Abstract
Background:
Recent research using serum high sensitivity C-reactive protein (hs-CRP) has evidenced existence of low grade systemic inflammation in asthmatics whose correlation with various clinical indices is not fully studied.
Objective:
To investigate the relationship between systemic inflammation and various clinical and treatment characteristics of asthma.
Materials and Methods:
Forty asthmatics (22 steroid inhaling and 18 steroid naïve) and 40 healthy subjects matched for age and sex were examined cross-sectionally. Along with clinical assessment, serum hs-CRP levels were measured for all subjects using latex enhanced immunoturbidometry method.
Results:
Serum hs CRP levels were significantly higher in steroid naïve asthmatics when compared to normal subjects (0.93 ± 1.18 vs 0.24 ± 0.31 mg/dL, respectively; Mann-Whitney U test, P < 0.001). This association persisted after adjusting for age, gender, body mass index (BMI), and socioeconomic status (adjusted odds ratio 10.47; 95% CI 1.88-58.3; P < 0.01). Steroid inhaling asthmatics had serum hs-CRP levels comparable with control group (0.17 ± 0.18 vs 0.24 ± 0.31 mg/dL respectively, P > 0.05). Among the clinical and treatment related variables, duration of inhaled steroids usage alone correlated significantly with serum hs-CRP levels (Pearson correlation coefficient r = 0.449, P < 0.05), which was independent of age, BMI, duration of illness, and frequency of emergency visits.
Conclusion:
This study confirms the existence of low grade systemic inflammation in asthma which is effectively controlled by inhaled steroids. Such an effect of inhaled steroids appears to be more pronounced in recent users than that of long-term users, possibly due to lower adherence rate among the latter.
Keywords: Asthma, high sensitivity C-reactive protein, inhaled steroids, systemic inflammation
INTRODUCTION
Asthma is characterized by airway inflammation and hyperresponsiveness. Besides local inflammation, asthma is also found to have systemic inflammation as evidenced by increased levels of plasma fibrinogen and serum amyloid A.[1] Serum C reactive protein (CRP) levels have been used extensively as a surrogate marker of systemic inflammation in several chronic diseases as well to predict adverse cardiovascular events.[2,3] Estimation of high sensitivity CRP (hs-CRP) with its enhanced sensitivity is useful in conditions with low grade systemic inflammation like asthma. Defining the role of hs-CRP in asthma has been a topic of interest in recent research.
Serum CRP levels were found to be correlating with asthma and its characteristics like bronchial hyperresponsiveness,[4] FEV1 level,[5] control of symptoms,[6] and sputum cytology.[7] Several other studies have denied some of the above mentioned associations,[8,9] thereby emphasizing the need for further evaluation of the unique role of hs-CRP in asthma. Also, the effect of inhaled steroids needs to be studied. Given the association of higher CRP levels with cardiovascular disease,[3,10] diabetes mellitus[11] and metabolic syndrome,[12,13] any potential ‘anti-inflammatory’ effect of inhaled steroids on CRP level will influence the long-term prospects of asthma in addition to controlling airway inflammation. This study attempts to characterize the relationship between hsCRP, asthma, and its treatment.
MATERIALS AND METHODS
This was a case-control study involving 40 asthmatic patients attending the chest clinic of a tertiary care center. Their diagnosis was established based on clinicoradiological and pulmonary function testing (PFT) criteria suggested by the Expert Panel Report III[14] of the National Institute of Health, USA. All asthmatic subjects were defined either as “steroid naοve” if never taken inhaled steroids in the past or “steroid inhaling” if taking inhaled steroids for at least 2 months prior to the recruitment. Forty normal subjects were recruited for control group. All study subjects were aged between 18 and 40 years and were never smokers. Asthmatics did not have acute exacerbation within 2 weeks prior to the recruitment nor did they have any clinically evident focus of infection. Subjects with current or past history of diabetes mellitus, cardiovascular diseases, malignancy, and systemic inflammatory disorders were excluded. Similarly subjects with current or recent history of intake of oral steroids more than 5 mg/day and/or any other anti-inflammatory drugs were excluded. This study was approved by institutional ethics committee and all subjects gave written informed consent before enrollment.
Sociodemographic variables and disease characteristics including duration of illness, comorbidities, exposure to environmental triggers, number of emergency department visits for acute wheezing during last 6 months prior to enrollment, usage of inhaled corticosteroids, and its duration were evaluated. Venous blood samples were taken from all study subjects after at least 4 h of fasting. The samples were allowed to clot and then centrifuged at 3,000 rpm for 5 min. Serum collected from the centrifuged sample was used to measure hs-CRP level by a high sensitivity CRP assay (latex immunoturbidometry method) with measurement range of 0.01-2 mg/dL (Cobas Integra 400 plus with Roche reagents, USA).
Statistical analysis
Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS) 17 software. The data was examined initially for normality of distribution and homogeneity of variance. The comparison of quantitative variables between the groups was carried out by using Student's t-test. Categorical variables were compared by using Chi-square test and odds ratio were evaluated. Correlations between serum hs-CRP and variables like age, body mass index (BMI), duration of illness, frequency of emergency department visits during last 6 months, and duration of usage of inhaled steroids were analyzed using Pearson's correlation tests. A P value of <0.05 was considered significant.
RESULTS
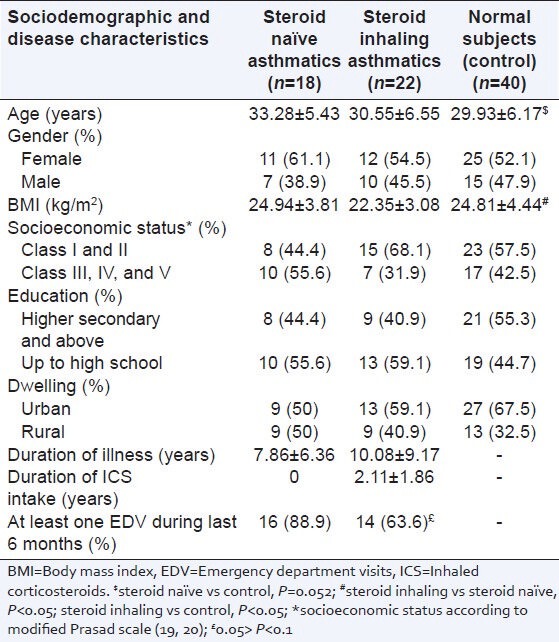
A total of 80 subjects completed the study whose data was as follows: 40 normal subjects were compared with 22 steroid inhaling asthmatics and 18 steroid naοve asthmatics. Their sociodemographic and disease characteristics are summarized in Table 1. Age and BMI were marginally different in asthma subgroups as compared with control subjects. Other characteristics like gender, socioeconomic status,[15,16] education level, and dwelling were matched across the groups. All of the study subjects had mixed diet habits and were nonsmokers. None reported any significant comorbid history. Comparing disease characteristics among asthmatics, mean duration of illness was 10.08 ± 9.17 and 7.86 ± 6.36 years among steroid naοve and steroid inhaling groups, respectively (P > 0.05). Mean duration of usage of inhaled steroids was 2.11 ± 1.86 years. Inhaled steroid dose among steroid inhaling asthmatics ranged from 200 to 400 μg per day. Sixteen (88.9%) of steroid naοve asthmatics reported to have visited emergency departments for wheezing at least once during last 6 months prior to the study, compared to 14 (63.6%) of steroid inhaling asthmatics reporting the same (P = 0.067).
Table 1.
Sociodemographic and disease characteristics of study subjects
Serum hs-CRP levels were significantly higher in steroid naïve asthmatics when compared to normal subjects (0.93 ± 1.18 versus 0.24 ± 0.31 mg/dL, respectively; Mann-Whitney U test, P < 0.001) [Figure 1]. After adjusting for age, gender, BMI, and socioeconomic status; serum hs-CRP level remained significantly associated with steroid naive asthma (adjusted odds ratio 10.47; 95% CI 1.88-58.3; P < 0.01). Serum hs-CRP levels among steroid inhaling asthmatics were comparable with control group (0.17 ± 0.18 versus 0.24 ± 0.31 mg/dL respectively, P > 0.05).
Figure 1.
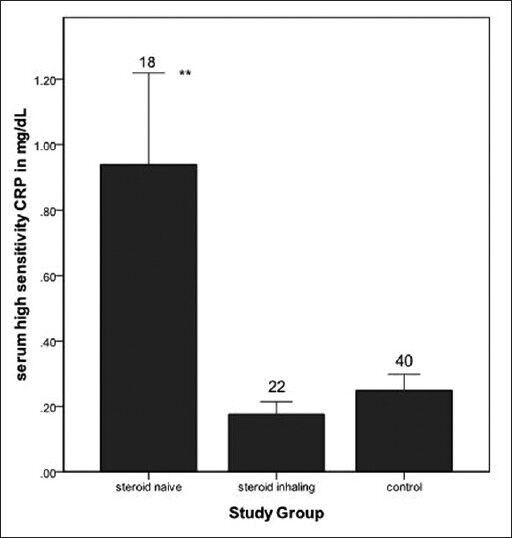
Bar chart representing mean value of serum high sensitivity C-reactive protein (hs-CRP) levels among three study groups. Error bars show standard error of the mean and number over error bar indicates sample size of the respective groups. Steroid naïve asthmatics had higher mean hs-CRP compared to healthy control (**P < 0.01)
Table 2 shows the correlation coefficients of serum hs-CRP with various clinical and treatment indices of asthma. Among steroid inhaling asthmatics, duration of inhaled steroids usage alone correlated significantly with serum hs-CRP levels (Pearson correlation coefficient r = 0.449, P < 0.05) [Figure 2]. This correlation persisted after controlling for age, BMI, duration of illness, and frequency of emergency department visits during last 6 months (r = 0.535, P < 0.05). Among steroid naïve asthmatics none of the studied indices correlated with hs-CRP levels.
Table 2.
Correlation between serum hs-CRP level and clinical and treatment characteristics among asthmatics
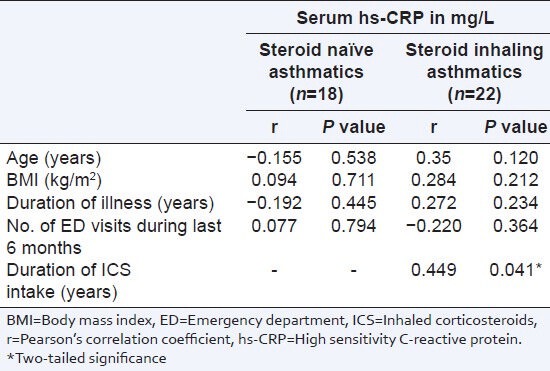
Figure 2.
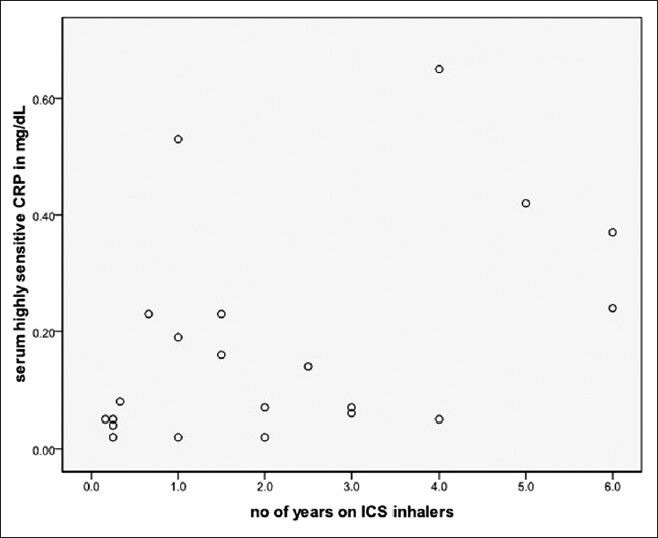
Graphical representation of correlation between serum hs-CRP level and duration of usage of inhaled corticosteroids among asthmatics (n = 22; Pearson's correlation coefficient r = 0.449, P < 0.05)
DISCUSSION
This study has confirmed the existence of low grade systemic inflammation in asthma and that it could be effectively controlled by intake of inhaled corticosteroids, to the level which is comparable with normal subjects. This study is also unique in evaluating the correlation between serum hs-CRP and various clinical variables related to the disease and its treatment, particularly the duration of usage of inhaled steroids.
Many researchers, using various biomarkers, have evidenced the existence of low grade systemic inflammation in addition to airway inflammation in asthma. Some of the studies have used hs-CRP as a surrogate marker for this systemic inflammation and found significantly elevated levels in asthmatics. Our study is one of the few that has demonstrated the beneficial effect of inhaled steroids in controlling this low grade systemic inflammation. Takemura et al.,[7] in their study compared 22 steroid naïve and 23 steroid inhaling asthmatics with 14 normal subjects and found that mean serum hs-CRP level was significantly higher in steroid naïve asthmatics, whereas the level in steroid inhaling asthmatics was comparable with normal subjects. Another study by Kasayama et al.,[17] showed similar results and the authors also found that treatment of asthma with inhaled steroids for 3 months significantly reduced serum CRP level. Our findings are consistent with the above mentioned studies, thereby clearly demonstrating the salutary effect of inhaled steroids upon the serum CRP levels. We found a 10.47-fold increase in risk of diagnosing steroid naïve asthma for every 1 standard deviation (SD) increase in the serum hs-CRP level above the mean value. This risk was independent of age, gender, BMI, and socioeconomic status.
We found that among steroid inhaling asthmatics hs-CRP significantly correlated with the duration of usage of inhaled steroids, which was independent of other important confounders like age, BMI, duration of illness, and asthma control as determined by frequency of emergency department visits during last 6 months. The correlation coefficient was positively oriented (r = 0.449, P < 0.05) indicating that higher hs-CRP levels were associated with longer duration of use of inhaled steroids. This finding appears to be paradoxical, since our results and other references cited earlier indicated that inhaled steroids lowered the CRP level in asthmatics. This paradox could partly be explained in our study population, by possible lower adherence rate in long-term users than those who were recently started on inhaled steroids. Such a behavior pattern is reported to be common among the patients with various chronic disease settings including asthma.[18,19] A recent study reported that adherence rates among asthmatics decreased over time, even in patients who had received the medication free of charge.[20] This behavioral pattern of compliance and other reasons need to be evaluated for its potential influence on the effect of inhaled steroids on hs-CRP level in asthmatics. Our initial study design did not envisage such an evaluation and hence we could not confirm the above proposed mechanism. Nevertheless, our findings on positive correlation between hs-CRP and duration of inhaled steroid use, is undeniable. To our knowledge this is the first report on differential effect of inhaled corticosteroids on high sensitivity C-reactive protein (hs-CRP) levels in asthma. Such a ‘confounding’ influence was never considered in any of the older studies that evaluated the relationship between hs-CRP and asthma, whose results may be challenged by this previously unknown effect. We recommend that future studies on systemic inflammation in asthma, should address this important confounder.
In the present study, meticulous steps were taken to minimize the confounding effects of various factors on the association between asthma and hs-CRP. All the subjects in our study were never smokers, and did not have past or current history of comorbidities that could have possibly influenced the CRP levels in serum. They were also free from recent or ongoing infections and asthma exacerbations. Our study also had the strength of 1:2 case/control comparison of asthmatic subgroups with 40 normal subjects which enhanced the power of the study. Despite above merits, this study has various limitations. Lung function testing, although was used for diagnosis of asthma prior to the study, it was not reevaluated during the study and hence its effect on hs-CRP level could not be studied. However, it is to be understood that asthma severity involves both the severity of underlying airway obstruction (as indicated by % of predicted forced expiratory volume in 1 second (FEV1)) and its responsiveness to treatment. Thus, assessment of severity of asthma based on lung function alone does not reflect the actual phenotype of disease. An assessment of asthma control based on symptomatology and response to treatment is more relevant. Global Initiative for Asthma guidelines 2012 recommends classifying asthma severity on the basis of intensity of treatment required for a good control whose defining criteria includes frequency of exacerbation.[21] We used frequency of emergency department visits prior to recruitment, as a marker of asthma control which did not correlate with hs-CRP. Smaller sample size prohibited conclusive interpretation of some of our important findings. However, the sample gave adequate power to the study calculated based on standard deviation and the difference between the mean values of the study groups. Initial hypothesis leading to the study design did not foresee the paradoxical finding of correlation between duration of inhaled steroid usage and hs-CRP level. Thus, the treatment compliance was not evaluated, which in ideal situations could have been of great use in interpreting this important finding. However, such evaluation is known to be notoriously unreliable and subjective. We also believe that onetime assessment during the study could not have reliably reflected the dynamics of treatment compliance through the duration of inhaled steroid usage, a factor that is important in control of airway and systemic inflammation.
Summarizing the results of our study, we conclude that low grade systemic inflammation exists in asthma which can be controlled effectively by use of inhaled corticosteroids. Such an effect of inhaled steroids appears to be more pronounced in recent users than that of long-term users and was independent of age, BMI, duration of illness, and frequency of emergency department visits for acute wheezing. Exact mechanism of above findings is not known, although a possible low level of adherence to inhaled steroids in long-term users may be speculated to cause such a differential effect on systemic inflammation. Further studies are required to confirm the above proposed mechanism and its clinical applications. We recommend future studies to consider this confounding effect while exploring systemic inflammation in asthma. Given the salutary effect of inhaled steroids on systemic inflammation we also recommend studies to investigate the role of hs-CRP as a surrogate marker of treatment adequacy and compliance in asthma.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol. 2002;89:381–5. doi: 10.1016/S1081-1206(10)62039-X. [DOI] [PubMed] [Google Scholar]
- 2.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–61. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 4.Kony S, Zureik M, Driss F, Neukirch C, Leynaert B, Neukirch F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): A population based study. Thorax. 2004;59:892–6. doi: 10.1136/thx.2003.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian FH, Zhang Q, Zhou LF, Liu H, Huang M, Zhang XL, et al. High sensitivity C reactive protein: A predictive marker in severe asthma. Respirology. 2008;13:664–9. doi: 10.1111/j.1440-1843.2008.01314.x. [DOI] [PubMed] [Google Scholar]
- 6.Navratil M, Plavec D, Dodig S, Jelcic Z, Nogalo B, Erceg D, et al. Markers of systemic and lung inflammation in childhood asthma. J Asthma. 2009;46:822–8. [PubMed] [Google Scholar]
- 7.Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, et al. High sensitivity C reactive protein in asthma. Eur Respir J. 2006;27:908–12. doi: 10.1183/09031936.06.00114405. [DOI] [PubMed] [Google Scholar]
- 8.Panaszek B, Liebhart E, Liebhart J, Pawlowicz R, Fal AM. Serum concentration of C reactive protein is not a good marker of bronchial hyperresponsiveness. Arch Immunol Ther Exp (Warsz) 2007;55:341–5. doi: 10.1007/s00005-007-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez D, Patel P, Casillas A, Cotelingam J, Boggs P, Bahna SL. Assessment of high sensitivity C-reactive protein as a marker of airway inflammation in asthma. Ann Allergy Asthma Immunol. 2010;104:485–9. doi: 10.1016/j.anai.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Wisniacki N, Taylor W, Lye M, Wilding JP. Insulin resistance and inflammatory activation in older patients with systolic and diastolic heart failure. Heart. 2005;91:32–7. doi: 10.1136/hrt.2003.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6 and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 12.Hassinen M, Lakka TA, Komulainen P, Gylling H, Nissinen A, Rauramaa R. C-reactive protein and metabolic syndrome in elderly women: A 12-year follow up study. Diabetes Care. 2006;29:931–2. doi: 10.2337/diacare.29.04.06.dc05-2508. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome and the risk of incident cardiovascular events: An 8-year follow-up of 14719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 14.Expert panel report 3: Guidelines for the diagnosis and management of asthma. 2007 NHLBI. [Last accessed on 2012 Jan 15]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf .
- 15.Prosad BG. Changes proposed in social classification of Indian families. J Indian Med Assoc. 1970;55:198–9. [PubMed] [Google Scholar]
- 16.Kumar P. Social classification: Need for constant updating. Indian J Community Med. 1993;18:60–1. [Google Scholar]
- 17.Kasayama S, Tanemura M, Koga M, Fujita K, Yamamoto H, Miyatake A. Asthma is an independent risk for elevation of plasma C-reactive protein levels. Clin Chim Acta. 2009;399:79–82. doi: 10.1016/j.cca.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Evans CD, Eurich DT, Lamb DA, Taylor JG, Jorgenson DJ, Semchuk WM, et al. Retrospective observational assessment of statin adherence among subjects patronizing different types of community pharmacies in Canada. J Manag Care Pharm. 2009;15:476–84. doi: 10.18553/jmcp.2009.15.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansur N, Weiss A, Hoffman A, Gruenewald T, Beloosesky Y. Continuity and adherence to long-term drug treatment by geriatric patients after hospital discharge: A prospective cohort study. Drugs Aging. 2008;25:861–70. doi: 10.2165/00002512-200825100-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lasmar L, Camargos P, Bousquet J, Goulart E, Sakurai E, Carvalhais M. Factors related to lower adherence rates to inhaled corticosteroids in children and adolescents: A prospective randomized cohort study. J Trop Pediatr. 2009;55:20–5. doi: 10.1093/tropej/fmn067. [DOI] [PubMed] [Google Scholar]
- 21.Global Strategy for Asthma Management and prevention. Updated 2012. [Last accessed on 2013 Aug 25]. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_March13.pdf .


