Abstract
Background
The general anesthetics isoflurane and sevoflurane cause developmental abnormalities in neonatal animal models via incompletely understood mechanisms. Despite many common molecular targets, isoflurane and sevoflurane exhibit substantial differences in their actions. We sought to determine whether these differences can also be detected at the level of neurodevelopmental effects.
Methods
Postnatal day 4–6 rats were exposed to 1.2% isoflurane or 2.1% sevoflurane for 1–6 hrs and studied for immediate and delayed effects.
Results
Isoflurane exposure was associated with weaker seizure-like electroencephalogram patterns than sevoflurane exposure. Confronted with a new environment at a juvenile age the sevoflurane- but not isoflurane-exposed rats spent significantly more time in an “immobile” state than unexposed rats. Electroencephalographic (55.5±12.80 s vs. 14.86 ± 7.03 s, mean± SE, P = 0.014, n = 6–7) and spontaneous behavior (F(2,39)= 4.43, P=0.018) effects of sevoflurane were significantly diminished by pretreatment with the Na+–K+–2Cl– co-transporter inhibitor bumetanide, whereas those of isoflurane were not . Pretreatment with bumetanide, however, diminished isoflurane-induced activation of caspase-3 in the cerebral cortex (F(2,23)= 22.697, P=0.001) and prevented impairment in sensorimotor gating function (F(2,36)= 5.978, P= 0.006).
Conclusions
These findings in combination with our previously reported results suggest that isoflurane and sevoflurane produce developmental effects acting via similar mechanisms that involve an anesthetic induced increase in neuronal activity. At the same time differences in their effects suggest differences in the mediating mechanisms and in their relative safety profile for neonatal anesthesia.
Introduction
Operative procedures for millions of preterm and sick babies with different pathophysiological conditions require general anesthesia and frequently repeated exposures are needed. Considering the immense brain plasticity during this period of life and the profound effects of general anesthetics on almost all aspects of central nervous system function it is not surprising that there is a high level of concern among professionals and knowledgeable parents that exposure of newborns to general anesthesia may alter the course of brain development.1–3 Animal studies across various species, from rodents to non-human primates, demonstrate that volatile anesthetics, including isoflurane and sevoflurane, may cause profound neuronal death if applied at early stages of postnatal brain development.4–15 Many of these studies reported long-term neurocognitive abnormalities detected using different experimental paradigms,4,7–9,13–15 though the link between neuronal death and delayed functional abnormalities remains unclear.16 The results of human retrospective epidemiological studies are less conclusive. Four of nine such studies did not detect long-term developmental abnormalities in children who had general anesthesia at a young age.17–25 It is obvious that, in addition to other factors, the design of human studies lacks focus because the mechanisms that mediate the adverse developmental effects of general anesthetics are incompletely understood even in animal models. It is plausible that some anesthetics are more harmful than others, just as some neonates may be more susceptible to neurodevelopmental problems, for example, if their diseases share mechanisms with the side effects of anesthetics. New studies will be needed to address these possibilities.
We have recently found that sevoflurane, administered to neonatal rats, causes not only brain function-related developmental effects, such as seizure-like electroencephalogram patterns, an increase in levels of activated caspase-3 in the cerebral cortex, and an impairment in sensorimotor gating function, measured as a decrease of the prepulse inhibition (PPI) of the acoustic startle response three weeks after exposure to anesthesia, but also a prominent increase in serum levels of aldosterone. 10,15 Aldosterone is a key component of the hypothalamic–pituitary–adrenal axis and the surgical stress response. Exogenous administration of aldosterone further aggravated developmental outcomes of neonatal anesthesia with sevoflurane.15
Sevoflurane and isoflurane, despite acting via common cellular mechanisms, such as an enhancement of γ-aminobutyric acid type A (GABAA) receptor activity and activation of two pore domain potassium leak channels, exhibit substantial differences in their actions.26 Among others, isoflurane inhibits a major component of the excitatory glutamatergic synaptic transmission, postsynaptic N-methyl-D-aspartate receptors,27–29 while sevoflurane may not produce this effect.30 The differences in the side effects of these two otherwise very similar anesthetics with relatively well studied mechanisms of action, if detected, would provide further insight in to the relative safety profile of the two anesthetics and the mechanisms mediating the side effects of general anesthesia in neonates. Therefore we compared acute and delayed effects of equipotent exposures to isoflurane and sevoflurane in a neonatal rat model.
Materials and Methods
Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee (Gainesville, FL). Sprague Dawley rats were studied. To control for litter variability we used several pups from each litter for different treatment conditions. At the beginning of each experiment, the pups were determined to be well nourished as judged by their stomachs being full of milk (detectable through the transparent abdominal wall). Different sets of animals were used in each given experiment.
Anesthesia and electroencephalogram recording
In order to study the effects of isoflurane and sevoflurane, anesthesia was induced in a small temperature controlled chamber with 3.4% isoflurane (6% sevoflurane) and 1.5 L/min oxygen over 3 min, and maintained with 1.2% isoflurane (2.1% sevoflurane) and 1.5 L/min oxygen over 60 min. Anesthesia gas monitoring was performed using a calibrated Datex side stream analyzer (Datex-Ohmeda, Helsinki, Finland) that sampled from the interior of the animal chamber. According to Orliaguet et al.,31 1.2% isoflurane and 2.1% sevoflurane lie near 0.6 minimum alveolar concentration for P4–P7 rats. At the doses of 2.1 % sevoflurane and 1.2% isoflurane, rat pups appeared to be fully anesthetized in the absence of surgical procedures. Blood glucose levels after 6 hrs of anesthesia with 1.2% isoflurane and 2.1% sevoflurane were 120.3±3.7 (n=6) and 125.3±3.8 (n=3), respectively. To study the effects of isoflurane and sevoflurane on cortical activity, rat pups ranging from postnatal day 4 to day 6 (P4–P6) were instrumented for electroencephalogram recording as described previously.10,15 In brief, during a 12–15 min long minor surgical procedure that was performed under isoflurane anesthesia (1.6–2.0%) four electrodes of the headmounts of the electroencephalogram /electromyogram system (Pinnacle Technology, Lawrence, KS) were implanted. No obvious differences in electroencephalographic activity were detected when electroencephalogram electrode implantation was done either immediately prior or 1 to 2 days before start of electroencephalogram recording. Electroencephalogram patterns characterized by an amplitude at least three times higher than the baseline and rhythmic (>2 Hz) activity that lasted for at least 3 s and abruptly reverted to baseline were defined as seizure-like electroencephalogram patterns.32 In most cases these patterns started as high frequency-low amplitude activity that developed to increased amplitude and decreased frequency and then abruptly reverted to baseline activity. The seizure-like electroencephalogram patterns were detected visually by at least three independent reviewers and consensus was reached for summary data. Animals that exhibited episode(s) of seizure-like electroencephalogram patterns before the start of anesthesia were not included in the data analysis.
Determination of activated cleaved caspase-3 using Western blot
The levels of activated caspase-3 in the cerebral cortex were determined as described previously.10,15 Western blot analysis for tissue samples from each animal was done in triplicate and reported as an average.
Measurements of acoustic startle response and prepulse inhibition of startle
The PPI of startle tests were performed using the SR-Lab startle apparatus (San Diego Instruments, San Diego, CA) as previously described by our laboratory.15 The %PPI for each prepulse intensity was calculated using the following formula: %PPI=100×[(pulse alone) – (prepulse + pulse)]/pulse alone.33 Data were collected as average amplitude of the 1000 ms long recording window.
Immobility behavior testing
Increased immobility in rodents is used as behavioral test for anxiety/depression behavior.34 The rats were video-recorded in a clear Plexiglas chamber (28 cm diameter by 30.5 cm high). Each rat was placed in the chamber alone during the video recording. A camera was focused on the rat, providing a close-up view of the rat’s body. The behavior of each rat was analyzed during a period of 10 minutes. Immobility was defined as a sudden pause of all locomotion lasting at least 10 seconds. Termination of immobility was defined as the consecutive movement of the two front paws in a walking manner. The data are presented as total time spent in an immobile state.
Drugs
Isoflurane and sevoflurane were manufactured by Minrad Inc. (Bethlehem, PA) and Fushimi-machi (Osaka, Japan), respectively. Bumetanide (Ben Venue Laboratories, Inc.) was purchased from Bedford Laboratories™ (Bedford, OH). Cleaved caspase-3 antibodies were acquired from (Cell Signalling, Danvers, MA), and horseradish peroxidase conjugated goat anti-rabbit and anti- γ-tubulin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Statistical Analysis
Values are reported as mean ± SEM. SigmaStat 3.11 software (Systat Software, Inc, Point Richmond, CA) was used for statistical analyses. Single comparisons were tested using the unpaired t test, whereas multiple comparisons among groups were analyzed using one way ANOVA, followed by Holm-Sidak tests. Changes in PPI of startle for three prepulse intensities in multiple groups were analyzed using two way repeated measures ANOVA, followed by Holm-Sidak tests. All comparisons were run as two-tailed tests. A p < 0.05 was considered significant.
Results
Anesthesia with isoflurane, in contrast to anesthesia with sevoflurane, is associated with few seizure-like electroencephalogram patterns, which were not further diminished by pretreatment with bumetanide
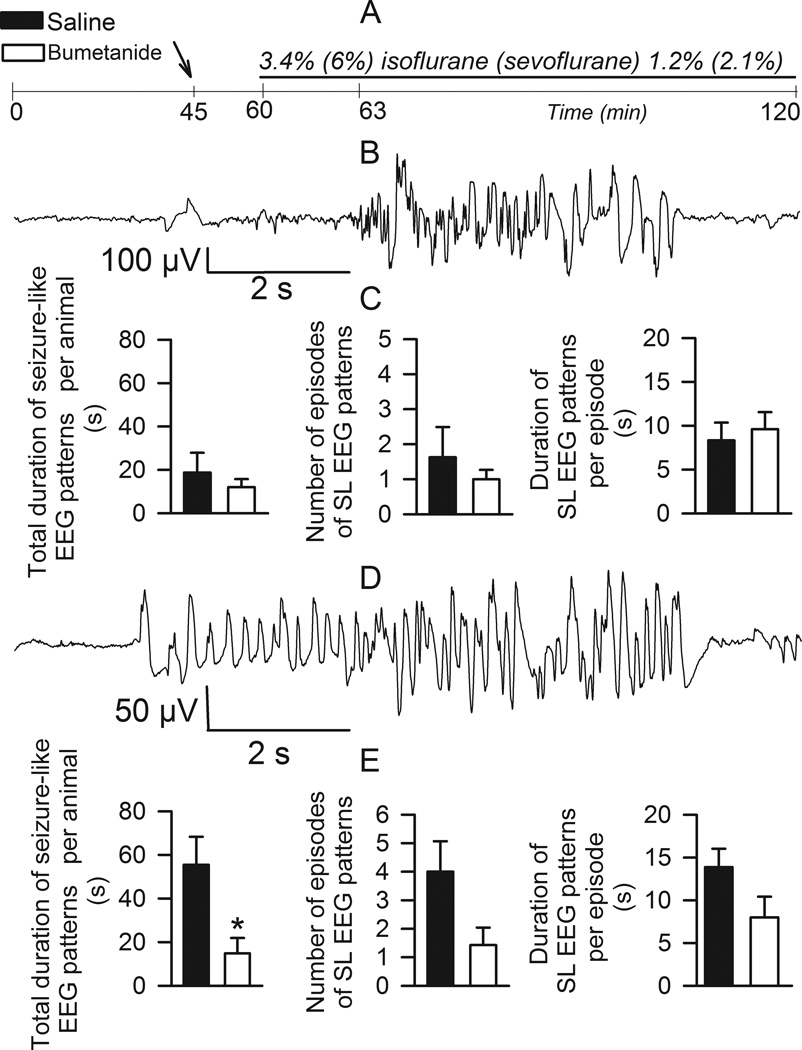
Electroencephalographic activity in postnatal day 4–6 (P4–P6) rats was recorded during two one-hour periods: one hr baseline prior to and then one hr during anesthesia with isoflurane or sevoflurane (Fig. 1A). Fifteen min prior to initiating the anesthetic half of the rats were treated with bumetanide (5 µmol/kg, i.p.) and half received an equal volume of saline. During the 3 min induction period with 3.4% isoflurane one of eight rats pretreated with saline had a 20 s long episode of electroencephalographic activity that met our criteria for a seizure-like electroencephalogram pattern. During the 57 min maintenance period with 1.2% isoflurane, seizure-like electroencephalogram patterns in this treatment group were detected in five of seven rats (Fig. 1B-D). This electroencephalogram-detectable seizure-like activity consisted of 1.9±0.8 episodes with single episodes lasting 9.2±2.1 s and total durations of 21.9±9.3 s. All analyzed parameters of seizure-like electroencephalogram patterns, e.g., total duration, number of episodes and single episode duration, tended to decrease in rats pretreated with bumetanide; however, these effects of bumetanide were not sufficient to achieve statistical significance (Fig. 1B). We compared the effects of bumetanide on sevoflurane-caused electroencephalogram detectable hyperexcitation events in an experiment of the same design, i.e., equipotent concentration of sevoflurane administered to the P4–P6 rats for the same duration of time. The seizure-like activity caused by sevoflurane was more pronounced (Fig. 1E); rats pretreated with bumetanide had significantly shorter total durations of seizure-like electroencephalogram patterns during one hour exposure to sevoflurane compared to rats that received saline prior to exposure to sevoflurane.
Figure 1.
Seizure-like electroencephalogram (EEG) patterns in neonatal rats anesthetized with isoflurane or sevoflurane. A: Illustration of the experimental protocol. B: An example of electroencephalogram recording of seizure-like electroencephalogram pattern in a P6 rat during 1.2% isoflurane anesthesia. C: Histograms showing properties of seizure-like electroencephalogram (SL EEG) patterns in neonatal rats anesthetized with 1.2% isoflurane that were pretreated with either bumetanide (5 µmol/kg, intraperitoneally, n=8, filled bars) or equal volume of saline (n=8, open bars). D: An example of electroencephalogram recording of seizure-like electroencephalogram pattern in a P6 rat during 2.1% sevoflurane anesthesia. E: Histograms showing properties of seizure-like electroencephalogram patterns in neonatal rats anesthetized with 2.1% sevoflurane that were pretreated with either bumetanide (5 µmol/kg, intraperitoneally, n=7, filled bars) or equal volume of saline (n=6, open bars). *, P<0.05 vs. total duration of seizure-like electroencephalogram patterns in the saline-pretreated rats.
Isoflurane increases levels of activated caspase-3 in the cerebral cortex, an effect partially diminished by pretreatment with bumetanide
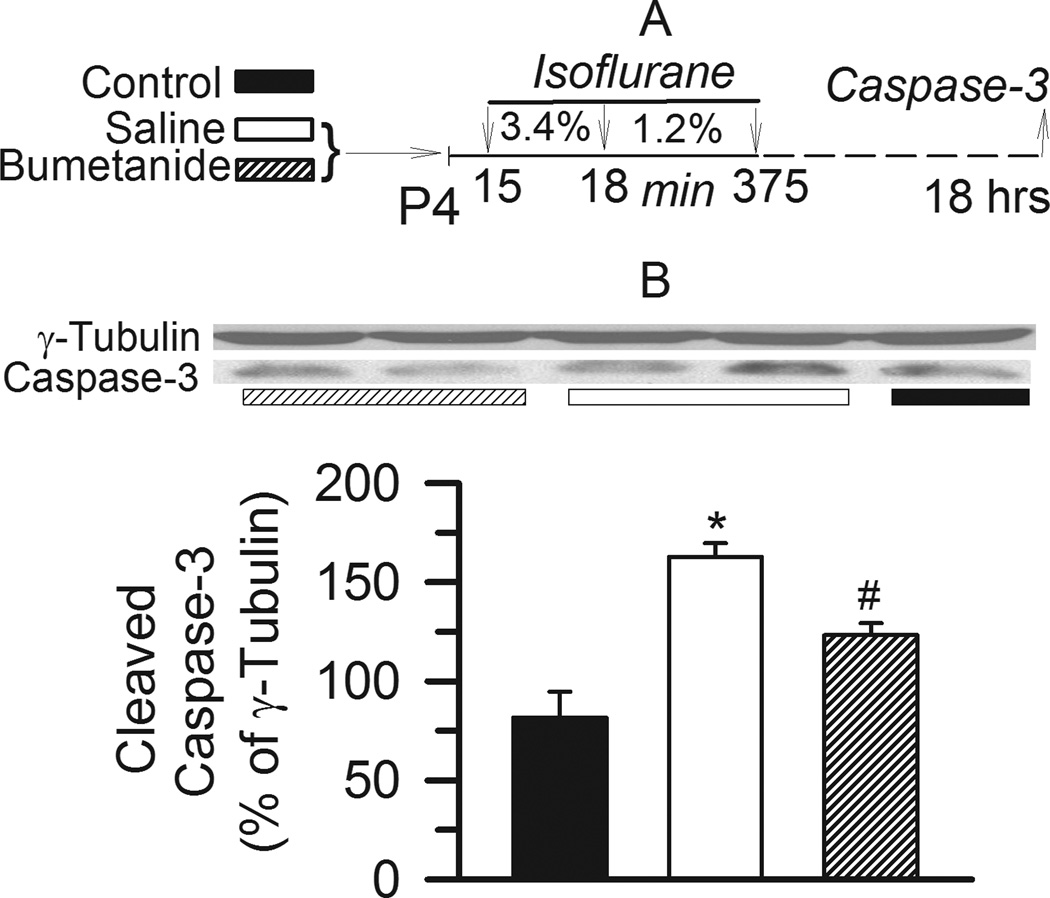
Similar to our recent published study with sevoflurane,15 Western blot analysis revealed increased levels of activated caspase-3 in the cerebral cortex of P5 rats exposed to 6 hrs of anesthesia with isoflurane one day earlier. The rats that were pretreated with bumetanide (5 µmol/kg, intraperitoneally) prior to exposure to isoflurane had lower levels of activated caspase-3 in comparison with those pretreated with saline and anesthetized with isoflurane (F(2,23)= 22.697, P=0.001 versus control and bumetanide groups; Fig. 2A,B). Pretreatment with bumetanide prior to anesthesia with isoflurane did not completely prevent the increase in levels of activated caspase-3 caused by isoflurane (P=0.002 versus control).
Figure 2.
Increased levels of activated caspase-3 in the cerebral cortex of neonatal rats that were anesthetized with isoflurane. A: Illustration of the experimental protocol. B: Representative Western blot (WB) images of cleaved caspase-3 and γ-tubulin blots and histogram showing results of the densitometric analysis of cleaved caspase-3 in the cortex tissue from three experimental groups. Rats in the two treatment groups were exposed to isoflurane anesthesia after they were pretreated with either bumetanide (5 µmol/kg, intraperitoneally, n=10) or equal volume of saline (n=10). Rats in the control group (n=6) did not undergo anesthesia on postnatal day 4. Densities of γ-tubulin blots from the same tissue sample were taken as 100%. *, P=0.001 vs control and bumetanide, #,P=0.002 vs control.
Anesthesia of neonatal rats with sevoflurane, but not isoflurane, results in bumetanide-sensitive delayed “immobile state” behavior
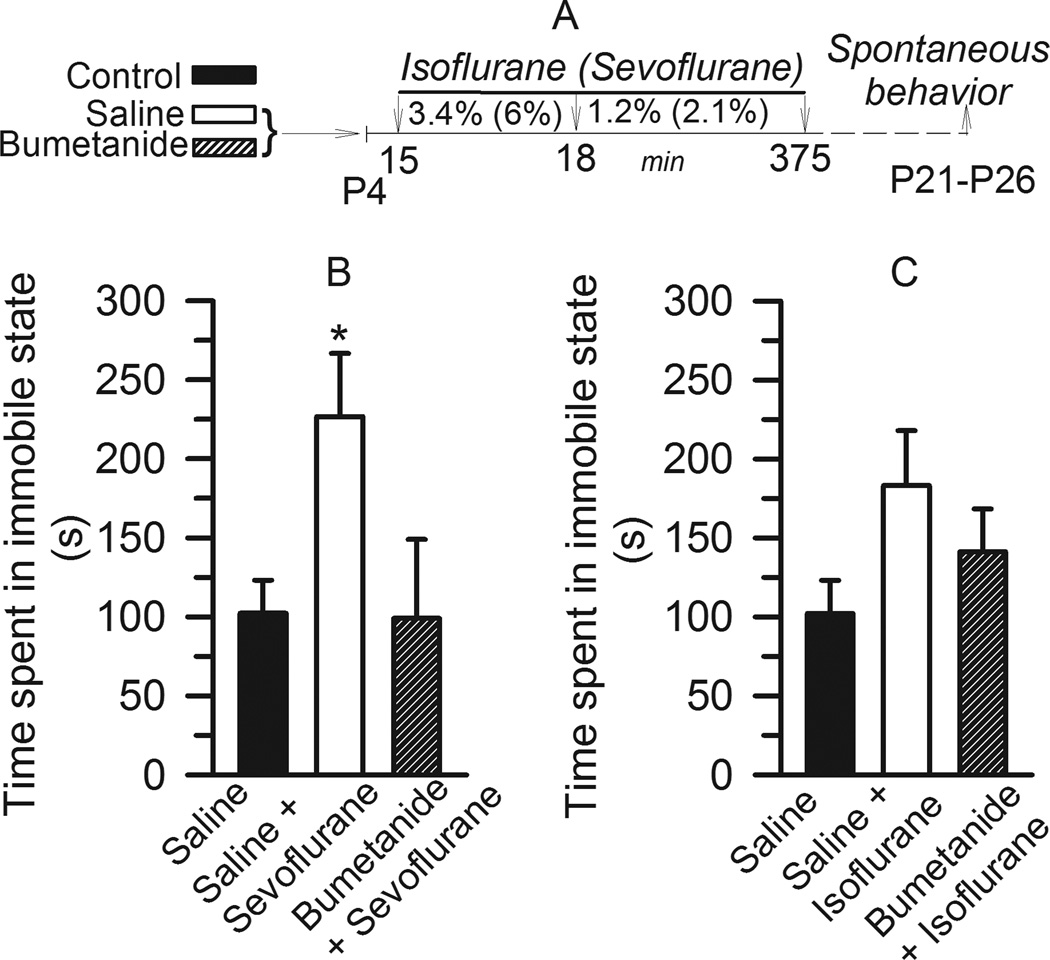
The effects of neonatal anesthesia with isoflurane and sevoflurane at P4–P5 on subsequent spontaneous behavior in rats was tested when the animals achieved 21–26 days of age. Most of the rats were 24 days of age at the time of testing. When exposed to a new environment, rats previously anesthetized with sevoflurane spent significantly longer time in an immobile state compared to non-anesthetized controls (F(2,39)= 4.43, P = 0.018 versus control and bumetanide groups; Fig. 3A,B). No significant differences were observed between the control rats not exposed to anesthesia and those pretreated with bumetanide prior to exposure to sevoflurane at P4–P5 (P=0. 949). Rats anesthetized with isoflurane at an early postnatal age also tended to spend more time in an immobile state when compared with rats that were never exposed to anesthesia or with those pretreated with bumetanide prior to exposure to isoflurane. However, the effects of neither isoflurane nor bumetanide were sufficient to achieve statistical significance (F(2,40)= 2.397, P=0.104; Fig. 3A,C).
Figure 3.
Rats that were anesthetized with sevoflurane on postnatal days 4–5 (P4–P5), but not isoflurane, spent significantly increased time in immobile state; an effect that was alleviated by pretreatment with bumetanide. A: Illustration of the experimental protocols. B: The effect of isoflurane. Histogram showing time spent in immobile state in three experimental groups: Rats in the two treatment groups were exposed to isoflurane anesthesia after they were pretreated with either bumetanide (5 µmol/kg, intraperitoneally, n=12 per) or equal volume of saline (n=12). Rats in the control group (n=19) did not undergo anesthesia at P4. C: The effect of sevoflurane. Histogram showing time spent in immobile state by three treatment groups: 1) control (n=19); saline + sevoflurane (n=13) and bumetanide + sevoflurane (n=10). *, P<0.05 vs Control. All non-anesthetized rats tested in spontaneous behavior experiments were combined in the control groups.
Isoflurane causes a long-term decrease of PPI of startle in control but not bumetanide-pretreated rat pups
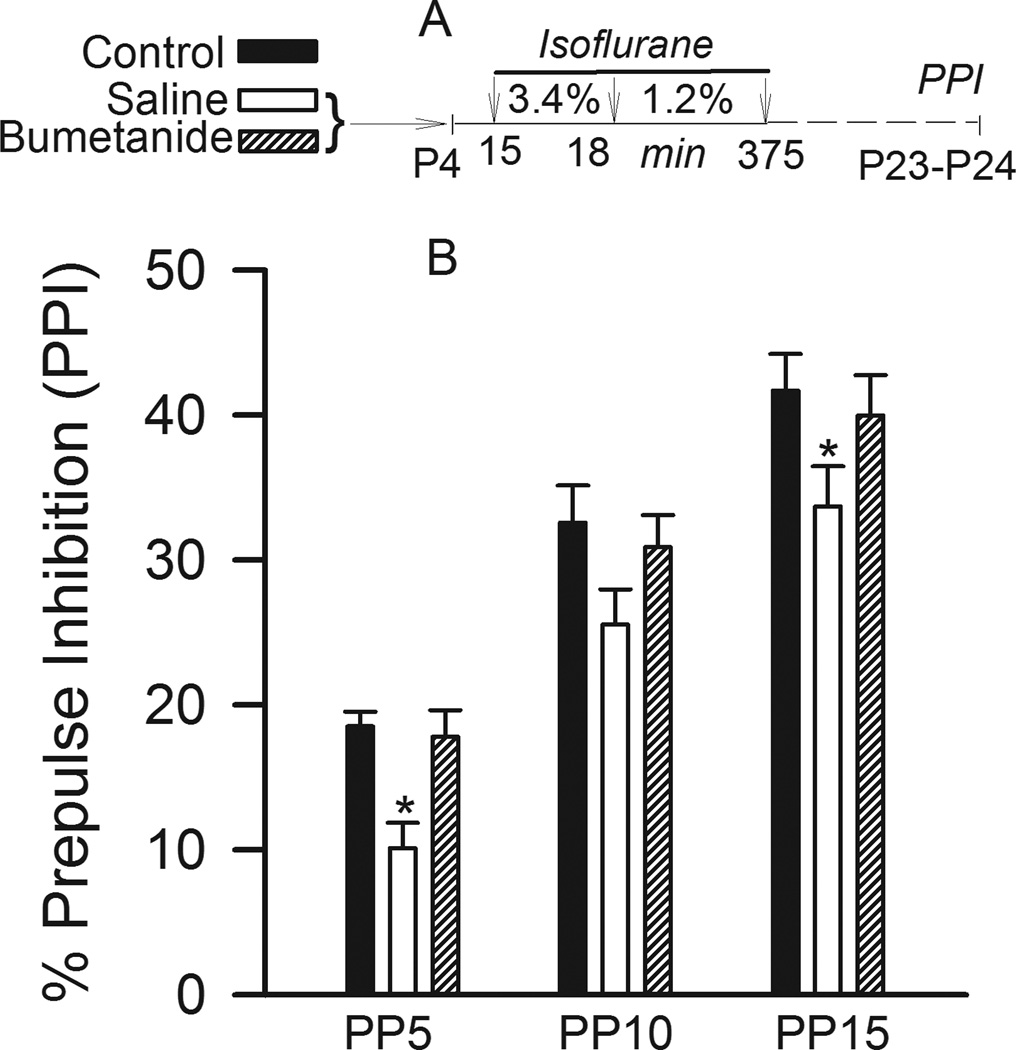
Startle stimuli by themselves caused similar responses in all three treatment groups. Startle response amplitudes were 15.8±1.2, 15.2±1.2 and 16.9±1.4 in non-anesthetized, anesthetized and bumetanide-pretreated anesthetized animals, respectively (Fig. 4A, F(2,36)= 0.506, P=0.607; one way ANOVA). Startle stimuli, presented in combination with preceding prepulse stimuli, inhibited startle responses, but prepulse inhibition of startle was less in animals exposed to isoflurane early after birth. Two way repeated measures ANOVA analysis of PPI of startle found significant effects of prepulse intensities (main effect, F(2,4)= 118.287, P<0.001), and treatment with isoflurane (main effect, F(2,36)= 5.978, P= 0.006, Fig. 4B). The rats that were exposed to isoflurane at P4 exhibited reduced PPI of startle at all prepulse intensities when compared to non-anesthetized animals, although a significant reduction was detected at prepulse intensities of 5 dB and 15 dB, but not of 10 dB. The effect of treatment did not depend on the prepulse intensities (P = 0.980). There was no significant reduction in PPI of startle in animals that received bumetanide (5 µmol/kg, intraperitoneally) prior to isoflurane anesthesia (P=0.571 versus non-anesthetized and P=0.011 versus isoflurane). We have previously demonstrated that a single dose of bumetanide alone without anesthesia did not result in any obvious changes in the acoustic startle response.15
Figure 4.
Isoflurane anesthesia on postnatal days 4–5 (P4–P5) causes abnormalities in prepulse inhibition (PPI) of the acoustic startle response evaluated at P23–P24. Bumetanide (5 µmol/kg, intraperitoneally), administered prior to anesthesia, alleviated the effect of isoflurane on PPI of acoustic startle. A: Illustration of the experimental protocol. Rats in the control groups did not undergo anesthesia on P4–P5. B: Histogram showing %PPI of startle in different treatment groups: control (n=11), saline + isoflurane (n=14) and bumetanide + isoflurane (n=15). *, P<0.05 vs Control. PP5–PP15 – prepulse intensities in dB above background.
Discussion
We have found that in neonatal rats isoflurane, similar to previously reported effects of sevoflurane,15 caused a number of both acute and delayed adverse effects: seizure-like electroencephalogram patterns, neuroapoptosis in the cerebral cortex (measured as increased levels of activated caspase-3), and impairment of sensorimotor gating function. The latter could be detected weeks after exposure to the anesthetic. Our results also indicate that the effects of isoflurane and sevoflurane show important differences. Isoflurane caused less pronounced seizure-like electroencephalogram patterns and non-significant increases in “immobility” behavior. Furthermore, bumetanide, the NKCC1 inhibitor, did not alter the effects of isoflurane on electroencephalographic activity in rats. Pretreatment with bumetanide, however, decreased the isoflurane-caused impairment of PPI of the startle response, as previously seen in the sevoflurane-anesthetized animals.15 Bumetanide partially diminished an increase in the levels of activated caspase-3 in the cerebral cortex caused by isoflurane.
Seizure-like electroencephalogram patterns detected during anesthesia of neonatal rats with sevoflurane and isoflurane indicate that the anesthetics may increase neuronal cortical activity at this age. These findings taken together with the effects of bumetanide in the isoflurane- and sevoflurane-anesthetized neonatal rats suggest that the anesthetic-caused apoptotic cell death and long-term functional abnormalities (impairment of sensorimotor gating function and increase in immobility patterns in rats previously anesthetized with sevoflurane) result, at least in part, from the anesthetic-caused increase of neuronal activity. Importantly, our findings do not link the anesthetic-caused seizure-like electroencephalogram patterns as prerequisites to neuronal death and functional effects, as well as neuronal death to delayed functional abnormalities. Although current understandings of the developmental neurophysiology, mechanisms of action of volatile anesthetics and neuronal effects of bumetanide make GABAA receptor-mediated signaling the most plausible candidate for mediating the anesthetic-induced neuronal stimulation in the developing brain, the results of our experiments with bumetanide do not exclude other potential mechanism(s) contributing to the anesthetic-caused increase of neuronal activity in the developing brain. Our pilot studies suggest that the anesthetic-activated hypothalamic–pituitary–adrenal axis may represent one of such mechanisms. It is plausible that the-anesthetic-increased neuronal activity to certain level, independent of the mediating mechanisms, is sufficient for the observed side effects to occur. Bumetanide, by decreasing GABAA receptor-mediated excitation or promoting GABAA receptor-mediated inhibition, decreases total neuronal activity in the developing brain and alleviates the anesthetic-caused side effects. In line with this, less pronounced seizure-like electroencephalogram patterns caused by isoflurane may be the result of less neuronal stimulation by isoflurane due to inhibitory actions of the anesthetic via additional pathways that are not engaged by sevoflurane. For example, sevoflurane may be less potent than isoflurane at inhibition of N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subtype of postsynaptic glutamate receptors 27–29 or may even not have any effect on functioning of these receptors.30 The inhibitory profile of isoflurane is further supported by its depression of presynaptic glutamate release.31 Still, the alleviating effects of bumetanide on isoflurane-caused increases in levels of activated caspase-3 and impairment in sensorimotor gating function suggests a role of the anesthetic-caused neuronal stimulation in its developmental effects. Previously, Sanders and colleagues35 observed cell death in mouse organotypic hippocampal slice cultures exposed to a combination of 0.75% isoflurane and 50 µM GABAA receptor antagonist SR95531 (gabazine), calling into question the involvement of GABAA receptor mediated-signaling in the neurotoxic effects of isoflurane. Given that slice cultures with substantially altered/eliminated extrinsic inputs/outputs and hormonal/neurotransmitter controls represent a simplified model of the in vivo environment, it is difficult to directly compare our in vivo measurements with bumetanide and the measurements with gabazine in slice cultures.35 Nonetheless, the results with gabazine may support our hypothesis on the role of increased neuronal activity in the adverse effects of isoflurane and sevoflurane in the developing brain, since treatment of P10–P11 hippocampal slices with gabazine in the presence of isoflurane is likely to increase neuronal activity, though gabazine alone in the absence of the anesthetic did not cause any effect.
A possibility that isoflurane may cause alterations in the sensorimotor gating function in neonatal rats by acting at GABAA receptors is indirectly supported by the findings reported by other laboratories that phenobarbital and allopregnanolone, both positive GABAA receptor modulators, caused long-term impairment of PPI of startle after single administration to neonatal rats.36,37 A recent study found that reduction of PPI of startle in juvenile subjects may be a predictor of predisposition to schizophrenia before other symptoms of the illness can be detected.38 Sensorimotor gating deficits are characteristic of a number of other human neuropsychiatric diseases, 39,40 raising the possibility that subjects predisposed to these disorders may be more susceptible to the developmental effects of isoflurane and sevoflurane.
In summary, these findings in combination with our previous reports10,15 suggest that isoflurane and sevoflurane may produce developmental effects acting via similar mechanisms involving an increase in neuronal activity. At the same time, substantial differences in the effects of isoflurane and sevoflurane in neonatal rats suggest differences in the mechanisms mediating their actions and, more importantly, potentially also on the relative safety profile of the two anesthetics for neonatal anesthesia. If these findings translate to human then by extension isoflurane may be preferred over sevoflurane as an anesthetic for very young pediatric patients, especially to those predisposed to epileptic seizures.
Final Boxed Summary Statement.
What we already know about this topic
-
*
Whether early posnatal exposure to volatile anesthetics produces long term neurodevelopmental abnormalities in children remains a matter of debate.
-
*
Sevoflurane and isoflurane, despite acting via common cellular mechanisms, exhibit substantial differences in their actions.
What this article tells us that is new
-
*
At subanesthetic concentrations isoflurane and sevoflurane produce developmental effects in neonatal rats acting via similar mechanisms that may involve an increase in neuronal activity. At the same time, substantial differences in the effects of the two drugs suggest differences in the mechanisms mediating their actions and in their safety profile for neonatal anesthesia.
Acknowledgment
We would like to thank Wengang Cao, M.D. (Senior Biological Scientist, University of Florida, Gainesville, Florida) for technical assistance. We would like to acknowledge technical contribution of Phillip Sussman, B.S. (Student, University of Florida, Gainesville, Florida), Nikolaus L. Gravenstein, B.S. (Medical Student, University of Florida, Gainesville, Florida) and Bruno Panzarini (Undergraduate Student, University of Florida, Gainesville, Florida).
Sources of financial support: Supported by grant No. R01 GM93036-01A1 from the National Institute of Health/ National Institute of General Medical Sciences, Bethesda, Maryland (to AEM), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (to NG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Department/institution to which the work is attributed: Department of Anesthesiology, University of Florida, Gainesville, Florida
Meetings at which the work has been presented: ASA Annual Meeting, Chicago, IL, October 16, 2011.
References
- 1.Davidson AJ. Neurotoxicity and the need for anesthesia in the newborn: Does the emperor have no clothes? Anesthesiology. 2012;116:507–509. doi: 10.1097/ALN.0b013e3182475673. [DOI] [PubMed] [Google Scholar]
- 2.Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–37. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loepke AW. Developmental neurotoxicity of sedatives and anesthetics: A concern for neonatal and pediatric critical care medicine? Pediatr Crit Care Med: 2010;11:217–226. doi: 10.1097/PCC.0b013e3181b80383. [DOI] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Xue Z, Sun A. Subclinical concentration of sevoflurane potentiates neuronal apoptosis in the developing C57BL/6 mouse brain. Neurosci Lett. 2008;447:109–114. doi: 10.1016/j.neulet.2008.09.083. [DOI] [PubMed] [Google Scholar]
- 6.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 7.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 8.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 9.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- 11.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 14.Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 15.Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012;117:791–800. doi: 10.1097/ALN.0b013e318266c62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 17.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–704. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimaggio C, Sun L, Li G. Early Childhood Exposure to Anesthesia and Risk of Developmental and Behavioral Disorders in a Sibling Birth Cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–812. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 22.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–253. doi: 10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 23.Sprung J, Flick RP, Wilder RT, Katusic SK, Pike TL, Dingli M, Gleich SJ, Schroeder DR, Barbaresi WJ, Hanson AC, Warner DO. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–310. doi: 10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 25.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 26.Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: Linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–3679. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 28.Brosnan RJ, Pham TL. Does anesthetic additivity imply a similar molecular mechanism of anesthetic action at N-methyl-D-aspartate receptors? Anesth Analg. 2011;112:568–573. doi: 10.1213/ANE.0b013e3182080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stucke AG, Zuperku EJ, Tonkovic-Capin V, Krolo M, Hopp FA, Kampine JP, Stuth EA. Sevoflurane depresses glutamatergic neurotransmission to brainstem inspiratory premotor neurons but not postsynaptic receptor function in a decerebrate dog model. Anesthesiology. 2005;103:50–56. doi: 10.1097/00000542-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–739. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–739. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 32.White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods. 2006;152:255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2003 doi: 10.1002/0471142301.ns0817s24. Chapter 8:Unit 8.17. [DOI] [PubMed] [Google Scholar]
- 34.Pálenícek T, Hlinák Z, Bubeníková-Valesová V, Votava M, Horácek J. An analysis of spontaneous behavior following acute MDMA treatment in male and female rats. Neuro Endocrinol Lett. 2007;28:781–788. [PubMed] [Google Scholar]
- 35.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 36.Darbra S, Pallarès M. Alterations in neonatal neurosteroids affect exploration during adolescence and prepulse inhibition in adulthood. Psychoneuroendocrinology. 2010;35:525–535. doi: 10.1016/j.psyneuen.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Bhardwaj SK, Forcelli PA, Palchik G, Gale K, Srivastava LK, Kondratyev A. Neonatal exposure to phenobarbital potentiates schizophrenia-like behavioral outcomes in the rat. Neuropharmacology. 2012;62:2337–2345. doi: 10.1016/j.neuropharm.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziermans TB, Schothorst PF, Sprong M, Magnée MJ, van Engeland H, Kemner C. Reduced prepulse inhibition as an early vulnerability marker of the psychosis prodrome in adolescence. Schizophr Res. 2012;134:10–15. doi: 10.1016/j.schres.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 40.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]