker for major depression and recent drug use. Biological psychiatry. 2005;58:955–962. doi: 10.1016/j.biopsych.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 3.El-Mowafy AM, Alkhalaf M. Resveratrol activates adenylyl-cyclase in human breast cancer cells: A novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis. 2003;24:869–873. doi: 10.1093/carcin/bgg015. [DOI] [PubMed] [Google Scholar]
- 4.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annual review of pharmacology and toxicology. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Moorman C, Plasterk RH. Functional characterization of the adenylyl cyclase gene sgs-1 by analysis of a mutational spectrum in caenorhabditis elegans. Genetics. 2002;161:133–142. doi: 10.1093/genetics/161.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tepe NM, Liggett SB. Transgenic replacement of type v adenylyl cyclase identifies a critical mechanism of beta-adrenergic receptor dysfunction in the g alpha q overexpressing mouse. FEBS Lett. 1999;458:236–240. doi: 10.1016/s0014-5793(99)01147-3. [DOI] [PubMed] [Google Scholar]
- 7.Petrashevskaya N, Gaume BR, Mihlbachler KA, Dorn GW, 2nd, Liggett SB. Bitransgenesis with beta(2)-adrenergic receptors or adenylyl cyclase fails to improve beta(1)-adrenergic receptor cardiomyopathy. Clin Transl Sci. 2008;1:221–227. doi: 10.1111/j.1752-8062.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timofeyev V, Porter CA, Tuteja D, Qiu H, Li N, Tang T, Singapuri A, Han PL, Lopez JE, Hammond HK, Chiamvimonvat N. Disruption of adenylyl cyclase type v does not rescue the phenotype of cardiac-specific overexpression of galphaq protein-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;299:H1459–H1467. doi: 10.1152/ajpheart.01208.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okumura S, Vatner DE, Kurotani R, Bai Y, Gao S, Yuan Z, Iwatsubo K, Ulucan C, Kawabe J, Ghosh K, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase enhances desensitization of cyclic adenosine monophosphate signal and increases akt signal with chronic catecholamine stress. Circulation. 2007;116:1776–1783. doi: 10.1161/CIRCULATIONAHA.107.698662. [DOI] [PubMed] [Google Scholar]
- 11.Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S, Chandrasekar B, Gu Y, Luo J, Hamid T, Hill BG, Prabhu SD. Downregulation of cuzn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res. 2007;74:445–455. doi: 10.1016/j.cardiores.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the caenorhabditis elegans longevity protein daf-16 by insulin/igf-1 and germline signaling. Nature genetics. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 14.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mrnas and consensus binding sequences for mouse daf-16 homologues. The Biochemical journal. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Chiu JF, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of foxo3a by akt in explanted vascular smooth muscle cells from old rats. J Biol Chem. 2006;281:40429–40439. doi: 10.1074/jbc.M606596200. [DOI] [PubMed] [Google Scholar]
- 16.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor foxo3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 17.Hu CL, Chandra R, Ge H, Pain J, Yan L, Babu G, Depre C, Iwatsubo K, Ishikawa Y, Sadoshima J, Vatner SF, Vatner DE. Adenylyl cyclase type 5 protein expression during cardiac development and stress. Am J Physiol Heart Circ Physiol. 2009;297:H1776–H1782. doi: 10.1152/ajpheart.00050.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park JY, Hong C, Gao S, Holle E, Morin D, Dhar SK, Wagner T, Berdeaux A, Tian B, Vatner SF, Depre C. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of stat3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124:406–415. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial nad(p)h oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 20.Peter PS, Brady JE, Yan L, Chen W, Engelhardt S, Wang Y, Sadoshima J, Vatner SF, Vatner DE. Inhibition of p38 alpha mapk rescues cardiomyopathy induced by overexpressed beta 2-adrenergic receptor, but not beta 1-adrenergic receptor. The Journal of clinical investigation. 2007;117:1335–1343. doi: 10.1172/JCI29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of mnsod reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 22.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of foxo transcription factors by the sirt1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 24.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 25.van der Horst A, Burgering BM. Stressing the role of foxo proteins in lifespan and disease. Nature reviews. Molecular cell biology. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 26.Iwatsubo K, Bravo C, Uechi M, Baljinnyam E, Nakamura T, Umemura M, Lai L, Gao S, Yan L, Zhao X, Park M, Qiu H, Okumura S, Iwatsubo M, Vatner DE, Vatner SF, Ishikawa Y. Prevention of heart failure in mice by an antiviral agent that inhibits type 5 cardiac adenylyl cyclase. American journal of physiology. Heart and circulatory physiology. 2012;302:H2622–H2628. doi: 10.1152/ajpheart.00190.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MU, Cheema Y, Shahbaz AU, Ahokas RA, Sun Y, Gerling IC, Bhattacharya SK, Weber KT. Mitochondria play a central role in nonischemic cardiomyocyte necrosis: Common to acute and chronic stressor states. Pflugers Arch. 2012;464:123–131. doi: 10.1007/s00424-012-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson AD, Ramanathan KB, McGee JE, Newman KP, Weber KT. Oxidative stress and cardiomyocyte necrosis with elevated serum troponins: Pathophysiologic mechanisms. Am J Med Sci. 2011;342:129–134. doi: 10.1097/MAJ.0b013e3182231ee3. [DOI] [PubMed] [Google Scholar]
- 29.Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wexler RK, Elton T, Pleister A, Feldman D. Cardiomyopathy: An overview. American family physician. 2009;79:778–784. [PMC free article] [PubMed] [Google Scholar]
- 31.Izem-Meziane M, Djerdjouri B, Rimbaud S, Caffin F, Fortin D, Garnier A, Veksler V, Joubert F, Ventura-Clapier R. Catecholamine-induced cardiac mitochondrial dysfunction and mptp opening: Protective effect of curcumin. American journal of physiology. Heart and circulatory physiology. 2012;302:H665–H674. doi: 10.1152/ajpheart.00467.2011. [DOI] [PubMed] [Google Scholar]
- 32.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 33.Dhalla NS, Adameova A, Kaur M. Role of catecholamine oxidation in sudden cardiac death. Fundam Clin Pharmacol. 2010;24:539–546. doi: 10.1111/j.1472-8206.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 34.Shaheen M, Cheema Y, Shahbaz AU, Bhattacharya SK, Weber KT. Intracellular calcium overloading and oxidative stress in cardiomyocyte necrosis via a mitochondriocentric signal-transducer-effector pathway. Exp Clin Cardiol. 2011;16:109–115. [PMC free article] [PubMed] [Google Scholar]
- 35.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richardson A. Knockout mice heterozygous for sod2 show alterations in cardiac mitochondrial function and apoptosis. American journal of physiology. Heart and circulatory physiology. 2001;281:H1422–H1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 37.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The nad(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, Pasinetti GM. Regulation of forkhead transcription factor foxo3a contributes to calorie restriction-induced prevention of alzheimer's disease-type amyloid neuropathology and spatial memory deterioration. Annals of the New York Academy of Sciences. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin SJ, Defossez PA, Guarente L. Requirement of nad and sir2 for life-span extension by calorie restriction in saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 40.Gerhart-Hines Z, Dominy JE, Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The camp/pka pathway rapidly activates sirt1 to promote fatty acid oxidation independently of changes in nad(+) Mol cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. Creb and chrebp oppositely regulate sirt1 expression in response to energy availability. EMBO reports. 2011;12:1069–1076. doi: 10.1038/embor.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of sirt1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. Foxo transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase ltp. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang HB, Pineda VV, Chan GCK, Wong ST, Muglia LJ, Storm DR. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. Journal of Neuroscience. 2003;23:9710–9718. doi: 10.1523/JNEUROSCI.23-30-09710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vadakkan KI, Wang HS, Ko SW, Zastepa E, Petrovic MJ, Sluka KA, Zhuo M. Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Molecular Pain. 2006:2. doi: 10.1186/1744-8069-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim KS, Kim J, Back SK, Im JY, Na HS, Han PL. Markedly attenuated acute and chronic pain responses in mice lacking adenylyl cyclase-5. Genes, brain, and behavior. 2007;6:120–127. doi: 10.1111/j.1601-183x.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 48.Lai NC, Tang T, Gao MH, Saito M, Takahashi T, Roth DM, Hammond HK. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.