Abstract
The present study investigated the effects of a single intravenous (i.v.) dose of Vitamin C (ascorbate, ASC) on spatial memory in APP/PSEN1 mice, an Alzheimer's disease model. First, we confirmed the uptake time course in ASC-depleted gulo (−/−) mice, which cannot synthesize ASC. Differential tissue uptake was seen based on ASC transporter distribution. Liver (SVCT1 & SVCT2) ASC was elevated at 30, 60 and 120 min post-treatment (125 mg/kg, i.v.), whereas spleen (SVCT2) ASC increased at 60 and 120 min. There was no detectable change in cortical (SVCT2 at choroid plexus, and neurons) ASC within the 2-hour interval, although the cortex preferentially retained ASC. APP/PSEN1 and wild type (WT) mice at three ages (3, 9, or 20 months) were treated with ASC (125 mg/kg, i.v.) or saline 45 min before testing on the Modified Y-maze, a two-trial task of spatial memory. Memory declined with age and ASC treatment improved performance in 9 month-old APP/PSEN1 and WT mice. APP/PSEN1 mice displayed no behavioral impairment relative to WT controls. Although dopamine and metabolite DOPAC decreased in the nucleus accumbens with age, and improved spatial memory was correlated with increased dopamine in saline treated mice, acute ASC treatment did not alter monoamine levels in the nucleus accumbens. These data show that the Modified Y-maze is sensitive to age-related deficits, but not additional memory deficits due to amyloid pathology in APP/PSEN1 mice. They also suggest improvements in short-term spatial memory were not due to changes in the neuropathological features of AD or monoamine signaling.
Keywords: aging, Alzheimer's disease, spatial memory, Modified Y-maze, dopamine, APP/PSEN1, vitamin C
1. Introduction
Vitamin C (ascorbate, ASC) is an antioxidant that plays a critical role in the brain, including synthesis of catecholamines [1, 2] and protection from oxidative stress. ASC is accumulated in cells by two isoforms of the sodium-dependent vitamin C transporter (SVCT). SVCT1 is involved in ASC homeostasis and is found primarily in liver, kidney, lung and intestines. The SVCT2 transporter is found in metabolically active tissues such as the brain, spleen, and liver, and protects against oxidative stress [3-5]. ASC does not freely cross the blood-brain barrier and instead cortical levels are maintained by a two-step mechanism whereby ASC is transported from plasma into cerebrospinal fluid in the choroid plexus and then into neurons [6].
Alzheimer's disease (AD) is a progressive neurodegenerative disease that is the most common type of dementia and the sixth-leading cause of death in the United States, with a prevalence of 5.2 million in 2013 [7]. Hallmarks of AD include cognitive impairment, accumulation of amyloid-β (Aβ) deposits, neurofibrillary tangles, elevated oxidative stress and neurotransmitter dysfunction [8-10]. The primary neurotransmitter target for AD treatment has been the cholinergic system, but other reports have suggested abnormalities in multiple neurotransmitters in AD, including monoamines such as dopamine, serotonin and noradrenaline [11, 12]. Plasma ASC levels are decreased in AD patients [13, 14] and increased dietary intake has been suggested to reduce the risk of developing AD [15], as well as to delay the aging process [16]. Given the variety of roles of ASC in the brain, the link between cognition and ASC, as well as a protective mechanism in AD are still unclear.
Previously, we have shown [17] that intraperitoneal (i.p.) administration of ASC (125 mg/kg) reversed some learning and memory deficits (Y-maze alternation rates and Morris water maze) in aged (12 mo) and very old (24 mo) APP/PSEN1 transgenic AD model mice [18-20]. No effects of ASC were found on Aβ, oxidative stress or ASC level in brain or liver after 12 daily treatments. Acetylcholinesterase (AChE) activity was decreased in APP/PSEN1 mice but not altered by ASC treatment. Short-term, pharmacological-like modulation of neurotransmitter function was hypothesized as a potential mechanism, and a subsequent study suggested ASC might act in part by modulating cholinergic signaling, as the same treatment reduced some scopolamine-induced spatial learning deficits in young wild type (WT) mice [21]. Other studies have suggested that ASC can improve memory, although the method of administration was i.p. in each case [22-25]. The effects of intravenous ASC on learning and memory have not been investigated, particularly in an AD model mouse.
ASC concentration in plasma and tissues after oral administration is tightly controlled. Bioavailability declines after single doses of 400 mg or higher, as the SVCT transporters saturate and any remaining ASC is excreted [26, 27]. Parenteral ASC administration bypasses the tight control and can increase plasma concentrations up to 70-fold [28]. Both intraperitoneal and intravenous administration of ASC lead to large and rapid increases in plasma ASC, although intravenous is faster and produces larger concentrations [29, 30].
The goal of the present study was to determine if the more rapid intravenous ASC administration could improve learning in aged APP/PSEN1 mice and WT controls. The experiment was designed to avoid repeated intravenous treatments and therefore limit any restraint stress and potential damage to veins limiting the effectiveness of injections, so a oneday memory task was chosen. The Modified Y-maze [31, 32] is a two-trial, hippocampus-dependent test of spatial memory [33]. This task involves free exploration and recognition of novelty, which are associated with dopamine signaling [34]. In addition, monoaminergic neurodegeneration is found in APP/PSEN1 mice [11], and ASC is critically involved in dopamine synthesis. Therefore, this study examined the relationship between dopamine, ASC and spatial memory in aged AD model mice and WT controls. AD neuropathology (Aβ) was also examined. Before assessing the effects of intravenous ASC on behavior, the time course of ASC uptake into tissues following intravenous administration was explored using ASC-depleted gulo (−/−) mice, which cannot synthesize ASC and require dietary supplementation to survive [35]. In the present study, young adult (3 mo), middle-aged (9 mo) and very old (20 mo) APP/PSEN1 mice and WT controls were given intravenous treatment of ASC or saline and tested on the Modified Y-maze. Following behavioral testing, a final treatment (ASC or saline) was administered and brain tissue, liver and spleen were assessed for changes in ASC and monoamine levels.
2. Material and methods
2.1. Subjects
Heterozygous gulo (+/−) mice were originally obtained from Mutant Mouse Regional Resource Centers (http://www.mmrrc.org, #000015-UCD) and were maintained on a C57BL/6J background (stock #000664; Jackson Laboratories, Bar Harbor, ME, USA). Gulo (−/−) mice were bred in house. These mice lack a functional copy of the gulonolactone oxidase gene responsible for the final step in ASC synthesis and are dependent on dietary intake of ASC [35]. Wild type-equivalent levels of ASC in tissues are maintained by providing de-ionized drinking water with 0.33 g/L ASC and 20 μl 0.5 M EDTA per liter to help maintain stability of ASC. APPSwe/PSEN1ΔE9 bigenic mice were obtained from Jackson (stock #005864) and maintained as double hemizygotes by crossing with wild type individuals on a C57BL/6J background strain (Jackson Laboratories stock # 000664). APP/PSEN1 bigenic mice harbor the Swedish double-mutation (K595N/M596L) in the APP gene, and a deletion in exon 9 of the PSEN1 gene. The original mice, maintained on a hybrid C3HeJ x C57BL/6J background, exhibit amyloid aggregation and amyloid-related neuropathology and cognitive decline, and age- and genotype-dependent cholinergic dysfunction [36-39]. APP/PSEN1 mice with C57BL/6 background also show amyloid accumulation [40, 41]. Mice were group-housed by sex in standard tub cages (26.5 × 17 × 12 cm) with fiber bedding under a 12/12-h light/dark cycle (lights on at 0600 h), with free access to food and water. 22 adult (3 mo) gulo (−/−) mice were used in Experiment 1 (10 male, 12 female). For Experiment 2, 38 (16 male, 22 female) wild type (WT) and 36 APP/PSEN1 (17 male, 19 female) mice were tested at one of three ages: 3 (M = 3.48, SD = 0.60), 9 (M = 9.43, SD = 0.43) or 20 mo (M = 19.99, SD = 0.66). Genotypes for all mice were confirmed using polymerase chain reaction (PCR). All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. ASC treatments
Gulo (−/−) mice were provided with 0.33 g/L ASC supplemented water at weaning. For seven weeks prior to the experiment, mice were given minimal supplementation (0.033g/L ASC) and depletion was ensured by providing non-supplemented tap water for five days preceding i.v. ASC treatment. Sodium ascorbate (125 mg/kg; Sigma, St. Louis, MO, USA) was dissolved in de-ionized water and injected via tail vein at a volume of 200 μL. Mice were euthanized 30, 60 or 120 min post-treatment (5-6 per group). Control mice received no treatment. Brain, liver and spleen were dissected and frozen at −80°C.
APP/PSEN1 and WT mice received an i.v. injection (125 mg/kg ASC or saline) 45 min before testing on the Modified Y-maze. A within-subjects design was used where each mouse received both a saline and ASC injection (separated by at least one week) and was tested twice on the Modified Y-maze.
2.3. Behavioral testing
The Modified Y-maze is a two-trial spatial memory task that uses an apparatus constructed of clear plastic with three arms (34 × 6.2 × 10 cm, L × W × H). Retention in the Modified Y-maze does not last more than a few hours [32] so mice could be tested twice without any carryover effects. Testing took place using the same apparatus in two different rooms with different distal and proximal cues. The treatment order, as well as injection-room pairings were randomized and counterbalanced. Black and white paper shapes were positioned approximately 5 cm from each vertex to serve as salient extra-maze cues and the characteristics of the testing room (tables, chairs, shelves and sink) were also visible to the mice. During Trial 1 (Training), one arm of the maze, chosen at random, was blocked and the mouse was placed at the end of one of the two other arms, with its head facing away from the center of the maze, and allowed to explore for 5 min. After a 5 min ITI the mouse was returned to the start arm of the maze and allowed to explore all three arms for 5 min (Trial 2; Retention). Recording of Trial 2 began when the mouse had left the start arm. The start and blocked arms were counterbalanced across groups. The entire maze was cleaned with 10% ethanol between trials and mice to minimize odor cues. The time in each arm, distance traveled and number and duration of visits to each arm was recorded by the ANY-Maze software (Stoelting, Wood Dale, IL, USA). Preferential exploration of the novel arm (blocked arm in Trial 1) was used as an indicator of spatial memory. All behavioral testing took place in the facilities of the Vanderbilt Neurobehavioral Core. One final treatment (saline or ASC, counterbalanced) was administered 45 min before mice were euthanized. Brain, liver and spleen were dissected and frozen at −80°C.
2.4. Measurement of Aβ42
Hippocampal Aβ42 was quantified in a randomly selected subset of mice at each age (APP/PSEN1 n = 6, WT n = 4) using a sandwich enzyme-linked immuno-sorbent assay (ELISA) kit (Life Technologies, Grand Island, NY, USA). As genotype had already been confirmed with PCR, only a subset of mice was used for Aβ42 quantification. Briefly, samples (5-10 mg) were homogenized using BSAT-DPBS with 1× protease inhibitor. Equal aliquots of raw homogenate were used to extract soluble and total Aβ. Soluble Aβ42 was extracted using cold 0.4% (v/v) diethylamine in 100mM NaCl and total Aβ42 was extracted using cold 5M guanidine in 50 mM Tris HCl. Protein concentration was measured using a Nanodrop 2000 (Thermo Scientific, Asheville, NC, USA) using BSA as a standard. Prior to the ELISA, samples were diluted to 100 μg/100 μl using the provided standard diluent buffer. Samples for older mice were further diluted (1:3 for 9 mo and 1:5 for 20 mo). Each sample was measured in duplicate and compared to a linear standard curve generated with known concentrations of human Aβ42 using a Synergy H4 plate reader (BioTek, Winooski, VT, USA) with an absorbance of 450 nm.
2.5. Measurement of ascorbate
ASC content of cortex, liver and spleen was determined by HPLC according to previously published methods [21, 42, 43] using the Dionex Ultimate 3000 and Coulochem III for ion pair HPLC with electrochemical detection (Thermo Scientific). Tissue was processed for all mice, with approximately half in each group receiving a final, pre-euthanasia treatment of either saline or 125 mg/kg ASC. Briefly, weighed tissue samples were homogenized in a combination of two solutions, 25% (w/v) aqueous metaphosphoric acid and 100 mM sodium phosphate buffer containing 5 mM EDTA, with a final ratio of 2:7. Samples were centrifuged and the supernatant was taken for assay of ASC as described above following appropriate dilution with deionized water.
2.6. Measurement of neurotransmitters and metabolites
The nucleus accumbens was isolated by taking a tissue slice between approximately 0.66 and 1.66 mm Bregma, according to a mouse brain atlas [44] using a Zivic Instruments Mouse Brain Slicer Matrix (Pittsburgh, PA, USA). One mm punches were taken from each hemisphere and frozen at −80°C. Catecholoamines, serotonin and their metabolites were quantified using HPLC by the Vanderbilt University Neurochemistry Core.
2.7. Statistical analysis
All data were analyzed using IBM SPSS Statistics Version 22 for Mac. Modified Y-maze data were analyzed using the repeated measures Analysis of Variance (ANOVA) to compare the effects of age, genotype and treatment on time spent in the novel arm. Neurochemical analyses were conducted using separate univariate ANOVAs. When appropriate, Bonferonni post-hoc tests were used. Prior to analysis of behavioral testing, data were examined for outliers. Mice either identified in SPSS as statistical outliers (performance scores at least 1.5 times greater than the interquartile range) or that received poor or incomplete injections were excluded (one 3 mo, six 9 mo and six 20 mo mice). Final group sizes for behavior were: 3 mo WT (n = 8), 3 mo APP/PSEN1 (n = 9), 9 mo WT (n = 5), 9 mo APP/PSEN1 (n = 4), 20 mo WT (n = 17), 20 mo APP/PSEN1 (n = 18). Initial analyses included sex as an additional between groups factor. No differences were found according to sex so data were collapsed and analyzed together.
3. Results
3.1. Experiment 1: Time course of IV ASC uptake
Time course uptake was performed in gulo (−/−) mice that were not also subject to behavioral testing. ASC levels in the liver increased significantly beginning at 30 min post-treatment (F(3, 18) = 189.70, p < .001) and remained elevated through 120 min (ps < .001, Figure 1A). Liver ASC values in ASC-treated gulo (−/−) were much larger than WT values at all time points examined. Less robust, but statistically significant increases in spleen (SVCT2 only) ASC were found (F(3, 18) = 14.33, p < .001), in addition to a slower uptake time course (Figure 1B). Spleen ASC levels were significantly elevated at 60 (p < .01) and 120 (p < .001) min post-treatment, but did not approach WT levels. There was no significant elevation in cortical ASC after treatment, although a numerical increase was seen at 120 min (Figure 1C). Cortical ASC levels were larger compared to the liver and spleen (but much smaller than WT levels) in depleted mice receiving no treatment, confirming that the brain preferentially retains ASC during periods of prolonged deprivation.
Figure 1.
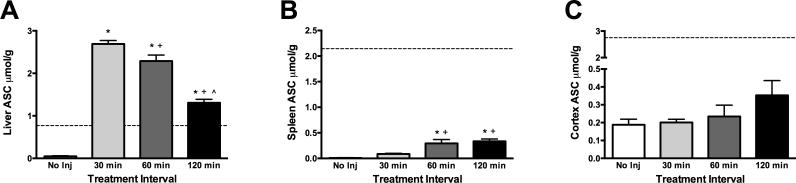
Intravenous ASC uptake time course in ASC-depleted gulo (−/−) mice. A) Liver ASC increased significantly above WT levels beginning 30 min post-treatment and remained elevated through 120 min. B) Spleen ASC did not return to WT levels but was significantly increased at 60 and 120 min post-treatment. C) Cortical ASC was not significantly increased at any time following treatment, although a slight increase was seen at 120 min. Group sizes were 5-6 for all analyses. Values are mean ± SEM. *different from No Inj; +different from 30 min; ^different from 60 min. -------- indicates ASC level of WT mice or gulo (−/−) mice supplemented with 1.0 g/L ASC.
3.2. Experiment 2: IV ASC improves spatial memory in middle-aged mice
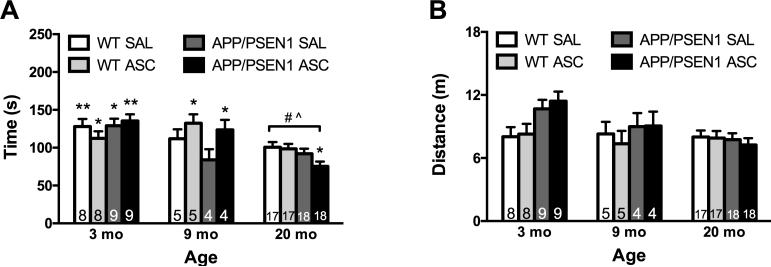
Overall, the oldest mice performed more poorly on the Modified Y-maze, spending less time exploring the novel arm during the retention trial (F(2, 55) = 17.65, p < .001). Post-hoc tests confirmed that mice aged 20 months were significantly impaired relative to mice aged 3 (p < .001) and 9 (p < .05) months (Figure 2A). Investigation of an Age x Treatment interaction revealed that ASC treatment improved spatial memory in 9 month-old mice (p < .05). No main effects of genotype were reported, as APP/PSEN1 mice were not significantly impaired at any age tested. In addition, we confirmed age- and treatment-related effects using multiple One-Sample T Tests. Performance in each group was compared to chance (100 sec). All of the young mice performed significantly above chance (although p = .053 for WT ASC mice), and only ASC-treated middle-aged mice performed significantly above chance. None of the oldest mice were significantly different from chance, except for the APP/PSEN1 ASC group, which was significantly below 100 sec. There were no significant effects of first treatment (saline or ASC) or testing room during the retention trial.
Figure 2.
Intravenous ASC improved short-term spatial memory in APP/PSEN1 and WT mice aged 9 months. A) The time spent exploring the novel arm of the Modified Y-maze decreased significantly with age as mice aged 20 months were significantly impaired relative to mice aged 3 (p < .001) and 9 months (p < .05). ASC treatment significantly improved spatial memory in 9 month-old APP/PSEN1 and WT mice (p < .05). No other effects of genotype or ASC treatment were reported. B) Total exploration was not affected by ASC treatment, although 3 month-old mice covered significantly more distance than 20 month-old mice (p < .05). This effect was mainly due to elevated activity in 3 month-old APP/PSEN1 mice. Values are mean ± SEM. Numbers on bars indicate group size. * p < .05; ** p < .01 in a One-Sample T Test comparing time in the novel arm to chance (100 sec). # p < .001 compared to all 3 mo mice; ^ p < .05 compared to all 9 mo mice.
Total exploration of the maze during the retention trial was not affected by ASC treatment, although a significant effect of age on total distance traveled was found (F(2, 55) = 3.88, p < .05, Figure 2B). Post-hoc tests revealed that 3 month-old mice covered significantly more distance than 20 month-old mice (p < .05), although this effect was likely driven by increased distance traveled in 3 month-old APP/PSEN1 mice (age x genotype interaction: F(2, 55) = 3.68, p = .05). Similar results were found when comparing distance traveled during the training trial—there was a significant effect of age (F(2, 55) = 11.27, p < .001) as 3 month-old mice covered significantly more distance than mice aged 20 months (p < .001). Again, the effect of age was driven by increased distance traveled in young APP/PSEN1 mice (data not shown).
3.3. Measurement of Aβ42
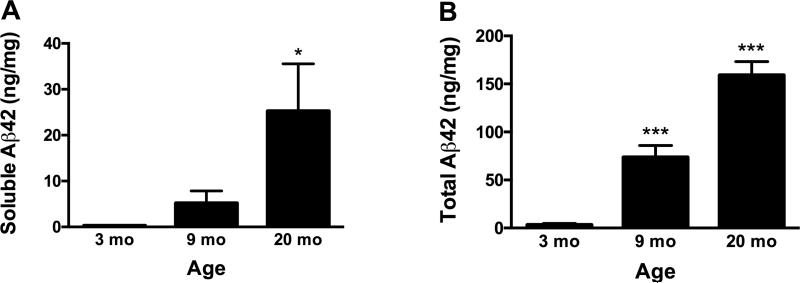
Aβ42 was measured in the hippocampus of 6 APP/PSEN1 and 4 WT mice from each age group. As WT mice have no human Aβ, the minimal amount of binding seen in the ELISA was considered background, and the average WT values were subtracted from APP/PSEN1 values. Soluble Aβ42 increased with age as expected (F(2, 12) = 3.96, p < .05; Figure 3A). Total Aβ42 increased significantly with age in APP/PSEN1 mice (F(2, 12) = 90.35, p < .001) and was significantly elevated at 9 (p < .001) and 20 (p < .001) months compared to 3 month-old mice (Figure 3B). The effect of final ASC treatment on total Aβ42 was significant, as ASC treated mice had significantly less Aβ42 (F(1, 12) = 9.2, p < .05). This result likely reflects the variability in total Aβ42 measured (9 mo range: 38.9-120.4 ng/mg protein; 20 mo range: 106.6-203.2 ng/mg protein), and does not indicate that ASC treatment 45 min before euthanasia significantly altered total hippocampal Aβ42. Therefore, data are presented by age group, collapsed across treatment groups.
Figure 3.
Hippocampal Aβ42 increased with age in APP/PSEN1 mice. A) Soluble Aβ42 increased with age, with significantly elevated amounts in mice aged 20 months (p = .05). B) Total Aβ42 was significantly elevated at 9 (p < .001) and 20 (p < .001) months compared to 3 months. ASC treated mice had significantly less Aβ42 (p < .05), though this result was likely due to variability in total Aβ42 measurements among mice. Values are mean ± SEM. n = 6 for each age group. * p < .05 compared to 3 mo; *** p < .001 compared to 3 mo.
3.4. Neurochemical analyses in APP/PSEN1 and WT mice
3.4.1. ASC
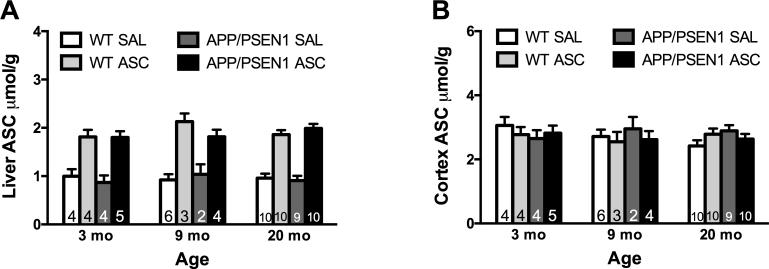
Intravenous treatment with ASC 45 min prior to euthanasia led to significant elevation in the liver in all ages and genotypes (F(1, 59) = 146.32, p < .001) but did not affect cortical levels of ASC (Figure 4A-B). Neither age nor genotype affected liver or cortical ASC levels. 3.4.2. Neurotransmitters and metabolites
Figure 4.
Intravenous ASC increased liver but not cortical ASC. A) Liver ASC was significantly increased 45 min post-treatment (p < .001). Overall, liver ASC was stable from 3-20 months of age. No genotype effects were reported. B) Cortical ASC levels were not altered by age, genotype or treatment. Values are mean ± SEM. Numbers on bars indicate group size.
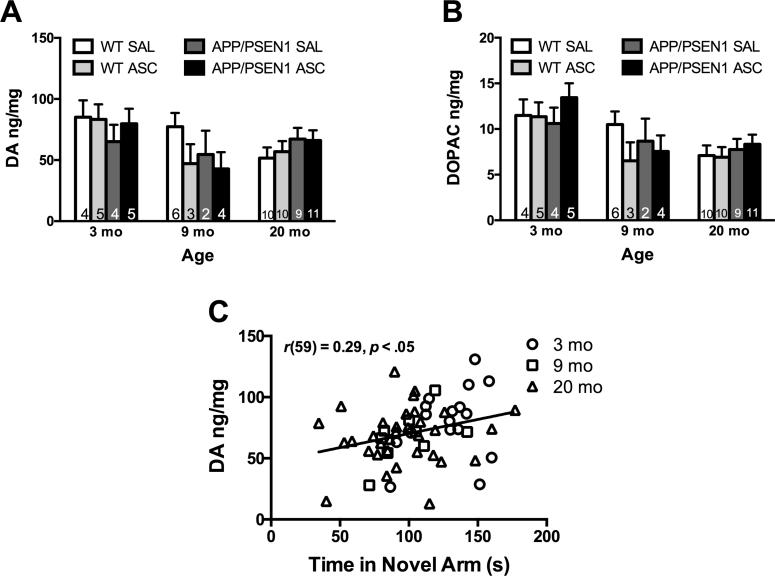
The amount of dopamine and its metabolite DOPAC decreased in the nucleus accumbens with age (dopamine F(2, 61) = 3.37, p < .05; DOPAC F(2, 61) = 8.96, p < .001, Figure 5A-B). Dopamine was lowest in 20 month-old mice and DOPAC was significantly lower in mice aged 9 (p < .05) and 20 months (p < .001) compared to 3 month-old mice. As there were no significant effects of the final ASC treatment on dopamine or DOPAC, all mice were considered in a comparison of dopamine and baseline spatial memory ability. In mice treated with saline 45 min before behavioral testing (collapsed across age), the amount of dopamine in the nucleus accumbens correlated with increased time in the novel arm (r(59) = 0.29, p < .05, Figure 5C), indicating an association between better memory and more dopamine. No other age-, genotype-, or treatment-related changes were found in dopamine metabolite HVA, serotonin or metabolite 5-HIAA.
Figure 5.
Changes in dopamine in the nucleus accumbens with age. A) Dopamine decreased with age (p < .05) but was not affected by genotype or ASC treatment. B) Dopamine metabolite DOPAC was significantly lower in mice aged 9 (p < .05) and 20 (p < .001) months compared to 3 month-old mice. The amount of DOPAC was not affected by genotype or ASC treatment. C) In saline treated mice, larger amounts of dopamine correlated significantly with better performance on the Modified Y-maze (p < .05). Values are mean ± SEM. Numbers on bars indicate group size.
4. Discussion
In the present study, acute intravenous administration of ASC led to significant increases in circulating and tissue ASC level beginning at 30 (liver) or 60 (spleen) min post-treatment but did not alter cortical ASC within the 2 hour period studied. In addition, a single intravenous treatment of ASC reversed short-term age-related spatial memory deficits in middle-aged (9 mo), but not old (20 mo) APP/PSEN1 and WT mice. ASC treatment effects were independent from changes in AD neuropathology or monoaminergic signaling.
In Experiment 1 we confirmed the time course of ASC uptake in ASC-depleted gulo (−/−) mice. Depleted mice that received no ASC treatment had virtually undetectable ASC content in the liver and spleen. ASC levels in the cortex were much lower than normal, but remained elevated compared to the liver and spleen. These results confirmed the preferential retention of ASC in the brain relative to other organs [45, 46]. The larger increase in the liver compared to spleen reflects the different distribution of ASC transporters, as well as the blood content of the two organs. Both the liver and spleen are filtration units for circulating blood, but the liver contains both SVCT1 and SVCT2, whereas the spleen contains only SVCT2 [3, 5]. SVCT1 has a higher capacity than SVCT2 [3], which allowed for a larger increase in ASC in the liver compared to the spleen. The delayed uptake time course in the cortex (slight increase in ASC at 120 min post-treatment) confirmed the slower, two-step SVCT2 mechanism, from blood into CSF at the choroid plexus, and then into neurons [6].
Although we did not find a detectable increase in stored ASC in Experiment 1, the time course for Experiment 2 was chosen based on therapeutic windows observed in previous studies using intraperitoneal administration [17, 29] and findings from Experiment 1 showing increased circulating ASC from 30-120 min after intravenous treatment. In addition, the time course of Experiment 2 was chosen so that both training and retention trials of the Modified Y-maze could be administered within this time frame. We report an age-related deficit in short-term spatial memory in middle-aged (9 mo) and old (20 mo) APP/PSEN1 and WT mice. The Modified Y-maze task has been used extensively with rats but also has been validated in number of inbred and hybrid mouse strains [31, 47, 48]. C57BL/6 mice (APP/PSEN1 background strain in the present study) show robust exploration of novelty and learning on the task. Age-related impairment in the Modified Y-maze has been reported in high responder rats [49], but few studies exist comparing this task in aging mice [50, 51].
We have confirmed that the Modified Y-maze is sensitive to age-related changes in APP/PSEN1 and WT mice; however, no genotype-specific impairment was detected. Young APP/PSEN1 mice displayed increased activity during training and retention trials; however, this result has not been reported in other studies using APP/PSEN1 AD model mice [52, 53]. These findings may not be meaningful and do not alter the interpretation of the retention trial, as additional exploration in young APP/PSEN1 mice did not lead to improved performance on the task. Importantly, acute ASC treatment did not affect overall exploration at any age tested. Few Modified Y-maze studies using AD model mice exist. Age- and genotype-related impairment has been reported at 16 months in TGF-β1 mice, a model for cerebral amyloidosis [54], although no genotype-related impairment has been reported up to 8-12 months in APP/PSEN1 mice [55]. APP/PSEN1 mice show spatial learning deficits in other tasks such as the Morris water maze [21, 37] and it is possible that increasing task difficulty in the present study may have revealed more robust genotype effects [56-58]. Increasing the ITI in the present study would have increased difficulty and perhaps revealed more subtle genotype-related differences, particularly since retention times of up to 2 hours have been reported in young C57BL/6 mice [31]. However, the experiment was designed so that mice would be under the effects of ASC treatments during both encoding and retention trials and longer ITIs may have required additional treatments.
ASC treatments in Experiment 2 replicated findings from Experiment 1 in depleted gulo (−/−) mice. At 45 min post-treatment, liver levels reflected the increased circulating ASC due to intravenous administration and cortical levels were unchanged. In addition, no age-related changes in cortical or liver ASC were reported. Stability of cortical ASC is frequently reported [59], though reports of depletion with age usually compare immature young animals to old animals [60]. The present study confirmed this notion, as no change was found when comparing young (but mature), middle-aged and old mice. Given the small cortical changes detected in the depleted gulo (−/−) mice, we did not expect to find detectable changes in ASC in the WT and APP/PSEN1 mice in this study. Our data are in line with two earlier studies in our lab that suggested AD model mice do not show differences in baseline ASC levels [17, 61] because mice can synthesize their own ASC and thus are able to maintain consistently high ASC levels. Other studies using AD model mice and ASC do not always report baseline ASC levels [62].
The Modified Y-maze task depends on the hippocampus [33] and several neurotransmitter systems have been implicated in behavior on this task, such as GABA [63], acetylcholine and glutamate [64]. Dopamine is often associated with spatial learning [65, 66] and our results confirmed that dopamine is involved in spatial learning assessed by the Modified Y-maze in APP/PSEN1and WT mice. In saline treated mice, improved spatial memory was associated with increased dopamine in the nucleus accumbens. These results suggest that whereas dopamine is important for the behavior assessed by the Modified Y-maze, it does not appear to be the mechanism by which ASC was effective.
Several potential mechanisms by which ASC could improve learning and memory were investigated in the present study. APP/PSEN1 mice had increased soluble and total Aβ42 with age, though we did not report impairment specific to the APP/PSEN1 genotype. Mice receiving a final ASC treatment were found to have decreased amounts of Aβ42, but it is unlikely that this result suggests a significant clearance of Aβ42 45 min after an acute treatment with ASC. The more probable explanation is that this result is due to the variability of Aβ42 deposits with age in APP/PSEN1 mice. We also examined the amount of monoamines—dopamine, serotonin and norepinephrine—and their metabolites in the nucleus accumbens across age and genotype. We reported age-related decreases in dopamine and metabolite DOPAC, but no changes in any neurotransmitter or metabolite as a function of ASC treatment. ASC is directly involved in catecholamine and serotonin synthesis [2]; however, the present results suggest that a mechanism for ASC-induced short-term spatial memory improvement does not involve dopamine or other monoamines. Low statistical power may have complicated interpretation of null neurochemical findings in Experiment 2, especially given the small sample sizes for 3 and 9 month-old mice. Based on the time course data from Experiment 1, though, it appears that any potential changes in neurotransmitter function may not have been captured at our 45 min interval, as brain levels of ASC had not significantly increased at this point. The Meredith and May study used embryos from genetically modified mice that had permanently increased and decreased ASC in brain. It is possible that such changes occur in long term ASC deficiency such as lack of dietary intake but that was not assessed in this study
Other potential mechanisms were not investigated in the current study. Our previous work suggested the cholinergic system might be implicated [21]; however, the time course data from the present study suggests changes in the cholinergic system would also not be detected at the current treatment interval. Another possible mechanism involves the effects of ASC on blood flow. ASC facilitates endothelial- and nitric oxide-dependent vasodilation [67, 68]. Vasodilation increases blood flow and provides additional nutrients and neuroprotective factors, which could allow for improved spatial processing through a highly metabolically active structure like the hippocampus. Similar effects are seen with physical exercise-induced increases in blood flow, including changes in neuroprotective factors such as brain-derived neurotrophic factor (BDNF), which have been found to mediate spatial memory improvement [69-71]. In normal aging, degradation and dysfunction in the cardiovascular system is found in humans [72]. Interestingly, a multi-nutrient diet (including ASC) was found to increase cerebral blood flow in APP/PSEN1 mice, which display reduced blood flow compared to WT controls [73], and improved search and swim efficiency in the Morris water maze [74].
ASC is also important for maintaining the general function of the blood brain barrier (BBB) [75, 76]. Breakdown or disruptions of the BBB are found in normal aging [77] and AD [78, 79] and are associated with memory loss [80, 81]. ASC has been found to tighten junctions between endothelial cells such as those that form the BBB, especially in cases of additional oxidative stress [82, 83]. Though we did not report any additional impairment in our APP/PSEN1 mice, it appears plausible that the benefits of ASC in middle-aged mice may be due to increased blood flow.
In the present study we expected to see detrimental effects of AD pathology and to determine if ASC could rescue AD-related deficits. We found no additional impairment in APP/PSEN1 mice but instead report that ASC improved learning in normally aging mice. Normal and pathological aging do not involve identical processes, and have different outcomes at a behavioral and neuroanatomical level. For example, normal aging does not include gross neuron loss in the hippocampus [84], whereas in AD there is a massive loss of neurons [85]. Normal aging in the hippocampus is associated with alterations in synapses [86] as well as reduced neurogenesis [87]. In addition, a different pattern of deficits is seen in normal aging and AD when using eyeblink classical conditioning [88]. There is great interest in identifying mechanisms specific to normal, rather than pathological, aging [89] and behavioral tasks sensitive to age-related memory impairment. Interestingly, our Modified Y-maze task was found to be more sensitive to normal aging and not AD, and ASC treatment did not affect AD pathology. Therefore, the Modified Y-maze may be better suited for studies of normal aging.
A final issue to consider is the absence of impairment in our APP/PSEN1 mice. At both 9 and 20 months of age, these mice had accumulated Aβ42 in the hippocampus but no additional decreases in dopamine or metabolites in the nucleus accumbens, despite reports of degeneration of VTA dopaminergic neurons that project to the forebrain [11]. There is considerable controversy regarding the presence and/or timing of learning and memory deficits in APP/PSEN1 mice [90]. There have been several reports of unimpaired spatial learning ability in AD model mice [91-93]. Within APP/PSEN1 mice the background strain can vary, including C57BL/6 [40, 41] and B6C3 hybrid mice [18, 19]. It is possible that the absence of a behavioral deficit in the present study may be due to the C57BL/6 background strain. C57BL/6 mice, in particular, have been reported to be robust learners in strain comparisons of spatial learning tasks [94, 95] and these findings may be due to differences in hippocampus physiology [96]. A strong learning ability from the background strain may obscure or delay the appearance of deficits. Therefore, genotype- or age-related comparisons may be complicated by changes in the appearance of learning and memory deficits.
The present study showed that ASC could improve short-term spatial memory in middle-aged (9 mo) mice; however, the action of ASC at 9 months is no longer sufficient to improve memory by 20 months of age. Our data suggest that middle-aged mice perform at the level of younger mice with a single intravenous ASC treatment but that any benefit of ASC on short-term spatial memory is independent of changes in Aβ42 burden or monoamine signaling. The precise mechanism for the beneficial effects of ASC and the differences between 9 and 20 month-old mice that render ASC ineffective still need to be investigated. In addition, our data have shown that the Modified Y-maze is a useful task for aging- and dopamine-related studies. As intravenous ASC is sometimes used as a treatment in cancer patients [97, 98] at doses of 1000 mg or more, our results using a dose relevant to and tolerated by humans (approximately 600 mg in a 60 kg person) [99] suggest that the cognition enhancing effects of intravenous ASC require further exploration.
Highlights.
Time course of ASC uptake varied among organs in ASC-depleted mice following IV ASC
IV ASC improved short-term spatial memory in middle-aged APP/PSEN1 and WT mice
Dopamine decreased with age and was correlated with poorer spatial memory
ASC effects were independent of beta-amyloid neuropathology and monoamine levels
Acknowledgments
This research was supported by NIH grant AG038739 to FEH. We thank Clare Gamlin, Shilpy Dixit and Lisa Moore for their contributions to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Englard S, Seifter S. The biochemical functions of ascorbic acid. Ann Rev Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 2.Meredith ME, May JM. Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid. Brain Res. 2013;1539:7–14. doi: 10.1016/j.brainres.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347–55. doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- 4.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–7. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 5.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X, Wang Y, et al. A family of mammalian Na+-dependentL-ascorbic acid transporters. Nature. 1999;399:70–5. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 6.Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res. 2007;85:1046–56. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- 7.Alzheimer's Association Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:1–68. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 9.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–9. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 10.Shah RS, Lee HG, Xiongwei Z, Perry G, Smith MA, Castellani RJ. Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother. 2008;62:199–207. doi: 10.1016/j.biopha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, et al. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2008;28:13805–14. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyness SA, Zarow C, Chui HC. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging. 2003;24:1–23. doi: 10.1016/s0197-4580(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 13.Charlton KE, Rabinowitz TL, Geffen LN, Dhansay MA. Lowered plasma vitamin C, but not vitamin E, concentrations in dementia patients. J Nutr Health Aging. 2004;8:99–107. [PubMed] [Google Scholar]
- 14.Riviere S, Birlouez-Aragon I, Nourhashemi F, Vellas B. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749–54. doi: 10.1002/(sici)1099-1166(1998110)13:11<749::aid-gps860>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements. Arch Neurol. 2004;61:82–8. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Ames BN. Micronutrients prevent cancer and delay aging. Toxicol Lett. 1998;102-103:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 17.Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav. 2009;93:443–50. doi: 10.1016/j.pbb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borchelt DR, Ratovitski T, Van Lare J, Lee MK, Gonzales VB, Jenkins NA, et al. Accelerated amyloid deposition in the brains of transgenic mice co-expressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–45. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 19.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–70. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 20.Lee MK, Borchelt DR, Kim G, Thinakaran G, Slunt HH, Ratovitski T, et al. Hyperaccumulation of FAD-linked presenilin 1 variants in vivo. Nat Med. 1997;3:756–60. doi: 10.1038/nm0797-756. [DOI] [PubMed] [Google Scholar]
- 21.Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res. 2009;205:550–8. doi: 10.1016/j.bbr.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Angelis L, Furlan C. The effects of ascorbic acid and oxiracetam on scopolamine-induced amnesia in a habituation test in aged mice. Neurobiol Learn Mem. 1995;64:119–24. doi: 10.1006/nlme.1995.1050. [DOI] [PubMed] [Google Scholar]
- 23.Parle M, Dhingra D. Ascorbic Acid: a promising memory-enhancer in mice. J Pharmacol Sci. 2003;93:129–35. doi: 10.1254/jphs.93.129. [DOI] [PubMed] [Google Scholar]
- 24.Pettenuzzo LF, Schuck PcF, Fontella F, Wannmacher CMD, Wyse ÂT, Dutra-Filho CS, et al. Ascorbic acid prevents cognitive deficits caused by chronic administration of propionic acid to rats in the water maze. Pharmacol Biochem Behav. 2002;73:623–9. doi: 10.1016/s0091-3057(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 25.Shahidi S, Komaki A, Mahmoodi M, Atrvash N, Ghodrati M. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull. 2008;76:109–13. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93:3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98:9842–6. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Intern Med. 2004;140:533–8. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104:8749–54. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009;47:32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Dellu F, Contarino A, Simon H, Koob GF, Gold LH. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem. 2000;73:31–48. doi: 10.1006/nlme.1999.3919. [DOI] [PubMed] [Google Scholar]
- 32.Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588:132–9. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- 33.Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine treatment. Behav Neurosci. 1996;110:1321–34. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–31. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 35.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000;97:841–6. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, et al. Elimination of GD3 synthase improves memory and reduces amyloid-β plaque load in transgenic mice. Neurobiol Aging. 2009;30:1777–91. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Lalonde R, Kim HD, Maxwell JA, Fukuchi K. Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+PS1/DeltaE9 mice with amyloid plaques. Neurosci Lett. 2005;390:87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Machova E, Jakubík J, Michal P, Oksman M, Iivonen H, Tanila H, et al. Impairment of muscarinic transmission in transgenic APPswe/PS1dE9 mice. Neurobiol Aging. 2008;29:368–78. doi: 10.1016/j.neurobiolaging.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 40.Aso E, Lomoio S, Lopez-Gonzalez I, Joda L, Carmona M, Fernandez-Yague N, et al. Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer's disease. Brain Pathol. 2012;22:636–53. doi: 10.1111/j.1750-3639.2011.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malm TM, Iivonen H, Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Kanninen K, et al. Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden. J Neurosci. 2007;27:3712–21. doi: 10.1523/JNEUROSCI.0059-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha- tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–9. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 43.Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- 44.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed. Academic Press; New York: 2008. [Google Scholar]
- 45.Lykkesfeldt J, Perez Trueba G, Poulsen HE, Christen S. Vitamin C deficiency in weanling guinea pigs: differential expression of oxidative stress and DNA repair in liver and brain. Br J Nutr. 2007;98 doi: 10.1017/s0007114507787457. [DOI] [PubMed] [Google Scholar]
- 46.Harrison FE, Green RJ, Dawes SM, May JM. Vitamin C distribution and retention in the mouse brain. Brain Res. 2010;1348:181–6. doi: 10.1016/j.brainres.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bekker A, Shah R, Quartermain D, Li YS, Blanck T. Isoflurane preserves spatial working memory in adult mice after moderate hypoxia. Anesth Analg. 2006;102:1134–8. doi: 10.1213/01.ane.0000198637.36539.c1. [DOI] [PubMed] [Google Scholar]
- 48.Rayatnia F, Javadi-Paydar M, Allami N, Zakeri M, Rastegar H, Norouzi A, et al. Nitric oxide involvement in consolidation, but not retrieval phase of cognitive performance enhanced by atorvastatin in mice. Eur J Pharmacol. 2011;666:122–30. doi: 10.1016/j.ejphar.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Dellu F, Mayo M, Vallee M, Le Moal M, Simon H. Reactivity to novelty during youth as a predictive factor of cognitive impairment in the elderly: a longitudinal study in rats. Brain Res. 1994;653:51–6. doi: 10.1016/0006-8993(94)90371-9. [DOI] [PubMed] [Google Scholar]
- 50.Albiston AL, Fernando RN, Yeatman HR, Burns P, Ng L, Daswani D, et al. Gene knockout of insulin-regulated aminopeptidase: loss of the specific binding site for angiotensin IV and age-related deficit in spatial memory. Neurobiol Learn Mem. 2010;93:19–30. doi: 10.1016/j.nlm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Da Silva Costa V, Duchatelle P, Boulouard M, Dauphin F. Selective 5-HT6 receptor blockade improves spatial recognition memory and reverses age-related deficits in spatial recognition memory in the mouse. Neuropsychopharmacology. 2009;34:488–500. doi: 10.1038/npp.2008.94. [DOI] [PubMed] [Google Scholar]
- 52.Lalonde R, Kim HD, Fukuchi K. Exploratory activity, anxiety, and motor coordination in bigenic APPswe + PS1/DeltaE9 mice. Neurosci Lett. 2004;369:156–61. doi: 10.1016/j.neulet.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 53.Sood A, Warren Beach J, Webster SJ, Terry AV, Buccafusco JJ. The effects of JWB1-84-1 on memory-related task performance by amyloid Abeta transgenic mice and by young and aged monkeys. Neuropharmacology. 2007;53:588–600. doi: 10.1016/j.neuropharm.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Lifshitz V, Weiss R, Benromano T, Kfir E, Blumenfeld-Katzir T, Tempel-Brami C, et al. Immunotherapy of cerebrovascular amyloidosis in a transgenic mouse model. Neurobiol Aging. 2012;33:432, e1–e13. doi: 10.1016/j.neurobiolaging.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, et al. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer's disease in a sex-dimorphic pattern. Neuroscience. 2006;141:1149–62. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 56.de Fiebre NC, Sumien N, Forster MJ, de Fiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. Age. 2006;28:235–53. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 58.Kennard JA, Woodruff-Pak DS. Age sensitivity of behavioral tests and brain substrates of normal aging in mice. Front Aging Neurosci. 2011;3:1–22. doi: 10.3389/fnagi.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwama M, Amano A, Shimokado K, Maruyama N, Ishigami A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J Nutr Sci Vitaminol. 2012;58:169–74. doi: 10.3177/jnsv.58.169. [DOI] [PubMed] [Google Scholar]
- 60.Lykkesfeldt J, Moos T. Age-dependent change in Vitamin C status: a phenomenon of maturation rather than of ageing. Mech Ageing Dev. 2005;126:892–8. doi: 10.1016/j.mad.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Harrison FE, Allard J, Bixler R, Usoh C, Li L, May JM, et al. Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 mice. Nutritional neuroscience. 2009;12:203–18. doi: 10.1179/147683009X423364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami K, Murata N, Ozama Y, Kinoshita N, Irie K, Shirasawa T, et al. Vitamin C restores behavioral deficits and amyloid-β oligomerization without affecting plaque formation in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2011;25:1–12. doi: 10.3233/JAD-2011-101971. [DOI] [PubMed] [Google Scholar]
- 63.Mayo W, Dellu F, Cherkaoui J, Chapouthier G, Dodd RH, Le Moal M, et al. Cognitive enhancing properties of β-CCM infused into the nucleus basalis magnocellularis of the rat. Brain Res. 1992;589:109–14. doi: 10.1016/0006-8993(92)91168-e. [DOI] [PubMed] [Google Scholar]
- 64.Le XT, Pham HT, Do PT, Fujiwara H, Tanaka K, Li F, et al. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem Res. 2013;38:2201–15. doi: 10.1007/s11064-013-1129-6. [DOI] [PubMed] [Google Scholar]
- 65.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 66.Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Rev. 2003;41:268–87. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 67.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 68.May JM, Harrison FE. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory, and visual regions of the brain in miniature swine. J Physiol. 2001;533:849–59. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–95. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 71.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 72.Marín J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79:71–114. doi: 10.1016/0047-6374(94)01551-v. [DOI] [PubMed] [Google Scholar]
- 73.Zerbi V, Jansen D, Wiesmann M, Fang X, Broersen LM, Veltien A, et al. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer's disease. Neurobiol Aging. 2013;35:600–13. doi: 10.1016/j.neurobiolaging.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 74.Wiesmann M, Jansen D, Zerbi V, Broersen LM, Garthe A, Kiliaan AJ. Improved Spatial Learning Strategy and Memory in Aged Alzheimer AβPPswe/PS1dE9 Mice on a Multi-Nutrient Diet. J Alzheimers Dis. 2013;37:233–45. doi: 10.3233/JAD-130179. [DOI] [PubMed] [Google Scholar]
- 75.May JM, Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun. 2011;404:701–5. doi: 10.1016/j.bbrc.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol. 2009;297:C169–78. doi: 10.1152/ajpcell.00674.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988;9:31–9. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- 78.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. Cell Transplant. 2007;16:285–99. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 79.Sharma HS, Castellani RJ, Smith MA, Sharma A. The blood-brain barrier in Alzheimer's disease: novel therapeutic targets and nanodrug delivery. Int Rev Neurobiol. 2012;102:47–90. doi: 10.1016/B978-0-12-386986-9.00003-X. [DOI] [PubMed] [Google Scholar]
- 80.Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1α impairs memory processing in mice: Dependence on blood-brain barrier transport into posterior division of the septum. JPET. 2001;299:536–41. [PubMed] [Google Scholar]
- 81.Kiyota T, Gendelman HE, Weir RA, Higgins EE, Zhang G, Jain M. CCL2 affects beta-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:1060–8. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.May JM, Qu ZC. Ascorbic acid prevents increased endothelial permeability caused by oxidized low density lipoprotein. Free Radic Res. 2010;44:1359–68. doi: 10.3109/10715762.2010.508496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.May JM, Qu ZC. Ascorbic acid prevents oxidant-induced increases in endothelial permeability. Biofactors. 2011;37:46–50. doi: 10.1002/biof.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, et al. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 2010;107:1624–9. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–9. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 86.Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–44. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 87.Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–40. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 88.Woodruff-Pak DS. Eyeblink classical conditioning differentiates normal aging from Alzheimer's disease. Integr Physiol Behav Sci. 2001;36:87–108. doi: 10.1007/BF02734044. [DOI] [PubMed] [Google Scholar]
- 89.Pavlopoulous E, Jones S, Kosmidis S, Close M, Kim C, Kovalerchik O, et al. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Transl Med. 2013;5:1–12. doi: 10.1126/scitranslmed.3006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pietropaolo S, Delage P, Lebreton F, Crusio WE, Cho YH. Early development of social deficits in APP and APP-PS1 mice. Neurobiol Aging. 2012;33:1002, e17–27. doi: 10.1016/j.neurobiolaging.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, et al. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 92.Bizon J, Prescott S, Nicolle MM. Intact spatial learning in adult Tg2576 mice. Neurobiol Aging. 2007;28:440–6. doi: 10.1016/j.neurobiolaging.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 93.Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: Lack of association with amyloid deposits. Behav Genet. 1999;29:177–85. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- 94.Ammassari-Teule M, Caprioli A. Spatial learning and memory, maze running strategies and cholinergic mechanisms in two inbred strains of mice. Behav Brain Res. 1985;17:9–16. doi: 10.1016/0166-4328(85)90003-8. [DOI] [PubMed] [Google Scholar]
- 95.Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–99. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 96.Passino E, Middei S, Restivo L, Bertaina-Anglade V, Ammassari-Teule M. Genetic approach to variability of memory systems: analysis of place vs. response learning and fos-related expression in hippocampal and striatal areas of C57BL/6 and DBA/2 mice. Hippocampus. 2002;12:63–75. doi: 10.1002/hipo.10007. [DOI] [PubMed] [Google Scholar]
- 97.Parrow NL, Leshin JA, Levine M. Parenteral Ascorbate As a Cancer Therapeutic: A Reassessment Based on Pharmacokinetics. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Riordan HD, Casciari JJ, Gonzalez MJ, Riordan NH, Miranda-Massari JR, Taylor P, et al. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. PR Health Sci J. 2005;24:269–76. [PubMed] [Google Scholar]
- 99.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]