Abstract
The evolution of early multicellular eukaryotes 400–500 million years ago required a defensive strategy against microbial invasion. Pore-forming proteins containing the membrane-attack-complex-perforin (MACPF) domain were selected as the most efficient means to destroy bacteria or virally infected cells. The mechanism of pore formation by the MACPF domain is distinctive in that pore formation is purely physical and unspecific. The MACPF domain polymerizes, refolds, and inserts itself into bilayer membranes or bacterial outer cell walls. The displacement of surface lipid/carbohydrate molecules by the polymerizing MACPF domain creates clusters of large, water-filled holes that destabilize the barrier function and provide access for additional anti-bacterial or anti-viral effectors to sensitive sites that complete the destruction of the invader via enzymatic or chemical attack. The highly efficient mechanism of anti-microbial defense by a combined physical and chemical strategy using pore-forming MACPF-proteins has been retargeted during evolution of vertebrates and mammals for three purposes: (1) to kill extracellular bacteria C9/polyC9 evolved in conjunction with complement, (2) to kill virus infected and cancer cells perforin-1/polyperforin-1 CTL evolved targeted by NK and CTL, and (3) to kill intracellular bacteria transmembrane perforin-2/putative polyperforin-2 evolved targeted by phagocytic and nonphagocytic cells. Our laboratory has been involved in the discovery and description of each of the three pore-formers that will be reviewed here.
Keywords: MACPF domain; Complement—extracellular bacteria; erforin-1—virus-infected cells, cancer; Perforin-2—intracellular bacteria
Control of extracellular bacteria
Complement
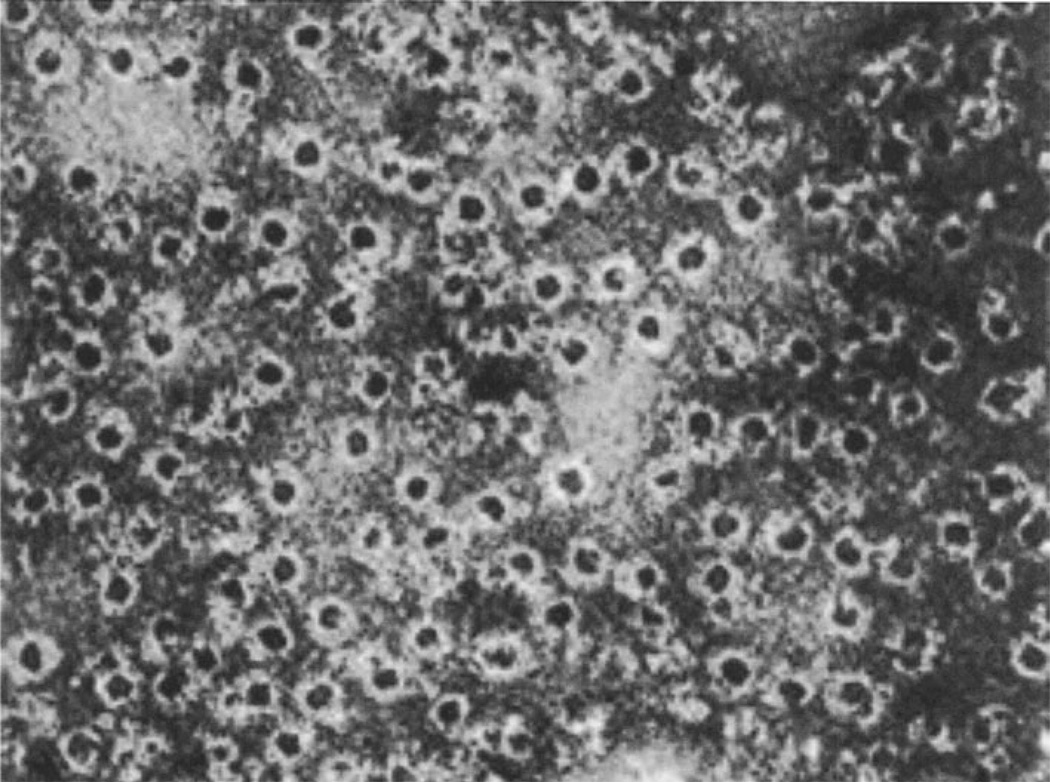
Blood serum is anti-bacterial. In 1896, Jules Bordet recognized that serum contains heat stable and heat labile components and that both are required to achieve bacterial killing. Paul Ehrlich introduced the term complement for the heat labile components of serum that were required to complement bacterial lysis by the heat stable antibodies. Complement is a complex, self-regulatory system of proteins that has evolved to attack and kill extracellular bacteria in body fluids. Humphrey provided the first clue to the mechanism of killing performed by complement in 1964 when he utilized electron microscopy to observe ‘complement lesions’ in cell membranes [1, 2] (Fig. 1). In these early days, the concept that cell membranes are lipid bilayers that generate a permeability barrier had just been developed with the help of electron microscopy. There was considerable controversy as to the molecular interpretation and implication of ‘complement lesions’ on cell membranes. In 1972, Mayer proposed the doughnut hypothesis that suggested that the terminal complement components C5, C8, C7, C8, and C9 form a complex that inserts into membranes in the form of a doughnut with a central, water-filled hole [3]. In 1978, Bhakdi and Tranum-Jensen [4] confirmed this model and identified the C5–9 complex as a hollow, cylindrical macromolecule with lipid-binding regions on one end of the cylinder that enable it to penetrate into the lipid bilayer and create transmembrane pores.
Fig. 1.
Complement lesions on erythrocyte membranes as originally described by Humphrey et al. in 1964
Since complement targets extracellular bacteria, any bystander activation imperils cells nearby, including erythrocytes, from attack by the MAC-polyC9 complex. For this reason, tightly regulated mechanisms exist to target the MAC proteins to the proper membranes. This targeting is accomplished via specific protein inhibitors at checkpoints in the complement system. Complement regulatory proteins are present in the plasma, as well as on the host cell surface, and target the components of the complement activation pathways as well as theMAC. Serum inhibitors specific for the C5b–C6 complex (SP-40, 40) and the C5b–7 complex (Vitronectin/S protein) prevent the complete formation of the activation complex. C8-binding protein and CD59 are expressed on the surface of all host cells binding C8 and thereby preventing C9 recruitment and subsequent pore-formation in host membranes [5–7]. A deficiency in these inhibitory pathways is demonstratedwith a genetic defect inCD59 expression, which leads to paroxysmal nocturnal hemoglobinuria (PNH). This disease is characterized by complement-induced hemolytic anemia, hematuria, and thrombosis [8]. Genetic defects in each of the terminal complex components are associated with susceptibility to infections with Neisseria meningitides and N. gonorrhoeae [9]. N. meningitidis is especially resistant to phagocytosis by complement-mediated opsonization making MAC-polyC9 formation the primary mechanism of innate immune defense against this pathogen [10].
A single protein, C9, polymerizes to form a hollow cylinder with lipid-binding regions
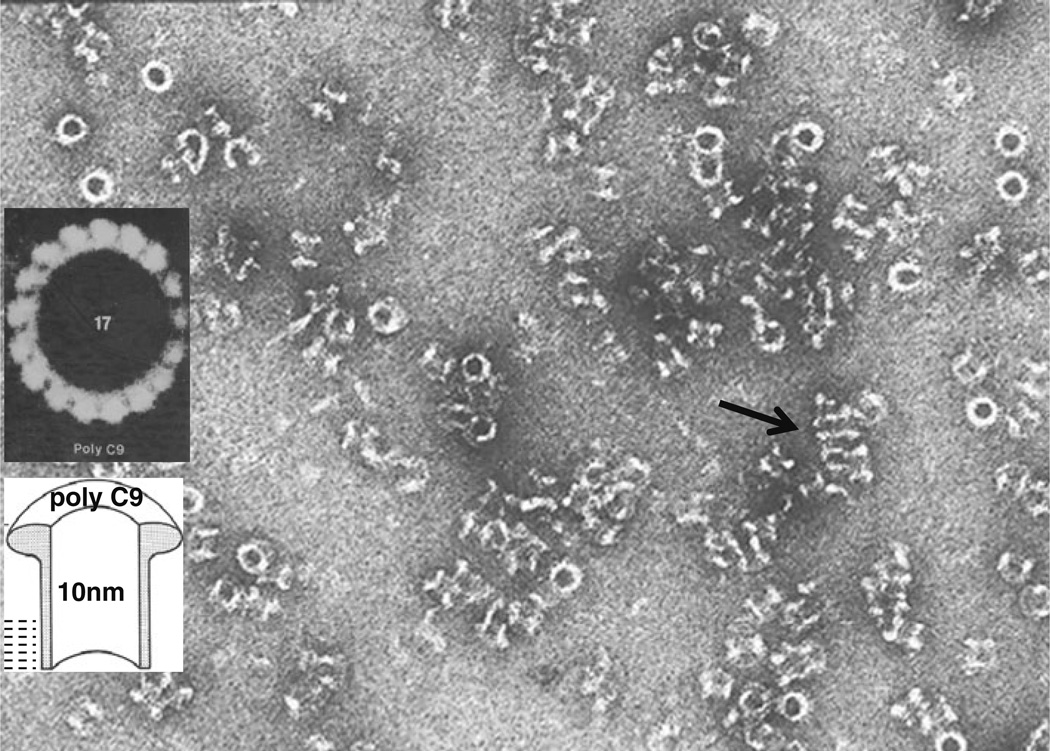
Tschopp and Podack (Fig. 2) in the laboratory of Muller-Eberhard at Scripps, La Jolla, CA, in 1982 discovered that purified C9 is capable of self-polymerizing at 37 °C creating hollow, cylindrical complexes of 16 nm length, with ~10 nm internal diameter and with a 5 nm long lipid-binding region on one end of the cylinder that resemble C5–9 complexes (Fig. 3) [11–13].
Fig. 2.
Jürg Tschopp
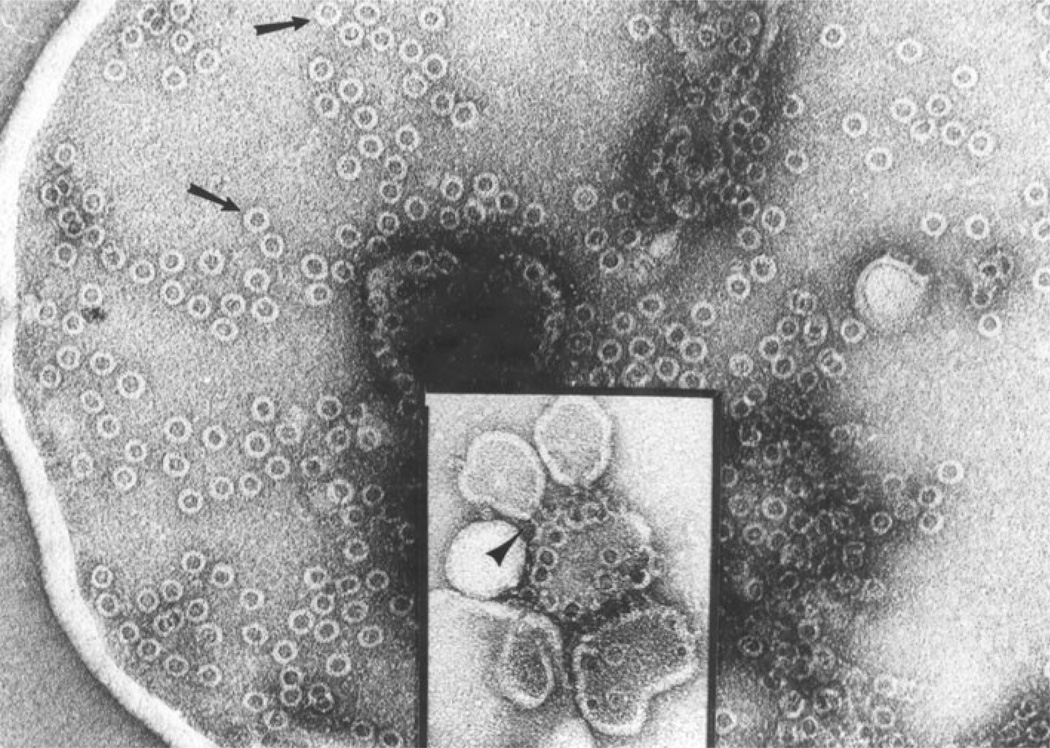
Fig. 3.
PolyC9 in solution. The cylindrical complexes are seen in top view as white rings and in side views as hollow, negativestain-filled cylinders. The arrow points to an array of polymers that interact via the hydrophobic lipid-binding domains allowing the determination of the length (~50 Å) of the hydrophobicity area. Inset: heptadecameric polyC9, image-enhanced by rotational averaging
The seminal discovery that, via polymerization, a single molecule can form a membrane inserted transmembrane pore of 100 Ådiameter provided crucial insights and had important consequences for future research.
Molecular mechanism
First, poly C9 provided a molecular mechanism for pore formation as illustrated in Fig. 4. The hydrophilic monomer C9 refolds during polymerization exposing a peptide loop that has a hydrophobic and hydrophilic surface on opposite sides. Insertion into membranes (or bacterial cell walls) through the hydrophobic forces on one surface of the loop will repel lipids from the other side of the loop. The polymerizing complex expels lipids while closing in on itself, as dictated by the geometry of the monomer, thereby creating a water-filled pore.
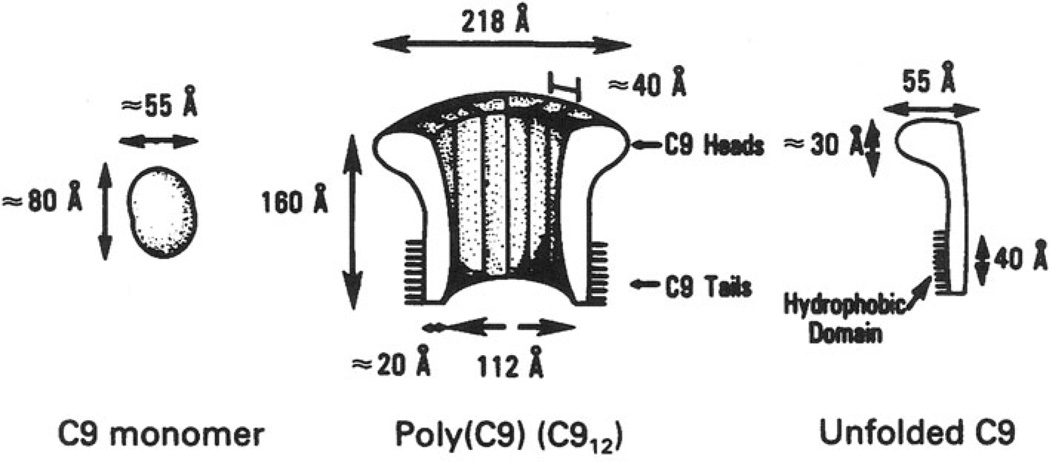
Fig. 4.
Model of monomeric and polymerized C9 with approximate dimensions and hydrophobic area, as published by us in 1982
The model (Fig. 4) suggests that at least two monomers must interact to create a small pore by displacing and repelling lipids from the hydrophilic surface of the loops. With the addition of monomers, the polymer and apparent pore size grows until the cylinder closes on itself and terminates the polymerization process. The final pore size is determined by the inner diameter of the cylinder whose curvature depends on the geometry of the monomer [14]. Various pore sizes have been reported for complement ‘channels’ [15, 16]. The full size pore is clearly not a typical channel with any similarity to ion channels but rather a large hole.
Pore-formation does not break covalent bonds
Second, pore formation by polymerization does not require or use enzymatic activity. The process is driven by tight physical interaction of the monomers during polymerization and hydrophobic interactions during insertion that provide the free energy to displace lipids and form the pore.
Lack of target specificity of pore formation
Third, the mechanism of forming pores on cell walls or membranes is unspecific, not requiring receptors or specific molecular interactions. The trigger for polymerization has to occur close to the surface of a membrane/cell wall that allows hydrophobic insertion of the peptide loop.
Trigger for pore-formation provides target information
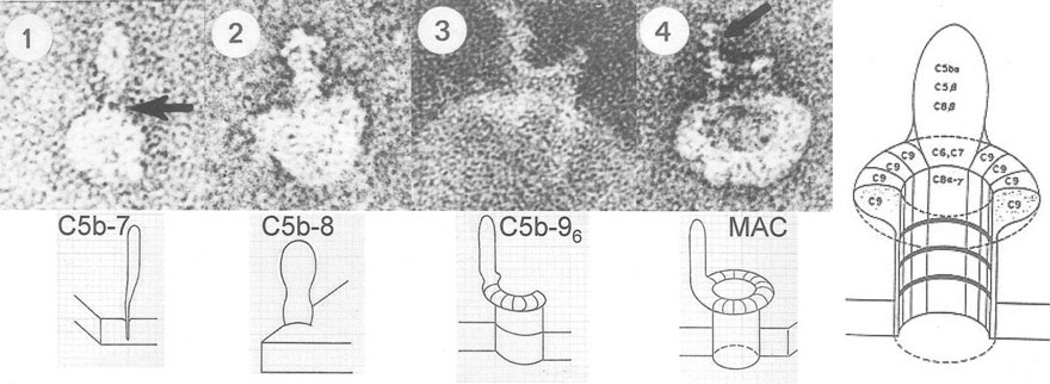
Fourth, the lack of target specificity of pore formation itself requires that the trigger for polymerization is targeted accurately. The trigger for C9-polymerization is targeted by C3b, which has the dual function as opsonin for phagocytosis and as initiator of the assembly of the membrane-attack complex on the target surface by cleavage of C5–C5b, the last enzymatic step in the sequence leading to pore formation. C5b forms a complex with C6 and C7, and the C5b–7 complex inserts itself into the target surface next to C3b (Fig. 5) [17–20]. After binding C8, the C5b–8 complex triggers and accelerates C9 polymerization and pore-formation on the target surface. Clustered pores by the MAC-polyC9 complex are used by lysozyme to penetrate to and degrade the peptidoglycan layer of bacteria resulting in bacterial collapse and lysis [21].
Fig. 5.
Images of the growing membrane-attack complex of complement inserted into a single-layer lipid vesicle. Arrow in one points to the knife like lipid-binding domain of C5b–7. The arrow in four points to the C5b–8 part of MAC-polyC9
C9 was the first of the terminal complement proteins to be cloned and sequenced [22]. C9 contains the membrane-attack-complex-perforin domain that was subsequently also found in C6, C7, C8α, and C8β. It is likely that the membrane-attack-complex-perforin (MACPF) domains enable the formation of the copolymer of the membrane-attack complex shown in Fig. 5.
Single-gene hypothesis for pore-formation
Fifth, perhaps the most important insight derived from the discovery of C9 polymerization was the realization that the product of a single gene is sufficient to encode a pore-forming molecule. At that time, it was known that NK cells and CTL are able to kill tumor target cells and virus-infected cells. However, the mechanism of killing was not known and highly controversial. The insight that the product of a single gene is sufficient for pore formation inspired a collaboration that led to the discovery of perforin-1 described below.
Control of virus infection and cancer
In 1982, it was known that NK cells are able to kill tumor cells [23] and that CTL can kill virus-infected cells that are recognized in a MHC-restricted mechanism [24]. The killing mechanisms of CTL and NK cells, however, were not clear [25, 26].
With the discovery of polyC9, it became clear that a single-gene product is sufficient to make large membrane pores by homopolymerization to hollow cylindrical membrane inserted complexes composed of 12–16 protomers. This is the process by which complement kills extracellular bacteria. Importantly, this type of physical process perforating the lipid barrier leaves imprints on the target: Membrane lesions made by cylindrical polymers that can be detected by electron microscopy.
Perforin-1 controls viral infection and cancer
We asked whether killer cells use a killing mechanism that creates membrane lesions detectable by electron microscopy. In other words, do NK and CTL express a killer molecule that can polymerize and kill cells? If that were the case, we should find membrane lesions on the killed target. We set out to answer this question.
At that time, it had just become possible to clone CTL with the help of supernatants of activated T-cells containing T cell growth factor, now known as IL-2. Gunther Dennert (Fig. 6) at the Salk Institute had established cloned NK-like and CTL-cell lines. We therefore asked whether membranes isolated from mixtures containing killed cells had the hallmark-membrane lesions detectable by electron microscopy; the answer was yes in the very first experiment (Fig. 7). Indeed, NK and CTL have a protein that we named perforin-1 due to its ability to perforate membranes. Perforin-1 polymerizes to form hollow cylindrical complexes of 160 Å (16 nm) inner diameter that are detectable as membrane lesions by electron microscopy [27, 28]. At the same time, Blumenthal et al. [29] described a protein in rat large granular lymphoma cells that released markers from liposomes with similar properties as perforin-1 and that was designated cytolysin. Cloned CTL and NK cells have large intracellular granules that move close to the contact area between killer and target cells. Isolated cytoplasmic granules of CTL efficiently kill any target cell including erythrocytes in a calcium-dependent reaction. These granules contain perforin-1 and it is perforin-1 that acts as the Ca-dependent lytic effector [29, 30].
Fig. 6.
Gunther Dennert
Fig. 7.
Membrane lesions on membranes of cells killed by NK cells or CTL as published in 1983. The arrows show the cylindrical polyperforin-1 complex in top view, the arrow head in the inset in side view. The inner diameter is 160 Å
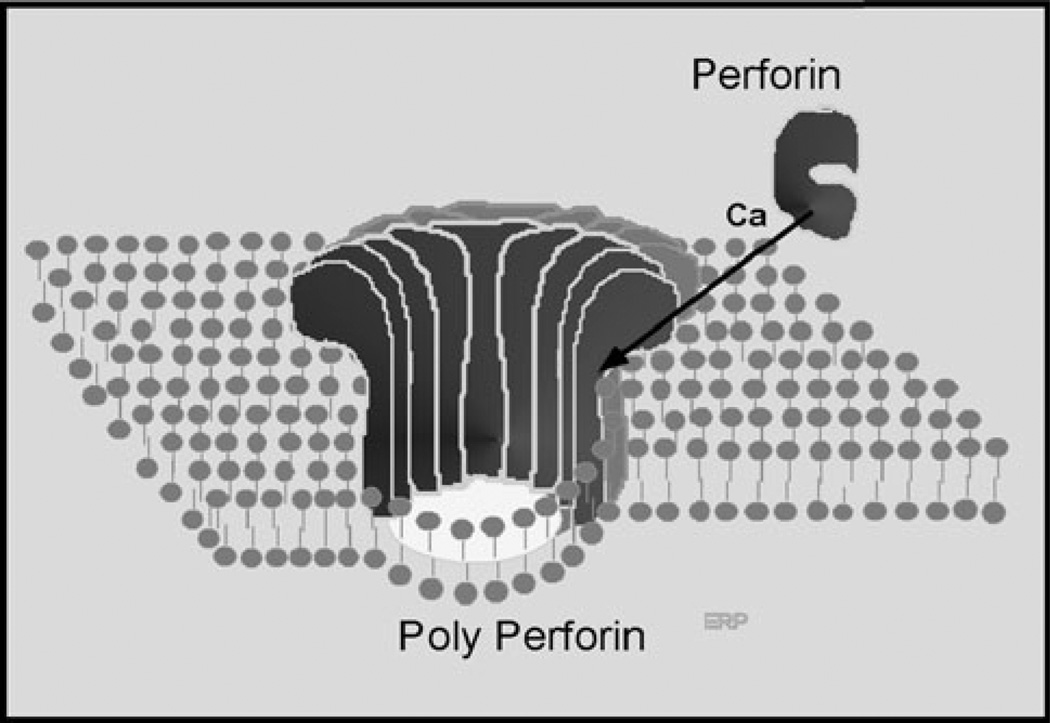
Isolated granules from CTL-cell lines demonstrated rapid and unspecific lysis of cells, while the intact CTL cell from which they were isolated mediated highly specific MHC and peptide restricted killing of virus-infected cells [14]. The finding that isolated granules have nonspecific killing properties compared with the high specificity of intact CTL reinforced our prior conclusion that the targeting and actual killing are independent events. In complement, the C5–8 complex is targeted to the target surface where it triggers C9-polymerization. Targeting of porepolymerization is performed by a mechanism distinct from triggering pore formation. NK and CTL provide targeting information for granule exocytosis through their membrane receptors recognizing and binding to the target cell. The granule membrane fuses with the plasma membrane of the killer cell at the time of exocytosis, thereby releasing the granule content into the synapse between the killer cell and the target cell. The trigger for perforin-1 polymerization is extracellular Ca2+ (Fig. 8) and the directionality of attack to the target cell membrane is assured via the protection of the membrane of the killer cell within the synapse from attack by perforin-1.
Fig. 8.
Model of perforin-1 polymerization and insertion into lipid bilayer triggered by extracellular calcium ions
Polymerization and lysis by purified perforin-1 is triggered by Ca2+. It is temperature dependent and pH sensitive, with the optimal conditions at 37 °C and neutral pH. Ca2+ ions trigger the polymerization of perforin-1 to a hollow cylindrical complex of 16 nm inner diameter with lipid-binding sites on one end. The final killing event, perforin-1 polymerization, membrane insertion and pore formation is strikingly similar to polyC9. Polyperforin-1 pores cause cell death by two separate effects. First, perforin-1-mediated membrane disruption via pore formation results in the rapid equilibration of small molecules causing colloid osmotic lysis of the target cell. Second, granules contain granzymes, in addition to perforin-1, that are released into the immunologic synapse. Polyperforin-1 pores deliver granzymes into the interior of the target cell where they mediate additional damage and cell death.
Granzymes are a family of lymphocytic serine proteases that, together with the proteoglycan chondroitin sulfate C, make up the bulk of the protein mass of the cytolytic granule. perforin-1 pores mediate entry of granzymes into the target cell by direct diffusion through the large hollow core of perforin-1 or indirectly by stimulating pinocytosis and uptake of granzymes [31–35]. Granzymes cause target cell death by DNA degradation via several mechanisms as well as by other, not yet identified, mechanisms unrelated to apoptosis.
Cloning and sequencing of perforin-1 [36–38] revealed that it had a ~300 amino acid domain in common with C9 which, as determined later, it also shared with C6, C7, C8α, and C8β. The domain therefore received the name MACPF as acronym for membrane-attack-complex-perforin domain.
The biologic importance of perforin-1 in the immune response against virus-infected and transformed cells was first demonstrated when it was discovered that perforin-1 knockout mice die following LCMV infection [39, 40]. This dramatic phenotype finally convinced the skeptics in the field that perforin-1 may indeed constitute an important effector mechanism in cytotoxic lymphocytes.
Perforin-1 in CTL also is essential for controlling and killing MHC class I-antigen-presenting cells in a negative feedback loop terminating activation of antigen-specific CD8 CTL [41]. Perforin-1 deficiency in mice and in humans is lethal; it leads to a disorder called familial hemophagocytic lymphohistiocytosis (FHL) characterized by hyperproliferation and activation of macrophages mimicking acute responses to infection. MHC I-antigen-presenting cells cannot be killed resulting in continued CTL activation and cytokine production causing the symptoms of the disease. FHL presents with fever, hepatosplenomegaly and enhanced hemophagocytosis in spleen, liver, and bone marrow usually early in life [42–44].
In mice, perforin-1 is important for tumor surveillance. Perforin-1-deficient mice are more susceptible to carcinogens and develop tumors spontaneously with aging. IFN-γ and perforin-1 expressed by NK and CTL are the most critical protectors against tumor growth [45, 46].
Control of intracellular bacteria
Bacteria in blood and body fluids are recognized and attacked by antibody and complement that opsonize bacteria for uptake by phagocytic cells and activate the membrane-attack pathway. If the MAC-polyC9 complex is unable to kill the bacteria, intracellular killing mechanisms within the phagocyte will be engaged to kill the bacteria. Reactive oxygen and nitrogen species and fusion of the bacterium-containing phagosome with the lysosome are thought to be the dominant bactericidal effectors.
As described below, we have identified a MACPF domain-containing membrane protein expressed by interferon-activated fibroblasts and named it perforin-2 based on the hypothesis that its MACPF domain attacks bacteria trapped within endosomes or phagosomes.
Perforin-2 is essential for control of intracellular bacteria
Cloning and sequencing of perforin 1 in 1987 showed that the pore-formers C9 and perforin-1 have the MACPF domain in common [36, 37]. I speculated that there may be additional pore-forming proteins used by the immune system and used BLAST searches with the MACPF sequence of the national data base. For the next ~ 18 years, no hits identifying novel proteins were obtained excluding complement proteins and perforin-1 in other species. With the publication and deposition into public databases in 1996 of the sequences of expressed sequence tags (ESTs) encompassing the complete cell-expressed human genome [47] our BLAST searches revealed ESTs that encoded MACPF domains unrelated to complement or perforin-1. Tracking the origin of the ESTs suggested that macrophages express an mRNA for a MACPF-protein. Using the regions upstream and downstream of the MACPF-sequence as search sequences, we were able to identify overlapping ESTs to assemble the entire predicted open-reading frame encoding a ~ 72 kD protein. We named the protein perforin-2 under the assumption that it is a novel pore-forming protein. The expression of a pore-forming protein in macrophages suggests that it may be used to kill intracellular bacteria. However, the paradigm at the time suggested that killing of intracellular bacteria is accomplished by ROS, NO, and lysosomes without the need for pore formation. It seemed likely therefore that perforin-2 may be needed only to kill bacteria that are resistant to the known effectors.
Perforin-2mRNA is not only expressed inmacrophages, as suggested by the name macrophage expressed gene 1 [48]. IFN-α, β, and γ rapidly induce perforin-2 mRNA in murine fibroblasts and in HeLa cervical epithelial cells [49, 50]. Perforin-2 mRNA expression is required to make the cells competent for the killing of intracellular bacteria while perforin-2 mRNA knockdown with siRNA abolishes the ability of IFN-activated fibroblasts to kill intracellular bacteria. Perforin-2 mRNA expression in fibroblasts is also induced by intracellular bacterial infection within 6–12 h. Intracellular bacteria initially replicate in naïve fibroblasts, however, when sufficient perforin-2 has been produced within the cell, intracellular killing of bacteria ensues unless blocked by perforin-2 siRNA.
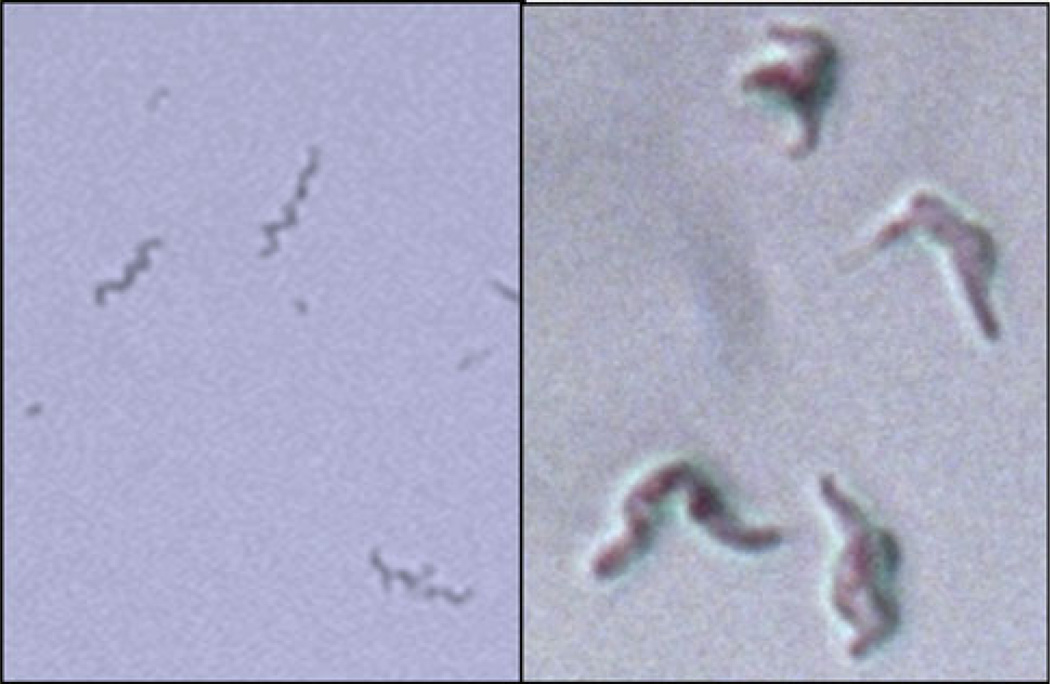
Perforin-2 in fibroblasts is able to kill intracellular pathogenic bacteria including clinical isolates of grampositive methicillin resistant Staphylococcus aureus (MRSA), gram-negative Salmonella typhimurium and Mycobacterium avium, and Mycobacterium smegmatis as well as many other bacterial species. Isolation of M. smegmatis from fibroblasts 4 h after infection and plating showed that they are grotesquely swollen but still intact but do not replicate (Fig. 9). Addition of low concentrations of lysozyme caused bacterial lysis and death. The data suggest that the action of perforin-2 damaged the outer shell of M. smegmatis by pore formation allowing penetration of water and osmotic swelling. However, the peptidoglycan skeleton appeared to still be intact, allowing for expansion of the bacteria and preventing osmotic lysis until lysozyme was added [50]. The data are consistent with pore formation by perforin-2 inflicting structural damage to the outer surface of the bacterial envelops. Perforin-2-mediated damage allows lysozyme to penetrate and degrade the peptidoglycan skeleton causing osmotic lysis. The data suggest that the bactericidal activity of lysozyme is dependent on pore formation and cell wall damage by perforin-2, similar to an earlier report for complement and lysozyme [21]. ROS and NO may similarly depend on perforin-2 because in its absence intracellular bacteria replicate despite the production of ROS and NO and the presence of lysosomes in fibroblasts.
Fig. 9.
Left: M. smegmatis from fresh liquid culture. Right: M. smegmatis isolated from IFN-activated fibroblasts 5 h after infection and then plated on agar overnight. Note the intact but grotesquely swollen body of M. smegmatis. Addition of lysozyme does not affect the images on the left but disintegrates M. smegmatis on the right to small fragments and debris (not shown)
The potent bactericidal activity of perforin-2 dictates that bacteria that want to establish even a temporary residence in host cells must suppress perforin-2 expression or prevent or interfere with activation and targeting of perforin-2. This expectation has been validated for Chlamydia trachomatis, which is a gram-negative obligate intracellular bacterium that preferentially infects epithelial cells. Professional phagocytes provide C. trachomatis only a limited ability to survive and are proficient killers of chlamydiae. Knockdown of perforin-2 in macrophages results in chlamydial growth that closely mirrored growth in HeLa cells.
Infection of HeLA cells with chlamydiae blocks perforin-2 induction but is relieved by chloramphenicol, suggesting an active suppression mechanism. Ectopic expression of perforin-2 in HeLa cells, however, does result in killing. The data suggest that chlamydiae have mechanisms that suppress perforin-2 induction. When perforin-2 is present, chlamydiae are killed and are unable to prevent its bactericidal action [49].
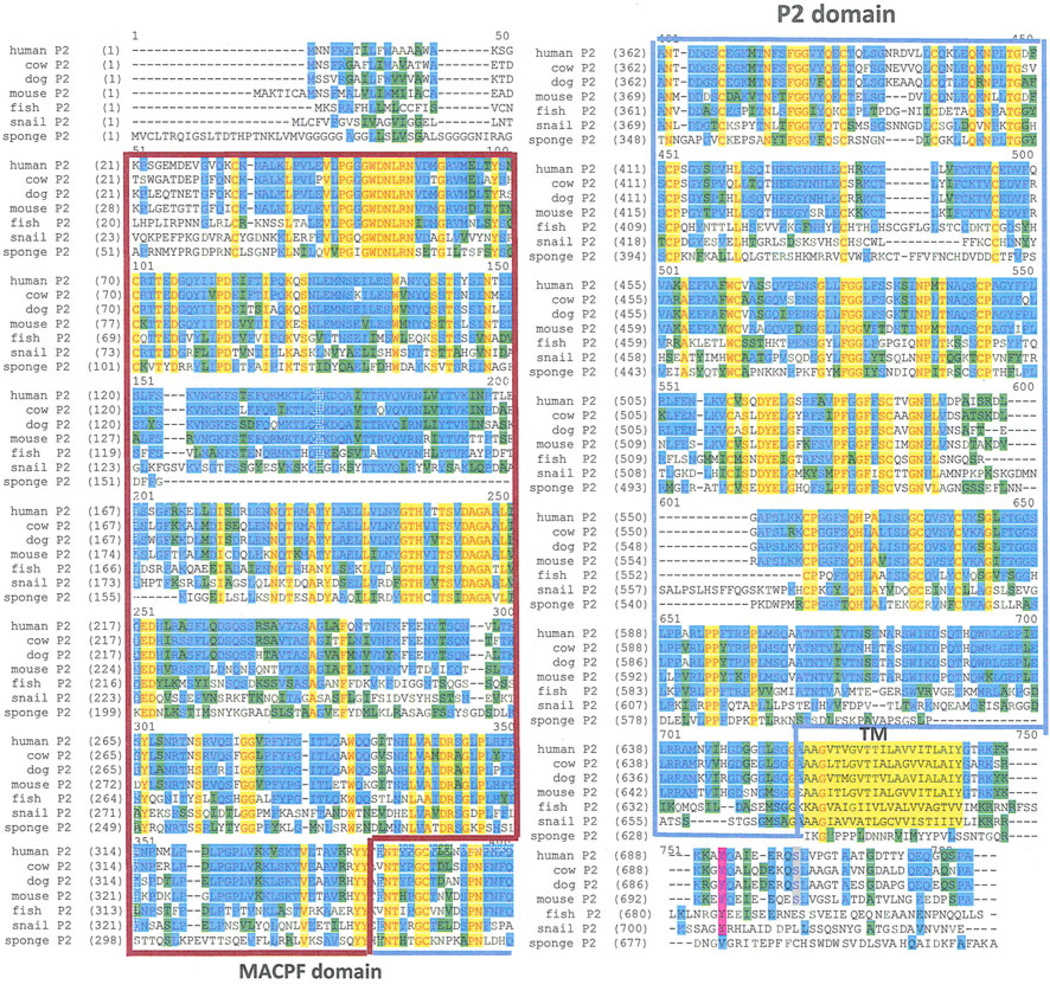
Perforin-2/Mpeg1 is an ancient intron-less gene that is highly conserved (Fig. 10) throughout evolution beginning with Porifera, one of the most ancient metazoan lineages, whose origin predates the divergence of protostomes and deuterostomes. Interestingly, perforin-2/Mpeg1 is not present in Drosophila or Caenorhabditis elegans genomes indicating that it was lost in protostomes following their divergence. There is evidence that the perforin-1 of CTL and NK cells originated following a duplication of a perforin-2/Mpeg1 gene, as perforin-2/Mpeg1 and perforin-1 are adjacent and in the same orientation in the Gnathostomata genome, the most ancient ancestor of modern perforin-1 [51]. There have been a number of studies investigating perforin-2/Mpeg1 expression and function in marine invertebrate species. Perforin-2/Mpeg1 from sponge [52] and abalone [53–55] is expressed by innate immune cells, and the transcript is upregulated following treatment with lipopolysaccharide (LPS) or by direct infection with bacteria. Recombinant perforin-2/Mpeg1, corresponding to the MACPF domain (aa158–358), from Crassostrea gigas (Pacific oyster) had potent bactericidal activity (60–80 % killing) against gram-positive and gram-negative bacteria including Escherichia coli (E. coli) and S. aureus [56].
Fig. 10.
Phylogenetic conservation of perforin-2 from sponge to man. Identity is indicated by red/yellow, conservative substitution is shown in blue and green The transmembrane domain is indicated as TM followed by the short cytoplasmic domain (Color figure online)
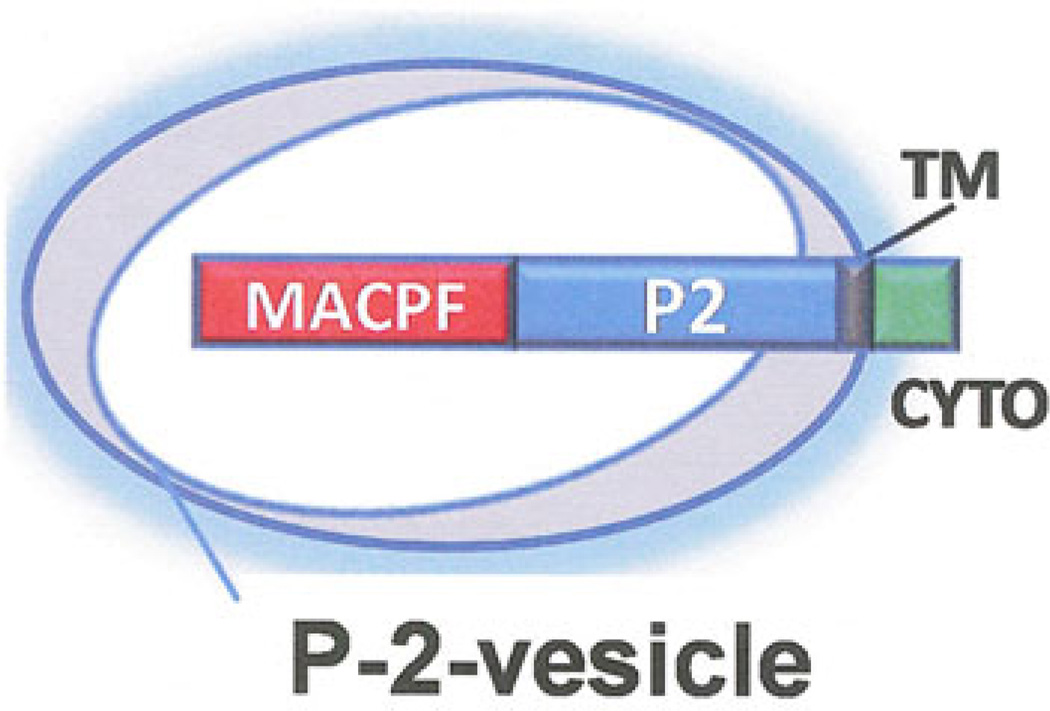
Perforin-2 is a 713 amino acid transmembrane protein. The MACPF domain is located at the N-terminus and the transmembrane domain and cytoplasmic domain at the C-terminus. These domains are separated by a unique-conserved domain only observed in perforin-2 proteins that we have designated P2 domain. The short 36 amino acid cytoplasmic is also highly conserved in vertebrates suggesting signaling function. The predicted transmembrane orientation places the MACPF domain inside membrane vesicles (Fig. 11).
Fig. 11.
Predicted transmembrane orientation of perforin-2 in membrane vesicles stored in the cytoplasm of perforin-2 expressing cells
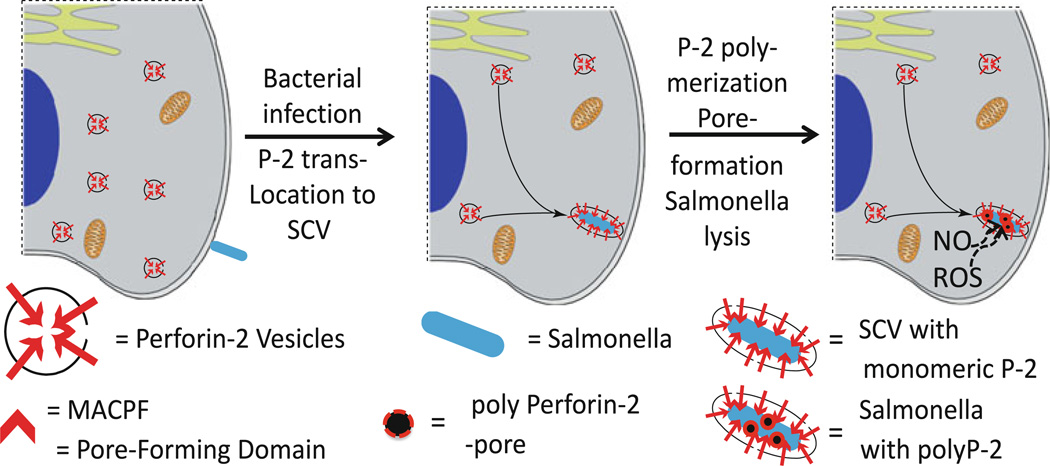
Our working hypothesis posits that upon bacterial infection, perforin-2 vesicles traffic to and fuse with the bacterium-containing endosome thereby orienting the MACPF domain toward the bacterium (Fig. 12). Triggering perforin-2 polymerization at this point initiates attack and pore formation on the bacterial surface thereby making the bacterium susceptible to additional bactericidal effectors such as lysozyme, ROS, and NO.
Fig. 12.
Hypothetical model of perforin-2 vesicles trafficking to and fusing with the bacterium-containing endosome/phagosome, perforin-2-polymerization and killing the bacterium by pore formation
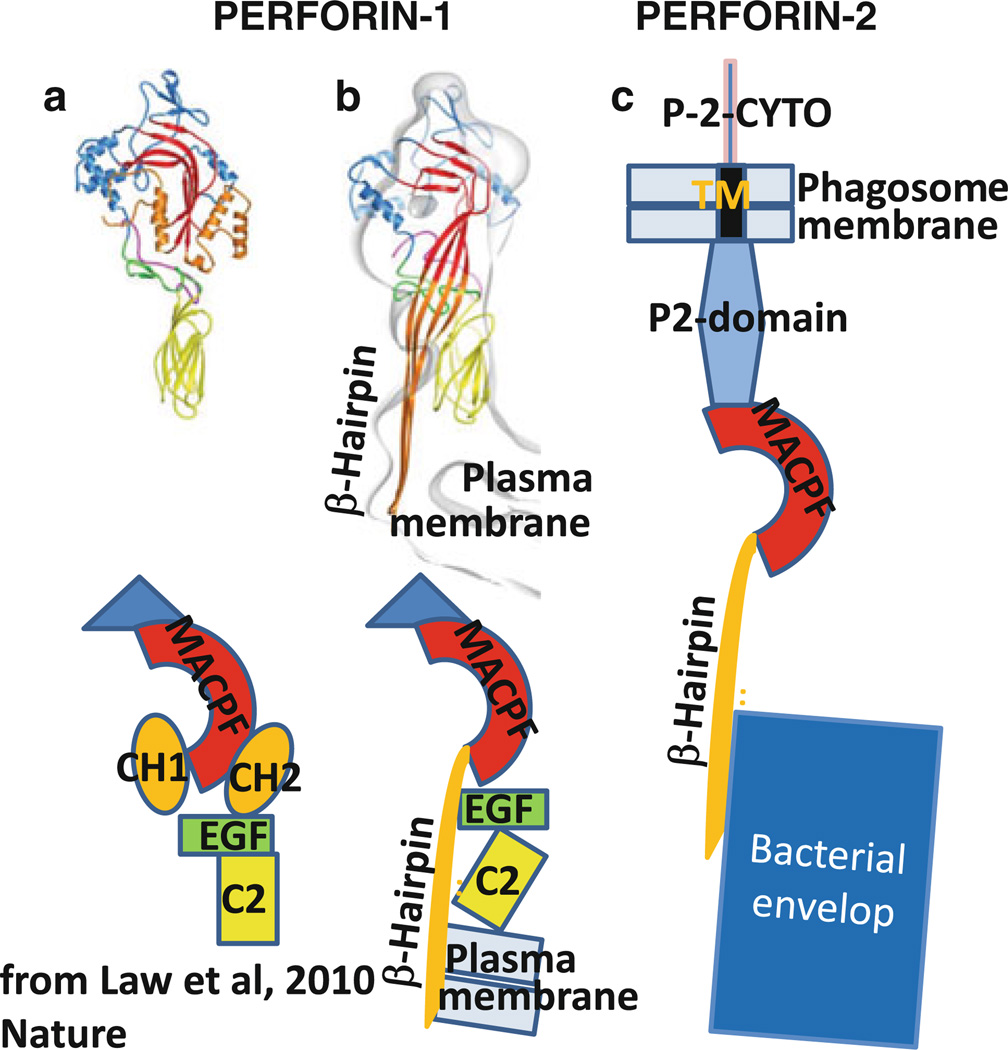
The MACPF domain initiates polymerization through ionic interaction [57] that triggers refolding of the α-helical CH1 and CH2 sequences to amphiphilic β-hairpins (Fig. 13). The crystal structure of the MACPF domain has been resolved from several proteins including the insect pathogen, Photorhabdus luminescens (Plu)-MACPF [58], human C8α [59, 60], and murine perforin-1 [61].
Fig. 13.
Model of perforin-2 refolding and killing bacterium based on crystal structure of perforin-1 (Law et al. 2010). a Crystal structure and schematic domain structure of perforin-1. b Refolded perforin-1 inserted into lipid bilayer (plasma membrane). c Refolded perforin-2 attacking bacteria inside phagosome. Note that perforin-2 remains tethered to the phagosome membrane via its transmembrane domain
The complement proteins, including C9 and perforin-1, are soluble proteins attacking bacteria or cells, respectively, in solution. Perforin-2, in contrast, is tethered via its transmembrane domain to membranes. As mentioned above, we suggest that perforin-2-vesicles traffic to and fuse with the membrane of endosomes or phagosomes containing the bacterium that needs to be killed. Based on the crystal and lipid-binding analysis of perforin-1 [61], we suggest the model shown in Fig. 13c how perforin-2 while being tethered to the phagosome membrane attacks the bacterium inside the phagosome.
Concluding remarks
Perforin-2 is already present in Porifera, one of the earliest multicellular organisms, and has been conserved through most species including humans in its original form. Sponges such as other multicellular organisms must protect their cells from bacterial invasion and replication. This important function has not changed throughout evolution since bacteria continue to be a threat for survival.
With further differentiation during evolution, it is likely that C9 and the other terminal complement components diverged from perforin-2 to protect body fluids and blood from extracellular bacterial replication.
A third protein, perforin-1, diverged from perforin-2 to kill cells that are virally infected or transformed to cancer by mutation.
Thus, each of the three major groups of microbial pathogens is attacked and eliminated by a pore-forming protein specially adapted for its target. The utility of pore-forming proteins for immune defense arises from their ability to inflict physical damage thereby damaging permeability barriers and structural protection. This physical damage is then used by additional effectors to penetrate sensitive sites of the target and complete its destruction. The combination of physical and chemical attack is highly efficient allowing our survival despite a continuous barrage of attacking microbes that have huge advantages in number and the ability to rapidly replicate and mutate.
Biographies

Ryan Mc Cormack

Lesley de Armas

Motoaki Shiratsuchi

Eckhard R. Podack
Contributor Information
Motoaki Shiratsuchi, Email: mshira@intmed3.med.kyushu-u.ac.jp.
Eckhard R. Podack, Email: EPodack@med.miami.edu.
References
- 1.Borsos T, Dourmashkin RR, Humphrey JH. Lesions in erythrocyte membranes caused by immune haemolysis. Nature. 1964;202:251–252. doi: 10.1038/202251a0. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey JH, Dourmashkin RR. The lesions in cell membranes caused by complement. Adv Immunol. 1969;11:75–115. doi: 10.1016/s0065-2776(08)60478-2. [DOI] [PubMed] [Google Scholar]
- 3.Mayer MM. Mechanism of cytolysis by complement. Proc Natl Acad Sci USA. 1972;69:2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci USA. 1978;75:5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalman LS, Wood LM, Muller-Eberhard HJ. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci USA. 1986;83:6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonermark S, Rauterberg EW, Shin ML, Loke S, Roelcke D, Hansch GM. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986;136:1772–1776. [PubMed] [Google Scholar]
- 7.Podack ER, Muller-Eberhard HJ. Isolation of human S-protein, an inhibitor of the membrane attack complex of complement. J Biol Chem. 1979;254:9808–9814. [PubMed] [Google Scholar]
- 8.Yamashina M, et al. Inherited complete deficiency of 20-kilodalton homologous restriction factor (CD59) as a cause of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1990;323:1184–1189. doi: 10.1056/NEJM199010253231707. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007;15:233–240. doi: 10.1016/j.tim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Podack ER, Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultra-structure resembling the membrane attack complex of complement. Proc Natl Acad Sci USA. 1982;79:574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschopp J, Muller-Eberhard HJ, Podack ER. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982;298:534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- 13.Tschopp J, Podack ER. Membranolysis by the ninth component of human complement. Biochem Biophys Res Commun. 1981;100:1409–1414. doi: 10.1016/0006-291x(81)91981-1. [DOI] [PubMed] [Google Scholar]
- 14.Young JD, Cohn ZA, Podack ER. The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T cells: structural, immunological, and functional similarities. Science. 1986;233:184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]
- 15.Ramm LE, Mayer MM. Life-span and size of the transmembrane channel formed by large doses of complement. J Immunol. 1980;124:2281–2287. [PubMed] [Google Scholar]
- 16.Sims PJ, Lauf PK. Steady-state analysis of tracer exchange across the C5b-9 complement lesion in a biological membrane. Proc Natl Acad Sci USA. 1978;75:5669–5673. doi: 10.1073/pnas.75.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer CH, Nicholson A, Mayer MM. On the mechanism of cytolysis by complement: evidence on insertion of C5b and C7 subunits of the C5b,6,7 complex into phospholipid bilayers of erythrocyte membranes. Proc Natl Acad Sci USA. 1975;72:5076–5080. doi: 10.1073/pnas.72.12.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podack ER, Muller-Eberhard HJ. Limited proteolysis of C5b-6: functional stability of the degraded complex. J Immunol. 1980;124:332–336. [PubMed] [Google Scholar]
- 19.Podack ER, Muller-Eberhard HJ. Membrane attack complex of complement. Evidence for its dimeric structure based on hybrid formation. J Biol Chem. 1981;256:3145–3148. [PubMed] [Google Scholar]
- 20.Preissner KT, Podack ER, Muller-Eberhard HJ. The membrane attack complex of complement: relation of C7 to the metastable membrane binding site of the intermediate complex C5b-7. J Immunol. 1985;135:445–451. [PubMed] [Google Scholar]
- 21.Schreiber RD, Morrison DC, Podack ER, Muller-Eberhard HJ. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med. 1979;149:870–882. doi: 10.1084/jem.149.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiScipio RG, Gehring MR, Podack ER, Kan CC, Hugli TE, Fey GH. Nucleotide sequence of cDNA and derived amino acid sequence of human complement component C9. Proc Natl Acad Sci USA. 1984;81:7298–7302. doi: 10.1073/pnas.81.23.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herberman RB. Overview on NK cells and possible mechanisms for their cytotoxic activity. Adv Exp Med Biol. 1982;146:337–351. doi: 10.1007/978-1-4684-8959-0_18. [DOI] [PubMed] [Google Scholar]
- 24.Doherty PC, Zinkernagel RM. H-2 compatibility is required for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. J Exp Med. 1975;141:502–507. doi: 10.1084/jem.141.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berke G, Clark WR. T lymphocyte-mediated cytolysis—a comprehensive theory. I. The mechanism of CTL-mediated cytolysis. Adv Exp Med Biol. 1982;146:57–68. doi: 10.1007/978-1-4684-8959-0_4. [DOI] [PubMed] [Google Scholar]
- 27.Dennert G, Podack ER. Cytolysis by H-2-specific T killer cells. Assembly of tubular complexes on target membranes. J Exp Med. 1983;157:1483–1495. doi: 10.1084/jem.157.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podack ER, Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. Nature. 1983;302:442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- 29.Blumenthal R, Millard PJ, Henkart MP, Reynolds CW, Henkart PA. Liposomes as targets for granule cytolysin from cytotoxic large granular lymphocyte tumors. Proc Natl Acad Sci USA. 1984;81:5551–5555. doi: 10.1073/pnas.81.17.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podack ER, Konigsberg PJ. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984;160:695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasternack MS, Verret CR, Liu MA, Eisen HN. Serine esterase in cytolytic T lymphocytes. Nature. 1986;322:740–743. doi: 10.1038/322740a0. [DOI] [PubMed] [Google Scholar]
- 32.Masson D, Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. 1987;49:679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- 33.Jenne DE, Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima H, Henkart PA. Cytotoxic lymphocyte granzymes trigger a target cell internal disintegration pathway leading to cytolysis and DNA breakdown. J Immunol. 1994;152:1057–1063. [PubMed] [Google Scholar]
- 35.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 36.Shinkai Y, Takio K, Okumura K. Homology of perforin to the ninth component of complement (C9) Nature. 1988;334:525–527. doi: 10.1038/334525a0. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenheld MG, et al. Structure and function of human perforin. Nature. 1988;335:448–451. doi: 10.1038/335448a0. [DOI] [PubMed] [Google Scholar]
- 38.Lowrey DM, et al. Cloning, analysis, and expression of murine perforin 1 cDNA, a component of cytolytic T-cell granules with homology to complement component C9. Proc Natl Acad Sci USA. 1989;86:247–251. doi: 10.1073/pnas.86.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kagi D, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 40.van den Broek ME, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spielman J, Lee RK, Podack ER. Perforin/Fas-ligand double deficiency is associated with macrophage expansion and severe pancreatitis. J Immunol. 1998;161:7063–7070. [PubMed] [Google Scholar]
- 42.Stepp SE, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. [PubMed] [Google Scholar]
- 43.Fadeel B, Orrenius S, Henter JI. Familial hemophagocytic lymphohistiocytosis: too little cell death can seriously damage your health. Leuk lymph. 2001;42:13–20. doi: 10.3109/10428190109097672. [DOI] [PubMed] [Google Scholar]
- 44.Goransdotter Ericson K, et al. Spectrum of perforin gene mutations in familial hemophagocytic lymphohistiocytosis. Am J Hum Genet. 2001;68:590–597. doi: 10.1086/318796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 46.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 47.Schuler GD, et al. A gene map of the human genome. Science. 1996;274:540–546. [PubMed] [Google Scholar]
- 48.Spilsbury K, O’Mara MA, Wu WM, Rowe PB, Symonds G, Takayama Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood. 1995;85:1620–1629. [PubMed] [Google Scholar]
- 49.Fields KA, McCormack R, de Armas LR, Podack ER. Perforin-2 restricts growth of Chlamydia trachomatis in macrophages. Infect Immun. 2013;81:3045–3054. doi: 10.1128/IAI.00497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormack R, de Armas LR, Shiratsuchi M, Ramos JE, Podack ER. Inhibition of intracellular bacterial replication in fibroblasts is dependent on the perforin-like protein (Perforin-2) encoded by macrophage-expressed gene 1. J Innate Immun. 2013;5:185–194. doi: 10.1159/000345249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Angelo ME, Dunstone MA, Whisstock JC, Trapani JA, Bird PI. Perforin evolved from a gene duplication of MPEG1, followed by a complex pattern of gene gain and loss within Euteleostomi. BMC Evol Biol. 2012;12:59. doi: 10.1186/1471-2148-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiens M, et al. Innate immune defense of the sponge Suberites domuncula against bacteria involves a MyD88-dependent signaling pathway. Induction of a perforin-like molecule. J Biol Chem. 2005;280:27949–27959. doi: 10.1074/jbc.M504049200. [DOI] [PubMed] [Google Scholar]
- 53.Mah SA, Moy GW, Swanson WJ, Vacquier VD. A perforin-like protein from a marine mollusk. Biochem Biophys Res Commun. 2004;316:468–475. doi: 10.1016/j.bbrc.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 54.Kemp IK, Coyne VE. Identification and characterisation of the Mpeg1 homologue in the South African abalone, Haliotis midae. Fish Shellfish Immunol. 2011;31:754–764. doi: 10.1016/j.fsi.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Wang GD, et al. Molecular cloning and responsive expression of macrophage expressed gene from small abalone Haliotis diversicolor supertexta. Fish Shellfish Immunol. 2008;24:346–359. doi: 10.1016/j.fsi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 56.He X, Zhang Y, Yu Z. An Mpeg (macrophage expressed gene) from the Pacific oyster Crassostrea gigas: molecular characterization and gene expression. Fish Shellfish Immunol. 2011;30:870–876. doi: 10.1016/j.fsi.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 57.Baran K, et al. The molecular basis for perforin oligomerization and transmembrane pore assembly. Immunity. 2009;30:684–695. doi: 10.1016/j.immuni.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Rosado CJ, et al. A common fold mediates vertebrate defense and bacterial attack. Science. 2007;317:1548–1551. doi: 10.1126/science.1144706. [DOI] [PubMed] [Google Scholar]
- 59.Hadders MA, Beringer DX, Gros P. Structure of C8alpha-MACPF reveals mechanism of membrane attack in complement immune defense. Science. 2007;317:1552–1554. doi: 10.1126/science.1147103. [DOI] [PubMed] [Google Scholar]
- 60.Slade DJ, Lovelace LL, Chruszcz M, Minor W, Lebioda L, Sodetz JM. Crystal structure of the MACPF domain of human complement protein C8α in complex with the C8γ subunit. J Mol Biol. 2008;379:331–342. doi: 10.1016/j.jmb.2008.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Law RH, et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468:447–451. doi: 10.1038/nature09518. [DOI] [PubMed] [Google Scholar]