Abstract
Chronic cocaine use produces long-lasting changes in reward circuits that may underlie the transition from casual to compulsive patterns of drug use. Although strong neuroadaptations within the mesocorticolimbic system are known to occur, the specific role of these drug-induced plasticities on sensitization remains to be elucidated. Here we investigate whether PKMζ, a protein involved in maintaining long-term potentiation (LTP), plays a role in these cocaine-induced changes in synaptic strengthening. We performed whole-cell voltage clamp recordings of putative ventral tegmental area (VTA) dopamine (DA) cells 24 hours after five days of 15 mg/kg i.p. cocaine or isovolumetric saline injections. We observed that superfusion of 5µM ZIP (PKMζ inhibitory peptide) decreased AMPA currents and AMPA/NMDA ratios only in cocaine sensitized rats. In vivo ZIP microinfusions (10 nmol) into the VTA after cocaine sensitization decreased locomotor activity on a subsequent cocaine challenge only if given ZIP is given before the withdrawal period. On the other hand, ZIP microinfusions into the nucleus accumbens (NAc) core after a seven days withdrawal period disrupt the expression of locomotor sensitization. The present data provide a potentially relevant region, and time-specific PKMζ-dependent brain mechanism that enables sensitization. Our results support the vision that addiction involves a pathological learning process. They imply that if this synaptic strengthening is reversed, changes in the behavioral response may also be overturned.
Keywords: Protein kinase Mzeta, VTA, LTP, cocaine sensitization, dopamine, AMPA/NMDA ratio
Introduction
Chronic cocaine users undergo a transition from casual to compulsive patterns of drug use (O'Brien & McLellan, 1996; Leshner, 1997; Volkow & Li, 2004) that might be caused by aberrant learning (Berke & Hyman, 2000; Hyman & Malenka, 2001; Nestler, 2001b). Underlying such pathological behavior are strong neuroadaptations within the mesocorticolimbic system (reviewed by (Thomas et al., 2000; Kalivas, 2004; Kauer & Malenka, 2007; Bowers et al., 2010; Schmidt & Pierce, 2010; Wolf & Ferrario, 2010). The specific role of these drug-induced plasticities in critical components of this circuit, particularly the ventral tegmental area (VTA) and the nucleus accumbens (NAc), remains to be elucidated.
Emerging evidence suggests that glutamatergic neurotransmission plays a central role in the changes that occur in the mesocorticolimbic system regulating the physiological action induced by drugs of abuse. In the VTA, a robust α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR)-mediated potentiation of excitatory synapses, typically measured as an increase in AMPA/NMDA ratio, occurs after exposure to cocaine, which can alter reward related behaviors (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004; Sarti et al., 2007; Chen et al., 2009). Similarly, chronic cocaine administration followed by a withdrawal period increases AMPA/NMDA ratio in the NAc (Kourrich et al., 2007a). Therefore, cocaine-induced changes in synaptic strengthening may be responsible for the persistent physiological alterations observed during and after discontinuing drug use and could form the basis for maintaining the sensitized response (Vanderschuren & Kalivas, 2000; Nestler, 2001a).
Recently, it has been shown that a variety of long-term memories in different regions of the brain are quickly erased by local inhibition of PKMζ, a persistently active protein kinase that maintains LTP (Pastalkova et al., 2006; Shema et al., 2007; Serrano et al., 2008; Kwapis et al., 2009; Shema et al., 2009; Hardt et al., 2010; Madronal et al., 2010; Migues et al., 2010; Sacco & Sacchetti, 2010; von Kraus et al., 2010). The potentiation of synaptic transmission by PKMζ in the hippocampus during LTP may be similar to the change in AMPA/NMDA ratio after cocaine in the VTA in that the plasticity occurs through increases in the AMPAR-mediated synaptic transmission (Ling et al., 2002; Ling et al., 2006; Migues et al., 2010). Thus we examined PKMζ’s effect on cocaine-related plasticities in the VTA and the NAc.
In our studies we used a cocaine sensitization model to examine the cellular and molecular changes that occur after chronic drug administration. Behavioral sensitization is characterized by a progressive increase in behavioral response to repeated injections of the same dose of a drug that persists after a withdrawal period (Segal, 1975; Robinson & Becker, 1986). We postulate that if drug exposure induces LTP-like changes in reward-related regions, it should be possible to prevent or even reverse behavioral responses with inhibitors of LTP. Here, we show that infusions of a PKMζ inhibitor alter AMPA-mediated currents and AMPA/NMDA ratio in the VTA and cocaine sensitization, reducing cocaine-induced changes in locomotor activity, first in the VTA, and after a withdrawal period in the NAc.
This work was published in a preliminary form at the Society for Neuroscience Meeting in 2009, 2010 and 2011 (Velez-Hernandez et al., 2009, 2010, 2011).
Materials and Methods
Animals
All experimental procedures were performed according to the US Public Health Service publication “Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Care and Use Committee at the University of Puerto Rico Medical Sciences Campus. Sprague-Dawley male rats (150–250g) were used as the experimental subjects. Animals were housed two per cage and maintained at constant temperature and humidity with a 12-hr light/dark cycle. Water and food was provided ad libitum. Rats were randomly assigned to the groups before the study began.
Whole-cell voltage-clamp recordings
Patch clamp experiments were performed in brain slices from rats (150–250g) that received a cocaine (15mg/kg) i.p or an isovolumetric saline injection daily for five days. Twenty-four hours after the last injection, rats were anaesthetized by an intraperitoneal injection of chloral hydrate (400 mg/kg) and decapitated. Horizontal slices (thickness: 220 μm) containing the VTA were prepared using a vibratome (VT1000S, Leica, Germany). Slices were cut in ice-cold (2–4 °C) oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): 127 NaCl; 2.5 KCl; 1.25 NaH2PO4; 25 NaHCO3; 2 CaCl2; 1 MgCl2; 25 D-glucose. The solution was previously saturated with a 95% O2 and 5% CO2 gas mixture to pH = 7.4. Slices were transferred to an intermediate chamber and incubated at 32 °C in the same ACSF solution for approximately 1 hr before transferring them to the recording chamber. In AMPA/NMDA sudies after 1 hr in the intermediate chamber half of the slice was placed in a ZIP incubation solution and the other half kept in the intermediate ACSF chamber. Whole-cell voltage-clamp recordings were obtained from visually identified neurons in the VTA area (Paxinos & Watson, 1998) using infrared microscopy with DIC (BX51WI Olympus, Japan) and water-immersion objectives. Putative VTA DA cells were identified by the presence of large Ih current. Ih is present in about 84% VTA DA neurons (Sarti et al., 2007) and VTA GABA cells do not express this conductance (Margolis et al., 2006) Therefore, the contribution of non-DA cells to our data is likely to be not significant.
The slice was submerged in a 500 µl recording chamber, which was connected to a superfusion system (1–2 mL per minute). The bath solution was the same used for slice preparation, with the chamber temperature maintained at 32 °C. Borosilicate glass patch pipettes (O.D. 1.5 mm, I.D. 1,0 mm; WPI, Sarasota, FL) were pulled to a final resistance of 3–6 MΩ when filled with (in mM): 120 cesium methanesulfonate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 5 TEA-Cl, 2.5 MgATP, and 0.25 NaGTP, pH 7.2–7.3 (270 –285mOsm) (Na)GTP and (Mg)ATP were prepared daily. Pharmacologically isolated (100 µM picrotoxin) EPSC’s were electrically evoked by placing a stimulating electrode 60–150 µm rostral to the patched cell. Once a stable EPSC was achieved 5mM ZIP (InVitrogen, CA) was superfused into the slide and EPSC’s were recorded for 14 minutes. Series resistance was monitored during the entire experiment and data were discarded if changes of more than 15% occurred.
To determine the role of ZIP administration on AMPA/NMDA ratios slices from rats treated with saline or cocaine for 5 days were cut in half and one portion was incubated in ZIP (cocaine/ZIP and saline/ZIP) and the other in ASCF (cocaine and saline) for 1 hr. NMDA and AMPA receptor traces were constructed by averaging 15 EPSCs elicited at +40 mV. NMDA receptor responses were calculated by subtracting the average response in the presence of 50 µM D-2-amino-5-phosphonovalerate (D-APV) (AMPA receptor-mediated only) from that recorded in its absence. Then the peak of the AMPA receptor-only EPSC was divided by the peak of the NMDA receptor-only EPSC to give an AMPA/NMDA ratio.
Data were collected with PClamp 9 software through a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, Ca), filtered at 10 kHz through a Bessel filter, digitized at 5 kHz using Digidata 1322A (Axon Instruments, Foster City, CA), and analyzed off line using Clampfit (Molecular Devices) software.
Placement of guide cannulas for intracranial injections
After five days of quarantine, animals (250–300 g) were anesthetized using 50 mg/kg of pentobarbital in 0.9% saline, i.p. A bilateral guide cannula (Plastics One, Roanoke, VA) was placed 1mm above the area of the VTA or the NAc core. Stereotaxic coordinates for the VTA were −5.3 mm from bregma and −7.0 mm ventral to the cortical surface and 2.3 from bregma, and −5.2 ventral to the cortical surface for the NAc using the Paxino’s and Watson Atlas, 1998.
Sensitization protocol and behavior
Animals were randomly divided into four groups (vehicle/saline, vehicle/cocaine, ZIP/saline, and ZIP/cocaine). Locomotor activity experiments started one week after surgery and were performed in an isolated acoustic chamber (Whisper Room Inc. Morristown, TN, USA). As shown on figure 1A, two days before the beginning of the experiment, each group was habituated (for 1 hour) to the infrared photocell box (Accuscan Instruments, Columbus, OH, USA). On experimental day one animals were habituated for 15 minutes after which rats were treated with either 15mg/kg i.p. cocaine (Sigma, St. Louis, MO, USA) or isovolumetric saline injections. Immediately after the injections, locomotion activity was assessed for one hour (Fig. 2A). This procedure was repeated for five days. Before the withdrawal period, on day 5, animals received an intra VTA 10nmol ZIP microinfusion (0.5uµL/side) or isovolumic vehicle after the locomotor assessment. On day 6, all groups received a cocaine challenge (15 mg/kg, i.p.) and locomotion was assessed for an hour. Animals were given a 7-day withdrawal period and on day 14 all animals received a second cocaine challenge (15 mg/kg, i.p.) and locomotor activity was again recorded for 1 hour. To determine the role of VTA- PKMζ on the expression of sensitization, animals received an intra-VTA 10nmol ZIP microinfusion (0.5uµL/side) or isovolumic vehicle on day 7 of the withdrawal period. On day 13, all groups received a cocaine challenge (15 mg/kg, i.p.) and locomotion was assessed for an hour.
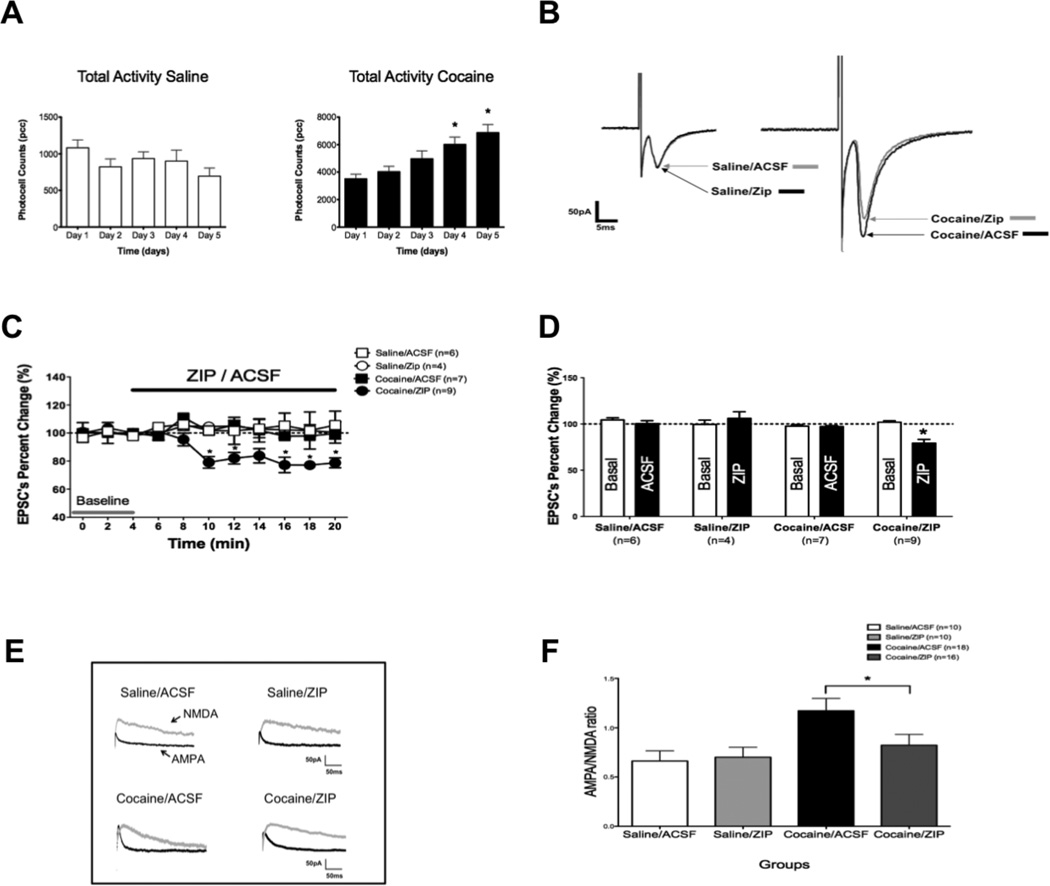
Figure 1. ZIP decreases AMPA currents and AMPA/NMDA ratio in cocaine-sensitized rats.
A. Mean total ambulatory activity (pcc/60 min, ± S.E.M.) was recorded each day for saline (white) and cocaine (black) injected animals. Asterisks (*) denote group significance when compared to Day 1. B Sample traces show AMPA EPSC’s reduction upon ZIP superfusion (5µM). Zip had no effect on saline treated animals (left). Notice the potentiated EPSC in cocaine treated rats, which was diminished by ZIP application (right). C: Time course showing that ZIP superfusion significantly decreases AMPA EPSC amplitudes in slices taken from cocaine treated animals (cocaine/ZIP) (*p < 0.05) and has no effect on saline treated animals (saline/ZIP) (p > 0.05; (Two-way ANOVA, followed by bonferroni post-test). ACSF superfusion has no effect on EPSC amplitude in control experiments (saline/ACSF and cocaine/ACSF) D: Bar graph shows AMPA EPSC’s reduction as percentage of change from basal (EPSC amplitude at 4 min). Continuous ZIP application was able to significantly reduce AMPA mediated transmission by ~20% (* p < 0.01 Student t-test) in cocaine treated animals when compared to basal but failed to do so in saline injected rats. ACSF superfusion had no effect in AMPA mediated currents in cocaine or saline injected animals. Data are percent changes ±SEM. E. Representative traces showing AMPA (black)/NMDA (gray) ratio from brain slices incubated in ZIP (5µM) or ACSF from saline or cocaine treated animals (scale: 50pA, 50 ms). F. Bar graph showing AMPA/NMDA ratios of saline and cocaine treated animals. The AMPA/NMDA ratio increased with cocaine injections. ZIP incubation significantly reduced the AMPA/NMDA ratio from cocaine treated rats but failed to do so in saline injected animals (* p < 0.05 One Way ANOVA Newman Keuls, ratios ± SEM).
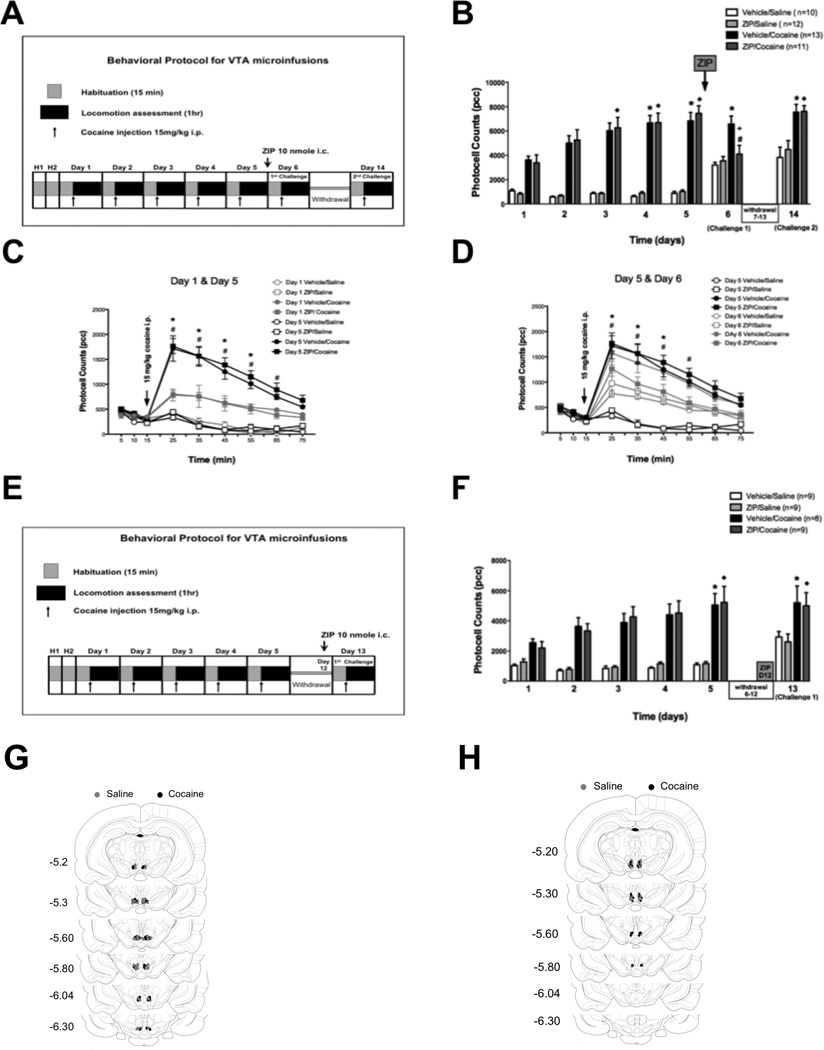
Figure 2. Intra-VTA ZIP microinfusions’s effect on behavioral sensitization.
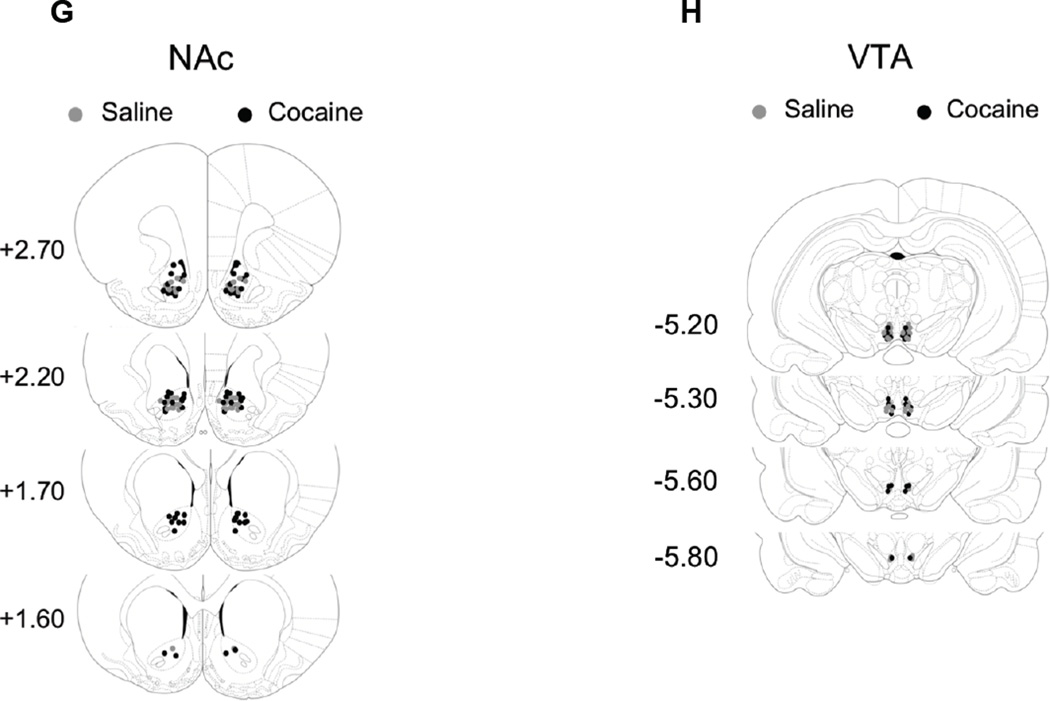
A: Diagram showing the cocaine sensitization protocol with the time point for intra VTA ZIP microinfusion. B: Mean total ambulatory activity (pcc/60 min, ± S.E.M.) was recorded each day for every group. Asterisks (*) and diamonds (♦) denote group significance when compared to Day 1 for they respective group. One day after ZIP treatment (Day 6) ZIP/cocaine treated animals showed a significant decrease (#) in locomotor activity compared to Day 5 (p < 0.01). Locomotion was also significantly different (+) from that of Vehicle/Cocaine animals on Day 6. On day 14, ZIP/cocaine pretreated animals showed a sensitized response. No statistical differences between ZIP/cocaine and vehicle/cocaine treated animals were observed on day 14 (p > 0.05). There was no change between ZIP/saline and vehicle/saline controls throughout the experiment (p > 0.05). C: Time course of locomotor responses after one (grey) and five (black) days of cocaine (15 mg/kg, i.p.) (filled) or saline (unfilled). Cocaine sensitization was manifested as an increase in locomotor activity primarily during the first 10–40 min (25–55 min) after cocaine injection. Significant differences (p < 0.05) are indicated by asterisks (*) for vehicle/cocaine and numeral (#) for ZIP/cocaine. Each time point represents the mean ± SEM. D: Time course for the locomotor response on days 5 (black) and 6 (grey). ZIP/cocaine injected animals (filled grey squares) show decreased locomotor activity (# p < 0.05) compared to Day 5. Asterisks (*) denote significance between ZIP/cocaine and vehicle/cocaine animals on day 6. Each point represents the mean ± SEM. E: Diagram showing the cocaine sensitization protocol with the time point for intra VTA ZIP microinfusion after a withdrawal period. F: On day 5 cocaine treated animals were sensitized to cocaine. One day after ZIP treatment (Day 12) ZIP/cocaine treated animals showed no significant change in locomotor activity compared to Day 5 or to vehicle/cocaine animals on Day 12 (p > 0.05). There was no change between ZIP/saline and vehicle/saline controls throughout the experiment (p > 0.05, Two-way ANOVA, followed by bonferroni post-test for all comparisons). G,H: Injection sites within the VTA for saline (grey circles) and cocaine (black circles) treated animals.
For intra NAc microinfusions animals were injected with 15mg/kg cocaine once a day for five days and locomotor activity was recorded for 1 hour (Fig. 3A). On days 6 and 7 of the withdrawal period (days 11 and 12 of the experiment) animals received either ZIP or vehicle microinfusions and no locomotor recordings were made. On day 13, all groups received a cocaine challenge (15 mg/kg, i.p.) (no microinjection given) and locomotion was measured for 1 hour to assess the expression of cocaine sensitization. Twenty-four hours after the termination of the experiments animals were euthanized and cannula placement was determined.
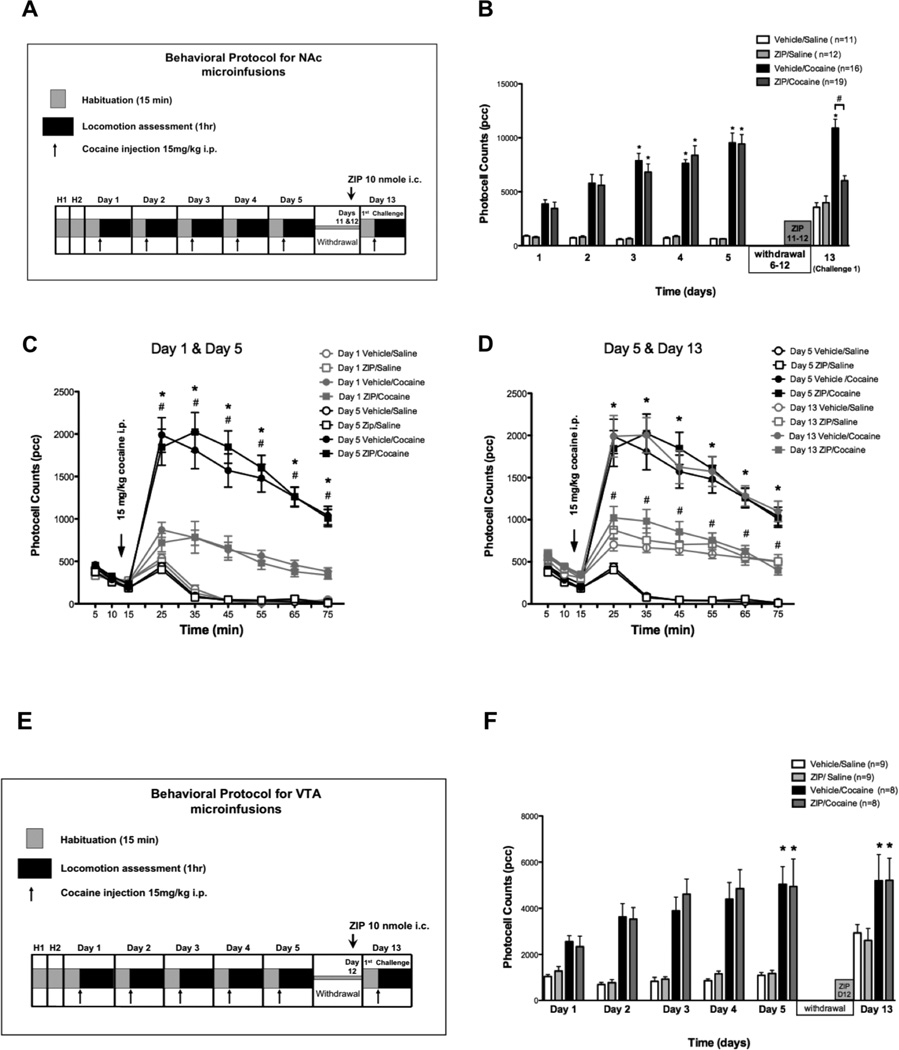
Figure 3. Intra NAc ZIP microinfusions decrease the expression of cocaine sensitization.
A: Diagram shows cocaine sensitization protocol with time points for intra NAc ZIP microinfusions. B: Mean total ambulatory activity (pcc/60 min, ± S.E.M.) was recorded for each group. Asterisks (*) denote significance of the group compared to corresponding Day 1 (p < 0.01). Numeral (#) denotes statistical significance between groups on Day 6. On Day 5 cocaine treated animals show sensitization to cocaine. On Day 13 ZIP/cocaine treated animals did not express sensitization (p > 0.05). Instead, they showed a significant decrease (Day 13) in locomotor activity when compared to Vehicle/cocaine animals (p < 0.05). There was no significance between ZIP/saline and vehicle/saline controls throughout the experiment (p > 0.05). C: Time course of locomotor responses after one (grey) and five (black) days of cocaine (15 mg/kg, i.p.) (filled) or saline (unfilled) administration. Cocaine sensitization was manifested as an increase in locomotor activity in all time points. Significant differences between day 1 and day 5 (p < 0.05) are indicated by asterisk (*) for vehicle/cocaine and # for ZIP/cocaine. Each time point represents the mean ± SEM. D: Time course for the locomotor response on Days 5 (black) and 13 (grey). ZIP/cocaine injected animals (filled grey squares) show decreased locomotor activity (* p < 0.05) compared to Day 5 (#). Asterisks (*) denote significance between ZIP/cocaine and vehicle/cocaine animals on day 13. Each point represents the mean ± SEM. (Two-way ANOVA, followed by bonferroni post-test for all comparisons). E: Injection sites within the NAc for saline (grey circles) and cocaine (black circles) treated animals.
Drug infusion
ZIP (Myr-SIYRRGARRGARRWRKL-OH, Anaspec) was dissolved in 100 mM Tris-saline solution (vehicle, pH 7.2). Intracranial bilateral infusions of 10 nmol ZIP and 2% Pontamine Sky Blue were made by inserting a 31-gauge injection needle 1 mm below the tip of the guide cannula. Using a 1 µl syringes (Hamilton, Reno, NV) and a microsyringe drive (Cole Palmer, Chicago IL) 0.5 μl/side were delivered at a rate of 0.1ul/min. The bilateral cannula was left in place for an additional minute to avoid drug backflow.
Histological Verifications of cannulas’ positions
At the end of the experiment, animals were anesthetized with 50mg/kg of pentobarbital and the injection site was marked with a pontamine sky blue 0.5 µl/side (2%) microinfusion. Cannula placement was determined using microscopic examination of Pontamine Sky Blue in coronal brain sections (20 microns, Fig. 2E and 3E). Animals that received microinfusions outside of the region of interest were excluded from data analyses.
Statistics
Total ambulatory activity, expressed as photocell counts (pcc), between groups were analyzed using One-Way or Two-way ANOVA followed by bonferroni post-test (numbers are presented as mean +/− standard error). A significant statistical difference of p < 0.05 when compared to day 1 was considered a successful sensitization protocol (Kalivas & Stewart, 1991). Two-way ANOVA followed by bonferroni post-test was used for statistical analysis of EPSC amplitude. One-Way ANOVA followed by Newman Keuls was used for AMPA/NMDA ratio and a value of p < 0.05 was considered statistically significant.
Results
ZIP superfusions decreases AMPA-mediated EPSCs in the VTA of cocaine-sensitized rats
PKMζ is thought to enhance synaptic transmission by persistently increasing postsynaptic AMPAR-mediated responses (Yao et al., 2008; Migues et al., 2010) thereby maintaining late-LTP (Serrano et al., 2005) in regions like the hippocampus. Excitatory synapses are potentiated after a single or multiple injections of cocaine (Ungless et al., 2001). Therefore, we examined the effect of the PKMζ inhibitor ZIP on AMPAR current in the VTA after five days of cocaine injections. Previous studies on cocaine-induced synaptic plasticity in the VTA were performed 24 h after an in vivo cocaine exposure (Ungless et al., 2001; Borgland et al., 2004; Bellone & Luscher, 2006). For this reason, we performed whole-cell voltage clamp recordings of putative VTA DA cells 24 hours after five days of 15 mg/kg cocaine or saline injections (Fig. 1A). Saline injected rats show no changes in behavioral response (1082 +/−109 counts on day 1 and 694 +/−110 counts on day 5, n=10; One-Way ANOVA p < 0.05; Fig. 1A). Cocaine treated animals show sensitization when day 1 and day 5 locomotor activity are compared (3514 +/− 330 counts on day 1 and 6880 +/− 587 counts on day 5, n=16; One-Way ANOVA p < 0.05; Fig. 1A). A time course shows a decrease in AMPAR-mediated EPSCs of cocaine-treated animals when ZIP is added to the bath solution (ANOVA, F 32,102 = 6.54, p < 0.05; Fig. 1C). Superfusion of 5 μM ZIP resulted in a significant decrease (81.10 +/− 4.58 pA; n=9) in AMPA-mediated EPSCs in cocaine-treated rats when compared with saline-treated (100.32 +/− 3.21 pA; n=4) or ACSF superfused animals (97.48 +/− 3.02; n=7; ANOVA F 7,44 = 6.91, p < 0.01; Fig. 1B, 1C and 1D).
AMPA/NMDA ratio
Slices from animals treated with saline or cocaine i.p. injections for 5 days were incubated in ACSF or ZIP for 1 hour before recording. There was a significant increase in AMPA/NMDA ratio in cocaine-treated (1.17 ± 0.12, n=18) compared to saline-injected animals (0.66 ± 0.10, n=10) after incubation in regular ACSF (Fig. 1E and 1F). However, incubation with 5 μM ZIP resulted in a decrease of AMPA/NMDA ratio only in cells from cocaine-treated rats (0.82 ± 0.11, n= 16; One-Way ANOVA p < 0.05). ZIP incubation had no effect on putative VTA DA cells from saline treated animals (0.69. ± 0.10 n=10; One-Way ANOVA p > 0.05; Fig. 1E and 1F).
Intra-VTA ZIP microinfusions before a withdrawal period decrease behavioral sensitization
To determine if PKMζ inhibition has behavioral consequences in vivo we examined the effect of intra-VTA ZIP microinfusions on behavioral sensitization to cocaine. Rats were chronically injected with 15 mg/kg cocaine or saline i.p. over 5 consecutive days to induce behavioral sensitization (Segal, 1975; Robinson & Becker, 1986; Kalivas & Duffy, 1993; Jimenez-Rivera et al., 2006). Cocaine-injected animals showed a significant increase in locomotor activity on day 5 (6830 ± 698 counts for vehicle/cocaine, n = 13 and 7443 ± 625 counts for ZIP/cocaine, n=11), compared to day 1 (3615 ± 318 counts for vehicle/cocaine and 3378 ± 649 counts for ZIP/cocaine; ANOVA F 27,287 = 23.06, p < 0.01; Fig. 2B). To determine whether inhibition of PKMζ affects the already established sensitization, 10 nmol ZIP was infused into the VTA after one hour of locomotion assessment on day 5. On day 6 a single cocaine challenge (15 mg/kg) was given to all animals and locomotion activity was assessed for one hour. This day, ZIP/cocaine animals (i.e., animals treated with ZIP the day before the cocaine challenge) showed a significant decrease in locomotion (4095 ± 724 counts; n=11) compared to vehicle/cocaine treated animals (6575 ± 652 counts; n=13; ANOVA F 27,287 = 23.06, p < 0.01; Fig. 2B). ZIP microinfusion on day 5 did not alter cocaine’s acute response since locomotor activity of ZIP/cocaine animals (4095 ± 724 counts; n=11) was not significantly different from those that received cocaine for the first time on day 6 (3545 ± 348 counts, n=12, for ZIP/saline and 3200 ± 221 counts, n=10 for vehicle/saline; ANOVA F27,287 = 23.06, p > 0.05; Fig. 2B).
Time course of locomotor responses on days 1 and 5 (Fig. 2C) compares locomotor activity after one (grey) and five (black) days of cocaine (15 mg/kg, i.p. filled) or saline (unfilled). Cocaine sensitization was manifested as an increase in locomotor activity primarily during the first 40 min after cocaine injection (ANOVA F71,774 = 22.97, p < 0.05). Figure 2D shows a decrease in locomotor activity primarily in the first 30 min after cocaine injection in ZIP/cocaine animals when compared to vehicle/cocaine rats on day 6 (*p < 0.05) and ZIP/cocaine on day 5 (ANOVA F 71,774 = 18.89, # p < 0.05). There is no statistical differences on any time point between ZIP/cocaine group and animals that received cocaine for the first time (vehicle/saline and ZIP/saline). Also, ZIP pre-treatment has no effect on the acute response to cocaine since there is no difference at any time point between vehicle/saline and ZIP/saline on day 6.
To determine whether ZIP’s effect on locomotion on day 6 (decreased locomotion) alters the expression of sensitization after withdrawal, we then tested the effect of a second challenge of cocaine (15 mg/kg i.p.) after a 7 day drug-free period. On day 14, ZIP/cocaine-pretreated animals showed no significant difference in locomotion (7542 ± 640 counts; n =13) compared to vehicle/cocaine pre-treated rats (7608 ± 475 counts; n=11; ANOVA F 27,287 = 23.06, p > 0.05; Fig. 2B). These results suggest that either intra-VTA ZIP’s effect on sensitization is temporary (short lived) or that a different mechanism independent of VTA’s PKMζ is active on day 5 that sustains expression of sensitization after a withdrawal period. Because previous studies have suggested a time-limited role for the VTA in cocaine-sensitization, which is later taken over by the NAc (Wolf, 2002b), we investigated whether inhibition of PKMζ in the VTA or the NAc, after a withdrawal period, modifies the expression of previously established sensitization.
Intra-VTA ZIP microinfusions after a withdrawal period do not alter the expression of behavioral sensitization
Rats were chronically injected with cocaine (15 mg/kg) or saline i.p. over 5 consecutive days to induce behavioral sensitization. Cocaine-injected animals showed a significant increase in locomotor activity on day 5 (5043 ± 762 counts, n=9 for vehicle/cocaine and 5217 ± 1079 counts, n=8 for ZIP/cocaine) compared with day 1 (2553 ± 256 counts for n=9 vehicle/cocaine and 2203 ± 423 counts for ZIP/cocaine, ANOVA F23,190 = 9.89, p < 0.05; Fig. 2F). ZIP (10 nmol) was microinfused into the VTA on the last day of the withdrawal period (day 12, Fig. 2E). A cocaine challenge (15 mg/kg i.p.) was given 24 hours later. On day 13, ZIP/cocaine-pretreated animals did not present any change in locomotion (4991± 878 counts, n=8) compared with vehicle/cocaine-pretreated rats (5193 ± 1133 counts, n=9, ANOVA F23,190 = 9.89, p > 0.05; Fig. 2F). This figure also shows that ZIP pre-treatment has no effect on the acute response to cocaine since there is no difference between ZIP/saline (n=9) and vehicle/saline animals (n=9) on day 13. Animals that received microinfusions outside of the VTA were excluded from data analyses (Fig. G-H)
Intra-NAc ZIP microinfusions after a withdrawal period decrease the expression of behavioral sensitization
We performed microinjections of PKMζ inhibitor ZIP directly into the NAc at the end of a withdrawal period following a cocaine sensitization scheme. Rats were chronically injected with 15 mg/kg cocaine or saline i.p. over 5 consecutive days to induce behavioral sensitization. Cocaine-injected animals showed a significant increase in locomotor activity on day 5 (9438 ± 871 counts, n=19 for ZIP/cocaine and 9535 ± 914 counts, n=16 for vehicle/cocaine) compared with day 1 (3456 ± 574 counts for ZIP/cocaine and 3865 ± 391 counts for vehicle/cocaine; ANOVA F23,319 = 25.43, p < 0.01; Fig. 3B). ZIP (10 nmol) was microinfused into the NAc on the last two days of the withdrawal period (days 11 and 12, Fig. 3A). A cocaine challenge (15 mg/kg i.p.) was given on day 13. On day 13, ZIP/cocaine-pretreated animals presented a significant decrease in locomotion (6027± 464 counts) compared with vehicle/cocaine-pretreated rats (10906 ± 811 counts; ANOVA F23,319 = 25.43, p < 0.05; Fig. 3B). Time course of locomotor responses on days 1 and 5 (Fig. 3C) compares locomotor activity after one (grey) and five (black) days of cocaine (15 mg/kg, i.p.) (filled) or saline (unfilled). Cocaine sensitization was manifested as an increase in locomotor activity for ZIP/cocaine (#p < 0.05) and vehicle/cocaine (*p < 0.05) animals during all time points after cocaine injection (ANOVA F71,1026 = 29.99, p < 0.05; Fig. 3C). Figure 3D shows that there is a decrease in locomotor activity of ZIP/cocaine animals in every time point on day 13 when compared to day 13 vehicle/cocaine (*p < 0.05) animals and when compared to ZIP/cocaine animals on day 5 (ANOVA F71,1026 = 23.34, #p < 0.05). This figure also shows that ZIP pre-treatment has no effect on the acute response to cocaine since there is no difference between ZIP/saline (n=12) and vehicle/saline (n=11) animals. Histological analysis confirmed cannula placements and animals that received microinfusions outside of the NAc were excluded from data analyses These results suggest that PKMζ may help maintain the expression of cocaine sensitization in NAc.
Discussion
In this study we demonstrated that ZIP decreased AMPA-mediated currents and AMPA/NMDA ratios in VTA-DA cells from cocaine-treated animals. Moreover, VTA-ZIP infusions before a withdrawal period decrease locomotor behavioral sensitization. On the other hand, intra VTA ZIP infusions after a withdrawal period, had no effect on the expression of cocaine sensitization. Administration of the PKMζ inhibitor into the NAc following a withdrawal decreases the expression of cocaine sensitization. These results indicate that the persistent activity of PKMζ in the VTA is critical for the maintenance of cocaine-induced behavioral sensitization only before a withdrawal period. They also suggest that the there is a need of a persistently active PKMζ in the NAc for the expression of behavioral sensitization after a withdrawal period.
Synaptic plasticity that occurs in the reward system as a consequence of addictive drugs is thought to contribute to addiction-related behaviors (Kauer & Malenka, 2007; Thomas et al., 2008; Kalivas, 2009; Bowers et al., 2010; Russo et al., 2010; Schmidt & Pierce, 2010; Wolf & Ferrario, 2010). In particular, excitatory synaptic transmission in the mesolimbic DA system has been shown to support some of the behavioral long-lasting drug effects (Kalivas, 1995; Wolf, 1998; Overton et al., 1999). However, to our knowledge, evidence demonstrating that selectively reversing these late LTP-like neuroadaptations after chronic cocaine treatment alters the enhanced behavioral response characteristic of sensitization is sorely missing.
One of the immediate molecular effects that underlies single and multiple injections of cocaine are long-term changes in synaptic plasticity in the VTA (Ungless et al., 2001; Borgland et al., 2004; Schumann et al., 2009). This area is implicated in the development of drug addiction (Ikemoto & Wise, 2004; Kauer, 2004; Self, 2004) and sensitization (Kalivas et al., 1993; Wolf & Xue, 1998). In addition, the NAc core, which contributes to drug-associated, cue-induced cocaine seeking and mediates the incentive value of reward-conditioned stimuli (Hollander & Carelli, 2005; 2007; Anderson et al., 2008; Goto & Grace, 2008; Owesson-White et al., 2009) also undergoes synaptic strengthening after repeated exposure to cocaine followed by a period of abstinence (Boudreau & Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007a; Ferrario et al., 2010). The specific molecular mechanisms that sustain these changes have been unknown, but may be related to those implicated in LTP.
Several protein kinases have been shown to be important for LTP. Recent studies have focused on the activity of enzymes critical within 30 minutes after titanic-stimulation, but not afterwards. Therefore, these proteins are thought to be important for the induction but not the maintenance of LTP. During LTP, a single PKC isozyme, PKMζ (Sacktor et al., 1993; Osten et al., 1996) is activated by a mechanism fundamentally different from that of the kinases important for LTP induction (Osten et al., 1996; Hernandez et al., 2003). PKMζ is a brain-specific protein kinase C isoform (Hernandez et al., 2003) that lacks, inhibition from a regulatory domain, thus, is persistently active (Sacktor et al., 1993). ZIP is a myristoylated PKMζ pseudosubstrate inhibitory peptide that specifically mimics the autoregulatory domain of PKCζ, therefore blocks PKMζ. For this reason, ZIP infusions produce retrograde amnesia of several long-term memories (Shema et al., 2007; Serrano et al., 2008; Kwapis et al., 2009; Shema et al., 2009; Hardt et al., 2010; Madronal et al., 2010; Migues et al., 2010; Sacco & Sacchetti, 2010; von Kraus et al., 2010; Gamiz & Gallo, 2011; He et al., 2011; Shema et al., 2011; Crespo et al., 2012; Kwapis et al., 2012; Pauli et al., 2012). Hence, PKMζ is the first enzyme known to sustain memory by maintaining LTP in the brain.
Cocaine administration has been recently shown to increase PKMζ in the VTA (Ho et al., 2012). Phosphorylation by PKMζ contributes to the late-phase of LTP, by increasing the number of AMPA receptors that are expressed at synapses, enhancing AMPAR-mediated synaptic transmission (Serrano et al., 2005; Ling et al., 2006; Yao et al., 2008). Thus PKMζ inhibition reverses additional AMPAR responses that contribute to potentiated synapses, but has no effect on baseline AMPAR responses, either in vitro (Serrano et al., 2005; Ling et al., 2006; Yao et al., 2008) or in vivo (Pastalkova et al., 2006). For this reason, we investigated whether ZIP selectively decreases AMPA currents from putative VTA-DA neurons of cocaine-sensitized animals and has no effect on AMPA currents of saline-injected rats (Fig. 1). The results confirmed that only potentiated synapses are altered by PKMζ inhibition and that the potentiation observed after cocaine administration is in part mediated by PKMζ. Since the basal or potentiated strength of excitatory synapses is difficult to compare between slices, we calculated the ratio between AMPA and NMDA currents as a normalization procedure (Thomas et al., 2001). We found an increase in AMPA/NMDA ratio in cocaine-treated rats (Fig. 1). This increase in synaptic strength is a key feature of LTP production in animals with repeated cocaine injections (Ungless et al., 2001; Borgland et al., 2004; Sarti et al., 2007; Argilli et al., 2008; Bird et al., 2010). ZIP incubation decreased AMPA/NMDA ratio only in cells from cocaine-sensitized rats (Fig. 1). This results are in agreement with recent evdence showing that inhibition of PKMζ blunts cocaine-enhanced AMPAR/NMDAR ratio (Ho et al., 2012).
In our studies, intra-VTA ZIP infusion on day 5 altered the established cocaine sensitization (Fig. 2); indicating a modification in the mechanism that maintains the long-lasting effects after chronic cocaine exposure (Kalivas, 1995). Since ZIP is thought to reverse already established LTP, these findings imply that LTP occurring in the VTA is PKMζ-dependent and critical for the maintenance of behavioral sensitization prior to a withdrawal period.
ZIP’s effect on behavioral sensitization, AMPA currents and AMPA/NMDA ratios could be due to removal of AMPA receptors from postsynaptic sites (Serrano et al., 2005; Ling et al., 2006; Yao et al., 2008). Potentiation in the VTA after cocaine administration is thought to be accompanied by an exchange of GluA2-containing for GluA2-lacking AMPA receptors (Bellone & Luscher, 2006; Brown et al., 2010; Good & Lupica, 2010). However, recent studies in the NAc show that GluA2-lacking receptors do not seem to mediate expression of cocaine sensitization since animals that have received non-contingent cocaine administration do not show the change from GluA2-containing to GluA2-lacking AMPA receptors, but still show sensitization to cocaine (Boudreau & Wolf, 2005; Kourrich et al., 2007a; Ghasemzadeh et al., 2009; Ferrario et al., 2010). Therefore, it might be possible that decreasing the number of GluA2-containing AMPA receptors left at the synapse can modify the synaptic strengthening in these regions and therefore alter sensitization. To confirm this hypothesis, and therefore find the specific mechanism of ZIP’s effect, further experiments exploring the presence or absence of GluA2 containing receptors before and after ZIP treatment should be performed.
Here, the expression of sensitization measured after a 7-day withdrawal period was not altered by ZIP infusions into the VTA on day 5 (Fig. 2). A simple explanation to this result might be that ZIP’s effect on sensitization is just temporary, as was shown in fear conditioning experiments (see Parsons and Davis 2011 but see (Gamiz, 2011 #1532). It is also possible that other drug-induced VTA neuroadaptations, independent of PKMζ, may exist and allows the expression after a withdrawal period. On the other hand numerous studies have shown that AMPA receptor-mediated activation in the VTA may be a key process responsible for the development of addiction (Kalivas & Stewart, 1991; Vanderschuren & Kalivas, 2000; Wolf et al., 2003; Kauer & Malenka, 2007; Kessels & Malinow, 2009), whereas the NAc mainly supports the mechanisms for expression (Vanderschuren & Kalivas, 2000; Nestler, 2001a). This change in anatomical location is thought to reveal a “transfer” of sensitization from the VTA to the NAc as one progress from initiation to expression (Wolf, 2002a). We believe that synaptic potentiation in the VTA, which eventually induces alterations in the NAc before the withdrawal period (Kourrich et al., 2007a; Conrad et al., 2008; Mameli et al., 2009) had already occurred by the time ZIP was infused into the VTA. For this reason, it might be too late for intra-VTA ZIP infusions to alter expression after withdrawal. The data suggest that elimination of PKMζ-dependent drug-evoked synaptic plasticity within the VTA is not necessary for the maintenance of cocaine induced synaptic changes in the NAc. It is consistent with previous observations that, following cocaine exposure, long-lasting neuroadaptations take place in the NAc even before withdrawal periods (Thomas & Everitt, 2001; Kourrich et al., 2007b).
To confirm that PKMζ-dependant potentiation in the VTA is not important for the expression of sensitization ZIP was microinfused into the VTA after a seven days withdrawal period. There was no change in locomotor activity after ZIP infusion. Therefore, PKMζ dependent potentiation in the VTA seems to not play a role in the expression of sensitization. This is also supported by previous studies that say that the synaptic potentiation in the VTA decays during the withdrawal period (Argilli et al., 2008).
Finally, we tested whether synaptic plasticities occurring in the NAc were also maintained by PKMζ. ZIP was infused into the NAc of sensitized rats on the last two days of the withdrawal period, immediately prior to a challenge dose of cocaine. This manipulation prevented the increase in locomotor activity normally elicited by a subsequent cocaine challenge (Fig. 3). Our NAc results are consistent with two recently published articles that show a role of PKMζ in maintaining addiction memories (Li et al., 2011; Shabashov et al., 2011). In addition, an increase in AMPAR has been shown to occur in the NAc at different time points of the withdrawal period (Boudreau et al., 2007; Boudreau & Wolf, 2005). This increase is thought to be responsible for the drug-evoked synaptic potentiation observed in the NAc (Boudreau & Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007a; Boudreau et al., 2009; Ghasemzadeh et al., 2009; Schumann & Yaka, 2009; Ferrario et al., 2010). Furthermore, GluA2-containing receptors are present in the NAc after a withdrawal period in non-contingent cocaine administration (McCutcheon et al., 2011). Thus, in our studies, PKMζ inhibition may be decreasing the number of GluA2-containing AMPA receptors at the NAc synapse. While further experiments should test the latter hypothesis, our results are in agreement with recent findings showing that NAc postsynaptic GluA2-containing receptors are relevant for the maintenance of memories for drug cues via PKMζ (Li et al., 2011).
Taken together, the present data provide a potentially relevant region and time-specific brain mechanism, dependant of PKMζ that enables sensitization. It suggests that PKMζ may maintain a VTA-dependent mechanism that underlies sensitization prior to a withdrawal period but that a different neuroadaptation, VTA-PKMζ insensitive, must come on-line after a withdrawal period, which mediates expression. We postulate that this VTA-PKMζ insensitive mechanism may be a PKMζ-dependent NAc synaptic plasticity. Our results confirm the vision that addiction involves a pathological learning process. They imply that if this learning process is reversed changes in the behavioral response may also be at least temporary overturned.
Acknowledgements
This work was supported by grants from NIGMS SC1GM084854 to CAJR and MBRS-RISE R25-GM061838 to MVH. The authors thank Juan Vicenty, Sharon González, José Nieves, Carina Jiménez, Carolina Jiménez, Jose Peña, Noel Sanchez, and Bermary Santos for their help on neurosurgical procedures. We also thank Dr. Gregory Quirk for suggestions on the experimental design. This research was part of the student’s dissertation requirement to obtain a PhD degree from the University of Puerto Rico, Medical Sciences Campus
Footnotes
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nature neuroscience. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature neuroscience. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bird MK, Reid CA, Chen F, Tan HO, Petrou S, Lawrence AJ. Cocaine-mediated synaptic potentiation is absent in VTA neurons from mGlu5-deficient mice. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13:133–141. doi: 10.1017/S1461145709990162. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. Journal of neurochemistry. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, Balland B, Dahan L, Lujan R, Deisseroth K, Luscher C. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PloS one. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung medical journal. 2009;32:148–154. [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Stockl P, Ueberall F, Jenny M, Saria A, Zernig G. Activation of PKCzeta and PKMzeta in the nucleus accumbens core is necessary for the retrieval, consolidation and reconsolidation of drug memory. PLoS One. 2012;7:e30502. doi: 10.1371/journal.pone.0030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamiz F, Gallo M. Intra-amygdala ZIP injections impair the memory of learned active avoidance responses and attenuate conditioned taste-aversion acquisition in rats. Learn Mem. 2011;18:529–533. doi: 10.1101/lm.2253311. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Good CH, Lupica CR. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7900–7909. doi: 10.1523/JNEUROSCI.1507-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends in neurosciences. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt O, Migues PV, Hastings M, Wong J, Nader K. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2010;20:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- He YY, Xue YX, Wang JS, Fang Q, Liu JF, Xue LF, Lu L. PKMzeta maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the infralimbic cortex. Neuropsychopharmacology. 2011;36:1972–1981. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Ho SY, Chen CH, Liu TH, Chang HF, Liou JC. Protein kinase mzeta is necessary for cocaine-induced synaptic potentiation in the ventral tegmental area. Biol Psychiatry. 2012;71:706–713. doi: 10.1016/j.biopsych.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Feliu-Mojer M, Vazquez-Torres R. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann N Y Acad Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Interactions between dopamine and excitatory amino acids in behavioral sensitization to psychostimulants. Drug and alcohol dependence. 1995;37:95–100. doi: 10.1016/0376-8716(94)01063-q. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Recent understanding in the mechanisms of addiction. Current psychiatry reports. 2004;6:347–351. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews. Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behavioural pharmacology. 1993;4:315–334. [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain research. Brain research reviews. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annual review of physiology. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007a;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007b;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Gilmartin MR, Helmstetter FJ. Intra-amygdala infusion of the protein kinase Mzeta inhibitor ZIP disrupts foreground context fear memory. Neurobiol Learn Mem. 2012;98:148–153. doi: 10.1016/j.nlm.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, Sacktor TC, Shaham Y, Lu L. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Madronal N, Gruart A, Sacktor TC, Delgado-Garcia JM. PKMzeta inhibition reverses learning-induced increases in hippocampal synaptic strength and memory during trace eyeblink conditioning. PLoS One. 2010;5:e10400. doi: 10.1371/journal.pone.0010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nature neuroscience. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2001a;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Neurobiology. Total recall-the memory of addiction. Science. 2001b;292:2266–2267. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mzeta in long-term potentiation. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport. 1999;10:221–226. doi: 10.1097/00001756-199902050-00004. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. The European journal of neuroscience. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pauli WM, Clark AD, Guenther HJ, O'Reilly RC, Rudy JW. Inhibiting PKMzeta reveals dorsal lateral and dorsal medial striatum store the different memories needed to support adaptive behavior. Learn Mem. 2012;19:307–314. doi: 10.1101/lm.025148.111. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain research. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Annals of the New York Academy of Sciences. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Matzner H, Michaeli A, Yaka R. NR2A/B-containing NMDA receptors mediate cocaine-induced synaptic plasticity in the VTA and cocaine psychomotor sensitization. Neuroscience letters. 2009;461:159–162. doi: 10.1016/j.neulet.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS. Behavioral characterization of d- and l-amphetamine: neurochemical implications. Science. 1975;190:475–477. doi: 10.1126/science.1166317. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabashov D, Shohami E, Yaka R. Inactivation of PKMzeta in the NAc Shell Abolished Cocaine-Conditioned Reward. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9671-7. [DOI] [PubMed] [Google Scholar]
- Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression. J Neurosci. 2001;21:2526–2535. doi: 10.1523/JNEUROSCI.21-07-02526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British journal of pharmacology. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:5581–5586. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Velez ME, Vazquez-Torres R, Jimenez-Rivera CA. Protein kinase Mzeta inhibitor ZIP decreases AMPA mediated currents of VTA neurons from cocaine sensitized rats 346. 17/U11 Society for Neurosciences Meeting; Chicago, IL. 2009. [Google Scholar]
- Vélez ME, Vázquez-Torres R, Velázquez-Martínez MC, Nieves-Meléndez JR, Sánchez-Díaz NG, Sacktor TC, Jiménez-Rivera CA. Protein kinase Mzeta inhibitor (ZIP) alters cocaine sensitization and decreases VTA AMPA mediated currents. Poster 43.5/G31, Society for Neurosciences Meeting; San Diego CA. 2010. [Google Scholar]
- Vélez ME, Vázquez-Torres R, Velázquez-Martínez MC, Sacktor TC, Jiménez -Rivera CA. Protein kinase Mzeta inhibitor (ZIP) alters cocaine sensitization initiation and expression and decreases AMPA/NMDA ratio in VTA DA. Poster 165.20/FF21, Society for Neurosciences Meeting; Washington DC. 2011. [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nature reviews. Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- von Kraus LM, Sacktor TC, Francis JT. Erasing sensorimotor memories via PKMzeta inhibition. PLoS One. 2010;5:e11125. doi: 10.1371/journal.pone.0011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Progress in neurobiology. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Molecular interventions. 2002a;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002b;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Annals of the New York Academy of Sciences. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ. Amphetamine and D1 dopamine receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: modification by repeated amphetamine administration. J Neurochem. 1998;70:198–209. doi: 10.1046/j.1471-4159.1998.70010198.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XM, Curran P, Baumgold J, Fishman PH. Modulation of adenylylcyclase by protein kinase C in human neurotumor SK-N-MC cells: evidence that the alpha isozyme mediates both potentiation and desensitization. J Neurochem. 1994;63:1361–1370. doi: 10.1046/j.1471-4159.1994.63041361.x. [DOI] [PubMed] [Google Scholar]