Abstract
Mullerian Inhibiting Substance (MIS) has been shown to inhibit ovarian cancer cells both in-vitro and in-vivo. Furthermore, recent evidence suggests that MIS may effectively target a putative ovarian cancer progenitor cell population enriched by a panel of CD44+, CD24+, Ep-CAM+, and E-cadherin-cell surface markers. In order to accommodate clinical testing of MIS in ovarian cancer patients, the production of recombinant human MIS must be optimized to increase yield and purity. Here we show that, compared to wild type, the substitution of the MIS leader sequence to that of human serum albumin, combined with a modification of the endogenous cleavage site from RAQR/S to a furin/kex2 RARR/S consensus site results in high expression, increased C-terminus cleavage and a reduction in unwanted cryptic internal cleavage products when produced in CHO cells. Purified MIS containing these alterations retains its capacity to induce regression of the Mullerian duct in fetal rat embryonic urogenital ridge assays.
INNOVATION
Various efforts to produce recombinant therapeutic proteins of the TGF-Beta family have proven to be challenging because they require a complex maturation process involving pre-pro protein cleavage, dimerization, and glycosylation. Previous attempts have been plagued by low production, poor cleavage, and lack of homogeneity, even in mammalian cells. Here we show the effect of C-terminal tagging, modifications of the cleavage site, and substitutions of the leader peptide on the production of MIS. Interestingly, the modification of the cleavage site to a more consensus kex-like sequence, and the addition of a serum albumin leader peptide, strongly increases the yield of cleaved mature C-terminal MIS, while reducing internal cryptic cleavage. The modifications herein described, which may be applicable to other TGF-beta family members, will help meet the scientific and clinical demand for active recombinant C-terminal MIS for applications such as cancer and neurodegenerative diseases.
INTRODUCTION
There is a growing need for a standard method of preparation and purification of Mullerian Inhibiting Substance (MIS) protein with documented bioactivity in order to judge preclinical efficacy1, since MIS is being measured in many infertility clinics around the globe, and clinical indications for its use are growing and compelling2. Although preparations expressed from E. coli are available commercially, their bioactivity in regression of the Mullerian duct has not been documented. The present study of MIS was undertaken to establish a preparation that demonstrated prohormone conversion, dimerization, improved production of a preclinical product with relative homogeneity in anticipation of FDA requirements, and, most importantly, one with retained bioactivity. We have previously produced human MIS (hMIS) from conditioned media from CHO cells transfected with a human wild type genomic clone3. The media has then been immunoaffinity purified4 using a mouse monoclonal antibody to human MIS5 or purified by serial chromatography6. Biological activity for this purification was confirmed in an embryonic organ culture Mullerian duct regression assay7 and concentration was measured by an ELISA5 using monoclonal and polyclonal antibodies raised to human MIS. The transfected CHO cells were subsequently adapted to serum free conditions and suspension culture (MacLaughlin, Stafford, Dean, Donahoe unpublished), clonally selected, scaled, and purified as above. However, Western analysis demonstrated only 25–30% cleavage to yield the homodimerized C-terminus bioactive moeity which was held in noncovalent association with the homodimerized N-terminus. Cleavage occurred at the Kex-like, primary cleavage site at p427–428, and secondary cleavage at position p227–228. Although bioactive, multiple bands were detected on reduced electrophoretic gels at 70, 55, 34, 22, and 12.5 kDa. Each band, however, was an MIS fragment as determined by amino acid sequencing4,6 and represented the predicted Kex and dibasic cleavage products. Their presence, nonetheless, was a strong deterrent to clinical scale up.
The present studies were undertaken using cDNA vectors to optimize production and cleavage, and tags were incorporated for both detection and purification. Position 428 was mutagenized previously to create a dibasic RAQR/R variant cleavage site which was bioactive8. Position 426 was more recently mutagenized9 to create a more consensus Kex cleavage site10,11, RARR/S, and an 8 amino acid Flag (DYKDDDDK) tag to aid in detection and purification was added just downstream of the C-terminal serine which was required for activity9. Although, the RARR/S Flag construct transfected into CHO cells displayed improved cleavage with preservation of bioactivity in HEK293 cells9, this preparation expressed poorly in CHO cells and could not be scaled in HEK cells. Further cloning in CHO cells was undertaken and a rare clone (1/400) was recovered successfully (CHO93) and expanded for production.
To scale expression, the MIS RARR/S Flag construct was further modified to substitute the endogenous MIS leader sequence with that of human serum albumin (HSA), the most abundant plasma protein which is secreted at a very high rate by the liver to achieve a blood concentration ranging from 3.4 to 5.4 g/dL12. The production and processing of HSA is finely tuned to allow efficient maturation and secretion of the protein. HSA, like MIS, is synthesized as a pre-pro protein, which contains a leader sequence that is subsequently cleaved during maturation. The HSA leader sequence consists of only 24 AA, has the advantage of being non-immunogenic in humans, and is removed during protein processing. Here we show that substitution of the MIS leader sequence with that of HSA increases production, and unexpectedly increases C-terminal cleavage while reducing unwanted cryptic cleavage, which results in increased potency of the recombinant human MIS product13. Furthermore, the smaller cDNAs are compatible with expression in viral backbones for gene therapy.
METHODS
Constructs and plasmid cloning
RF-MIS: pcDNA 3.1 and pAAV-IRES-NEO vectors containing MIS cDNA with native MIS leader sequence, modified cleavage site (R), and flag tag (F). The coding sequence of MIS, present in a pcDNA3.1 vector containing full-length human MIS cDNA sequence previously described9, was subcloned into a pAAV-IRES-Neo expression vector at an ECORV site. This coding sequence contains a FLAG-epitope inserted after a modified cleavage site at position 428 (RARR/S) FLAG.
LR-MIS: pcDNA 3.1 vector containing MIS cDNA with human albumin leader sequence (L) and modified cleavage site (R). The pcDNA3.1 vector containing a full-length human MIS cDNA sequence containing the RARR/S modified cleavage site was used to incorporate the albumin leader sequence and cloned in place of the MIS leader using a forward primer containing an EcoRV site CGAGATACATGAAGTGGGTGAGCTTCATCAG-CCTGCTGTTCCTGTTCAGCAGCGCTTACTC-C C G C G G TG T G T T C C GGCGC A G A G C A G A G - GAGCCAGCTGTG and a backward primer at position 451-432 of MIS GCTCCTGGAACCTCAGCGAG.
LRF-MIS: pcDNA 3.1 vector containing MIS cDNA with human albumin leader sequence (L), modified cleavage site (R), and Flag tag (F). The pcDNA3.1 vector containing a full-length human MIS cDNA sequence containing the modified cleavage site and a FLAG tag was used to incorporate the albumin leader sequence as described above.
WT-MIS: pBG311 vector with genomic MIS (WT-MIS). This vector, originally constructed from the genomic sequence of human MIS and sub-cloned into a pBG311 expression vector, was used as a control3.
Transfections and cloning
-
RF-MIS
The RF-MIS construct (in pAAV-IRES-NEO) was transfected in CHO-S cells using Fugene 6 (Roche) according to the manufacturer’s protocol and the CHO93 stably expressing clone was selected under neomycin selection (550 μg/ml) as the highest producers of the rare number of expressers, as determined by western blot.
-
LR-MIS
The LR-MIS construct (in pcDNA3.1) was transfected in CHO-K1 cells using lipofectamine 2000 (Invitrogen). Multiple clones were selected in 800 μg/ml of G418, and the highest expressers, as determined by western blot (LR8, 11, and 22), were chosen for further study.
-
LRF-MIS
The LRF-MIS construct (in pcDNA3.1) was transfected in CHO-K1 cells using lipofectamine 2000. Multiple clones were selected in 800 μg/ml of G418, and the highest expressers, as determined by western blot (LRF8, 18, and 22), were chosen for further study.
-
WT-MIS
The WT-MIS construct (pBG311) along with pSV2DHFR was transfected in DHFR-deficient CHO cells and the B9 clone was selected as the highest expresser, as previously described3 for use as a control.
Media and culture conditions
-
RF-MIS: CHO93 clone
CHO93 was grown in fluted roller bottles (1700 cm2) (Corning) with 200 ml of DMEM:F12 supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich), 550 μg/ml of G418, 2 nM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen); the cells remained confluent for several months in 5% CO2, at 37°C, while media was collected every 3–4 days and media spun to remove cells and debris.
Media was screened by western using the monoclonal antibody to FLAG M2 (Sigma Aldrich) or to human MIS C-terminus (Santa Cruz), and N-terminus (MGH4 and MGH6 polyclonal antibodies) and by MIS ELISA using an additional 6E11 mouse monoclonal antibody to holo-MIS5 to monitor and measure production. Media with sustained production by western was stored at −20°C for further purification.
-
LR-MIS and LRF-MIS: LR8, 11, 22 and LRF8, 18, 22 clones
Both LR and LRF clones were grown in fluted roller bottles (1700 cm2) with 200 ml of DMEM supplemented with 10% FCS, 800 μg/ml of G418, 2 nM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen); the cells were maintained at confluency for several months in 5% CO2, at 37°C, while media was collected every 3–4 days. Media was spun to remove cells and debris, screened by western and MIS ELISA to monitor and measure production, and stored at −20°C.
-
WT MIS: B9 clone
B9 was grown in fluted roller bottles (1700 cm2) with 200 ml of alpha MEM-supplemented with 5% female fetal calf serum (FFCS) (Biologos) to avoid contamination by bovine endogenous MIS, 0.24 μM methotrexate, 2 nM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen) and remained confluent for several months in 5% CO2, at 37°C while media was collected every 3–4 days.
Purification of MIS
-
Immunoaffinity purification using anti-Flag beads
RF-MIS and LRF-MIS, which contain a FLAG tag, were isolated from serum-containing media collected from roller bottles of stably expressing clones of CHO (CHO93, LRF8, LRF18, LRF22) as described above. Collected media was spun down to discard cells and the supernatant collected into 500 ml or 250 ml containers and stored in −20°C until purification. Only batches of media validated by western blot to have high levels of C-terminal MIS were selected for purification. For purification, media was thawed at 4°C overnight and then incubated with anti-FLAG agarose beads (SIGMA, 500 μl packed beads/500 ml media), mixing with rotation overnight at 4°C in two steps, first 250 ml with 500 μl packed beads, then another 250 ml with the same beads. Subsequently, the beads were spun at 4000 rpm, for 5 minutes and washed extensively (7X) with cold 1X Tris Buffered Saline (TBS) (SIGMA). All reagents are kept on ice. RF-MIS and LRF-MIS was eluted with 50 μg of 3X FLAG peptide (SIGMA)/500 μl beads in 500 ul 1X TBS at 25°C for 45 minutes with rotation. The beads are spun at 13,000 rpm for 10 seconds at room temperature and the supernatant containing the FLAG MIS collected, aliquoted at 200 μl, and stored in low protein binding Eppendorf tubes (VWR) at −80°C. Only those with 3 to 5+ bioactivity in the Mullerian duct regression assay were maintained for subsequent use.
-
Immunoaffinity purification using anti-MIS 6E11 column
The 6E11 MIS monoclonal 5 ml immunoaffinity antibody column was constructed using 50 mg of protein A/G-sepharose (Sigma Chemical Co., St Louis, MO)- purified mouse monoclonal anti-human rhMIS antibody5 and modified as follows. 6E11 producing cells were first adapted to grow in serum free media (CD hybridoma media, GIBCO) and the protein A/G purified 6E11 antibody dialyzed and confirmed by gel electrophoresis, then covalently attached to 5 ml packed Affigel-10 agarose resin (Biorad Laboratories, Richmont, CA) per manufacturer’s instructions (up to 80% coupling efficiency). The column was blocked with 0.1 M ethonalamine and equilibrated with 50 ml of 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes), pH 7.4 and 200 ml concentrated (10X, serum free) conditioned media loaded at 1 column vol/h at 4°C4,5. Alternatively, one can use the batch method where the 6E11 beads are directly incubated with the conditioned media at a ratio of 1 ml of packed beads per 500 ml of media and rotated at 4°C for 12 h, and proceed identically as described bellow. After loading, the column was washed with 10 column volumes of 20 mM Hepes, pH 7.4. A pre-elution step employed 1 column volume containing 0.5 M NaCl in 20 mM Hepes, pH 7.4. Elution of bound rhMIS was achieved using 1 M acetic acid in 20 mM Hepes, pH 3.0. The majority of the rhMIS eluted in a 2–5 ml fraction, after a 2 ml void volume fraction. Eluted rhMIS was immediately neutralized with NaOH to a pH between 7.0 and 7.4 and the acid eluted fractions dialyzed overnight versus PBS. The resulting rhMIS from both purification methods was analyzed for total protein by the Bradford method and for rhMIS concentrations by ELISA5, then examined by polyacrylamide gel electrophoresis, and western blot analysis. Bioactivity was then confirmed in vitro in Mullerian duct regression bioassays7, and only those with 3 to 5+ regression maintained for subsequent use.
ELISA
ELISAs were performed using the antibody “sandwich” method using plates coated with anti-holo MIS 6E11 mouse monoclonal antibody (which recognizes cleaved and uncleaved products) and the MGH6 rabbit polyclonal anti holo-MIS (which recognizes cleaved and uncleaved products) as reported previously5. In detail, Dynatech Immulon 2HB Elisa 96-well flat bottom plates (Thermoscientific, Rochester, NY) were coated overnight at 4°C with mouse monoclonal 6E11 anti-hrMIS antibody (described above) in 0.05 M sodium bicarbonate buffer pH 9.6 (5 μg/ml; 50 μl/well). The plates were washed five times with washing buffer (phosphate buffered saline (PBS)/0.1% Tween 20) (150 μl/well) and blocked with 1% BSA in PBS (IgG-free, protease-free) (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours at room temperature or overnight at 4°C. This blocking buffer was used for all subsequent dilutions wherever mentioned. Plates were sealed to prevent dehydration. The blocking buffer was discarded and the plates were washed with washing buffer. The concentration of the MIS standards were determined by Bradford. MIS standards or unknowns were diluted in blocking buffer and were incubated overnight at 4°C. All sample incubations were done at a volume of 50 μl per well. After five washes with washing buffer, the rabbit polyclonal anti MIS antibody (MGH6; developed in our laboratories) was added at 1:4000 dilution in blocking buffer and was incubated for 1 hour at room temperature. The plates were washed five times with washing buffer. Donkey anti rabbit IgG conjugated to horseradish peroxidase (HRP) (Jackson ImmunoResearch Laboratories, West Grove, PA) was added at 1:70,000 dilution in PBS, and the plates were incubated for 1 hour at 4°C. After five washes with washing buffer, 50 μl of 0.42 mM 3,3′,5,5′,-Tetramethylbenzidine TMB in 0.1 M sodium acetate citric acid (citrate buffer) pH 4.9/0.044% H2O2 was added to each well and the reaction was monitored 12.5 minutes at room temperature in the dark. The reactions were quenched by the addition of 2 N sulfuric acid, and absorbances were read at 595 nm on a microplate reader (Victor2 1420, Perkin Elmer Lifesciences, Shelton, CT). The ELISA of the LR and LRF clones was performed on the media of confluent plates incubated for 24 hours, after which the number of cells was counted to estimate the production (pg/cell/day) which was displayed as the mean of five experiments.
Electrophoresis and western blotting
Samples for gel electrophoresis were reduced with 100 mM dithiothreitol in 1x Laemmli buffer (0.0625 mM Tris pH 6.8, 2% (w/v) SDS stock, 10% (v/v) glycerol, 0.002% (w/v) bromophenol blue) and heat denatured on a thermoblock at 70°C for 10 minutes. Samples were run on a 4–12% Tris-Bis NuPage Novex “mini” gel (Invitrogen) at 130 V with 1X 2-(N-morpholino)ethanesulfonic acid (MES) running buffer (Invitrogen). Gels were stained with Lumitein™ (Biotium) according to the manufacturer’s protocol.
For Western blot analysis, gels were transferred onto PVDF (Millipore) membranes, previously equilibrated in 1x NuPage transfer buffer (Invitrogen) containing 12% (v/v) methanol, at 25 V for 45 minutes and at 35 V for another 45 minutes. Membranes were blocked with 1x PBS, 0.1% Tween-20 containing 5% nonfat dry milk for 30 minutes at room temperature and probed with horseradish peroxidase conjugated mouse monoclonal anti-FLAG M2 antibody (SIGMA) (1:1000), goat C20 anti-holo and C-terminus MIS antibody (Santa Cruz) (1:200), or rabbit MGH4 anti-holo and N-terminus MIS antibody (custom) (1:1000). Blots were washed two times, 5 minutes each at room temperature with 1x PBS, Tween-20 0.1%, and incubated with appropriate secondary antibody if necessary, and then washed three times 5 minutes again. Proteins bands were visualized with the ECL kit detection system (Perkin-Elmer) onto Kodak Biomax MR film. ImageJ (NIH, http://imagej.nih.gov/ij/) was used to perform densitometry to quantify the protein bands to compare cleavage of different constructs14 which was averaged over at least three independent western blots.
Animals and organ cultures
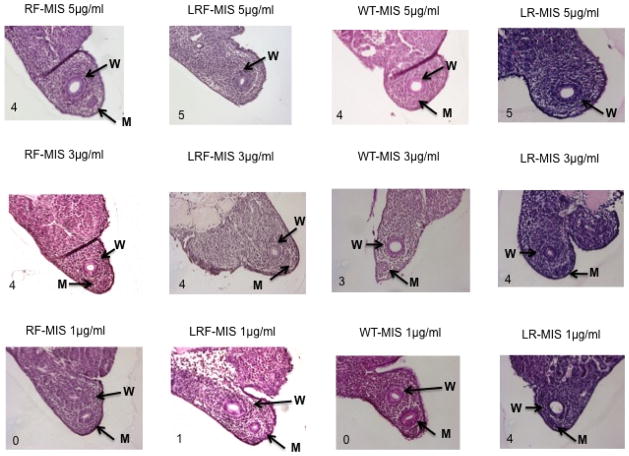
The organ culture bioassay for Mullerian Inhibiting Substance (MIS) was adapted7 as follows. Briefly, female urogenital ridges containing ovary, Wolffian and Mullerian ducts, and intervening mesenephros from timed pregnant rats at E14.5 (Harlan) were dissected. The urogenital ridge from one side of the fetus was used for testing, while the contralateral side of the same fetus was used as a control to ensure that only female ridges were treated. Ridges were carefully transferred for culture on agar coated stainless steel grids mounted above fortified Cambridge Medical Research Laboratories (CMRL) 1066 media (Life Technologies) supplemented with 10% FFCS (to avoid an effect of bovine MIS in male serum), 1% penicillin/streptomycin, 1% L-glutamine, 1% Fungizone (Invitrogen), and 5 nM testosterone (Sigma). After incubation for 72 hours in humidified 5% CO2 at 37°C, the specimens were fixed in Zamboni buffer (15% formaldehyde solution, and 5% picric acid), embedded in paraffin, and processed through a series of fixation and dehydration steps on an automated tissue processor (Technicon) overnight. Approximately 40 sections (8 μm) of the cephalic end (most sensitive) were cut and stained with hematoxylin and eosin. The sections were then scored from 0 (no regression) to 5 (complete regression), by two experienced blinded observers. Cultures were carried out with conditioned media (mock) and with replicates (N of at least 3) of purified RF-MIS, LRF-MIS, LR-MIS, or WT-MIS at a final concentration of 5 μg/ml and at lower doses of 3 μg/ml, and 1 μg/ml. LR-MIS was also tested at concentrations of 0.5 μg/ml and 0.2 μg/ml.
RESULTS
Design of novel recombinant MIS constructs, and isolation of CHO clones
We have developed new constructs using the human cDNA sequence of MIS to improve upon the original wild type (WT) genomic MIS constructs in an effort to increase production of recombinant MIS with a sequence size more amenable to other applications such as viral gene therapy. Three modifications were evaluated herein: a Q426R amino acid substitution in the C-terminal maturation cleavage site annotated as “R”, the addition of a FLAG-tag on the N-terminus of the C-terminal mature peptide at amino acid (AA) position 429 annotated as “F”, and a substitution of the endogenous MIS leader peptide with the human serum albumin leader (HSAL) peptide directly upstream of AA position 0 annotated as “L” (Table 1). The resulting constructs which incorporate these modifications are thus referred to as RF-MIS (566AA), LRF-MIS (567AA) and LR-MIS (559AA) (Fig. 1). The 1AA difference between (568AA) and LRF-MIS results from the fact that the HSAL peptide is 1AA shorter than that of MIS, which otherwise shares 20% identity (Fig. 1). Both the WT genomic MIS, referred to as “WT-MIS”, and the RF-MIS constructs have been previously described9,14. The novel LR-MIS and LRF-MIS transgenes were cloned in a pcDNA3.1 mammalian expression vector, and stably transfected into CHO-K1 cells (Table 2). The three highest expressing clones for the LR-MIS construct (LR8, LR11, LR18), and the LRF-MIS construct (LRF8, LRF18, LRF22) were selected from hundreds of screened clones by comparing MIS level in the media of each clone by western blot (data not shown).
Table 1.
List of modifications to the MIS peptide sequence and corresponding nomenclature.
| Notation | Native | Modification | Position (AA) | Purpose |
|---|---|---|---|---|
| R | RAQR/S | RARR/S | 423–428 | Furin/kex2 consensus site for improved cleavage9,11. |
| F | N/A | Flag tag (DYKDDDDK) | 429–437 | C-terminus flag tag for easier purification and tracking. |
| L | MIS Leader (25AA) | Albumin Leader (24AA) | −25–0 | Increased production, secretion, and cleavage. |
Figure 1.
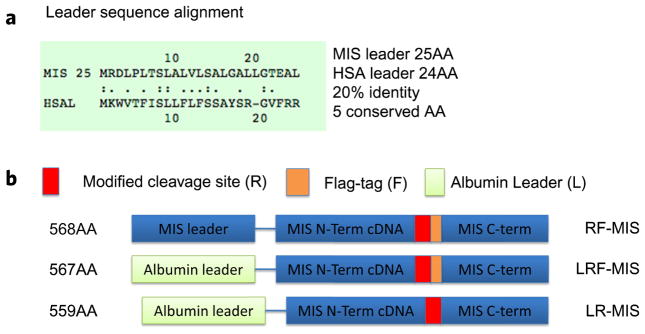
Design of new recombinant MIS constructs with the albumin leader sequence. (a) The leader sequence of MIS (25AA) and albumin (24AA) have 20% identity and 5 conserved AA. (b) The design of the RF, LRF, and LR constructs including the placement of the flag tag (F), the modified cleavage site (R), and the albumin leader (L).
Table 2.
List of constructs and cell line clones producing MIS and corresponding purification methods.
| Constructs | Clones | Vector | Cell line | Purification |
|---|---|---|---|---|
| WT-MIS | B9 | MIS WT genomic sequence in pBG311 plasmid. | CHO cells lacking the DHFR gene | Immunoaffinity using 6E11 monoclonal antibody against MIS. |
| RF-MIS | CHO93 | MIS cDNA sequence inserted into pAAV-IRES-Neo plasmid. | CHO-S | Immunoaffinity using M2 monoclonal antibody against the flag tag. |
| LR-MIS | LR8 LR11 LR18 | MIS cDNA sequence inserted into pcDNA3.1 plasmid. | CHO-K1 | Immunoaffinity using 6E11 monoclonal antibody against MIS. |
| LRF-MIS | LRF8 LRF18 LRF22 | MIS cDNA sequence inserted into pcDNA3.1 plasmid. | CHO-K1 | Immunoaffinity using M2 monoclonal antibody against the flag tag. |
Comparison of expression of MIS-producing clones
The L and R modifications to the peptide sequence were introduced in an effort to enhance secretion, cleavage, and purity, while the F modification is intended to facilitate purification and tracking of the active C-terminal MIS. To examine the results of these modifications we compared levels of expression of the CHO clones. When cultured 24 hours in flasks, the concentration of MIS, as detected in the media by ELISA, is greater in the serum containing media of WT-MIS clone B9 (16.821 ± 3.393 μg/ml) than in the media of LR-MIS clones (LR8: 0.865 ± 0.640 μg/ml; LR11: 4.866 ± 1.238 μg/ml; LR22 1.287 ± 1.027 μg/ml), LRF-MIS clones (LRF8 0.049 ± 0.068 μg/ml; LRF18 2.149 ± 0.479 μg/ml; LRF22 0.054 ± 0.020 μg/ml), and the RF-MIS clone CHO93 (1.236 ± 0.772 μg/ml), with p < 0.001 by one-way ANOVA with Tukey’s post-test. For concentrations of MIS in serum free media, the reference B9 clone (1.528 ± 0.105 μg/ml) produced significantly more than CHO93 (0.223 ± 0.063 μg/ml) (p < 0.01) and LRF18 (0.455 ± 0.254 μg/ml) (p < 0.05), but was not significantly different than LR11 (1.411 ± 0.249 μg/ml) by one-way ANOVA with Tukey’s post-test.
The data for clones B9, CHO93, and the highest expressing clones LR11 and LRF18 are summarized in Table 3. When corrected for the number of cells, the highest producing clone of LR-MIS, LR11, secretes 1.142 ± 0.482 pg/cell/day of MIS, significantly less (p = 0.01) than the WT-MIS clone B9 with 7.597 ± 1.378 pg/cell/day in serum containing media. Furthermore, the highest expressing clone of LRF, LRF18, has both a significantly higher concentration, 2.149 ± 0.479 μg/ml (p = 0.03), and a significantly higher production, 0.430 ± 0.177 pg/cell/day (p = 0.04), than the RF clone CHO93 with 1.236 ± 0.772 μg/ml and 0.254 ± 0.184 pg/cell/day respectively in serum containing media. These trends of expression as detected by ELISA are recapitulated in larger culture vessels (200 ml roller bottles), and are consistent with the amount of C-terminal MIS observed in the media by western blot (Fig. 2), with the highest MIS concentrations achieved in roller bottles attaining up to 20 μg/ml for B9, 25 μg/ml for LR11, 4 μg/ml for LRF18, and 2 μg/ml for CHO93.
Table 3.
Purification yield of MIS from various clones.
| B9 | CHO93 | LRF18 | LR11 | |
|---|---|---|---|---|
| MIS concentration (μg/ml) at 24 h in serum-containing media | 16.821 ± 3.393 | 1.236 ± 0.772 | 2.149 ± 0.479 | 4.866 ± 1.238 |
| Production of MIS (pg/cell/day) in serum-containing media | 7.597 ± 1.378 | 0.254 ± 0.184 | 0.430 ± 0.177 | 1.142 ± 0.482 |
| MIS concentration (μg/ml) at 24 h in serum-free media | 1.528 ± 0.105 | 0.223 ± 0.063 | 0.457 ± 0.254 | 1.411 ± 0.249 |
| Percent cleavage at 24 h in serum-free media (%) | 25 ± 5 | 50 ± 19 | 37 ± 28 | 79 ± 5 |
| Purification yield (% w/w) | 15 | 20 | 20 | 15 |
Values represent the average of at least 3 experiments ± standard deviation.
Figure 2.
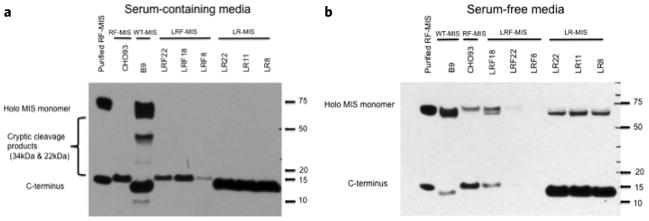
Conditioned media screening by western blot. MIS production and cleavage in CHOK1 clones stably transfected with LR-MIS and LRF-MIS constructs. (a) Western blot of media (10% serum) supernatant after 72 h in culture using an anti-MIS goat polyclonal antibody targeting the C-terminus of MIS (1:200). (b) Western blot of media supernatant after 24 h in culture in serum-free media, using an anti-MIS goat polyclonal antibody targeting the C-terminus of MIS (1:200). Purified RF-MIS is shown as a positive control.
Comparison of cleavage in MIS-producing clones
To estimate the amount of cleavage of the C-terminal MIS, MIS-producing clones were grown in serum-free media since the albumin in the serum interferes with the detection of holo-MIS by western blot (Fig. 2b). When examining the ratio of bands using a C-terminus antibody by densitometry analysis of western blot of conditioned serum free media, the LR-MIS clone LR11 displays over 79% cleavage, while WT-MIS produced by clone B9 shows only 25%. Comparatively, the Flag-containing clones, LRF18 and CHO93, have 37% and 50% cleavage respectively (Table 3). The cleavage was calculated using at least 4 independent experiments by western blot and indicates that the cleavage of LR11 is significantly higher than that of B9 or LRF18 (p < 0.01). The cleavage of MIS in the media translates into an increase in cleaved C-terminal MIS, and a complete absence of unwanted internal cryptic cleavage product in the immunoaffinity purified recombinant LR-MIS and LRF-MIS as observed by western blot analysis of 0.1 μg of purified material, or in gel electrophoresis with non-specific protein staining using 1 μg of purified material (Fig. 3).
Figure 3.

Purified recombinant MIS analyzed by western blot and gel staining. Purified WT-MIS, LR-MIS and LRF-MIS and RF-MIS is compared: (a) By western blot of 0.1 μg of each MIS type, and blotting with an antibody against the N-terminus (MGH4, above) which can recognize the holoenzyme, the cleaved N-terminus and cryptic cleavage products containing part of the N-terminus, and with an antibody against the C-terminus (Santa Cruz, below), which can recognize the holoenzyme, the cleaved C-terminus and cryptic cleavage products containing part of the C-terminus; (b) By staining a polyacrilamide gel with 1 μg of each type MIS with a non-specific protein stain (Lumitein™).
Bioactivity of purified MIS
To verify that the modifications to the protein sequence do not interfere with the activity of MIS, purified MIS was tested in an ex-vivo urogenital ridge culture assay. The ridges, dissected from female rat fetuses of E14.5 of age, contain gonadal tissue, Mullerian and Wolffian ducts, and much smaller mesonephric ducts. Ridges are incubated 72 hours at the air/media interface on grids containing an agarose substrate; the media is supplemented with 1, 3, or 5 μg/ml of MIS (Fig. 4), or down to 0.5 and 0.2 μg/ml for LR-MIS (Supplementary Fig. 1) for the treated ridge, while the contralateral ridge is left untreated to use as a control. Using this gold standard bioassay, regression of the Mullerian duct is qualitatively measured and scored on a scale of 0–5, where 0 represents no regression, while 5 is complete regression. Using this bioassay, we tested MIS purified from media of CHO93 (RF-MIS) and LRF18 (LRF-MIS) using anti-flag immunoaffinity, or MIS purified from media of LR11 (LR-MIS), and B9 (WT-MIS) using anti-MIS 6E11 immunoaffinity. All four preparations of WT-MIS, LR-MIS, RF-MIS, and LRF-MIS retain their ability to induce regression of the Mullerian duct at 5 μg/ml and at 3 μg/ml, whereas only LR-MIS still displays significant regression at 1 μg/ml with a score of 4 (Fig. 4). Activity of LR-MIS was preserved down to concentrations of 0.5 μg/ml with a score of 4, and still had residual activity at 0.2 μg/ml with a score of 3 (Supplementary Fig. 1).
Figure 4.
Comparing RF-MIS, LRF-MIS, WT-MIS, and LR-MIS in a Mullerian duct regression bioassay. MIS was incubated 72 h with fetal rat urogenital ridges and representative sections from the treated ridge are compared for Mullerian duct regression. The scores are indicated at the bottom left corner of each picture, with 5 being full regression and 0 no regression. W, Wolffian duct; M, Müllerian duct. Microscopy pictures were taken with a 200X objective.
DISCUSSION
Efforts to improve and scale the production of human recombinant MIS, a process essential for its predicted use in the clinic, led us recently to develop a new construct featuring the cDNA of hMIS with a modified cleavage site at position 427/428 inserted into pcDNA3.19; however, production levels and cleavage efficiency were too low to contemplate scaling of production in preparation for clinical trials. To overcome low expression yields, the backbone vector of RF-MIS was switched to pAAV-IRES-Neo, cloned into CHO-S cells, and screened under high G418 concentration. The resulting expression vector is polycistronic and includes an internal ribosomal entry site (IRES) driving expression of the neomycin resistance cassette downstream of MIS, allowing for better selection of high expressers. The highest expressing clone, CHO93, was scaled subsequently for production in roller bottles which can be accommodated in an academic setting, and recombinant RF-MIS was purified using anti-flag M2 immunoaffinity beads (Table 2). While RF-MIS′ increased cleavage of the active C-terminus, and reduced internal cryptic cleavage (Fig. 2,3), can be achieved with retained bioactivity, the yield and production of the cDNA clone CHO93 (0.254 pg/cell/day) remains significantly lower (p < 0.001) than that of the genomic clone B9 (7.597 pg/cell/day), underlining the need for better constructs (Table 3). It is unclear whether this lower production is due to the expression vector (pAAV-IRES-neo versus pBG311), the type of CHO cells (CHO-S versus CHO DHFR-), the nature of the drug selection (G418 versus Methotrexate), the type of message produced (cDNA versus genomic MIS), or the presence of the FLAG tag (Table 2). To circumvent these barriers we decided, because of its more manageable size, to focus on cDNA, and test the effects of adding or removing the FLAG tag and/or modifying the leader sequence of the background RF-MIS construct.
To improve production, the original R-MIS and RF-MIS constructs in pcDNA3.1 vectors were modified by substituting the 24 AA of the HSA leader sequence (pre-pro peptide) (noted as L in constructs) for the 25AA MIS leader to create the LR and LRF constructs (Table 1) (Fig. 1). HSA leader sequence fusion has been shown to increase production of recombinant interleukins15 and TNF-alpha16, and has been suggested as a way to produce proteins otherwise difficult to express and to scale. Furthermore, the HSA leader is known to enhance also secretion of target proteins in non-mammalian expression systems, such as the case of human lysozyme produced in yeast using the Pichia pastoris strain17. The rat albumin leader sequence has been successfully used to increase expression of TGF-β1 in CHO cells, which led to a 10–30 fold improvement, suggesting this modification may be applicable to other TGF-β superfamily members18.
The three highest stably expressing clones in CHOK1 were selected for further analysis: LR8/11/18 and LRF8/18/22 (Fig. 2). Both cloning efficiency and expression levels were greater for the LR clones than the LRF clones, suggesting the Flag tag, although helpful for tracking and purification, may make expression of MIS less efficient (Fig. 2, Table 3). These studies are important, however, in demonstrating that the bioactive C-terminus can host an “N-terminal” FLAG-tag while maintaining bioactivity and cleavage, which has not been true of other internal or C-terminal tagging attempts6. Similar to CHO93, all LR and LRF clones have reduced peptide fragments resulting from internal cryptic cleavage at position 229, when compared to B9. Cryptic cleavage of WT-MIS by the B9 clone is especially prevalent in serum-containing media, where MIS is expressed at much higher rate (Fig. 2). Unexpectedly, LR also appears to have a greater proportion of cleaved C-terminus (Fig. 2,3, Table 3). This increased cleavage could be explained by the strong evolutionary pressures on the albumin leader for efficient processing in the trans-golgi network and transport to secretory vesicles, since albumin is endogenously secreted at much higher rates than MIS19, or by differences in the clonal cell lines between CHOK1 and DHFR- CHO.
LRF18 was chosen for bioactivity characterization since it is the highest expressing LRF clone, and can be purified and tracked using the Flag-tag (Table 2). Both LRF-MIS purified from LRF18 and RF-MIS purified from CHO93 were purified by FLAG immunoaffinity, and both retained bioactivity and induced regression of the Mullerian ducts in the UGR bioassay at 5 μg/ml and 3 μg/ml (Fig. 4), suggesting the FLAG-tag does not interfere with activity. Finally both LR-MIS and WT-MIS displayed activity in the bioassay at doses of 5 μg/ml and 3 μg/ml, which are comparable to historical values using purified MIS1,20. Interestingly, LR-MIS was active at 1 μg/ml, a concentration at which other preparations of MIS are too dilute to cause significant regression (Fig. 4), and this activity was maintained down to concentrations of 0.5 μg/ml with some residual effect at 0.2 μg/ml (Supplementary Fig. 1).
While clone LR11 lags clone B9 when produced in serum, the differences are not statistically significant in serum-free media (an FDA requirement) (Table 3), and LR-MIS has several other key advantages. LR11 cells grow in a much more compact fashion and achieve a much higher percentage of cleavage (79%) which is significantly higher than B9 (25%) (p < 0.01), resulting theoretically in a greater abundance of cleaved C-terminus MIS in the media of LR11 than B9. To estimate the amount of C-terminus in the serum free media after 24 hours (where we know the amount of cleavage), we use the following formula: Concentration (holo MIS)/molecular mass (holoMIS) × Cleavage (%) × molecular mass (C-terminus) = concentration (C-terminus). For example, the B9 clone produces 1.528 μg/ml in serum free media over 24 hours, which corresponds approximately to 2.18 × 10−8 M of total MIS (0.001528 g/L divided by 70 kDa), which adjusted for 25% cleavage should theoretically yield 0.068 μg/ml of cleaved C-terminus (2.18 × 10−8 M times 26% times 12.5 kDa). Comparatively, LR11 produces 1.411 μg/ml, which corresponds to 2.01 × 10−8 M of total MIS, which adjusted for 79% cleavage should theoretically yield 0.199 μg/ml of cleaved C-terminus, a 2.91 fold increase over B9. The observed production of C-terminus by densitometry of western blot in serum free conditions, suggests an actual increase of 4 fold over B9 (Fig. 2).
The highest expressing clone of LRF, LRF18, has both a higher concentration, 2.149 ± 0.479 μg/ml (p = 0.03), and a higher production (p = 0.04), 0.430 ± 0.177 pg/cell/day, than the RF clone CHO93 with 1.236 ± 0.772 μg/ml and 0.223 ± 0.063 pg/cell/day respectively, but does not display any statistically significant differences in cleavage, and is produced in serum-containing media at an order of magnitude less than B9 (p = 0.004), and less than half the level of LR11 (p = 0.01) (Table 3). These data suggest that the addition of the FLAG-tag, while allowing for ease of purification and detection, may be interfering with protein stability or expression leading to the observed decrease in production between the otherwise identical LR and LRF clones and should discourage use of this modification in future clinical productions, particularly since the FLAG tag may be immunogenic. Another important advantage of the LR and LRF clones is the reduction in unwanted internal cryptic cleavage products as exemplified by the western blot comparison of media and purified MIS from clones CHO93, LR11, LRF18, and clone B9 (Fig. 2,3).
Since the C-terminus of MIS has previously been shown to be the active moiety21,22, and the cleaved C-terminus is responsible for the binding to the MIS type II receptor (MISR2) resulting in dissociation of the non-covalently bound N-terminus13, increased cleavage should correlate with greater clinical activity. As expected, we observe retention of bioactivity in the UGR assays of LR-MIS at doses three times lower than other purified MIS preparations which have lower cleavage ratios. Interestingly, conditioned media collected from B9 and LR clones was shown to induce partial regression of the Mullerian duct without the need to further purify, allowing for a quick validation of production of MIS from high expressing clones (data not shown).
Using the LR-MIS clone LR11, we observe both a high production rate (significantly greater than CHO93 or LRF18), and a significantly higher percentage of cleaved C-terminus MIS compared to all other clones (p < 0.01) with fewer internal cryptic cleavage products; making it a model candidate for scaling, and improved by these important criteria over the WT-MIS clone B9. Taken together, these data indicate that the LR-MIS construct promises greater yield with increased cleavage and higher bioactivity or potency, paving the way for industrial production of a more homogenous cleaved and bioactive recombinant MIS for clinical applications. Furthermore this combination of modifications will make the difference as to whether or not this important therapeutic can be made generally available as a standard preparation so that experiments and treatments can be appropriately compared and validated across the scientific and pharmaceutical communities.
Supplementary Material
Acknowledgments
We would like to thank D. MacLaughlin for his suggestions and critical reviews of this manuscript and C. Coletti for editorial support. This work was funded in part by the Ovarian Cancer Research Fund Ann Schreiber Mentored Investigator Award (D.P.), a Sudna Gar Foundation fellowship (D.P.), the Harvard Ophthalmology Department Support (D.G.V., M.H.), a Research to Prevent Blindness unrestricted grant to MEEI and an RPB Physician Scientist Award (D.G.V.), Foundation Lions Eye Research Fund (D.G.V.), the Yeatts Family Foundation (D.G.V.); a Department of Surgery grant (P.K.D.), a Department of Defense translational grant (P.K.D.); and gifts from the McBride Family Foundation, the Austen Foundation, and Commons Development (P.K.D.).
References
- 1.Pieretti-Vanmarcke R, Donahoe PK, Szotek P, Manganaro T, Lorenzen MK, et al. Recombinant human Mullerian inhibiting substance inhibits long-term growth of MIS type II receptor-directed transgenic mouse ovarian cancers in vivo. Clin Cancer Res. 2006;12:1593–1598. doi: 10.1158/1078-0432.CCR-05-2108. [DOI] [PubMed] [Google Scholar]
- 2.MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance/anti-Müllerian hormone: A potential therapeutic agent for human ovarian and other cancers. Future Oncol. 2010;6:391–405. doi: 10.2217/fon.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 4.Ragin RC, Donahoe PK, Kenneally MK, Ahmad MF, MacLaughlin DT. Human müllerian inhibiting substance: Enhanced purification imparts biochemical stability and restores antiproliferative effects. Protein Expr Purif. 1992;3:236–245. doi: 10.1016/1046-5928(92)90020-w. [DOI] [PubMed] [Google Scholar]
- 5.Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, et al. An immunoassay to detect human müllerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab. 1990;70:16–22. doi: 10.1210/jcem-70-1-16. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo HK, Teixeira J, Pahlavan N, Laurich VM, Donahoe PK, et al. New approaches for high-yield purification of Müllerian inhibiting substance improve its bioactivity. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;766:89–98. doi: 10.1016/s0378-4347(01)00436-4. [DOI] [PubMed] [Google Scholar]
- 7.Donahoe PK, Ito Y, Hendren WH., 3rd A graded organ culture assay for the detection of Mullerian inhibiting substance. J Surg Res. 1977;23:141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- 8.Kurian MS, de la Cuesta RS, Waneck GL, MacLaughlin DT, Manganaro TF, et al. Cleavage of Müllerian inhibiting substance activates antiproliferative effects in vivo. Clin Cancer Res. 1995;1:343–349. [PubMed] [Google Scholar]
- 9.Papakostas TD, Pieretti-Vanmarcke R, Nicolaou F, Thanos A, Trichonas G, et al. Development of an efficiently cleaved, bioactive, highly pure FLAG-tagged recombinant human Mullerian inhibiting substance. Protein Expr Purif. 2010;70:32–38. doi: 10.1016/j.pep.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachtigal MW, Ingraham HA. Bioactivation of Müllerian inhibiting substance during gonadal development by a kex2/subtilisin-like endoprotease. Proc Natl Acad Sci USA. 1996;93:7711–7716. doi: 10.1073/pnas.93.15.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, et al. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991;266:12127–12130. [PubMed] [Google Scholar]
- 12.Farrugia A. Albumin usage in clinical medicine: Tradition or therapeutic? Transfus Med Rev. 2010;24:53–63. doi: 10.1016/j.tmrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, et al. Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-beta. Mol Endocrinol. 2010;24:2193–2206. doi: 10.1210/me.2010-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter J, Zhang J, Dang TL, Hasegawa H, Cheng JD, et al. Fusion partners can increase the expression of recombinant interleukins via transient transfection in 2936E cells. Protein Sci. 2010;19:357–362. doi: 10.1002/pro.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda Y, Soda M, Ito K, Sato K. Efficient production of active TNF-alpha by albumin signal peptide. Biochem Mol Biol Int. 1997;42:825–832. doi: 10.1080/15216549700203261. [DOI] [PubMed] [Google Scholar]
- 17.Xiong R, Chen J, Chen J. Secreted expression of human lysozyme in the yeast Pichia pastoris under the direction of the signal peptide from human serum albumin. Biotechnol Appl Biochem. 2008;51:129–134. doi: 10.1042/BA20070205. [DOI] [PubMed] [Google Scholar]
- 18.Zou Z, Sun PD. Overexpression of human transforming growth factor-beta1 using a recombinant CHO cell expression system. Protein Expr Purif. 2004;37:265–272. doi: 10.1016/j.pep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- 20.Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF, Jr, et al. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res. 1999;5:3488–3499. [PubMed] [Google Scholar]
- 21.Pepinsky RB, Sinclair LK, Chow EP, Mattaliano RJ, Manganaro TF, et al. Proteolytic processing of mullerian inhibiting substance produces a transforming growth factor-beta-like fragment. J Biol Chem. 1988;263:18961–18964. [PubMed] [Google Scholar]
- 22.MacLaughlin DT, Hudson PL, Graciano AL, Kenneally MK, Ragin RC, et al. Mullerian duct regression and antiproliferative bioactivities of mullerian inhibiting substance reside in its carboxy-terminal domain. Endocrinology. 1992;131:291–296. doi: 10.1210/endo.131.1.1612008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.