Abstract
One major strategy by which plants adapt to temperature change is to decrease the degree of unsaturation of membrane lipids under high temperature and increase it under low temperature. We hypothesize that this strategy cannot be adopted by plants in ecosystems and environments with frequent alterations between high and low temperatures, because changes in lipid unsaturation are complex and require large energy inputs. To test this hypothesis, we used a lipidomics approach to profile changes in molecular species of membrane glycerolipids in two plant species sampled from alpine screes and in another two plant species grown in a growth chamber, with the temperature cycling daily between heat and freezing. We found that six classes of phospholipid and two classes of galactolipid showed significant changes, but the degree of unsaturation of total lipids and of three lysophospholipid classes remained unchanged. This pattern of changes in membrane lipids was distinct from that occurring during slow alterations in temperature. We propose two types of model for the adaptation of plants to temperature change: (1) remodeling of membrane lipids but maintenance of the degree of unsaturation are used to adapt to frequent temperature alterations; and (2) both remodeling and changes in the degree of unsaturation to adapt to infrequent temperature alterations.
Keywords: alpine screes, double bond index, high temperature, lipid remodeling, lipid unsaturation, lipidomics, low temperature, membrane glycerolipids, plant adaptation, temperature alteration
INTRODUCTION
Climatic stresses, which include high and low temperature, are the major factors that limit the geographic distribution of plants. Plants have evolved multiple strategies to adapt to climatic stresses, for example through adaptations in terms of phenology, morphology, and microhabitat preference, and adjustments in physiological and biochemical states (Levitt 1980; Körner 1999; Penfield 2008). From physiological and biochemical viewpoints, it is generally agreed that maintaining the integrity and fluidity of membranes is of fundamental importance for plants to survive high or low temperature stress (Levitt 1980; Sakai & Larcher 1987; Wallis & Browse 2002). In alpine ecological systems, the maintenance of membrane fluidity is of particular biological significance because plants are exposed to high and low temperature stresses within the same day (Körner 1999).
Membranes, particularly plasma and chloroplast membranes, are sensitive to environmental stimuli. Glycerolipids are the major constituents of membranes. In response to changes in temperature, plants can adjust the glycerolipid composition of their membranes to maintain the integrity and optimal fluidity of these membranes. For example, at temperatures < 0°C, the amount of lysophospholipids (lysoPLs) increases rapidly by 5–10-fold (Welti et al. 2002). Changes in the degree of unsaturation of membrane glycerolipids, which affect membrane fluidity, are well known and occur when plants encounter low or high temperatures. At low temperatures, the degree of unsaturation of fatty acids is increased through complex biosynthesis pathways (Harwood 1998). Cold acclimation increases the ratio of unsaturated to saturated fatty acids (Sakai & Larcher 1987). For example, in chickpea (Cicer arietinum), low temperature results in a 31% increase in the degree of unsaturation of fatty acids, from a double bond index (DBI) of 1.18 per fatty acid to one of 1.54 (Bakht, Bano & Dominy 2006). Plants with defects in desaturases display inhibited growth or even die at nonfreezing low temperatures of 6–12°C (Hugly & Somerville 1992; Miquel et al. 1993; Falcone, Ogas & Somerville 2004). In contrast, high temperatures result in a reduction in the degree of unsaturation of fatty acids in plants. For example, Arabidopsis plants grown at 36°C display a DBI of 1.46, as compared with a DBI of 2.39 for plants grown at 17°C, a decrease of 39% (Falcone et al. 2004). In addition, a decrease in unsaturation enhances thermotolerance in tobacco (Murakami et al. 2000). Galactolipids, which are the dominant component of thylakoid membranes, harbor more trienoic fatty acids than other membrane phospholipids, and therefore are major contributors to membrane unsaturation (Murakami et al. 2000).
The desaturation of lipids in plants starts with 16:0 and 18:0 (carbon number:double bond number) fatty acids (Buchanan, Gruissem & Jones 2002; Wallis & Browse 2002). Desaturation is mediated by a series of desaturases that are located in the endoplasmic reticulum and chloroplasts and have similar catalytic sequences within their active sites (Buchanan et al. 2002). To date, little is known about the enzymatic mechanism by which desaturated fatty acids are saturated. However, desaturation and its reverse process involve oxidation–reduction, and consume energy and additional resources (Harwood 1998).
In alpine and desert ecosystems, large and frequent daily fluctuations in temperature occur. Plants in these ecosystems need to switch their physiological status from coping with high temperature to coping with low temperature within a very short time, and such switching is usually necessary for several weeks and even throughout entire seasons. If plants adapted to these changes in temperature by frequently changing the degree of saturation of membrane lipids, it would be physiologically costly and a poor trade-off between survival and development, particularly given that resources can be limited in alpine and desert ecosystems (Körner 1999). As a consequence, we hypothesize that plants maintain the same degree of unsaturation of membrane lipids and instead vary the lipid composition of the membrane by mechanisms that require less energy, to adapt to environments with frequent changes between high and low temperatures.
Plant lipidomics is based on electrospray ionization–tandem mass spectrometry (ESI-MS/MS) analysis, which makes it possible to measure hundreds of lipid molecular species in vivo with small samples and in a short time (Welti et al. 2002). Several studies have employed lipidomics to profile changes in molecular species at low temperatures, and to characterize the function of genes that encode lipolytic enzymes, in combination with genetics approaches (Welti et al. 2002; Li et al. 2004; Devaiah et al. 2006; Devaiah et al. 2007; Li et al. 2008; Hong et al. 2009; Zhang et al. 2009).
The purpose of the current study was to use lipidomics to test the hypothesis raised above, and to characterize the changes in plant lipids that occur in environments with frequent alterations between high and low temperatures. Field and laboratory experiments were both used in this study. The field experiments were conducted in an alpine scree ecosystem of the Hengduan Mountains, southwest China, which has extreme environmental conditions, in particular, high daytime and low night-time temperatures (Deng & Zhou 2004). To exclude the potential effects of strong solar and UV radiation, which can occur in the field, a laboratory experiment was conducted in a growth chamber with daily temperature cycles of heat during the day and freezing during the night. Changes in the molecular species of membrane lipids were profiled in two representative species of alpine scree plants (Saussurea medusa and Solms-Laubachia linearifolia), as well as one alpine plant species (Crucihimalaya himalaica) and the model plant Arabidopsis thaliana, during alterations between high and low temperatures. Two working models for the way in which plants utilize changes in membrane lipids to adapt to environments with frequent or infrequent alterations between high and low temperatures are proposed.
MATERIALS AND METHODS
Site descriptions
We chose a study site that comprised an alpine scree at an altitude of 4560 m on Baima Snow Mountain in the Hengduan Mountains, a mountain range in southwest China in the southeast Qinghai–Tibet Plateau. The chain of mountains runs roughly north to south, defining the eastern edge of the Tibetan Plateau, and is situated in Sichuan, Yunnan, and Tibet. Baima Snow Mountain is in De Qin, Yunnan. Screes are special ecosystems in alpine areas; they are usually located above 4000 m and comprise steep slopes that are composed of rough-edged stones (1–2.5 cm in length) (Tsukaya, Fujikawa & Wu 2002; Deng & Zhou 2004). In this ecosystem, the solar radiation is extraordinarily strong, the daily temperature changes dramatically and frequently, the soil is very barren, and oxygen levels are low. The gravel on the sandy slopes is very loose and often rolls down. There is rich plant diversity in alpine screes, with 519 seed plants in 103 genera and 29 families (Deng & Zhou 2004). We chose the alpine subnival plants S. medusa and S.-L. linearifolia as representative species because they are typical species of the ecosystem (Deng & Zhou 2004) and have totally different morphology to each other (Yang, Körner & Sun 2008).
Plant materials
S. medusa (Compositae) is a perennial herb and a monocarpic plant (Tsukaya et al. 2002; Yang et al. 2008). It has a caudex that is covered with the dark-brown petioles of previous years and rosettes of low stature during the vegetative phase. The leaves are approximately 10 cm long, 0.5–3 cm wide, and covered densely with white hairs on both sides. Except for its flowers, S. medusa is covered with white hairs. The native habitat of S. medusa is generally high-elevation screes or rock fields. S.-L. linearifolia (Cruciferae) is also a perennial herb. It has sparse to dense straight trichomes that are 1.5 mm long, a caudex that is covered with the petioles of previous years, and rosettes of low stature during the vegetative phase. The leaves are blade linear or oblanceolate linear, approximately 3.5–5 cm long and 2–4 mm wide. The leaves are sparsely to densely pilose ciliate. The native habitat of S.-L. linearifolia is generally steep stony slopes that are composed of rough-edged stones. C. himalaica (Cruciferae) (Al-Shehbaz, Steve & Price 1999) is an annual or biennial herb. Its native habitat is rocky hillsides, grassy meadows, sandy slopes, flood plains, scree, and pastures at altitudes of 2600–4400 m. C. himalaica has a close genetic relationship with the model plant A. thaliana (Al-Shehbaz et al. 1999) and can be grown in our laboratory, which is located at an altitude of 1900 m.
Measurements of environmental parameters
All environmental parameters were measured on clear days in October 2005. Parameters in three Octobers in 2005, 2007, and 2008 had been recorded. Air temperature was recorded at 30-min intervals over an entire 24-h period, and diurnal variations in total solar radiation and solar UV radiation (data not shown) were recorded at 15-min intervals from 07:00 to 19:00 h with a data logger (LI-1400, LI-Cor, Lincoln, NE, USA) and UV radiometer (type UV-B, Beijing Normal University Photoelectric Instrument Factory, China). Statistical analysis was performed using Origin 7.0 (OriginLab Corporation, Northampton, MA, USA).
Field sampling strategies
Field sampling was performed at the alpine scree study site on one randomly chosen day in October 2005, because the daily temperature fluctuation was usually the largest in October as compared with other months. The sampling procedure followed was as described previously (Welti et al. 2002; Li et al. 2008) with minor modifications. The leaves of S. medusa and S.-L. linearifolia were harvested at 00:00, 06:00, 13:00, and 18:00 h (Fig. 1). Five replicates were harvested for each sampling time and each plant species. The leaves were transferred immediately into 3 mL of isopropanol with 0.01% butylated hydroxytoluene in a boiling water bath, at an altitude of 4500 m, for at least 15 min.
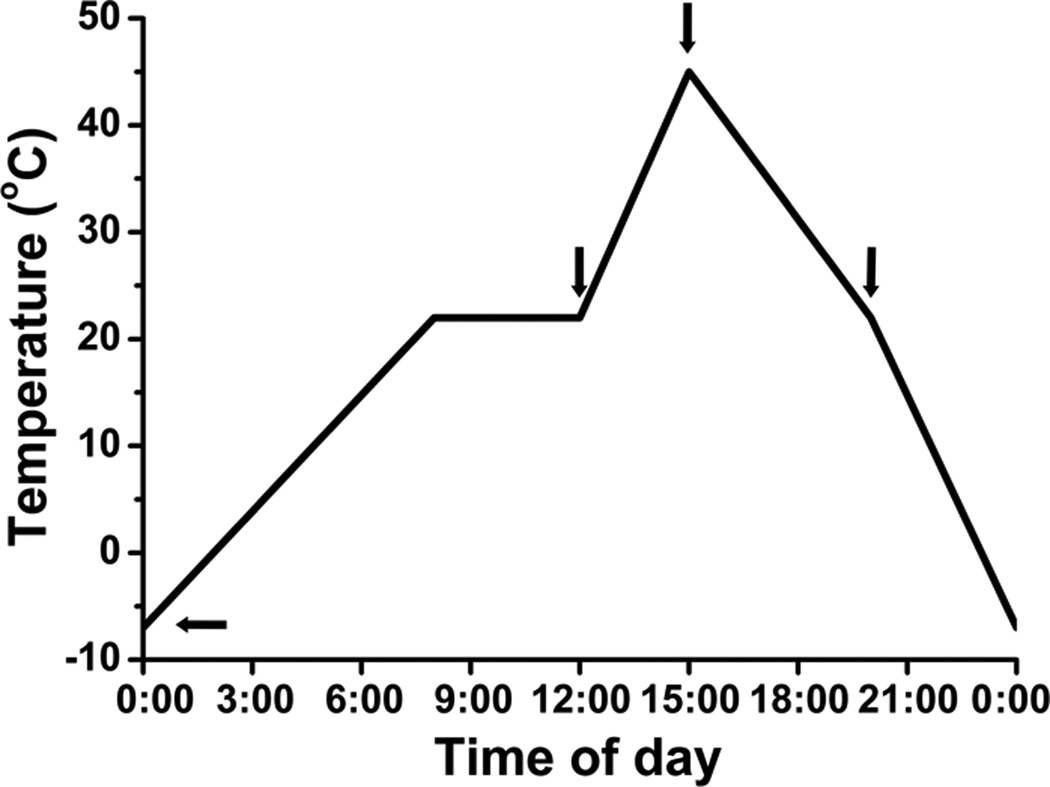
Figure 1.
Variation in air temperature with time of day and sampling time (indicated by arrows) in alpine screes of the Hengduan Mountains in October. The temperatures at the sampling times were −1°C at 00:00 h, −3°C at 06:00 h, 32°C at 13:00 h, and 6°C at 18:00 h.
Plant growth and treatments
Seeds of A. thaliana (Columbia ecotype) and C. himalaica were surface sterilized and then sown in 0.7%-agar-solidified plates that contained MS medium and 1% sucrose. After 2 d at 4°C, the seeds were germinated and grown at 22°C under 120 µmol m−2s−1 light for 5 d. Plates with seedlings were moved to the growth chamber with a programmed daily temperature cycle of −7°C at 00:00 h, 22°C at 08:00–12:00 h, 45°C at 15:00 h, and 22°C at 20:00 h (Fig. 2), for 30–40 d under a photoperiod of 12/12 h day/night. The temperature changes in the chamber were set to mimic those in the field but to be more extreme. Two-thirds of the plants survived and grew very slowly, and these were harvested for lipid analysis. After 30 d of growth, sampling was carried out on a single day at 00:00, 12:00, 15:00, and 20:00 h (Fig. 2). No significant changes in dry weight were detected in Arabidopsis during the 24-h sampling period.
Figure 2.
The daily temperature cycle within the growth chamber. The arrows indicate the sampling times after A. thaliana and C. himalaica had been grown in the chamber for 30 d. The temperatures at the sampling times were −7°C at 00:00 h, 22°C at 12:00 h, 45°C at 15:00 h, and 22°C at 20:00 h.
Lipid extraction, ESI-MS/MS analysis and data processing
Lipid extraction, ESI-MS/MS analysis, and quantification were performed as described previously, with minor modifications (Welti et al. 2002; Welti et al. 2007) (Kansas Lipidomics Research Center, http://www.k-state.edu/lipid/lipidomics). Leaves (field experiment) or three or four different plants (laboratory experiment) were harvested at the sampling time and, to inhibit lipolytic activity, were transferred immediately into 3 mL of isopropanol with 0.01% butylated hydroxytoluene in a boiling water bath (field experiment at an altitude of 4500 m) or a 75°C water bath (laboratory experiment). The tissue was extracted three times with chloroform/methanol (2:1), with 12 h of agitation each time. The remaining plant tissue was dried overnight at 105°C and weighed to give the dry weight of the plants. Lipid samples were analyzed on a triple quadrupole MS/MS equipped for ESI. Data processing was performed as described previously (Welti et al. 2002, 2007). The lipids in each class of head group were quantified in comparison to two internal standards for the class. Five replicates of each treatment for each genotype were analyzed. The Q test was performed on the total amount of lipid in each class, and data from discordant samples were removed (Welti et al. 2002). The data were subjected to one-way analysis of variance (ANOVA) with SPSS 13.0. Statistical significance was tested by Fisher’s least significant difference (LSD) method. DBI were calculated with the formula: DBI = (Σ[N × mol% lipid])/100, where N is the total number of double bonds in the two fatty acid chains of each glycerolipid molecule (Rawyler et al. 1999).
Results
S. medusa and S.-L. linearifolia growing on alpine screes at 4500-m altitude experienced temperatures of 32°C during the day and −3°C at night
First, we investigated the microclimates experienced at the alpine scree study site in October. We examined mainly the variations in air temperature (Fig. 1), solar radiation, and UV radiation within a 24-h period on a clear day (data not shown), and then randomly chose a day to harvest samples. During the sampling day, there was strong solar radiation and a high air temperature. The temperature reached a maximum of 32°C at 13:00 h and started to decrease at 16:00 h. During the night, the temperature was low and reached a minimum of −3°C at 05:00 h. It started to increase at 09:00 h. The difference between the minimum and maximum temperature was 35°C (Fig. 1). We had observed that the variation in temperature was close to 40°C in October (data not shown). The most dramatic changes in temperature occurred during the 9-h period from 09:00–18:00 h, when the temperature increased from 0 to 32°C and then decreased from 32 to 6°C. The average rate of change in temperature during this period was 6.6°C h−1 (Fig. 1). We conducted our sampling of S. medusa and S.-L. linearifolia at 00:00, 06:00, 13:00, and 18:00 h, when the temperature was −1, −3, 32, and 6°C, respectively. These time points enabled the responses of the plants to environmental conditions of low, rising, high, and falling temperatures to be analyzed (Fig. 1).
Membrane glycerolipids in S. medusa and S.-L. linearifolia changed significantly with alterations between high and low temperatures
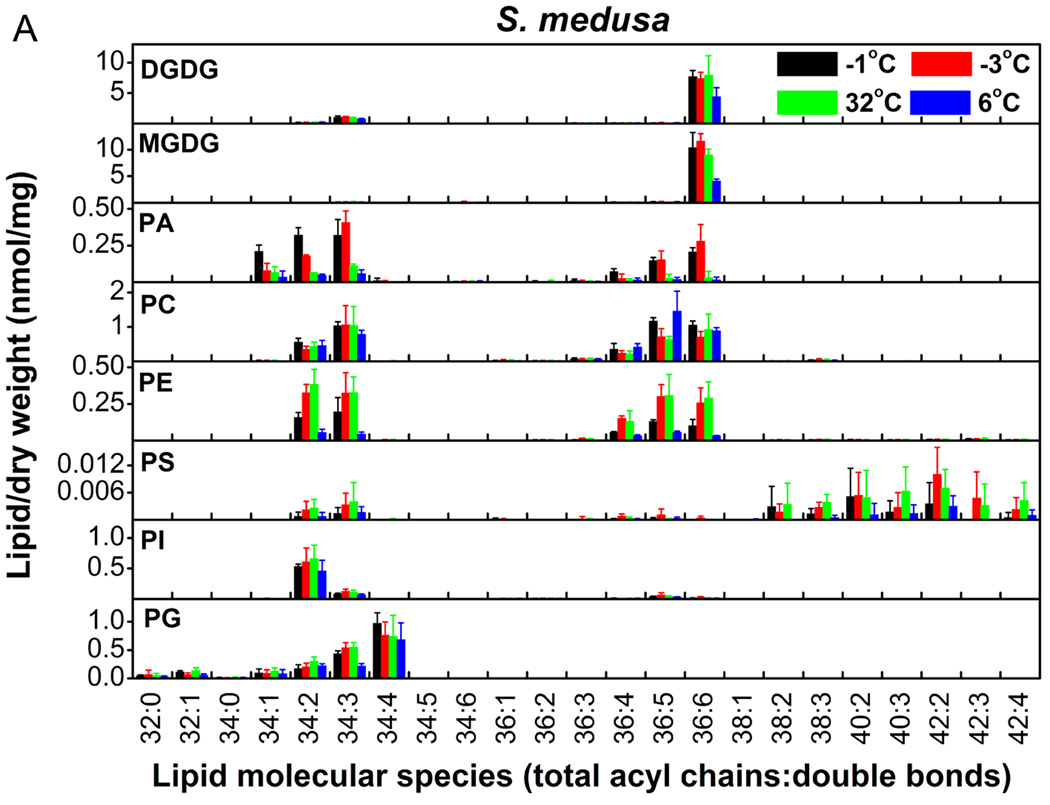
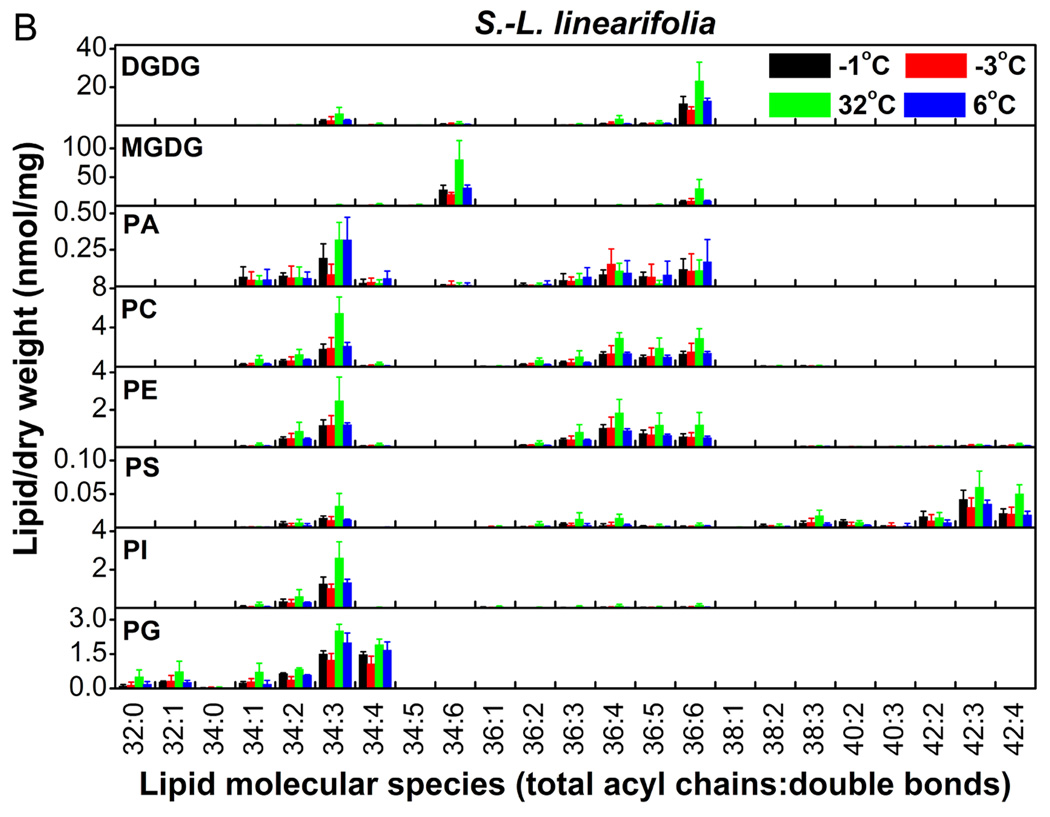
We profiled the molecular species of membrane glycerolipids in S. medusa and S.-L. linearifolia using a lipidomic approach based on ESI-MS/MS (Welti et al. 2002; Li et al. 2008). Eleven classes that contained 108 molecular species of membrane glycerolipids were detected, including two classes of galactolipids, six of phospholipids (Fig. 3A and 3B), and three of lysoPLs (Fig. 4). The data for the lysoPLs are shown separately (Fig. 4) because they were present at much lower levels than the other lipid classes. S.-L. linearifolia contained both 34:6 and 36:6 monogalactosyldiacylglycerol (MGDG) molecules (Fig. 3B), which meant that it was a 16:3 plant that harbored both prokaryotic and eukaryotic pathways of lipid synthesis. In contrast, S. medusa contained only 36:6 MGDG molecules (Fig. 3A), and therefore, it was an 18:3 plant that harbored only the eukaryotic lipid synthesis pathway (Buchanan et al. 2002). Thus, we could use S.-L. linearifolia and S. medusa as representative plants with two distinct lipid synthesis pathways, to examine their responses to changes in environmental temperature.
Figure 3.
Changes in the molecular species of membrane lipids in S. medusa (A) and S.-L. linearifolia (B) during alterations between high and low temperatures in alpine screes. Values are means ± SD (n = 3, 4, or 5).
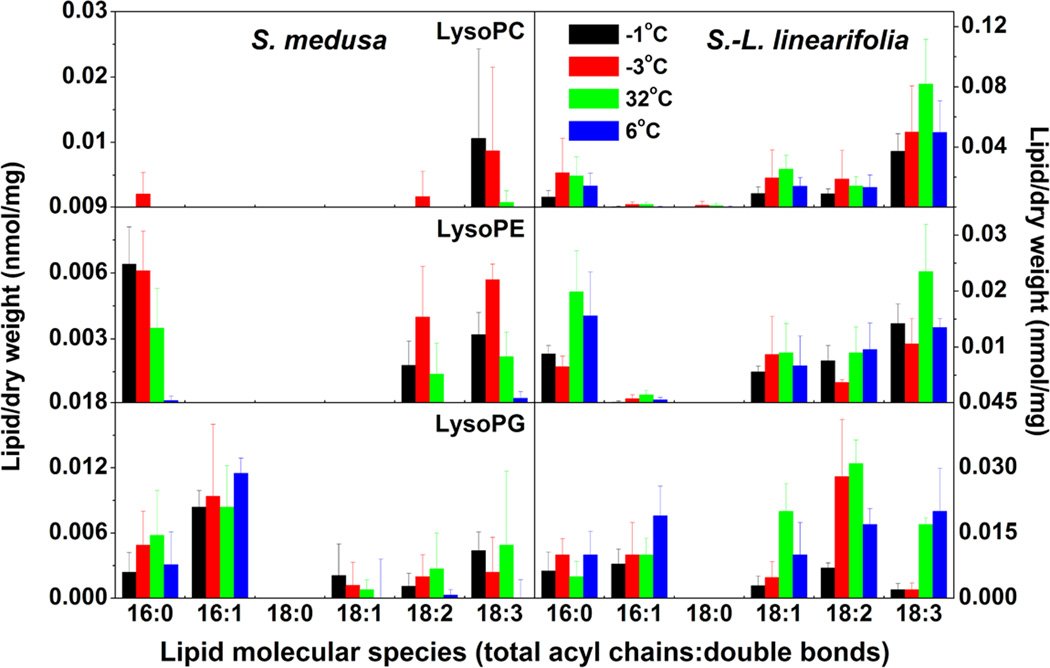
Figure 4.
Changes in the molecular species of lysoPLs in S. medusa and S.-L. linearifolia during alterations between high and low temperatures in alpine screes. Values are means ± SD (n = 3, 4, or 5).
Taking an overview (Fig. 3A and 3B), the levels of most lipid species changed dramatically in S. medusa and S.-L. linearifolia as the temperature changed. The lipid classes digalactosyldiacylglycerol (DGDG), MGDG, and phosphatidylethanolamine (PE) changed significantly in both species (Table 1). For example, DGDG decreased by 26.2% in S. medusa as the temperature decreased from 32 to 6°C, and increased by 126% in S.-L. linearifolia as the temperature increased from −3 to 32°C. The relative change in MGDG was 65.8% in S. medusa as the temperature increased from −3 to 32°C and then decreased to 6°C, whereas in S.-L. linearifolia MGDG increased by 142% as the temperature increased from −3 to 32°C. PE increased almost twofold when S. medusa and S.-L. linearifolia experienced a change in temperature from <0 to 32°C. There were differences in the changes in lipid between S. medusa and S.-L. linearifolia. Phosphatidic acid (PA) showed fivefold differences between temperatures >0°C and <0°C in S. medusa, but remained unchanged in S.-L. linearifolia (Table 1). The level of phosphatidylcholine (PC) remained the same in S. medusa as the temperature increased but showed a twofold increase in S.-L. linearifolia. These results suggested that the membrane glycerolipids in S. medusa and S.-L. linearifolia changed significantly during rapid alterations in temperature in alpine screes.
Table 1.
Amount of lipid in each head group class and total lipids in S. medusa and S.-L. linearifolia during high and low temperature alteration in alpine screes. The relative change (RC) in lipids from −3 to 32°C is the percentage value for the difference between the values at −3 and 32°C over the value at −3°C; the largest relative change (LRC) in lipids is the percentage value for the difference between the maximum and minimum values over the minimum values. Values in the same row with different letters are significantly different (P < 0.05). Values are means ± SD (n = 3, 4, or 5).
| Lipid class |
Plant species | Lipids/dry weight (nmol/mg) | RC (%) |
LRC (%) |
|||
|---|---|---|---|---|---|---|---|
| −1°C | −3°C | 32°C | 6°C | −3 to 32°C |
|||
| DGDG | S. medusa | 7.7 ± 3.0a | 8.2 ± 2.0a | 8.2 ± 4.2a | 6.5 ± 2.5a | 0.0 | 26.2 |
| S.-L. linearifolia | 16.6 ± 5.1bc | 11.6 ± 2.7c | 26.2 ± 4.1a | 18.5 ± 2.3b | 126 | 126 | |
| MGDG | S. medusa | 9.9 ± 4.2a | 12.1 ± 1.8a | 10.4 ± 6.3a | 7.3 ± 4.0a | −14.0 | 65.8 |
| S.-L. linearifolia | 36.9 ± 10.1bc | 27.4 ± 4.8c | 66.2 ± 13.3a | 41.7 ± 6.5b | 142 | 142 | |
| PA | S. medusa | 1.2 ± 0.3a | 1.0 ± 0.1a | 0.3 ± 0.0b | 0.2 ± 0.0b | −70.0 | 500 |
| S.-L. linearifolia | 0.7 ± 0.3a | 0.4 ± 0.3a | 0.8 ± 0.5a | 0.8 ± 0.7a | 100 | 100 | |
| PC | S. medusa | 3.6 ± 0.8a | 3.1 ± 1.9a | 4.7 ± 2.8a | 3.0 ± 1.7a | 51.6 | 56.7 |
| S.-L. linearifolia | 7.9 ± 0.7b | 6.1 ± 0.4b | 12.7 ± 2.7a | 7.6 ± 1.1b | 109 | 109 | |
| PE | S. medusa | 0.5 ± 0.3bc | 1.1 ± 0.7ab | 1.3 ± 0.8a | 0.1 ± 0.1c | 18.2 | 1200 |
| S.-L. linearifolia | 4.7 ± 1.1ab | 3.7 ± 1.5b | 6.3 ± 2.1a | 4.5 ± 0.5ab | 69.8 | 69.8 | |
| PS | S. medusa | 0.02 ± 0.02bc | 0.04 ± 0.03a | 0.04 ± 0.01ab | 0.01 ± 0.00c | 0.0 | 300 |
| S.-L. linearifolia | 0.15 ± 0.04ab | 0.06 ± 0.02c | 0.19 ± 0.08a | 0.11 ± 0.02ab | 217 | 217 | |
| PI | S. medusa | 0.7 ± 0.0a | 1.0 ± 0.5a | 1.0 ± 0.4a | 0.6 ± 0.2a | 0.0 | 66.7 |
| S.-L. linearifolia | 1.8 ± 0.6b | 1.5 ± 0.0b | 2.9 ± 0.7a | 1.7 ± 0.2b | 93.3 | 93.3 | |
| PG | S. medusa | 1.7 ± 0.6ab | 1.6 ± 0.6ab | 2.1 ± 0.97a | 1.1 ± 0.5b | 31.3 | 90.9 |
| S.-L. linearifolia | 4.2 ± 0.3bc | 3.2 ± 0.9bc | 6.4 ± 0.8a | 4.8 ± 0.9b | 99.9 | 99.9 | |
| Total lipids/dry weight (nmol/mg) | |||||||
| Total lipids | S. medusa | 25.4 ± 8.22a | 26.8 ± 9.7a | 28.1 ± 13.1a | 18.8 ± 8.64a | 4.9 | 49.5 |
| S.-L. linearifolia | 72.4 ± 18.5bc | 53.5 ± 10.7c | 122 ± 22.5a | 80.1 ± 9.3b | 128 | 128 | |
Total degree of unsaturation of membrane glycerolipids was maintained during alterations between high and low temperatures in S. medusa and S.-L. linearifolia
We employed the DBI to indicate the degree of unsaturation of membrane glycerolipids (Rawyler et al. 1999). The DBI is the average number of double bonds in the fatty acid chains of a glycerolipid molecular species; a high DBI indicates the presence of more highly unsaturated membrane lipids, and vice versa. For all glycerolipid classes, the changes in DBI were small, except for PA in both S. medusa and S.-L. linearifolia (Table 2). MGDG and DGDG, which were the major lipid constituents of the membrane (Table 1), showed the smallest changes in DBI among all lipid classes; their largest relative change was only 2.6% during all temperature changes. The DBI of PA changed by 29% but PA was only a minor constituent of the membrane glycerolipids (Table 1); hence, PA contributed little to the DBI of the total membrane lipids (Table 2). Between the lowest (−3°C) and the highest (32°C) temperatures, the DBI increased in some lipid classes but decreased in others. The DBI of the total lipids (Table 2) remained at 5.1 in S. medusa and at 5.0 in S.-L. linearifolia. The relative changes in DBI as the temperature increased from −3 to 32°C were 0.0% and −2.0% in S. medusa and S.-L. linearifolia, respectively, and the largest relative changes during all temperature changes were only 0.6% and 3.4%. These results suggested that the degree of unsaturation of the membrane glycerolipids was maintained in S. medusa and S.-L. linearifolia during the rapid alteration of temperature in alpine screes. The differences in morphology and lipid biosynthesis pathways between these two species did not affect their maintenance of the degree of glycerolipid unsaturation.
Table 2.
Double bond index (DBI) of membrane lipids in S. medusa and S.-L. linearifolia during high and low temperature alteration in alpine screes. DBI = (Σ[N × mol% lipid])/100, where N is the total number of double bonds in the two fatty acid chains of each glycerolipid molecule. The relative change (RC) in DBI from −3 to 32°C is the percentage value for the difference between the DBI at −3 and 32°C over the DBI at −3°C; the largest relative change (LRC) in DBI is the percentage value for the difference between the maximum and minimum DBI over the minimum DBI. Values in the same row line with different letters are significantly different (P < 0.05). Values are means ± SD (n = 5).
| Lipid class |
Plant species | Double bond index | RC (%) |
LRC (%) |
|||
|---|---|---|---|---|---|---|---|
| −1°C | −3°C | 32°C | 6°C | −3 to 32°C |
|||
| DGDG | S. medusa | 5.55 ± 0.08a | 5.41 ± 0.15a | 5.44 ± 0.21a | 5.41 ± 0.17a | 0.6 | 2.6 |
| S.-L. linearifolia | 5.30 ± 0.07a | 5.29 ± 0.05a | 5.26 ± 0.04a | 5.30 ± 0.03a | −0.6 | 0.8 | |
| MGDG | S. medusa | 5.93 ± 0.01a | 5.90 ± 0.05ab | 5.89 ± 0.05ab | 5.86 ± 0.05b | −0.2 | 1.2 |
| S.-L. linearifolia | 5.90 ± 0.02a | 5.90 ± 0.01a | 5.85 ± 0.01b | 5.89 ± 0.01a | −0.9 | 0.9 | |
| PA | S. medusa | 3.27 ± 0.46ab | 3.65 ± 0.28a | 2.83 ± 0.72b | 2.98 ± 0.68ab | −23 | 29 |
| S.-L. linearifolia | 3.61 ± 0.28a | 3.73 ± 0.39a | 3.09 ± 1.04a | 3.79 ± 0.42a | −17 | 23 | |
| PC | S. medusa | 4.10 ± 0.14b | 4.26 ± 0.23ab | 4.15 ± 0.07b | 4.39 ± 0.11a | −2.6 | 7.1 |
| S.-L. linearifolia | 3.80 ± 0.08b | 3.93 ± 0.09a | 3.80 ± 0.04b | 3.78 ± 0.07b | −3.3 | 4.0 | |
| PE | S. medusa | 3.45 ± 0.33b | 4.07 ± 0.28a | 4.08 ± 0.50a | 3.19 ± 0.61b | 0.2 | 28 |
| S.-L. linearifolia | 3.75 ± 0.05a | 3.74 ± 0.05a | 3.70 ± 0.02a | 3.70 ± 0.05a | −1.1 | 1.4 | |
| PS | S. medusa | 2.22 ± 0.77a | 2.64 ± 0.27a | 2.73 ± 0.38a | 2.74 ± 0.40a | 3.4 | 23 |
| S.-L. linearifolia | 2.96 ± 0.04c | 3.12 ± 0.11ab | 3.17 ± 0.04a | 3.07 ± 0.08b | 1.6 | 7.1 | |
| PI | S. medusa | 2.32 ± 0.02bc | 2.43 ± 0.11a | 2.31 ± 0.05b | 2.36 ± 0.07ab | −4.9 | 5.2 |
| S.-L. linearifolia | 2.80 ± 0.09b | 2.87 ± 0.03ab | 2.89 ± 0.02a | 2.85 ± 0.03ab | 0.7 | 3.2 | |
| PG | S. medusa | 3.09 ± 0.08a | 2.88 ± 0.25a | 2.92 ± 0.19a | 2.89 ± 0.22a | 1.4 | 7.3 |
| S.-L. linearifolia | 2.88 ± 0.08a | 2.85 ± 0.06a | 2.74 ± 0.07b | 2.93 ± 0.06a | −3.9 | 6.9 | |
| Total double bond index | |||||||
| Total lipids | S. medusa | 5.12 ± 0.09a | 5.14 ± 0.10a | 5.14 ± 0.05a | 5.15 ± 0.06a | 0.0 | 0.6 |
| S.-L. linearifolia | 5.03 ± 0.17a | 5.05 ± 0.09a | 4.95 ± 0.27a | 5.12 ± 0.12a | −2.0 | 3.4 | |
A. thaliana and C. himalaica grown under a 45°C/−7°C daily cycle showed marked changes in membrane lipids
In alpine screes, the high temperature during the day results from strong solar radiation and is associated closely with UV radiation. To exclude the effects of strong solar and UV radiation on alterations in membrane glycerolipids, we conducted a laboratory experiment that simulated the rapid changes between high and low temperatures, but without solar and UV radiation. We profiled the molecular species of membrane lipids in the model plant A. thaliana and the wild species C. himalaica when they were both grown on plates in an artificial environment that included frequent and rapid alterations between high and low temperatures. C. himalaica is a close relative of A. thaliana and is distributed in alpine areas. The daily temperature fluctuation was 52°C, and the average rates of temperature change were 3.5°C h−1 as the temperature increased and 5.8°C h−1 as the temperature decreased. Two-thirds of the plants survived and grew slowly. We randomly chose one day to harvest whole plants for lipid extraction at four time points during the day: 00:00 (−7°C), 12:00 (22°C), 15:00 (45°C), and 20:00 (22°C) h (Fig. 2).
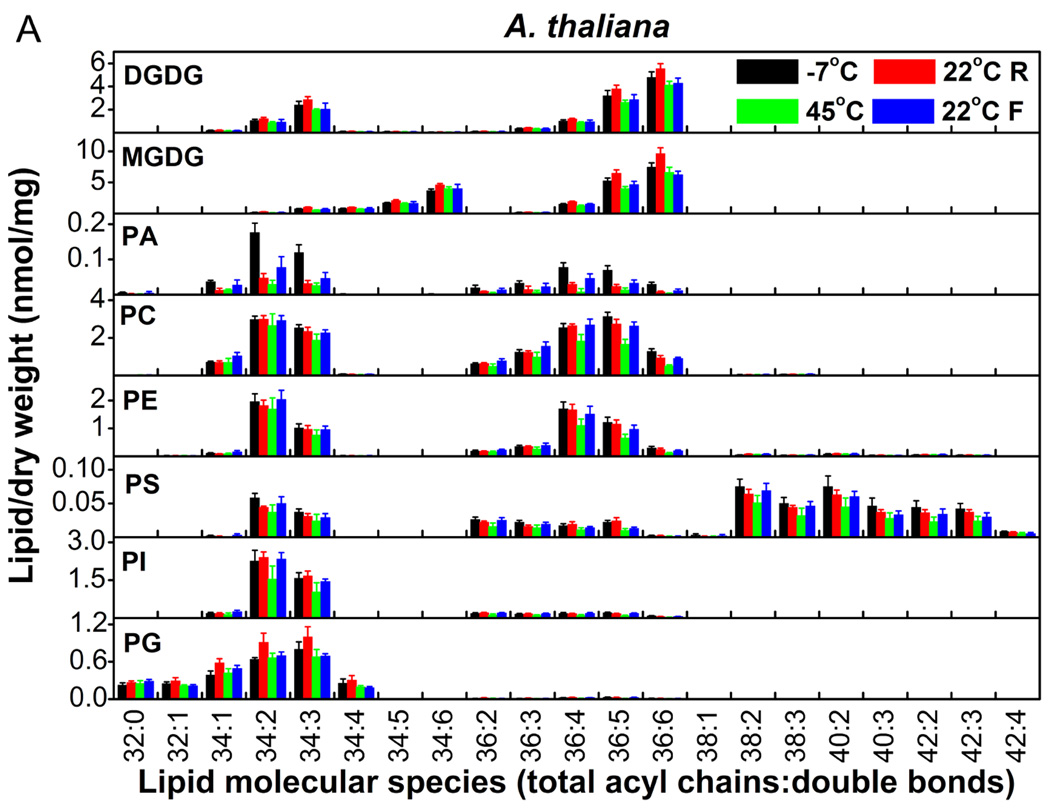
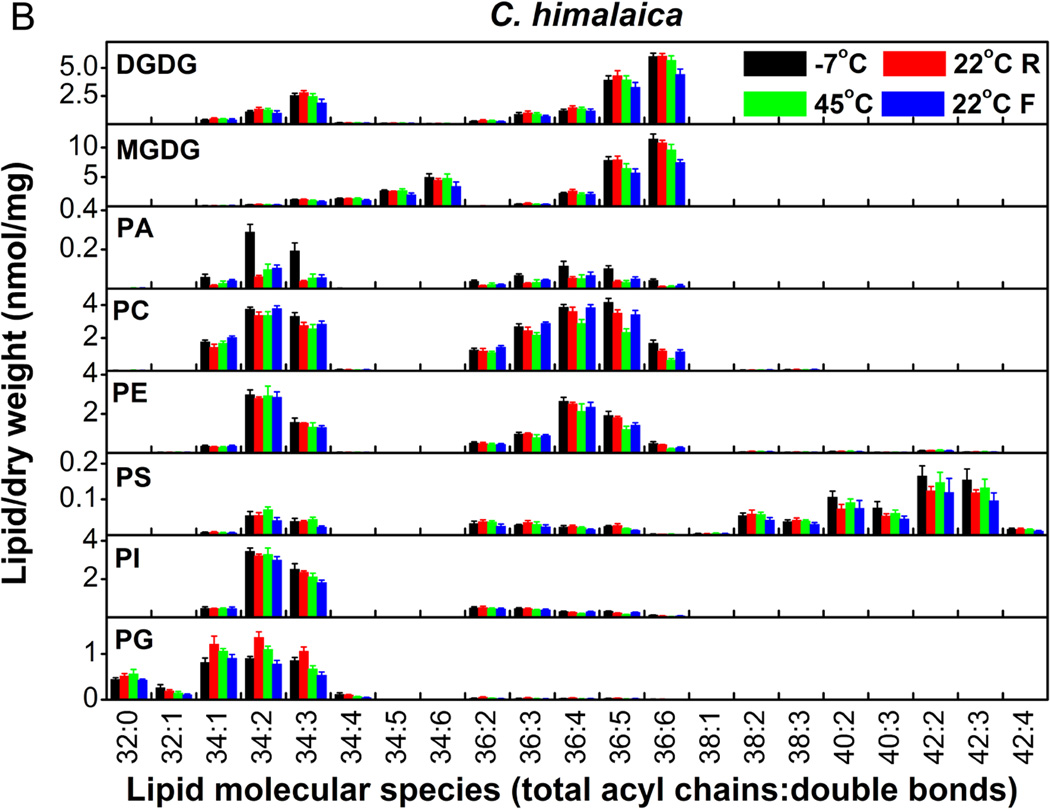
The molecular species of membrane lipids in A. thaliana and C. himalaica changed markedly with changes between high and low temperatures (Fig. 5A and 5B). The patterns of lipid changes were similar in both species. All lipid classes displayed significant changes (Table 3). PA showed some of the most dramatic changes with 2–4-fold changes. MGDG and DGDG changed by approximately 40%. Total membrane lipids changed by 40% in A. thaliana and 20% in C. himalaica (Table 3), which indicated that membrane glycerolipids in A. thaliana and C. himalaica changed significantly during frequent and rapid temperature alterations. These results also suggested that, among temperature, solar radiation, and UV radiation, temperature was the major factor that caused the changes in glycerolipid composition that were observed under the conditions experienced in alpine screes.
Figure 5.
Changes in the molecular species of membrane lipids in A. thaliana (A) and C. himalaica (B) during alterations between high and low temperatures in the growth chamber. ‘R’ and ‘F’ indicate that the temperature was rising or falling, respectively. Values are means ± SD (n = 4 or 5).
Table 3.
Amount of lipid in each head group class and total lipids in A. thaliana and C. himalaica during high and low temperature alteration in the growth chamber. ‘R’ and ‘F’ indicate that the temperature was rising and falling, respectively. The relative change (RC) in lipids from −7 to 45°C is the percentage value for the difference between the values at −7 and 45°C over the values at −7°C; the largest relative change (LRC) in lipids is the percentage value of the difference between the maximum and minimum values over the minimum values. Values in the same row with different letters are significantly different (P < 0.05). Values are means ± SD (n = 4 or 5).
| Lipid class |
Plant species | Lipids/dry weight (nmol/mg) | RC (%) |
LRC (%) |
|||
|---|---|---|---|---|---|---|---|
| −7°C | 22°C R | 45°C | 22°C F | −7 to 45°C |
|||
| DGDG | A. thaliana | 13.3 ± 1.6b | 15.6 ± 1.0a | 11.3 ± 0.7c | 11.9 ± 1.9bc | −15 | 38.1 |
| C. himalaica | 16.5 ± 1.1a | 18.0 ± 1.4a | 16.6 ± 1.3a | 13.1 ± 1.6b | 0.6 | 37.4 | |
| MGDG | A. thaliana | 21.4 ± 0.7b | 27.2 ± 1.7a | 19.1 ± 1.2c | 19.9 ± 2.2bc | −10.7 | 42.4 |
| C. himalaica | 32.2 ± 1.0a | 31.6 ± 1.4a | 28.5 ± 2.9b | 23.0 ± 2.4c | −11.5 | 40.0 | |
| PA | A. thaliana | 0.57 ± 0.07a | 0.18 ± 0.05c | 0.11 ± 0.04c | 0.28 ± 0.11b | −80.7 | 418 |
| C. himalaica | 0.90 ± 0.13a | 0.26 ± 0.03c | 0.33 ± 0.10bc | 0.40 ± 0.05b | −63.3 | 246 | |
| PC | A. thaliana | 15.2 ±1.0a | 14.4 ± 0.9a | 10.9 ± 2.1b | 15.0 ± 1.5a | −28.3 | 39.4 |
| C. himalaica | 22.8 ± 1.2a | 19.8 ± 1.5b | 17.0 ± 1.4c | 21.7 ± 0.9a | −25.4 | 34.1 | |
| PE | A. thaliana | 7.23 ± 1.05a | 6.94 ± 0.82a | 5.18 ± 1.21b | 6.87 ± 1.16a | −28.4 | 39.6 |
| C. himalaica | 11.9 ± 1.19a | 11.4 ± 0.37ac | 9.80 ± 1.65b | 10.3 ± 0.95bc | −17.6 | 21.4 | |
| PS | A. thaliana | 0.54 ± 0.08a | 0.45 ± 0.03a | 0.33 ± 0.09b | 0.44 ± 0.07a | −38.9 | 63.6 |
| C. himalaica | 0.85 ± 0.14a | 0.75 ± 0.09a | 0.81 ± 0.09a | 0.59 ± 0.13b | −4.7 | 44.1 | |
| PI | A. thaliana | 4.81 ± 0.83a | 5.04 ± 0.42a | 3.19 ± 1.10b | 4.86 ± 0.52a | −33.7 | 58.0 |
| C. himalaica | 8.19 ± 0.70a | 7.65 ± 0.24ab | 7.14 ± 0.62bc | 6.76 ± 0.47c | −12.8 | 21.2 | |
| PG | A. thaliana | 2.66 ± 0.18b | 3.48 ± 0.49a | 2.50 ± 0.33b | 2.67 ± 0.11b | −6.0 | 39.2 |
| C. himalaica | 3.57 ± 0.15b | 4.74 ± 0.52a | 3.78 ± 0.31b | 2.95 ± 0.20c | 5.9 | 60.7 | |
| Total lipids/dry weight (nmol/mg) | |||||||
| Total lipids | A. thaliana | 66.0 ± 4.5b | 73.5 ± 5.0a | 52.7 ± 5.6c | 62.1 ± 1.3a | −20.2 | 39.5 |
| C. himalaica | 97.1 ± 4.2a | 94.4 ± 4.5a | 84.1 ± 4.8b | 79.0 ± 3.7b | −13.4 | 22.9 | |
Total degree of unsaturation of membrane glycerolipids was maintained during alterations between high and low temperatures in both A. thaliana and C. himalaica
The changes in DBI for all classes of lipid were very small in A. thaliana and C. himalaica (Table 4), and the patterns of changes in DBI were similar in both species. The largest relative changes in DBI for MGDG and DGDG were only 1.5% over all temperature changes. This phenomenon was similar to that observed for S. medusa and S.-L. linearifolia in the field (Table 2). When the temperature increased from −7 to 45°C, the DBI of most lipid classes decreased only slightly. The DBI of the total lipids remained at around 4.1 and 4.0 and relative changes in DBI for total lipids were only 0.5 and −2.5% in A. thaliana and C. himalaica, respectively, over this temperature increase (Table 4). The largest relative changes for total lipids during all temperature alterations were only 4.5 and 5.2% in A. thaliana and C. himalaica, respectively. Considering that exposure to high temperature for 25 d can induce a 39% decrease in DBI in A. thaliana (Falcone et al. 2004), and exposure to low temperature for 14 d can induce a 31% increase in DBI in chickpea (Bakht et al. 2006), the difference between the minimum and maximum DBI could be >70% under slow changes between extreme temperatures. Therefore, our results indicated that the degree of unsaturation of membrane glycerolipids was maintained in A. thaliana and C. himalaica during the frequent and rapid changes between high and low temperatures.
Table 4.
Double bond index (DBI) of membrane lipids in A. thaliana and C. himalaica during high and low temperature alteration in the growth chamber. ‘R’ and ‘F’ indicate that the temperature was rising and falling, respectively. DBI = (Σ[N × mol% lipid])/100, N is the total number of double bonds in the two fatty acid chains of each glycerolipid molecule. The relative change (RC) in DBI from −7 to 45°C is the percentage value for the difference between the DBI at −7 and 45°C over the DBI at −7°C; the largest relative change (LRC) in DBI is the percentage value for the difference between the maximum and minimum DBI over the minimum DBI. Values in the same row with different letters are significantly different (P < 0.05). Values are means ± SD (n = 5).
| Lipid class |
Plant species |
Double bond index | RC (%) |
LRC (%) |
|||
|---|---|---|---|---|---|---|---|
| −7°C | 22°C R | 45°C | 22°C F | −7 to 45°C |
|||
| DGDG | A. thaliana | 4.56 ± 0.04a | 4.55 ± 0.07a | 4.56 ± 0.05a | 4.57 ± 0.07a | 0.0 | 0.4 |
| C. himalaica | 4.54 ± 0.06a | 4.45 ± 0.06a | 4.47 ± 0.06a | 4.47 ± 0.10a | −1.5 | 2.0 | |
| MGDG | A. thaliana | 5.29 ± 0.03b | 5.28 ± 0.01b | 5.35 ± 0.02a | 5.27 ± 0.01b | 1.1 | 1.5 |
| C. himalaica | 5.26 ± 0.03a | 5.21 ± 0.04ab | 5.25 ± 0.03bc | 5.18 ± 0.05c | −0.2 | 1.5 | |
| PA | A. thaliana | 3.04 ± 0.07a | 3.07 ± 0.06a | 2.83 ± 0.15b | 2.98 ± 0.12a | −6.9 | 8.5 |
| C. himalaica | 3.00 ± 0.09b | 3.18 ± 0.02a | 2.89 ± 0.10b | 2.98 ± 0.11b | −3.7 | 10.0 | |
| PC | A. thaliana | 3.50 ± 0.03a | 3.41 ± 0.04b | 3.20 ± 0.07c | 3.32 ± 0.03d | −8.6 | 9.4 |
| C. himalaica | 3.38 ± 0.03a | 3.34 ± 0.04a | 3.09 ± 0.03c | 3.22 ± 0.04b | −8.6 | 9.4 | |
| PE | A. thaliana | 3.35 ± 0.01a | 3.33 ± 0.03a | 3.11 ± 0.04c | 3.18 ± 0.02b | −7.2 | 7.7 |
| C. himalaica | 3.30 ± 0.01a | 3.28 ± 0.01a | 3.09 ± 0.01c | 3.17 ± 0.03b | −6.4 | 6.8 | |
| PS | A. thaliana | 2.61 ± 0.03b | 2.66 ± 0.04a | 2.60 ± 0.05bc | 2.55 ± 0.02c | −0.4 | 4.3 |
| C. himalaica | 2.56 ± 0.01ab | 2.59 ± 0.01a | 2.53 ± 0.01bc | 2.52 ± 0.05c | −1.2 | 2.8 | |
| PI | A. thaliana | 2.59 ± 0.03a | 2.53 ± 0.03b | 2.50 ± 0.03c | 2.51 ± 0.02bc | −3.5 | 3.6 |
| C. himalaica | 2.53 ± 0.02a | 2.48 ± 0.01b | 2.41 ± 0.02c | 2.48 ± 0.02b | −4.7 | 5.0 | |
| PG | A. thaliana | 2.14 ± 0.14a | 2.09 ± 0.07ab | 1.99 ± 0.06bc | 1.96 ± 0.04c | −7.0 | 9.2 |
| C. himalaica | 1.77 ± 0.06a | 1.76 ± 0.03a | 1.57 ± 0.02b | 1.58 ± 0.06b | −11.3 | 12.7 | |
| Total double bond index | |||||||
| Total lipids | A. thaliana | 4.14 ± 0.05a | 4.21 ± 0.01a | 4.16 ± 0.10a | 4.03 ± 0.10b | 0.5 | 4.5 |
| C. himalaica | 4.05 ± 0.02a | 4.01 ± 0.04ac | 3.95 ± 0.07bc | 3.85 ± 0.06d | −2.5 | 5.2 | |
No major changes in lysoPLs occurred during the changes between high and low temperatures in the four plant species tested
LysoPLs, which include lysophosphatidylcholine, lysophosphatidylglycerol, and lysophosphatidylethanolamine, are derived from the hydrolysis of phospholipids at the sn-1 or sn-2 position of the glycerol backbone. In comparison with other membrane glycerolipids, the content of lysoPL in A. thaliana membranes is low but very sensitive to stresses such as freezing, heat-shock, and dehydration. Upon exposure to stress, lysoPLs usually increase by 5–20-fold within hours or even minutes (Welti et al. 2002; Li et al. 2008, unpublished data). In the present study, 11, 16, 15, and 15 lysoPL species were detected in S. medusa, S.-L. linearifolia, and A. thaliana and C. himalaica, respectively (Fig 4 and 6). However, there were no significant changes in the levels of lysoPL species during changes between high and low temperatures, except for some individual molecular species, such as 16:0 and 18:3 lysoPG. These results indicated that the responses of lysoPLs to alterations between high and low temperatures differed from their responses to high or low temperature alone. This suggested that plants display different patterns of changes in membrane glycerolipid composition in response to different patterns of temperature alteration.
Figure 6.
Changes in molecular species of lysoPLs in A. thaliana and C. himalaica during alterations between high and low temperatures in the growth chamber. ‘R’ and ‘F’ indicate that the temperature was rising or falling, respectively. Values are means ± SD (n = 4 or 5).
Discussion
The responses of plants to high or low temperatures have been investigated extensively (Levitt 1980; Penfield 2008). The roles of membranes under extreme temperatures, and in particular the changes that occur in the glycerolipid composition of membranes, have been one focus of research (Murakami et al. 2000; Li et al.,2008; Penfield 2008). However, in many ecosystems, such as alpine screes and desert, plants can frequently encounter both high and low temperatures within a short time; thus, the effect of high and low temperature can have a combined effect on plants. As a consequence, a fundamental question that arises is whether the patterns of changes in membrane lipids that are observed in response to high or low temperature alone also apply to rapid changes between high and low temperature. In the present study, we profiled the molecular species of lipid in four plant species and calculated the DBI of plant membrane glycerolipids under conditions of frequent alterations between high and low temperatures in field and laboratory experiments. Our results indicated two major differences with respect to lipid composition during changes between high and low temperatures, as compared with high or low temperatures alone. The first distinction was that the degree of unsaturation of glycerolipids in the membrane was maintained, which confirmed our hypothesis. This maintenance of the level of unsaturation was affected neither by solar and UV radiation nor by differences in plant morphology and lipid biosynthesis pathways. However, the composition of the membrane glycerolipids did change significantly and the relative changes were as large as 21.2–1200% (Tables 1 and 3). This phenomenon was particularly noticeable with respect to the lipids in chloroplast membranes. MGDG and DGDG are known to be plastidic lipids and were found to be highly unsaturated in this study. The levels of MGDG and DGDG changed by 26.2–142% over the temperature range (Tables 1 and 3), but the values of DBI were maintained within a narrow range of 0.4–2.6% (Tables 2 and 4). These results demonstrate that chloroplast membranes have distinct properties in response to temperature stresses as compared with other cell membranes.
Two factors might limit the temperature-induced change in the degree of saturation and unsaturation during frequent changes between temperature extremes. One factor is the time limitation. For example, in the alpine screes investigated, the environmental temperature increased and decreased again within a period of 9 h (Fig. 1). This time was too short to allow plants to alter the degree of saturation and unsaturation of membrane lipids, because the two opposite biochemical processes involve complex reactions (Harwood 1998). Another potential factor is energy limitation. The energy required to form a double bond (C=C) from a single bond (C–C) is 171 kJ/mol (as estimated by chemical reaction heat). Saturation and desaturation are multiple-step reactions (mainly oxidation–reduction reactions) (Buchanan et al. 2002), and they take place respectively in response to decreases and increases in temperature that can be separated by hours or days. The exothermal energy that is released from breaking double bonds cannot be used in the subsequent formation of double bonds. Saturation and desaturation require a greater energy input than the turnover reactions involved in head group exchange (see below). In alpine screes, frequent changes between high and low temperatures are routine and usually occur throughout the spring and autumn. Performing saturation and desaturation reactions frequently over long periods would require substantial amounts of energy and is a poor trade-off between survival and development. In the laboratory experiments described herein, A. thaliana and C. himalaica grew very slowly on plates under conditions of alteration between high and low temperatures. This was possibly partly due to the amount of energy required to cope with the frequent alterations in temperature.
In the absence of changes in the degree of unsaturation, how do plants maintain their membrane functions during alterations in environmental temperature? An alternative solution is the turnover of head groups among the classes of lipid, which was observed in all plant species investigated and in both field and laboratory experiments. In cells, lipid turnover is a one-step reaction by which glycerolipids can exchange their head groups (Murphy 2005). During the exchange of head groups, bonds are broken and new bonds are formed synchronously and the properties of the original and new bonds are very similar. For example, when the head group of PC is exchanged for that of PE, the original bond RO−3PO–CH2CH2N+(CH3)3 is very similar to the new bond RO−3PO–CH2CH2N+H3. Thus, the endothermic and exothermic processes of bond breaking and bond formation, respectively, involve very similar amounts of absolute energy and the net energy cost of this reaction is close to zero. As a consequence, the energy requirement for head group exchange is much lower than that for desaturation and saturation reactions (see above). Head group exchange occurs constantly and is an important process during lipid synthesis, degradation, and/or homeostasis in plants (Murphy 2005).
Different head groups confer different spatial and electronic properties upon lipid molecules within the membrane matrix. The exchange of head groups might result in substantial changes in the proportion of different lipid classes and thus change the physical and biochemical properties of the membrane. The in vivo evidence for the relationship between the distribution of lipid head groups and the physical properties of membranes is limited. However, it has been shown that an increase in the proportion of PA, a cone-shaped molecule, favors the formation of the hexagonal II (HII) phase in the plasma membrane and chloroplast envelope membranes during freezing (Uemura, Josephy & Steponkus 1995). HII phase is speculated to be a freezing-induced membrane lesion. The results of our previous work demonstrated that freezing induced a significant increase in PA levels and that suppression of PA by genetic knockdown of phospholipase Dα1 conferred an increased tolerance to freezing on plants (Welti et al. 2002). Indeed, we observed in the present study that, under freezing conditions, PA levels in S. medusa, A. thaliana, and C. himalaica were 3–5 fold higher than at temperatures above zero (Table 1 and 3). These results were in agreement with the relationship between PA and freezing-induced membrane lesions and therefore suggested that head group exchange has a role in the adaptation of plants to changes in temperature through affecting the physical properties of membranes. However, there are two points that should be noted: 1) a freezing-induced increase in PA did not occur in S.-L. linearfolia. This suggests that the responses of alpine plants to environmental temperature are complicated and not fully understood yet. 2) The lipid molecules involved in head group exchange are not limited to the 11 classes that we analyzed. For example, the phosphate head group of PA can be exchanged with that of diacylglycerol (DAG) or diacylglycerol pyrophosphate (DGPP) (Munnik et al. 1996), and these two types of lipid were not measured in this study. In spite of these considerations, our results supported the idea that the remodeling of membranes through the exchange of head groups among different lipid classes provides a rapid response to changes in environmental conditions and requires relatively little energy. Therefore, we propose that the turnover of head groups enables plant membranes to be remodeled and optimized in a timely fashion during frequent alterations between high and low temperature.
The second distinct characteristic of membrane composition during alterations between high and low temperatures was that lysoPLs remained unchanged. This was an interesting finding because lysoPLs are considered to be a sensitive indicator of stress responses in plants (Welti et al. 2002). The content of lysoPL increases quickly and dramatically under extreme conditions of temperature, water deficit, and salinity (Welti et al. 2002; Li et al. 2008; unpublished data). For the alpine scree plants S. medusa and S.-L. linearifolia, the stability of lysoPL levels implied that the range of temperature variation detected in autumn was not a stress factor, but a regular condition. This interpretation supports the idea that, in terms of adaptive evolution, plants that have grown in alpine screes for a long period have adapted to the environment, not only at the levels of life cycle, morphology, and physiology, but also at the biochemical level. Therefore, the changes in classes of lipid and lack of variation in the degree of fatty acid unsaturation in membrane lipids under conditions of frequent temperature change could be an environmental adaptation in alpine plant species, and not a response to stress. The stable lysoPL level observed in A. thaliana and C. himalaica that were exposed to alteration between high and low temperatures for 30 d suggests that these plants had acclimated to the adverse conditions.
In nature, temperature change can be divided into two types: (1) frequent temperature alteration in which the temperature rises and falls rapidly and there is a daily temperature cycle that switches between high and low temperatures and lasts for several seasons, such as those of alpine screes and deserts; and (2) infrequent temperature alteration in which the temperature rises and falls slowly, and change between high and low temperatures is a seasonal cycle, as for temperate zones. We propose two working models in which the turnover of membrane lipids and the degree of unsaturation of these lipids play different roles and allow plants to respond to these two types of temperature change at the biochemical level. We found that plants employed lipid remodeling to adjust their membrane composition to cope with frequent alterations in temperature, because lipid turnover via the exchange of head groups is rapid and incurs a low energy cost. In contrast, plants employed both lipid remodeling and changes in the degree of unsaturation to adjust the membrane composition to cope with infrequent temperature change.
Acknowledgments
The study was supported by grants from the National Basic Research Program of China (2007CB411604), Knowledge Innovation Program of CAS (KSCX2-YW-N-014), NSFC (30670474 and 30870571), Fund of State Key Laboratory of Phytochemistry and Plant Resources in West China (0807B01211 and 097C1211Z1), Innovation teams of Yunnan Province, Germplasm Bank of Wild Species and CAS Innovation Program of Kunming Institute (540806321211), and 100-Talents Program of CAS.
The Kansas Lipidomics Research Center (KLRC) was supported by NSF grants MCB 0455318 and DBI 0521587, and NSF EPSCoR grant EPS-0236913 with matching support from the State of Kansas through the Kansas Technology Enterprise Corporation and Kansas State University. The KLRC is also supported by K-INBRE (NIH Grant P20 RR16475 from the INBRE program of the National Center for Research Resources).
The authors would like to thank Mary Roth for the acquisition and processing of the ESI-MS/MS data and Dr. Xuemin Wang and Dr. Ruth Welti for their critical reading of the manuscript.
Abbreviations
- DAG
diacylglycerol
- DBI
double bond index
- DGDG
digalactosyldiacylglycerol
- ESI
electrospray ionization
- lysoPL
lysophospholipid
- MS/MS
tandem mass spectrometry
- MGDG
monogalactosyldiacylglycerol
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
References
- Al-Shehbaz IA, Steve L, O'Kane J, Price RA. Genetic placement of species excluded from Arabidopsis (brassicaceae) Novon. 1999;9:296–307. [Google Scholar]
- Bakht J, Bano A, Dominy P. The role of abscisic acid and low temperature in chickpea (Cicer arietinum) cold tolerance. II. Effects on plasma membrane structure and function. Journal of Experimental Botany. 2006;57:3707–3715. doi: 10.1093/jxb/erl120. [DOI] [PubMed] [Google Scholar]
- Buchanan B, Gruissem W, Jones R. Biochemistry & molecular biology of plants. John Wiley & Sons; 2002. pp. 456–527. [Google Scholar]
- Deng M, Zhou Z-K. Seed plant diversity on screes from Northwest Yunnan. Acta Botanica Yunnanica. 2004;26:23–34. [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a Phospholipase Dα1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Devaiah SP, Pan X, Hong Y, Roth MR, Welti R, Wang X. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis. The Plant Journal. 2007;50:950–957. doi: 10.1111/j.1365-313X.2007.03103.x. [DOI] [PubMed] [Google Scholar]
- Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biology. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood JL. Plant lipid biosynthesis : Fundamentals and agricultural applications. New York: Cambridge Unviersity Press; 1998. pp. 95–110. [Google Scholar]
- Hong Y, Devaiah SP, Bahn SC, Thamasandra BN, Li M, Ruth W, Wang X. Phospholipase Dε and phosphatidic acid enhance Arabidopsis nitrogen signaling and growth. The Plant Journal. 2009;58:376–387. doi: 10.1111/j.1365-313X.2009.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S, Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiology. 1992;99:197–202. doi: 10.1104/pp.99.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. Alpine plant life : Functional plant ecology of high mountain ecosystems. New York: Springer Verlag; 1999. pp. 101–114. [Google Scholar]
- Levitt J. Responses of plants to environmental stresses. New York: Academic Press; 1980. pp. 163–166.pp. 194–196.pp. 375–376. [Google Scholar]
- Li W, Li M, Zhang W, Welti R, Wang X. The plasma membrane-bound phospholipase Ddelta enhances freezing tolerance in Arabidopsis thaliana. Nature Biotechnology. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- Li W, Wang R, Li M, Li L, Wang C, Welti R, Wang X. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. Journal of Biological Chemistry. 2008;283:461–468. doi: 10.1074/jbc.M706692200. [DOI] [PubMed] [Google Scholar]
- Miquel M, James D, Jr, Dooner H, Browse J. Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proceedings of the National Academy of Sciences U S A. 1993;90:6208–6212. doi: 10.1073/pnas.90.13.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, de Vrije T, Irvine RF, Musgrave A. Identification of diacylglycerol pyrophosphate as a novel metabolic product of phosphatidic acid during G-protein activation in plants. Journal of Biological Chemistry. 1996;271:15708–15715. doi: 10.1074/jbc.271.26.15708. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K. Trienoic fatty acids and plant tolerance of high temperature. Science. 2000;287:476–479. doi: 10.1126/science.287.5452.476. [DOI] [PubMed] [Google Scholar]
- Murphy DJ. Plant lipids : Biology, utilisation, and manipulation. Boca Raton, FL: CRC Press; 2005. pp. 131–141. [Google Scholar]
- Penfield S. Temperature perception and signal transduction in plants. New Phytologist. 2008;179:615–628. doi: 10.1111/j.1469-8137.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- Rawyler A, Pavelic D, Gianinazzi C, Oberson J, Braendle R. Membrane lipid integrity relies on a threshold of ATP production rate in potato cell cultures submitted to anoxia. Plant Physiology. 1999;120:293–300. doi: 10.1104/pp.120.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A, Larcher W. Frost survival of plants : Responses and adaptation to freezing stress, Ecological studies v. 62. New York: Springer Verlag; 1987. pp. 124–131. [Google Scholar]
- Tsukaya H, Fujikawa K, Wu S-G. Thermal insulation and accumulation of heat in the downy inflorescences of Saussurea medusa (asteraceae) at high elevation in Yunnan, China. Journal of Plant Research. 2002;115:63–268. doi: 10.1007/s10265-002-0030-1. [DOI] [PubMed] [Google Scholar]
- Uemura M, Joseph RA, Steponkus PL. Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions) Plant Physiology. 1995;109:15–30. doi: 10.1104/pp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JG, Browse J. Mutants of Arabidopsis reveal many roles for membrane lipids. Progress in Lipid research. 2002;41:54–278. doi: 10.1016/s0163-7827(01)00027-3. [DOI] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. Journal of Biological Chemistry. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Welti R, Shah J, Li W, Li M, Chen J, Burke JJ, Fauconnier M-L, Chapman K, Chye M-L, Wang X. Plant lipidomics: discerning biological function by profiling plant complex lipids using mass spectrometry. Frontiers in Bioscience. 2007;12:2494–2506. doi: 10.2741/2250. [DOI] [PubMed] [Google Scholar]
- Yang Y, Körner C, Sun H. The ecological significance of pubescence in Saussurea medusa, a high-elevation himalayan "Woolly plant". Arctic, Antarctic, and Alpine Research. 2008;40:250–255. [Google Scholar]
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. The Plant Journal. 2009;21:2357–2377. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]