Abstract
Aim and Objectives:
The present study was undertaken in 20 healthy human volunteers to evaluate the effect of a herbal bioenhancer, Carum carvi on pharmacokinetics of rifampicin, isoniazid, and pyrazinamide in fixed dose combination (FDC).
Materials and Methods:
It was a prospective, two-period, open-label, cross-over experiment on 20 healthy human male volunteers. The volunteers were administered a single dose of FDC containing rifampicin (450 mg), isoniazid (300 mg), and pyrazinamide (1000 mg) and after 10 days washout period the same FDC along with C. carvi extract (100 mg) was administered. Blood samples were collected at different time-points and analyzed by high-performance liquid chromatography (HPLC). Detailed pharmacokinetic parameters were calculated, which included Cmax, area under curve (AUC), time to reach maximum plasma concentration (Tmax), clearance (Cl), volume of distribution (Vd), and half-life (t½).
Results:
Additions of C. carvi extract lead to increase in plasma levels of rifampicin, isoniazid, and pyrazinamide. The bioavailability indices Cmax of rifampicin increased from 4.57 ± 0.19 to 5.95 ± 0.19 (P = 0.000) and AUC increased from 40.11 ± 1.69 to 53.01 ± 1.88 (P = 0.000). Similarly, Cmax of isoniazid increased from 2.66 ± 0.16 to 3.62 ± 0.16 (P = 0.000) and AUC from 17.72 ± 0.78 to 22.87 ± 0.94 (P = 0.000). The bioavailability indices of pyrazinamide also revealed an increase in Cmax from 18.81 ± 0.79 to 25.06 ± 1.14 (P = 0.000) and AUC from 107.65 ± 4.42 to 137.71 ± 5.92 (P = 0.000), respectively.
Conclusion:
C. carvi acts as a bioenhancer and modifies the kinetics of antitubercular treatment (ATT) favorably.
Keywords: Bioavailability, bioenhancer, Carum carvi, isoniazid, pyrazinamide, rifampicin, tuberculosis
INTRODUCTION
Tuberculosis (TB) remains a global public health threat. In India, it accounts for nearly one third of prevalent cases worldwide and remains a major cause of morbidity and mortality.[1] A fixed dose combination (FDC) containing rifampicin (R), isoniazid (I), and pyrazinamide (Z) is the mainstay of the treatment of TB. Although FDC products provide many advantages, poor and variable bioavailability of antitubercular treatment (ATT) drugs produce a challenge to successful antitubercular program.[2,3,4,5,6,7]
The low bioavailability of these drugs often results in insufficient therapeutic plasma levels of the drugs leading to treatment failure and emergence of resistance. Further drug-induced hepatotoxicity and potentially serious adverse effects add to the problem, and adverse effects often negatively affect the compliance.
Thus increasing the oral bioavailability of these drugs remains the priority of the treating clinician. A lot of strategies have been advocated for improving the bioavailability of these drugs. The concept of bioenhancers is derived from the traditional age-old system of Ayurvedic therapies and numerous plants have been evaluated for their potential bioavailability enhancing activity. In ayurveda, black pepper, long pepper, and ginger are collectively known as trikatu, which has been used as a bioenhancer.[8] Carum carvi is one such herbal bioenhancer that has been extensively studied along with antibiotics, antifungals, antivirals, anticancer, anti-inflammatory, antitubercular, antihistaminics.[9] Although, a few recent studies have proved the bioenhancing property of C. carvi with antitubercular drugs in animals,[10,11] to the best of our knowledge there is no study evaluating its bioenhancing property in humans. Thus, the present study was undertaken to evaluate the effect of C. carvi extract, a herbal bioenhancer on pharmacokinetics of antitubercular drugs in humans.
MATERIALS AND METHODS
Fixed dose combination (FDC) containing rifampicin (450 mg), isoniazid (300 mg), and pyrazinamide (1000 mg) (Rifacept® b.n. 6007; Concept Pharmaceuticals, Mumbai, India) and Capsule containing C. carvi extract (100 mg) was supplied by Indian Institute of Integrative Medicine (IIIM), Jammu. The preparation and standardization of test material (extraction of C. carvi) was done at IIIM, Jammu, as per the method described and used by Sachin et al.,[11] in their animal study evaluating pharmacokinetic interaction of some antitubercular drugs with caraway seeds.
High-performance liquid chromatography (HPLC; Shimadzu, Japan) was used to determine the plasma levels of rifampicin, isoniazid, and pyrazinamide. HPLC conditions were Column RP-18, 5 μm (length 250 ×4 nm); λmax 271nm; mobile phase, 50 mM phosphate buffer (pH 5.0); acetonitrile (60:40). The flow rate of rifampicin was 0.8 mL/min, whereas for isoniazid and pyrazinamide, it was 0.5 mL/min. The retention times of rifampicin, isoniazid, and pyrazinamide were 5.952, 5.397, and 5.890 min, respectively. No interfering peaks were observed at these retention times.
Formulations
FDC formulation used in this study was three drug FDC consisting of rifampicin (450 mg), isoniazid (300 mg), and pyrazinamide (1000 mg). This was designated as test formulation-A (TF-A) in the study. Test formulation-B (TF-B) contained the same three drugs FDC and capsule of C. carvi extract (100 mg).
Experimental design
This was a prospective, two-period, open-label, cross-over experiment on healthy human male volunteers. The study was approved by Institutional Ethics Committee and was conducted in collaboration with IIIM, CSIR, Jammu. The study was carried out in the Postgraduate Department of Pharmacology and Therapeutics of Govt. Medical College, Jammu.
Inclusion criteria
Before initiation of study, a group of volunteers was screened by performing physical examination, liver function tests, hemogram, lipid profile, renal function tests, chest X-ray, stool and urine examination, electrocardiogram, and ultrasonography. Healthy volunteers having normal laboratory values, aged between 20 and 40 years and weighing between 60 and 70 kg with no history of drug or alcohol abuse, liver, kidney, or gastrointestinal disorders were selected based on the screening results. The scope of the study was explained to all the volunteers and each one signed an informed consent form before onset of the study.
Dosing schedules
Trial was conducted in two sessions. On each experiment session, test formulations were swallowed on an empty stomach with a glass of water (200 mL) after overnight fasting. A light breakfast and lunch were provided after 2 and 6 h, respectively. The second test formulation was administered after a washout period of 10 days.
Collection of blood samples
Venous blood samples (3 mL) were collected using indwelling catheter in tubes having 5% ethylenediaminetetraacetic acid (EDTA) at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h. After collection, blood samples were immediately centrifuged at 10,000 rpm for a period of 10 min. Plasma was separated into tubes and stored at −80°C till analysis.
Calculation of pharmacokinetic parameters
All the pharmacokinetic parameters were determined by noncompartmental analysis. The concentration versus time profiles of antituberculosis drugs alone and in presence of C. carvi extract were plotted on a semi-log scale as function of time and the kinetics of absorption and elimination for all samples taken till 24 h after administration were calculated as given in the following paragraphs.
The hypothetical concentration at zero hour (C0) was directly read from the graph. The maximum plasma concentration (Cmax) and time to reach maximum plasma concentration (Tmax) were directly read from the concentration time plots. The half-life (t½) was calculated directly from the plasma concentration profile by reading the time needed for the concentration to decrease by one-half from any arbitrary point on a log concentration: time plot.[12]
Rate constant (k) was calculated from the formula: k = t½/0.693[12]
The area under the time concentration curve (AUC0-24) was calculated by the trapezoidal method where the area was divided into small trapezoids and the cumulative area under curve AUC(0-24) was then calculated by the formula:
Area = (½)(C1 + C2) (t2 − t1) +……….+ (½) (Cn− 1 + Cn) (tn − tn− 1).[13]
The volume of distribution (Vd) was obtained from the formula:
Vd = dose/C0[12]
Where dose is the amount of drug given orally and C0 is the concentration at zero time.
The plasma clearance (Cl) was calculated from the formula: Cl = Vd × k.[12]
Statistical analysis
The results obtained were analyzed using statistical analysis method. Paired t test was applied between two treatment groups. P < 0.05 were considered as statistically significant.
RESULTS
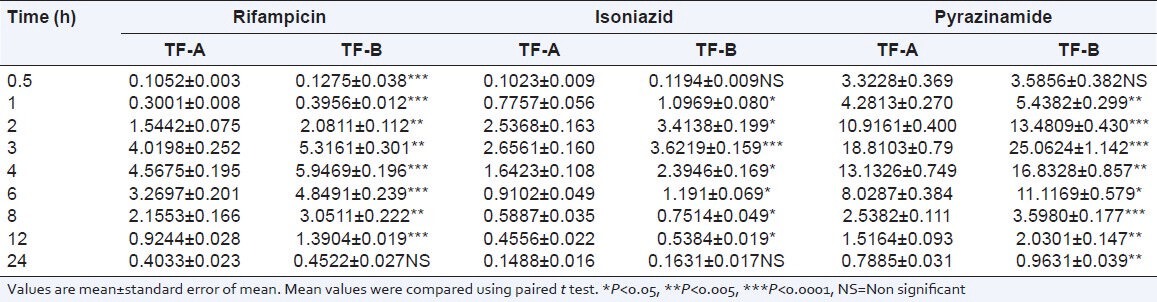
The plasma levels of rifampicin, isoniazid, and pyrazinamide after test formulation A and test formulation B are shown in Table 1.
Table 1.
Plasma levels (μg/ml) of rifampicin, isoniazid, and pyrazinamide with TF-A and TF-B
Pharmacokinetic parameters
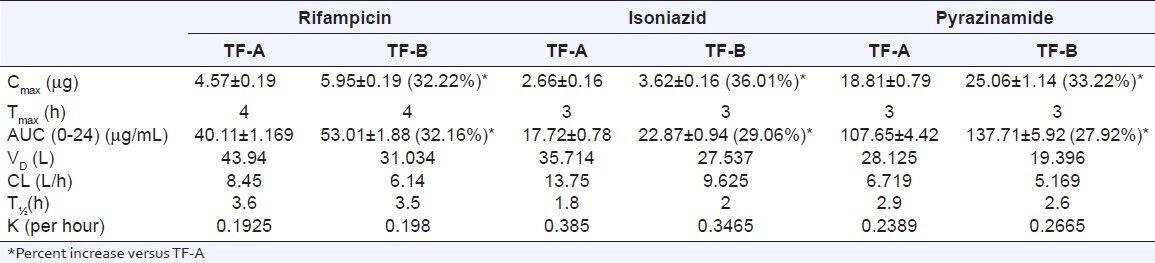
It was observed that in presence of C. carvi extract Cmax of rifampicin increased from 4.57 ± 0.19 to 5.95 ± 0.19 (32.22%) (P = 0.000) and AUC (0-24) from 40.11 ± 1.69 to 53.01 ± 1.88 (32.16%) (P = 0.000), respectively. Whereas volume of distribution (Vd) decreased from 43.94 to 31.03 and clearance (Cl) from 8.45 to 6.14. Tmax remained constant at 4 h. Half-life (t½) was not altered significantly.
The pharmacokinetic parameters of isoniazid showed an increase in Cmax from 2.66 ± 0.16 to 3.62 ± 0.16 (36.01%) (P = 0.000), AUC (0-24) from 17.72 ± 0.78 to 22.87 ± 0.94 (29.06%) (P = 0.000). Volume of distribution (Vd) decreased from 35.71 to 27.54 and clearance (Cl) from 13.75 to 9.62. Tmax was constant at 3 h. And half-life (t½) was not altered significantly.
Cmax of pyrazinamide showed an increase from 18.81 ± 0.79 to 25.06 ± 1.14 (33.22%) (P = 0.000), AUC(0 − 24) increased from 107.65 ± 4.42 to 137.71 ± 5.92 (27.92%) (P = 0.000). Volume of distribution (Vd) decreased from 28.12 to 19.39 and clearance (Cl) from 6.719 to 5.17. Tmax was 3 hrs with both test formulations. Half-life (t½) was not significantly different [Table 2].
Table 2.
Pharmacokinetic parameters of rifampicin, isoniazid, and pyrazinamide with TF-A and TF-B
DISCUSSION
Tuberculosis is a complex socioeconomic disease that apart from its alarming death statistics in developing countries is also a cause of concern for industrialized nations. Its treatment poses many difficulties in the form of poor and variable bioavailability of antitubercular drugs. Of many approaches applied for increasing bioavailability of these drugs, one is use of herbal bioenhancers. Earlier reports in rats indicate bioenhancing potential of C. carvi when it was administered along with rifampicin.[10] A recent report has also shown its bioenhancing action on rifampicin, isoniazid, and pyrazinamide in rats.[11] The current study has indicated bioenhancing potential of C. carvi along with ATT therapy for the first time in humans. Thereby there exists the possibility of ameliorating the dose-related toxicity of ATT by allowing reformulation of dose reduction.
C. carvi has been used since ages for many ailments in different parts of the world. It is a prized culinary herb, which is extensively used across different cultures. This herb has been mentioned in Ayurveda and other Indian systems of medicine prescriptions for a variety of ailments. It has been used as a carminative, stomachic, aromatic, and diuretic.[14]
There has been no study till date using C. carvi as bioenhancer along with antitubercular drugs in humans. The present study is the first of its kind to determine in humans the bioenhancing potential of C. carvi along with antitubercular drugs.
In our study, the various pharmacokinetic parameters were comprehensively studied. The results show that addition of C. carvi extract led to increase in plasma levels of rifampicin, which peaked at 4 h. Similar increase was observed with isoniazid and pyrazinamide levels, which increased in same fashion with peak at 3 h (P < 0.0001) [Table 1].
These observations state clearly that C. carvi acts as a bioenhancer and modifies the kinetics of antitubercular drugs favorably. This increase in the absorption of antitubercular drugs by C. carvi extract could be possibly attributed to the enhancement of mucosal to serosal permeation. Another factor responsible for this action of C. carvi extract could be the modification of permeation characteristics of the intestine. Besides, a possible reason could be its influence on the P-glycoprotein.[11] Moreover, there have been encouraging results on the use of this extract in animals.[11] These probable mechanisms could explain the beneficial effects of C. carvi on the kinetics of antitubercular drugs as revealed in our current study.
The outcome of the present study can be of immense clinical utility while managing patients of tuberculosis. There exists a possibility of developing a reformulated low-dose FDC regimen comprising these drugs. The use of herbal bioenhancer will be immensely useful in formulating dosage regimens for antitubercular drugs, which cause high toxicity on long-term use. Because ours is a preliminary study, which has been done in healthy volunteers, the results need to be carefully extrapolated in the context of patients of tuberculosis. Moreover, the present trial is a single-dose study and because tuberculosis patients require treatment for a long term, it remains to be seen how this bioenhancer behaves in these situations. Therefore, further research is suggested to see the bioavailability effects of C. carvi on antitubercular drugs in patients of tuberculosis.
CONCLUSION
The present study has shown that C. carvi acts as a bioenhancer and modifies the kinetics of antitubercular drugs favorably.
ACKNOWLEDGMENT
We are grateful to Dr. G.N. Qazi (Ex Director IIIM, CSIR, Jammu) and Dr. R.K Johri, Dr. S.C. Sharma, Mr. A. Tickoo, Mr. M. Tickoo, and Mr. S. Bhusari (from IIIM, CSIR, Jammu) for their immense help and support.
Footnotes
Source of Support: Department of Pharmacology IIIM, Canal Road, Jammu.
Conflict of Interest: None declared.
REFERENCES
- 1.Khatri GR, Frieden TR. Controlling tuberculosis in India. N Engl J Med. 2002;347:1420–5. doi: 10.1056/NEJMsa020098. [DOI] [PubMed] [Google Scholar]
- 2.Acocella G. Human bioavailability studies. Bull Int Union Tuberc Lung Dis. 1989;64:38–40. [PubMed] [Google Scholar]
- 3.Pillai G, Fourie PB, Padayatchi N, Onyebujoh PC, McIlleron H, Smith PJ, et al. Recent bioequivalence studies on fixed-dose combination anti-tuberculosis drug formulations available on the global market. Int J Tuberc Lung Dis. 1999;3(Suppl 3):S309–16. [PubMed] [Google Scholar]
- 4.Shishoo CJ, Shah SA, Rathod IS, Savale SS, Vora MJ. Impaired bioavailability of rifampicin in presence of isoniazid from fixed dose combination (FDC) formulation. Int J Pharm. 2001;228:53–67. doi: 10.1016/s0378-5173(01)00831-6. [DOI] [PubMed] [Google Scholar]
- 5.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet. 2001;40:327–41. doi: 10.2165/00003088-200140050-00002. [DOI] [PubMed] [Google Scholar]
- 6.McIlleron H, Wash P, Burger A, Folb P, Smith P. Widespread distribution of a single drug rifampicin formulation of inferior bioavailability in South Africa. Int J Tuberc Lung Dis. 2002;6:356–61. [PubMed] [Google Scholar]
- 7.Singh S, Mariappan T, Sharda N, Kumar S, Chakraborti A. The reason for an increase in decomposition of rifampicin in the presence of isoniazid under acid conditions. Pharmacy Pharmacol Comms. 2000;6:405–10. [Google Scholar]
- 8.Johri RK, Zutshi U. An ayurvedic formulation ‘Trikatu’ and its constituents. J Ethnopharmacol. 1992;37:85–91. doi: 10.1016/0378-8741(92)90067-2. [DOI] [PubMed] [Google Scholar]
- 9.United States Patent Application Publication. 2007. Jan 25, Paged as Bioavailability enhancing activity of Carum carvi extracts and fractions thereof. Pub. No: US 2007/0020347 AI. [Google Scholar]
- 10.Sachin BS, Sharma SC, Sethi S, Tasduq SA, Tikoo MK, Tikoo AK, et al. Herbal modulation of drug bioavailability: Enhancement of rifampicin levels in plasma by herbal products and a flavonoid glycoside derived from Cuminum cyminum. Phytother Res. 2007;21:157–63. doi: 10.1002/ptr.2046. [DOI] [PubMed] [Google Scholar]
- 11.Sachin BS, Monica P, Sharma SC, Satti NK, Tikoo MK, Tikoo AK, et al. Pharmacokinetic interaction of some antitubercular drugs with caraway: Implications in the enhancement of drug bioavailability. Hum Exp Toxicol. 2009;28:175–84. doi: 10.1177/0960327108097431. [DOI] [PubMed] [Google Scholar]
- 12.Niazi S. Textbook of biopharmaceutics and clinical pharmacokinetics. In: Niazi S, editor. Pharmacokinetic principles. New York: Appleton-Century-Crofts; 1979. pp. 141–213. [Google Scholar]
- 13.Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. In: Gibaldi M, Lea, Febiger, editors. Introduction to Pharmacokinetics. 3rd ed. Philadelphia: New York: Raven Press; 1984. pp. 315–6. [Google Scholar]
- 14.Mhaskar KS, Blatter E, Caius JF. Kirtikar and Basu's illustrated Indian medicinal plants. Vol. 5. New Delhi: Satguru Publications; 2000. pp. 1670–1. [Google Scholar]


