Abstract
Human small heat shock proteins are molecular chaperones that regulate fundamental cellular processes in normal unstressed cells as well as in many cancer cells where they are over-expressed. These proteins are characterized by cell physiology dependent changes in their oligomerization and phosphorylation status. These structural changes allow them to interact with many different client proteins that subsequently display modified activity and/or half-life. Nowdays, the protein interactomes of small Hsps are under intense investigations and will represent, when completed, key parameters to elaborate therapeutic strategies aimed at modulating the functions of these chaperones. Here, we have analyzed the potential pro-cancerous roles of several client proteins that have been described so far to interact with HspB1 (Hsp27) and its close members HspB5 (αB-crystallin) and HspB4 (αA-crystallin).
Keywords: human small Hsps, HspB1, HspB5, HspB4, Hsp27, alphaA-crystallin, alphaB-crystallin, clients, cancer
1. Introduction
The literature is filled with reports describing the overexpression of heat shock proteins (Hsps) in many types of cancer cells [1,2]. The phenomenon is probably linked to the drastic changes in protein homeostasis resulting of the accumulation of mutated proteins in cancer cells [2,3,4]. Hsps have been known for a long time as being able to prevent protein aggregation by binding polypeptide clients destabilized during cellular stress, such as heat shock. Among Hsps, the ATP-independent molecular chaperones HspB1, HspB5 and HspB4 belong to the group of small Hsps characterized by a common alpha-crystallin domain in their C-terminal part. Recently, small Hsps have been described to have an incredible number of crucial roles in normal unstressed cells as well as in pathological cells where they are usually expressed to high levels. These proteins are now considered as important therapeutic targets, particularly in cancer pathologies [5,6]. Indeed, they display anti-apoptotic and tumorogenic properties and trigger host anti-cancer response inhibition, such as senescence [7,8,9,10,11,12,13]. This results in aggressive cancer cell growth [14,15,16], metastasis formation and dissemination [12,17,18,19] and poor prognosis [2,20,21]. We have proposed that the large panel of cellular functions of small Hsps resulted of their interaction with numerous client proteins whose activity and/or half-life was modified by these chaperones [22,23,24,25]. Small Hsps protein interactomes appear therefore as fundamental parameters to consider when studying the complex activities of these proteins. Here, we have reviewed the pro-oncogenic client proteins interacting with HspB1, HspB5 or HspB4 that can play crucial roles in human cancer pathologies.
2. Small Hsps in Human Cancer Pathologies
2.1. HspB1
Consequently of the deleterous resistance and enhanced aggressivity brought to cancer cells by HspB1, a high level of expression of this molecular chaperone usually results in poor clinical outcome in the case of gastric, uterine, breast, prostate, ovarian, kidney and head/neck cancers as well as in tumors from the urinary and nervous systems [2,20,21,26,27]. HspB1 is also deeply involved in the invasion of tumor cells into surrounding tissues and formation of metastatic colonies [12,17,18,19,28,29]. Moreover, high levels of HspB1 expression affect tumor susceptibility to adjuvant cancer treatments, including chemotherapy, hyperthermia and radiation therapies [19]. Many client proteins are targeted by HspB1 to promote resistance to cell death and malignant phenotype [23,24,25]. Of particular interest, elevated levels of HspB1 have also been detected in several cancer stem cells, such as those from lung and breast cancers [30,31]. In these cells, HspB1 participates in their maintenance since its elimination suppresses epithelial-mesenchymal transition (EMT) signatures linked to snail, vimentin, E-cadherin and NF-κB [31] and induces long-term dormancy [32]. Snail is a well-defined client protein of HspB1 protected against a rapid degradation consequently of its interaction with this chaperone [33]. A relationship between HspB1 expression and the loss of heterozygosity (LOH) has also been detected supporting the hypothesis that this protein could act as a sensor of genetic imbalances (haploinsufficiency) [34]. Indeed, it was observed that in oligodendroglial tumors HspB1 is a marker of LOH of 1p, information that could help to predict the disease prognosis. Another worth noting point relates to HspB1 ability to enhance cancer cells resistance to a large panel of anti-cancer drugs [35,36,37,38,39,40,41,42,43]. For example, in MCF-7 breast cancer cells exposed to doxorubicin, survival cells are those that overexpress HspB1 consequently of the increased activity of the POU4F2/Brn-3b transcription factor [44]. This factor, which is elevated in more than 60% of breast cancers, modifies growth and behavior of cancer cells by regulating the expression of several target genes [45]. Hence, HspB1 inhibition of drug-induced apoptosis by further enhancing HspB1 expression also increases the cellular resistance to many anti-cancer drugs [46]. Contradictory findings have also been reported. For example, in pancreatic cancer cells, HspB1 has been linked with increased sensitivity [47] as well as with increased resistance [48] to gemcitabine. These opposite observations were reconciled by analyzing an essential parameter of HspB1, that is its phosphorylation status [49]. Crucial effect of phosphorylation has also been observed in Her-2/neu positive breast cancers, where serine 78 phosphorylated HspB1 correlates with Her-2/neu status and lymph node positivity [50]. In that respect, we have recently described the major roles played by the dynamic phosphorylation of HspB1 in the regulation of the anti-apoptotic strategies developped by this protein [51]. While RNAi strategies aimed at therapeutically decrease HspB1 level as well as the search for inhibiting drugs are nowdays deeply active, the use of HspB1 in the clinic is still mainly as a prognostic indicator of specific cancer types [52].
2.2. HspB5 and HspB4
The major lens structure proteins HspB5 and HspB4 play essential roles in maintaining normal cellular structure and physiology of both ocular and some non-ocular tissues. Mutations in these polypeptides are responsible of various human diseases such as cataract, neural disorders, and cardiovascular diseases. Of great interest was the recent discovery that abnormal over-expression of these molecular chaperones often occurs in tumors [41,53,54]. The first report describing the association of HspB5 and cancer was by studying breast carcinoma progression [55]. It was originaly observed that HspB5 expression was closely associated with advanced tumor grade progression, lymph vesicular invasion and mortality [53,56]. The phenomenon was particularly intense in “triple-negative” breast tumors [55,56,57], consequently HspB5 is now considered as a biomarker in the diagnosis of breast cancers, especially in those with advanced grade [57,58]. Concerning brain cancer, elevated levels of HspB5 were clearly demonstrated to occur in the more aggressive stages of gliomas [59,60]. Similarly, high levels of HspB5 are associated with low survival rate in hepatocellular carcinoma [61], lung adenocarcinoma [62] and prostatic carcinoma [63]. However, exceptions exist since tumorogenesis can occur in absence of HspB5 upregulation [64]. For example, the role of this protein in kidney tumorogenesis is not clear since an increased level of expression of HspB5 generally occurs in low grade tumors [65], a phenomenon which is in deep contrast to that observed in most breast and brain tumors. HspB5 was also reported to be downregulated in anaplastic thyroid carcinomas [66], nasopharyngeal carcinomas [67], cutaneous squamous cell carcinoma of the head and neck with clinical perineural invasion [68], oral cancer [69,70] as well as human ovarian cancer [71] and testicular tumors [63]. How HspB5 expression can be either up or down regulated in some cancers? At least in breast cancers, HspB5 up regulation may be linked to transcriptional activation. Indeed, HspB5 gene promoter contains an Ets1 binding site that can be activated by the oncogenic transcription factor Ets1 [72]. This Ets1-mediated event appears linked to poor survival.
Compared with HspB1, the functions of HspB5 and HspB4 in tumorogenesis are less known. As mentioned above, HspB5 can promote tumorogenesis while the effects mediated by HspB4 are still equivocal, at least in the few studies that have already been performed [26,73]. Interesting observations have nevertheless been made which showed the dual effect of HspB4 that is either up- or down-regulated depending of the tumor type. For example, elevated levels of HspB4 together with HspB5 are observed in many of the lens tumor cells, while γ-crystallin is selectively reduced [74]. Similarly, HspB4 is also detected in retinoblastoma [41] and in eyelids with sebaceous carcinoma [75]. In these tumors, HspB4 is supposed to prevent apoptosis of neoplastic cells and thus promotes tumorogenesis. In contrast, HspB4, which is expressed at moderate level in normal pancreas, is significantly downregulated in different types of pancreatic carcinoma cells where genetically forced expression of this protein revealed that it can act as a negative regulator that blocks cell transformation and retards cell migration [73]. The effect appears to occur through a modulation of ERK MAP kinase activity [54]. Hence, HspB4 and HspB5 appear to function differentially during tumorogenesis. In contrast to HspB1, therapeutic targetings of HspB5 or HspB4 are still in the stage of experimental exploration [5].
3. HspB1, HspB5 and HspB4 Clients and Their Potential Role in Cancer Pathologies
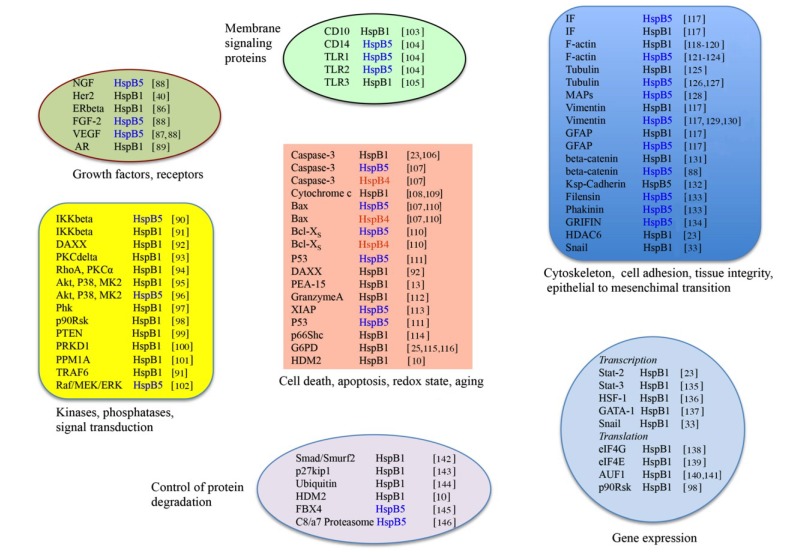
The literature is filled with reports describing the incredible number of roles played by small Hsps, particularly HspB1 and HspB5 in cancer cells. How can these proteins have such a vast spectrum of activities? We recently proposed, in view of our recent results, that their apparent pleiotropic activities probably result of their ability to bind, chaperone and modulate the activity or half-life of many protein targets involved in crucial cellular regulations, such those involved in apoptotic cell death, tumorogenic and metastatic processes [21,23,24,25]. Small Hsps are characterized by their complex oligomeric structures allowing them to interact with each other to form homo and hetero-oligomeric structures of dynamic size (up to 700 kDa) [76,77,78]. Moreover, HspB1, HspB5 and HspB4 contain several serine sites that can be phosphorylated by specific kinases, including stress and MAP kinases. The phosphorylation and oligomeric organizations of these proteins are dynamic and deeply modified by changes in the cellular environment [15,21,37,77,79,80,81,82,83]. Indeed, these structural modifications are reversible and probably act as sensors of the cellular environment. For example, depending on the apoptotic inducer, HspB1 differently reorganizes its phosphorylation and oligomerization status suggesting that multiple strategies are set up by this protein to counteract apoptosis [15,21,51,77]. Changes in small Hsps structure can lead to at least 300 different stoichiometries to allow them to interact with putative client proteins [84]. Their dynamic structural plasticity would then favor the recognition of the more appropriated pro-cancerous client proteins, hence allowing the cancer cell to grow and disseminate. The clients of HspB1, HspB5 and HspB4 that could participate in the escape to death, growth, survival and aggressivity of cancer cells are presented below and in Figure 1.
Figure 1.
HspB1, HspB5 and HspB4 client proteins and tumor cell growth. The client proteins are listed in function of their tumor-prone activities.
Abbreviations are the following: CD10: 100 kDa transmembrane metallo-endopeptidase; CD14: cluster of differentiation 14; TLR1: Toll-like receptor 1; TLR2: Toll-like receptor 2; TLR3: Toll-like receptor 3; NGF-beta: Nerve growth factor beta; Her2: Human Epidermal Growth Factor Receptor-2; ERbeta: Estrogen receptor beta; FGF-2: Fibroblast growth factor 2; VEGF: vascular endothelial growth factor; IKKbeta: inhibitor of nuclear factor kappa-B kinase subunit beta; DAXX: death domain-associated protein 6; PKC delta: protein kinase C delta; RhoA: Ras homolog gene family member A; PKCalpha: protein kinase C alpha; Akt: protein kinase B; P38: P38 mitogen-activated protein kinases; MK2: Mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2); Phk: Phosphorylase kinase (PhK); PTEN: phosphatase and TENsin homolog; PRKD1: Serine/threonine-protein kinase D1; PPM1A: protein phosphatase 1A (formerly 2C), magnesium-dependent, alpha isoform; TRAF6: tumor necrosis factor receptor-associated factor 6; Raf, MEK, ERK: Mitogen activated protein (MAP) kinase pathways; Bax: Bcl-2 associated X protein; PEA-15: astrocytic phosphoprotein PEA-15; XIAP: X-linked inhibitor of apoptosis; p66Shc: 66 kDa isoform of ShcA (Src homology 2 domain containing transforming protein 2); G6PD: glucose 6-phosphate dehydrogenase; HDM2: Human double minute 2 homolog; IF: intermediate filaments; MAPs: microtubule-associated proteins; GFAP: Glial fibrillary acidic protein; GRIFIN: galectin-related interfiber protein; HDAC6: histone deacetylase 6; STAT2 and 3: signal transducer and activator of transcription 2 and 3; HSF-1: heat shock factor 1; GATA-1: globin transcription factor 1; Snail: zinc finger protein that binds and inhibits E-cadherin promoter to induce epithelial mesenchymal transformation; eIF4E: eukaryotic translation initiation factor 4E; eIF4G: eukaryotic translation initiation factor 4G; AUF1: AU-rich element (ARE)-binding protein; p90 ribosomal S6 kinase; Smad-Smurf2: Smad ubiquitination regulatory factor 2; p27kip1: cyclin-dependent kinase inhibitor p27kip1; Fbx4: Fbox only protein 4.
3.1. Clients as Receptors and Growth Factors Stimulating Cell Growth
HspB1 and HspB5 have been reported to stimulate constitutive cell division by interacting with different cytoplasmic receptors, such as VEGF, FGF-2 and Her2, leading to an enhanced activity of the MAPK kinase/MEK/ERK pathway. However, the first report of the interaction of a small heat shock protein with a receptor concerned HspB1 being an estrogen receptor-beta (ERbeta) associated protein that could act as a co-repressor of estrogen signaling [85,86]. More recently, it was shown that, in human breast cancer cells, HspB1 increases Her-2/neu protein stability through its ability to chaperone this receptor. This resulted in an indirect negative effect of HspB1 towards Herceptin susceptibility [40]. Towards growth factors, one interesting small Hsps-client interaction has been detected during retinal angiogenesis. Indeed, in this tissue, HspB5 can chaperone vascular endothelial growth factor A (VEGF-A) and thus avoid its rapid degradation by the ubiquitin-proteasome machinery. The phenomenon results in a drastic stimulation of angiogenesis [87].
3.2. Clients Modulating Survival Signaling Pathways and Apoptosis
Apoptosis is a genetically programmed cell death negatively modulated by Hsps [7,38,147]. In contrast to the increased expression of Hsps by environmental insults, such as heat shock, no up-regulation of these proteins is observed in cells committed to apoptosis and only constitutively expressed pre-existing Hsps can be effective. This is particularly the case in human cancer cells where constitutively expressed small Hsps can counteract apoptotic process mediated by the immune system or therapeutic drugs. It is now believed that HspB1, HspB5 and HspB4 act by interacting with specific client proteins and modulate their activity in the initiation and execution phases of apoptosis [2,148].
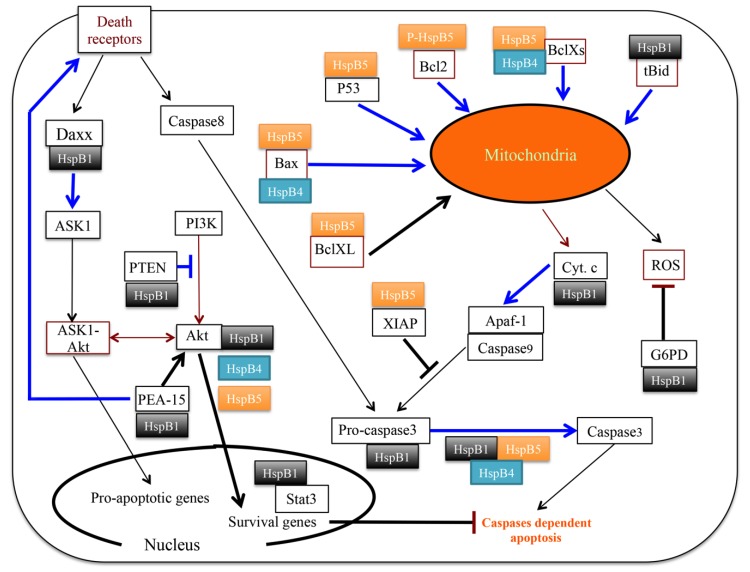
In cells committed to apoptosis, HspB1 is characterized by dynamic reorganization of its structural organization, particularly at the level of its phosphorylation and oligomerization status. These dynamic changes are signal transduction-dependent, hence favoring the hypothesis that this chaperone has multiple strategies, based on its interaction with specific targets, to counteract intrinsic and extrinsic apoptosis [51,77]. See Figure 2 for an illustration of these activities. Upstream of the intrinsic pathway, it was shown that by interfering with F-actin disruption and t-Bid translocation, HspB1 indirectly decreases the efficiency of the release of cytochrome c [149] and Smac-diablo [150] from mitochondria. Then, it interacts with cytosolic cytochrome c leading to impaired apoptosome and caspase-9 activation [108,151]. Downstream of these events, phosphorylated small oligomers of HspB1 interact with procaspase-3 and negatively regulate its activation [23,24,106]. It is also worth mentioning that the interaction of pro-caspase-3 with HspB1 counteracts the rapid degradation of this caspase by the ubiquitin-protasome machinery [23]. Hence, in addition of being able to inhibit a potent caspase effector, HspB1 has the surprizing property to protect it against a rapid proteolytic degradation. In the extrinsic pathways, HspB1 counteracts TNFαlpha, Fas and Trail induced apoptosis [7,11,152]. One well-defined target of HspB1 in the Fas pathway is Daxx [92,153]. Phosphorylated dimers of HspB1 interact with Daxx and indirectly prevent its association with both apoptotic signaling kinase1 (Ask1) and Fas; a phenomenon that blocks Daxx-mediated apoptosis. The cell survival kinase Akt is also a major target of HspB1 [95,154]. In fact, HspB1 is now considered as a key component of the Akt signaling cascade: Akt, BAD, mitogen-activated protein kinase kinase-3,6 and Forkhead transcription factors (Figure 2). By phosphorylating Bad, Akt abolishes its pro-apoptotic activity promoting cell survival. Another interesting protein recognized by HspB1 is the tumor suppressor PTEN that, by acting as a protein tyrosine phosphatase and a phosphatidylinositol phosphate (PIP) phosphatase, can negatively regulate the survival PI3K/Akt signaling pathway. In human breast cancer cells expressing high levels of HspB1, the interaction of PTEN with this chaperone further enhances the survival of these pathological cells [99]. In fact, it appears that HspB1 interaction with PTEN decreases the level of this phosphatase and therefore counteracts the PI3K-Akt survival pathway. Consequently, a geneticaly induced downregulation of HspB1 up-regulates PTEN level and blocks the survival pathway of cancer cells. This is an interesting example where HspB1 stimulates the degradation of a client protein instead of inhibiting its degradation. In gliomas, HspB1 was also described to participate in the signaling pathway that promotes cancer cell survival [155,156]. Another study showed that HspB1 silencing, which destroyed the interaction with PEA-15, coordinately inhibited proliferation and promoted Fas-induced apoptosis by regulating the PEA-15 molecular switch [13]. Hence, PEA-15 is an additional survival protein regulated by HspB1.
Figure 2.
Cartoon that summarizes the apoptotic and survival pathways controlled by HspB1, HspB5 and HspB4. Large blue arrows are indicative of an inhibition of the corresponding pathways consequently of the binding of the clients to HspB1, HspB5 or HspB4. Black arrows or lines indicate that small Hsps either favorize the indicated pathways or just override them.
HspB5 and HspB4 are well-known anti-apoptotic polypeptides [7,157] whose major property is to negatively regulate the pro-apoptotic members of the Bcl-2 family, Bcl-XL and Bax as well as caspase-3 [107]. HspB5 and HspB4 directly interact with caspase-3, Bax and Bcl-XS [107,110]. Moreover, in addition to interacting with these clients, HspB4 and HspB5 suppress their translocation from the cytosol into mitochondria and thereby prevent stress-induced apoptosis [110]. Similarly, HspB5 interacts with p53 to sequester its translocation to mitochondria and therefore indirectly inhibits its pro-apoptotic action towards anti-apoptotic Bcl-2 molecules [111] (see Figure 2). HspB5 was also found to abrogate calcium-activated Raf/MEK/ERK signaling pathway mediated p53-dependent apoptosis through inhibition of RAS activation [102]. In addition, both HspB4 and HspB5 could prevent UVA apoptosis through complex interactions with protein partners involved in regulating signaling PKCαlpha and Raf/MEK/ERK pathways [96]. Recently HspB5 was also reported to interact with XIAP, a most potent endogenous suppressor of apoptosis that acts by inhibiting caspases through a direct binding to these enzymes [113]. Thus, in spite of some common properties towards cell apoptosis, cell proliferation and tumor metastasis yet unknow molecular mechanisms have been described to explain the different role played by HspB4 and HspB5 in pancreatic carcinogenesis [54]. In that respect, it is important to recall that phosphorylation could be the culprit. One example supporting this point concerns serine 59 phosphorylation which shifts HspB5 activity from anti-apoptotic to pro-apoptotic since this phosphorylated isoform of HspB5 surprizingly sequesters Bcl-2 and blocks its translocation to mitochondria [158].
3.3. Senescence
In addition of protecting against programmed cell death, HspB1 can also counteract other host anti-cancer responses, such as the oncogene-induced senescence (OIS) pathway that acts as roadblock in cancer development but which is often lost upon cancer progression. The phenomenon which is p53 dependent has been detected by down-regulating HspB1 expression [10]. Indeed, depletion of HspB1 expression in cancer cells induces a senescent-like phenotype characterized by several morphological changes. Among them one can note a drastic multi-nucleation which results of the degradation of HDAC6, a specific client of HspB1 interacting with HspB1 oligomers phosphorylated at the level of serine 82 [23,24]. HDAC6 is a powerful modifier of carcinogenesis that contributes to oncogenic pathways activation [159]. A recent study performed in colorectal adenomas also suggests a possible role of HspB1 in overcoming PI3K/AKT induced OIS in tumours [160]. OIS depends on the activation of the p53 tumor suppressor protein pathway and induction of p21waf expression leading to cell cycle arrest [10]. Of interest, HspB1 interacts with HDM2, which serves as ubiquitin ligase (E3) to target p53 for degradation, and modulates its activity [10,161]. HspB1 down-regulation which leads to a senescent morphology appears therefore to result of the destabilization and degradation or inactivation of several client proteins involved in the senescent pathways, such as PI3K/AKT-PTEN, HDAC6 as well as the p53 stabilizator HDM2.
3.4. Clients Involved in Tumor Progression, Epithelial-to-Mesenchymal Transition, Metastasis
Additional to its role in protecting cancer cells by suppressing host anti-cancer response, such as apoptosis and senescence, other major pro-cancerous activities of HspB1 are linked to its tumorogenic property which promotes tumor growth and progression [9,14], invasion of tumor cells into surrounding tissues and dissemination to form metastatic colonies [17,18,19,28,29,162,163]. In spite of the fact that the molecular mechanism that drives HspB1 to promote tumor progression and metastasis is still not well understood, several different HspB1 clients have already been identified that participate in the achievement of these particular endeaviors. First, it is well known that HspB1 can directly modulate cytoskeleton integrity and indirectly extracellular matrix organization [23,78,117,118,164,165,166,167]. Indeed, recent observations clearly favor an important role played by HspB1 at the level of the cellular matrix suggesting that the relation between cancer cells and the normal cells that form the niche where the tumor grow is under the influence of small Hsps [19]. For example, HspB1 overexpression drives β-catenin from the cell membrane to the cytoplasm where both proteins interact with each other and consequently modulates cadherin–catenin cell adhesion proteins [131]. β-catenin also interacts with other proteins including heat shock transcription factor 1 (HSF1), P-cadherin, and caveolin-1. Disease-free survival were significantly shorter for patients with cytoplasmic expression of β-catenin consequently of the presence of HspB1. Hence, the interactions of β-catenin with HspB1 appears to play a key role in the molecular pathways that influence tumor cell survival. Another important role played by phosphorylated HspB1 in prostate cancer relates to the TGFbeta-induced activation of MMP-2 (matrix metalloproteinase type 2), an enzyme that digests components of the extracellular matrix surrounding tumor masses and subsequently stimulates human prostate cancer cells invasion [168]. TGFbeta-mediated increase in MMP-2 and cell invasion are inhibited in cells devoid of MAPKAPK2 or HspB1 expression as well as following the inhibition of HspB1 phosphorylation. Hence, both MAPKAPK2 and HspB1 appear necessary for TGFbeta-mediated cell invasion but the responsive clients of phosphorylated HspB1 acting in this pathway are still unknown. It was also shown that abrogation of 4T1 murine tumor migration induced by HspB1 down-regulation is associated with the repression of matrix metalloproteinase 9 expression together with a concomitant upregulation of its antagonist, tissue inhibitor metalloproteinase 1 [162]. This suggests that several metalloproteinases are under the control of HspB1. SPARC (secreted protein, acidic and rich in cysteine), a polypeptide playing an important role in cell-matrix interactions, is an additional polypeptide that uses HspB1 to modulate cell adhesion and migration. In colorectal cancer, stromal SPARC expression positively correlates with HspB1 expression and inversely with VEGF-A expression [169]. SPARC was also reported to promote invasion in gliomas through the up-regulation of the p38 MAPK/MAPKAPK2/HspB1 signaling pathway. This pathway promotes cancer cell survival by up-regulating the survival kinase pAKT activity through its interaction with HspB1 [155,156,170]. On the immunological side, high levels of HspB1 are associated with the repression of proteasome function and poor antigen presentation. By decreasing HspB1 level the proteasome activity is back to normal as well as the CD8+ T-cell-mediated tumor killing and the memory responses [171].
Back in 1997, a direct correlation between elevated level of HspB1 in the later stage of tumor progression and the invasive and metastatic potential of human breast cancer cells was confirmed in vivo in an assay measuring the number of lung metastases in mice injected with HspB1-transfected cells [17]. This study also reported that HspB1 overexpression decreases cell motility, but enhances invasiveness, adhesion, and growth in Matrigel. Consequently, suppression of HspB1 expression increases cell motility, but decreases in vitro invasiveness. Several recent studies have now confirmed that HspB1 is a key parameter in the emergence of metastasis [19]. A recent striking example is the promoting effect of HspB1 in breast cancer cells to metastasize and grow in the skeleton as bone tumours [12]. The epithelial-to-mesenchymal transition (EMT) is a fundamental parameter that controls metastasis formation, however the mechanisms regulating the phenomenon are still not well understood. In that respect, HspB1 participates in the maintenance of breast cancer stem cells through regulation of EMT and NF-κB transcription factor [31]. In prostate cancer, HspB1 also appears as an important player that promotes EMT since its depletion is associated with decreased cell migration, invasion, and MMP-2 activity [6]. The mechanisms involved in the promotion of the metastatic phenotype by HspB1 are not solved yet. It has been proposed, that HspB1 could modulate the expression of pro-metastatic genes [19], such as those dependent of the STAT3/Twist signaling [6]. Of interest, HspB1 and Twist expression are both elevated in high-grade prostate cancer tumors and HspB1 enhances the binding of the transcription factor STAT3 to the promoter of the Twist gene [6], an event that subsequently upregulates N-cadherin and downregulates E-cadherin expression, two hallmarks of EMT. However, how HspB1 mediates these effects is still not known (see below paragraph 3.7.1.).
HspB5 is well known for its involvment in cytoskeleton integrity [117,128]. Moreover, it also plays a role, at least in the kidney, for maintaining tissue integrity by interacting with Ksp-cadherin-16 and promoting the connection of this polypeptide to the cytoskeleton [132]. In cancer pathologies, HspB5 has been described to promote cell migration and invasion by modulating signal transduction pathways. For example, it induces EGF- and anchorage-independent growth of human breast basal-like tumors through the constitutive activation of the MAPK kinase/ERK (MEK/ERK) pathway [53]. Moreover, HspB5 can also act as an oncoprotein since, in nude mice, it has the intriguing property to transform immortalized human mammary epithelial cells in invasive mammary carcinomas that have the same aspect as basal-like breast tumors [53,172]. HspB5 expression in basal-like breast tumors is associated to poor patient survival independently of other prognostic markers. At least in pancreatic cancer cells, these properties are apparently not shared by HspB4 that acts, in spite of its protective property, as a negative regulator of carcinogenesis that retards cell migration and prevents tumor growth [73].
3.5. Clients on Cell Surface and Outside of Cells, Immunosurveillance, Angiogenesis and Autoantibodies
In spite of their major intracellular localization, recent reports mention that, similarly to the large Hsps such as Hsp70 [173], the small Hsps can localize in plasma membrane and be exported in the extracellular milieu [174,175,176]. Of interest, high levels of HspB1 cell surface expression were found to correlate with tumor growth and cell ability to metastasize [18]. Hence, do small Hsps, as the high molecular weight Hsps, associate with immunogenic peptides to bring an immune response to cancer cells [177] or are immunosupressive [178]? In that regard, an immunoregulatory activity has been associated to extracellular HspB1 that could contribute to immunopathology through the ability of this chaperone to interact with monocyte-derived dentritic cells and alter their activity, hence favoring immunosuppression. Extracellular HspB1 has also been described to act as a signaling molecule that activates NF-κB in macrophages [179]. In addition, a proangiogenic effect of HspB1 has been recently reported that depends on NF-κB activation [105]. Indeed, recombinant human HspB1 can recognize and interact with Toll-like receptor 3 (TLR3) at the surface of human microvascular endothelial cells (HMECs) grown as monolayers or spheroids and favors spheroid sprouting, NF-κB dependent VEGF gene transcription and promotes secretion of VEGF-activating VEGF receptor type 2 and angiogenesis. Hence, TLR3 can now be considered as a novel HspB1 interacting protein that indirectly promotes tumor growth.
An important point to keep in mind relates to the fact that circulating extracellular small Hsps can have pathology-dependent roles, similarly to their intracellular counterparts, that is they can either be deleterious or beneficial to patients. For example, circulating HspB1 is well known for its atheroprotective effect [176]. It also appears associated with micro- and macrovascular complications in type1 diabetic patients and considered as a novel marker for diabetic neuropathy [180]. The role of these extracellular proteins in normal physiological conditions will remain to be determined before therapeutic approaches are launched to target these circulating Hsps.
Another intriguing phenomenon concerns the presence of antibodies targeting HspB1 and HspB5 in the aqueous humor of normal tension glaucoma patients [181]. More surprizing is the fact that exogenously applied HspB1 antibody can penetrate in human retina neuronal cells and promote neuronal apoptosis by counteracting HspB1 anti-apoptotic activity [182]. This suggests that autoantibodies to small Hsps may impair cell survival, an activity that could be beneficial to cancer patients and be useful parameters in tumor diagnosis [26]. Circulating Hsps and anti-Hsps antibodies are now considered as being part of the recently defined “stress observation system” [173].
3.6. Clients Involved in Inflammation and in Controling Intracellular Redox State
Fledgling tumors are known to hijack inflammation and use it to accelerate the progression towards full-blown cancer [183]. Activation of NF-κB and a network of signaling molecules, which are key modulators in driving inflammatory cells to cancer cells, facilitate angiogenesis and promote the growth, invasion, and metastasis of tumors. In that respect, it is of great interest to note that HspB1 is essential for pro-inflammatory cell signaling and the expression of pro-inflammatory genes [184]. This protein is indeed required for both IL-1 and TNF-induced signaling pathways for which the most upstream common signaling protein is the pivotal kinase TAK1. Hence, downstream signalling by p38 MAPK, JNK and their activators (MKK-3, -4, -6, -7) and IKKbeta as well as expression of the pro-inflammatory mediators, cyclooxygenase-2, IL-6, and IL-8 are all dependent on HspB1. Unfortunately, the client(s) targeted by HspB1 are still unknown but should reside at the level or more upstream of TAK1. A surprizing finding has been made recently concerning HspB5 interaction, in the plasma of patient suffering of multiple sclerosis, with at least 70 different pro-inflammatory mediators (acute phase proteins, members of the complement cascade, and coagulation factors). Interaction with HspB5 reduces the concentration of these polypeptides leading to decreased inflammation [185]. Whether such a phenomenon occurs in patients suffering from cancer pathologies should urgently be tested. Another anti-inflammatory pathway linked to extracellular HspB5 concerns the activation of an immune-regulatory response in macrophages via endosomal/phagosomal CD14 and Toll-like receptors 1 and 2, two new HspB5 interacting proteins [104]. These reports clearly show that HspB1 and HspB5 act differently towards inflammation.
Inflammation is also linked to oxidative stress and, in this regard, expression of HspB1 or HspB5 is known for quite a while to induce a pro-reducing state in cells [78,83,115,152,186,187,188,189,190,191,192,193,194,195,196,197]. Indeed, decreased levels of intracellular reactive oxygen species and nitric oxide as well as iron uptake are observed in HspB1 and HspB5 expressing cells concomitantly to the upholding of mitochondrial membrane potential (∆Φm) and glutathione in its reducing form [115,152,186,187,194,198]. Consequently, protein oxidation, lipid peroxidation, DNA damages and cytoskeleton architecture disruption by oxidative stress are attenuated [186,189,196,199,200]. At least in the case of HspB1, these protective effects appear to result of the up-regulated activity of anti-oxidant enzymes [115,193], the major one being glucose 6-phosphate deshydrogenease (G6PD), a client of HspB1 [25,116], an enzyme that provides the reducing power of the cell consequently of its ability to transform NADP+ in NADPH+H+ [201], hence leading to oxidoresistance [202]. We recently showed that highly phosphorylated small homo-oligomers of HspB1 are responsive of the interaction of this chaperone with G6PD [25]. Consequently, drugs that interfere with the pro-reducing capability of HspB1 and HspB5 by interfering with their interaction with anti-oxidant enzymes may improve the killing efficiency of anti-cancer therapeutic drugs or conditions which depend on the intracellular redox state or the production of reactive oxygen species for their action, such as the Hsp90 inhibitor 17AAG or X-ray irradiation [196,203].
3.7. Clients Regulating Gene Expression
3.7.1. Transcription
STAT3 is a crucial transcription factor whose persistant activation is involved in the maintenance and anti-apoptotic status of many cancerous cells through the activation of anti-apoptotic genes, such as the one encoding survivin [204,205,206,207]. Interaction between STAT3 and HspB1, reported already in 2004 [208], was confirmed recently [23,135]. STAT3 interacts with many different polypeptides and is transcriptionaly activated by tyrosine phosphorylation by either Jack2, c-Src, epidermial growth factor or other tyrosine kinases in response to various cytokines and growth factors [209,210]. It then forms homo- or hetero-dimers that translocate to the cell nucleus and bind to DNA. Of great interest, down-regulation of HspB1 expression decreases the level of phosphorylated STAT3 [23]. This suggests that the half-life of activated STAT3 is dramatically expanded through its interaction with HspB1. Hence, the transcriptional activation of STAT3, consequently of its phosphorylation and nuclear translocation, appears to require the presence of HspB1 [23]. Moreover, HspB1 has also been reported to be essential for the binding of STAT3 to the Twist promoter, hence promoting EMT and metastasis [6]. However, the role of HspB1 towards STAT3 is probably more complex than it has been originally thought since, depending upon the mutational background of the tumor, STAT3 can either be pro-oncogenic or have a tumor suppressor activity [211]. For example, in colorectal cancer cells STAT3 down-regulates Snail-1 expression levels and thus suppresses EMT and metastasis [212]. Hence, depending on the tumor, the relation between HspB1 and STAT3 may have different consequences. Whether HspB1 phosphorylation plays a role in this phenomenon will have to be tested. HspB1 also interacts with the transcription factor STAT2 and avoid its degradation by the ubiquitin proteasome pathway [23]. STAT2 is mainly involved in the control of anti-viral responses through its activation by interferon-γ [205]. STAT2 was shown to specifically interact with serine 78 and 82 phosphorylated HspB1 large oligomers [23,24]. HspB1 also plays a role towards GATA1, a transcription factor essential for erythroid differentiation, which is heavily mutated in almost all megakaryoblastic leukemias in patients with Down syndrome [213]. HspB1 interacts with GATA1 and down-regulates its content and activity by inducing its ubiquitination and proteasomal degradation during terminal erythroid differentiation [137]. The role played by HspB1 towards GATA1 in these pathologies will have to be determined. An additional target of HspB1 is HSF1 (heat shock factor 1), the transcription factor responsible for Hsps expression that plays an important role in tumorogenesis [4]. In that regard, HspB1 is involved in SUMO-2/3 modification of HSF1 [136]. In the nucleus, large oligomers of HspB1 interact with HSF1 and induce its modification by SUMO-2/3 on lysine 298. This modication results of the interaction of HspB1 with the SUMO-E2-conjugating enzyme Ubc9 and takes place downstream of HSF1 phosphorylation. This modification does not affect HSF1 DNA-binding ability but blocks its transactivation function. Future studies will have to test whether SUMO-2/3 favors HSF1 activity as a signal modulator stimulating kinase activity, regulating energy metabolism and permitting the development of polyploidy in cancer cells or as an inhibitor of transcription which, in cooperation with NuRD family factors, can repress genes that oppose metastasis [4].
HspB5 has not yet been described to play a role in transcriptional modifications favoring the development of cancer cells. In contrast, HspB4, which can act as an inhibitor of tumorogenesis, has been proposed to halt cell transformation and retard cell migration by modulating the expression and activity of the transcription factor AP-1 [54].
3.7.2. Translation
HspB1 is well known for its activity as an inhibitor of translation during heat shock that limits the accumulation of unfolded proteins. In heat shock treated cells, HspB1 interacts with the initiation factor eIF4G, but not with the other factors of the cap-binding initiation complex such as eIF4E, eIF4A, eIF4E kinase Mnk1 and poly (A)-binding protein [138]. HspB1-eIF4G interaction facilitates the dissociation of cap-initiation complexes and promotes translation inhibition. In cancer cells, active translation machinery is a prerequisite for cell growth and proliferation and once again HspB1 plays a major role in the protein translational initiation process. A study performed in prostate cancer showed that HspB1 up-regulation, which is particularly intense after androgen withdrawal, confers broad-spectrum treatment resistance including chemotherapy. Analysis of the enhanced cell survival during cell stress induced by castration or chemotherapy resistance revealed that it resulted mainly of HspB1 protection of the protein synthesis initiation process. In that context, HspB1 was found to interact with the translation initiation factor eIF4E [139]. Moreover, HspB1 downregulation decreased eIF4E expression at the protein, but not mRNA, level. The phenomenon clearly shows that HspB1 acts as a chaperone that stabilizes eIF4E half-life by counteracting its degradation by the ubiquitin-proteasome pathway. Hence, by protecting translational initiation HspB1 can indirectly enhance cancer cell survival through an efficient translation of mRNAs encoding polypeptides. No activities of HspB5 or HspB4 have yet been detected in that particular field of investigation.
4. Peptides, Natural Molecules and Drugs that Inhibit the Proliferation of Human Cancer Cells through Their Ability to Interact with Small Hsps, Therapeutic Approaches
Through their ability to interact with each other small Hsps form complex homo- and hetero-oligomeric structures that deeply modify the activity of the parental molecules. For example, it has been observed that some exogenous mutants of HspB1 can be dominant negative knocking out the activity of endogenous wild-type HspB1 through formation of inactivated hetero-oligomeric complexes that cannot recognize protein partners. In that respect, the substitution of the only cysteine residue in HspB1 sequence by an alanine is very efficient [108,214]. We also recently reported the drastic dominant negative effect mediated by the R120G mutant of HspB5 towards HspB1 [78]. Hence, a mutated small Hsp can alter the protective activity of other members of this family of chaperones expressed in the same cell. This probably occurs through a modifcation of their ability to recognize specific client polypeptides [25,78]. In spite of these observations, the use of mutants as therapeutic approaches to modulate the activity of some small Hsps is not a realistic therapeutic approach. On the contrary, the use of peptides derived from crucial domains that mimick their structural organization and chaperone activity is more reasonable. Only few examples have been reported in the litterature about the effect of peptides mimicking HspB1 and HspB5 activities. One example concerns the interaction of HspB1 with seven amino acids of the PKC delta-V5 heptapeptide region (residues 668 to 674, E-F-Q-F-L-D-I) of Protein Kinase C delta (PKCdelta). This PKC region can sensitize human cancer cells by sequestring HspB1, hence inhibiting its interaction with pathological protein partners [93,200]. This resulted in the inhibition of HspB1-mediated resistance against drugs, such as cisplatin and DNA damaging agents. On the other hand, peptides can stimulate small Hsps activity such as in the case of HspB5 where peptides derived from crucial domains of this chaperone mimicked its positive action against the fibrillation of amyloidogenic proteins [215]. The search for peptides that, instead of mimicking small Hsps, directly modulate their interactions with client proteins is another approach that can lead to the discovery of potent peptidomimetic drugs. Peptide aptamers belong to this second class of peptides [16,216,217,218,219,220]. In that regard, we have described HspB1 interacting peptide aptamers that could either down-regulate or stimulate the anti-apoptotic and cytoprotective activities of this stress protein, hence confirming the importance of the structural organization of this chaperone [16,221]. Moreover, in vivo head and neck tumor xenograft studies revealed that aptamers could reduce tumor growth more efficiently than HspB1 depletion [16]. Aptamer poisoning altered HspB1 structural organization and probably its abberant pro-cancerous interactome. These observations support the hypothesis that, in addition of drugs, anti-senses or RNAis strategies that decrease HspB1 levels [6,13,222], therapeutic chemical drugs could be designed to directly destabilize small Hsps oligomeric structure and their pro-cancerous interactome networks.
Drugs that decrease HspB1 expression are well known but usually poorly specific. One can cite quercitin [223], berberin derivatives (EPO Patent 0813872-A1), paclitaxel [224], KNK437 [225] and interferon-γ which suppresses HspB1 basal transcription and promoter activity thus enhancing hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo [226,227]. Far more interesting drugs are those that directly interact with HspB1 and modulate its activity, here are some of them:
-
(1)
Biphenyl isooxazoles such as 5-(5-ethyl-2-hydroxy-4-methoxyphenyl)-4-(4-methoxyphenyl) isoxazole (KRIBB3) inhibit tumor cell migration and invasion by blocking protein kinase C-dependent phosphorylation of HspB1 through their direct binding to HspB1 [228].
-
(2)
RP101 (E)-5-(2-bromovinyl)-deoxyuridine, BVDU, brivudine), a 2'-deoxyuridine derivative (thymidine analogue). This well-known anti-viral drug, particularly efficient againt Herpes simplex virus (HSV-1) and varicella zoster virus (VZV), was recently reported to drastically enhance the efficiency of human pancreatic cancer chemotherapy. RP101 acts by inhibiting HspB1 interaction with pro-cancerous binding partners, such as cytochrome c, pro-caspase-3, and Akt1 [229]. This results in a stimulation of the activity of caspase-9 and apoptosis efficiency. In addition of being incorporated into viral DNAs and inhibiting the action of DNA polymerase and viral replication, RP101 can bind to a specific site of the N-terminal domain of HspB1 characterized by two phenylalanine residues (Phe29 and Phe33). This probably interferes with the dynamic oligomerization of this chaperone and its ability to recognize client proteins. In addition of being approved as an anti-viral drug, RP101 efficiency towards pancreatic cancer patients is actually in clinical phase II/III. Of interest, an increased efficiency has recently been reported for novel HspB1-targetting derivatives of RP101 [230].
-
(3)
Clerodane diterpenoids form a class of terpene derivatives biosynthesized in plants, particularly those of the Lamiaceae family. Among them, neo-clerodane diterpenoids from Salvia spp. were recently described as compounds inhibiting the proliferation of several human cancer cell lines. Chemical proteomics approach analyzing the cellular interactome of a representative clerodane diterpenoid molecule, hardwickiic acid (HAA), revealed HspB1 as a major HAA interacting protein partner in U937 leukemic cells [231]. The interaction was confirmed by several other approaches, including surface plasmon resonance measurements, limited proteolysis, and biochemical assays. Hence, the antitumor potential of clerodane diterpenoids may be linked to the inhibition HspB1 oncogenic properties. Moreover, this observation may be crucial for the future discovery of potent HspB1 diterpenoid-based chemical inhibitors.
Analyzing crucial modifications in the structure of small Hsps that play a role in cancer pathologies is another approach that could lead to the discovery of new drugs. In that respect, argpyrimidine modification could be of interest since this modification decreases HspB1 ability to bind cytochrome c and consequently alters its anti-apoptotic property [109]. The phenomenon is probably linked to the important structural role played by arginine residues in the conserved α-crystallin domain of small Hsps [76,78,232,233].
5. Conclusions
Additional to their up-regulated levels and broad molecular chaperone activities in stress conditions, small Hsps are now described to have an incredible number of fundamental functions in unstressed human cells [5,27,83,234]. The phenomenon is particularly intense in pathological cells where they show an apparent high level of constitutive expression. These multiple cellular functions result of the fundamental property of Hsps, first discovered in stressed cells, which consists in their interaction with client polypeptides to control folding. This activity can have several consequences: if the folding of the clients is not adapted to cellular conditions, small Hsps can participate in their refolding or degradation. Small Hsps can also artificially maintain their half-life by permanently interacting with them. The folding property of small Hsps can also modulate the activity of enzymes and/or trigger specific modifications to better adapt them to changes in cell physiology. Hence, the polypeptides that are controled by small Hsps could have crucial functions, particularly in pathological cells. For example, the protective role of small Hsps towards some clients could be beneficial against cell degeneration [22]. In contrast, other clients can be deleterious consequently of their ability to help cells to evade death and proliferate, such as cancer cells. In vew of all these observations, it was concluded that small Hsps are therapeutic targets. However, they are tricky ones since, depending on the pathology, their activities will have to be either stimulated or abolished.
The major drawback to understand the chaperone functions of small Hsps is the still not well characterized role played by their highly complex and dynamic combinatorial phospho-oligomeric structures that allow them to interact with a specific polypeptide in a define cellular condition. Despite this unsolved problem, the number of proteins that are nowdays discovered to interact with small Hsps is growing exponentially. Here, we have analyzed the putative pro-cancerous role of the major polypeptides that interact with HspB1, HspB5 and HspB4. It is of particular interest to note that these proteins are mainly engaged in processes that drastically modulate the development of tumors and their metastatic dissemination. For example, they can promote constitutive cell division by interacting with cytoplasmic receptors (VEGF, FGF-2, Her2) leading to enhanced activity of MAPK kinase/MEK/ERK pathway. HspB1, HspB5 and HspB4 are also particularly effective in inhibiting extrinsic and intrinsic apoptosis by using their own specific strategies. In that respect, they knock down key pro-apoptotic factors (cytochrome c, caspase-3, pro-apoptotic Bax, BclXs, tBid, P53, DAXX) and stimulate or override the anti-apoptotic activity of other factors (PEA-15, XIAP). Another important phenomenon highly stimulated by HspB1 is the survival pathway while the oncogene induced senescence (OIS) pathway appears inhibited. In that respect, the major targets are PTEN, Akt and the transcription factor STAT3 that induces expression of anti-apoptotic genes and promotes survival through cross talk with the Wnt/beta-catenin pathway [204,235]. Finally, HspB1 and HspB5 have been described to promote tumor progression, EMT and metastasis. In that regard, small Hsps are well-known regulators of the cytoskeleton and cellular matrix organization. Other crucial targets are SPARC, matrix metalloproteinase type 2 and β-catenin as well as still unknown ones involved in the up-regulation of pro-metastatic genes through STAT3/Twist signaling. Recently, reports have mentioned that tumor growth and cell ability to metastasize correlated with a fraction of the cellular content of HspB1 being localized in the plasma membrane and extracellular milieu, leading to a possible immunoregulatory or signaling activity such as the proangiogenic effect recently described to be associated to this circulating protein. Many other polypeptides are targeted, including modulators of inflammation, transcription and translation factors, regulators of protein stability and anti-oxidant enzymes, that all can have an impact on the development and maintenance of tumors. Hence, these studies lead to the apparent view that small Hsps interactomes could be controled by some cancer cells to promote their development. Further analysis of the phosphorylation patterns of HspB1, HspB5 and HspB4 will be required to test whether these modifications modulate their ability to negatively or positively modulate the pro-cancerous activity of their clients. Another parameter that generates even more complexity is the fact that, when expressed in the same cells, small Hsps can interact [236,237] and form multiple combinatorial chimeric hetero-oligomeric complexes that are differentially phosphorylated, such as HspB1 and HspB5 which are highly prone to interact [25,78]. Nothing is known concerning the interactomes of these chimeric structures that seem to have lost the properties associated to the parental homo-oligomers. Hence, these examples clearly illustrate the complexity associated to the interaction of small Hsps with defined polypeptides targets. More in-depth structural studies are urgently needed before comprehensive interactomes could be elaborated and used to search for specific therapeutic anti-cancer drugs.
Acknowledgments
We thank Patrick Mehlen for his continuous support.
Author Contributions
The bibliographical data were analyzed by André-Patrick Arrigo and Benjamin Gibert. André-Patrick Arrigo conceived and wrote the review and created the figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sherman M., Multhoff G. Heat shock proteins in cancer. Ann. NY Acad. Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- 2.Ciocca D.R., Arrigo A.P., Calderwood S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013;87:19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trepel J., Mollapour M., Giaccone G., Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderwood S.K. HSF1, a versatile factor in tumorogenesis. Curr. Mol. Med. 2012;12:1102–1107. doi: 10.2174/156652412803306675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrigo A.P., Simon S., Gibert B., Kretz-Remy C., Nivon M., Czekalla A., Guillet D., Moulin M., Diaz-Latoud C., Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Shiota M., Bishop J.L., Nip K.M., Zardan A., Takeuchi A., Cordonnier T., Beraldi E., Bazov J., Fazli L., Chi K., et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013;73:3109–3119. doi: 10.1158/0008-5472.CAN-12-3979. [DOI] [PubMed] [Google Scholar]
- 7.Mehlen P., Schulze-Osthoff K., Arrigo A.P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- 8.Kamradt M.C., Chen F., Cryns V.L. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 9.Garrido C., Brunet M., Didelot C., Zermati Y., Schmitt E., Kroemer G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:22. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 10.O’Callaghan-Sunol C., Gabai V.L., Sherman M.Y. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang H., Jiang W., Cheng W., Qian K., Dong W., Cao L., Huang Q., Li S., Dou F., Chiu J.F., et al. Down-regulation of HSP27 sensitizes TRAIL-resistant tumor cell to TRAIL-induced apoptosis. Lung Cancer. 2009;68:27–38. doi: 10.1016/j.lungcan.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Gibert B., Eckel B., Gonin V., Goldschneider D., Fombonne J., Deux B., Mehlen P., Arrigo A.P., Clezardin P., Diaz-Latoud C. Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumour formation in vivo. Br. J. Cancer. 2012;107:63–70. doi: 10.1038/bjc.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi N., Peacock J.W., Beraldi E., Zoubeidi A., Gleave M.E., Ong C.J. Hsp27 silencing coordinately inhibits proliferation and promotes Fas-induced apoptosis by regulating the PEA-15 molecular switch. Cell Death Differ. 2012;19:990–1002. doi: 10.1038/cdd.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido C., Fromentin A., Bonnotte B., Favre N., Moutet M., Arrigo A.P., Mehlen P., Solary E. Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res. 1998;58:5495–5499. [PubMed] [Google Scholar]
- 15.Bruey J.M., Paul C., Fromentin A., Hilpert S., Arrigo A.P., Solary E., Garrido C. Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene. 2000;19:4855–4863. doi: 10.1038/sj.onc.1203850. [DOI] [PubMed] [Google Scholar]
- 16.Gibert B., Hadchity E., Czekalla A., Aloy M.T., Colas P., Rodriguez-Lafrasse C., Arrigo A.P., Diaz-Latoud C. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene. 2011;34:3672–3681. doi: 10.1038/onc.2011.73. [DOI] [PubMed] [Google Scholar]
- 17.Lemieux P., Oesterreich S., Lawrence J.A., Steeg P.S., Hilsenbeck S.G., Harvey J.M., Fuqua S.A. The small heat shock protein hsp27 increases invasiveness but decreases motility of breast cancer cells. Invasion Metastasis. 1997;17:113–123. [PubMed] [Google Scholar]
- 18.Bausero M.A., Page D.T., Osinaga E., Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004;25:243–251. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaraja G.N., Kaur P., Asea A. Role of human and mouse HspB1 in metastasis. Curr. Mol. Med. 2012;12:1142–1150. doi: 10.2174/156652412803306701. [DOI] [PubMed] [Google Scholar]
- 20.Calderwood S.K., Khaleque M.A., Sawyer D.B., Ciocca D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Arrigo A.P., Gibert B. HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Curr. Mol. Med. 2012;12:1151–1163. doi: 10.2174/156652412803306693. [DOI] [PubMed] [Google Scholar]
- 22.Arrigo A., Simon S. Dual, Beneficial and Deleterous, Roles of Small Stress Proteins in Human Diseases: Implications for Therapeutic Strategies. In: Arrigo A.P., Simon S., editors. Small Stress Proteins in Human Diseases. Nova Sciences; New York, NY, USA: 2010. pp. 457–476. Book Serie: Protein Science Engineering. [Google Scholar]
- 23.Gibert B., Eckel B., Fasquelle L., Moulin M., Bouhallier F., Gonin V., Mellier G., Simon S., Kretz-Remy C., Arrigo A.P., et al. Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins. PLoS One. 2012;7:e29719. doi: 10.1371/journal.pone.0029719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arrigo A.P., Gibert B. Protein interactomes of three stress inducible small heat shock proteins: HspB1, HspB5 and HspB8. Int. J. Hyperth. 2013;29:409–422. doi: 10.3109/02656736.2013.792956. [DOI] [PubMed] [Google Scholar]
- 25.Arrigo A.P. Human small heat shock proteins: Protein interactomes of homo- and hetero-oligomeric complexes: An update. FEBS Lett. 2013;587:1959–1969. doi: 10.1016/j.febslet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Ciocca D.R., Calderwood S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrigo A.P. Pathology-dependent effects linked to small heat shock proteins expression. Scientifica. 2012;2012:19. doi: 10.6064/2012/185641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackburn R.V., Galoforo S.S., Berns C.M., Armour E.P., McEachern D., Corry P.M., Lee Y.J. Comparison of tumor growth between Hsp25- and Hsp27- transfected murine L929 cells in nude mice. Int. J. Cancer. 1997;72:871–877. doi: 10.1002/(SICI)1097-0215(19970904)72:5<871::AID-IJC26>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Katoh M., Koninkx J., Schumacher U. Heat shock protein expression in human tumours grown in severe combined immunodeficient mice. Cancer Lett. 2000;161:113–120. doi: 10.1016/S0304-3835(00)00601-7. [DOI] [PubMed] [Google Scholar]
- 30.Hsu H.S., Lin J.H., Huang W.C., Hsu T.W., Su K., Chiou S.H., Tsai Y.T., Hung S.C. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2011;117:1516–1528. doi: 10.1002/cncr.25599. [DOI] [PubMed] [Google Scholar]
- 31.Wei L., Liu T.T., Wang H.H., Hong H.M., Yu A.L., Feng H.P., Chang W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res. 2011;13:R101. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straume O., Shimamura T., Lampa M.J., Carretero J., Oyan A.M., Jia D., Borgman C.L., Soucheray M., Downing S.R., Short S.M., et al. Suppression of heat shock protein 27 induces long-term dormancy in human breast cancer. Proc. Natl. Acad. Sci. USA. 2012;109:8699–8704. doi: 10.1073/pnas.1017909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wettstein G., Bellaye P.S., Kolb M., Hammann A., Crestani B., Soler P., Marchal-Somme J., Hazoume A., Gauldie J., Gunther A., et al. Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- 34.Castro G.N., Cayado-Gutierrez N., Moncalero V.L., Lima P., de Angelis R.L., Chavez V., Cuello-Carrion F.D., Ciocca D.R. Hsp27 (HSPB1): A possible surrogate molecular marker for loss of heterozygosity (LOH) of chromosome 1p in oligodendrogliomas but not in astrocytomas. Cell Stress Chaperones. 2012;17:779–790. doi: 10.1007/s12192-012-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huot J., Roy G., Lambert H., Chretien P., Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252. [PubMed] [Google Scholar]
- 36.Richards E.H., Hickey E., Weber L.A., Master J.R. Effect of overexpression of the small heat shock protein HSP27 on the heat and drug sensitivities of human testis tumor cells. Cancer Res. 1996;56:2446–2451. [PubMed] [Google Scholar]
- 37.Arrigo A.P. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol. Biol. Paris. 2000;48:280–288. [PubMed] [Google Scholar]
- 38.Garrido C., Gurbuxani S., Ravagnan L., Kroemer G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 39.Kamada M., So A., Muramaki M., Rocchi P., Beraldi E., Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol. Cancer Ther. 2007;6:299–308. doi: 10.1158/1535-7163.MCT-06-0417. [DOI] [PubMed] [Google Scholar]
- 40.Kang S.H., Kang K.W., Kim K.H., Kwon B., Kim S.K., Lee H.Y., Kong S.Y., Lee E.S., Jang S.G., Yoo B.C. Upregulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer. 2008;8:286. doi: 10.1186/1471-2407-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kase S., Parikh J.G., Rao N.A. Expression of heat shock protein 27 and alpha-crystallins in human retinoblastoma after chemoreduction. Br. J. Ophthalmol. 2009;93:541–544. doi: 10.1136/bjo.2008.145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Shen X. Heat shock protein 27 protects L929 cells from cisplatin-induced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clin. Cancer Res. 2007;13:2855–2864. doi: 10.1158/1078-0432.CCR-06-2090. [DOI] [PubMed] [Google Scholar]
- 43.Chen R., Dai R.Y., Duan C.Y., Liu Y.P., Chen S.K., Yan D.M., Chen C.N., Wei M., Li H. Unfolded protein response suppresses cisplatin-induced apoptosis via autophagy regulation in human hepatocellular carcinoma cells. Folia Biol. Praha. 2011;57:87–95. doi: 10.14712/fb2011057030087. [DOI] [PubMed] [Google Scholar]
- 44.Fujita R., Ounzain S., Wang A.C., Heads R.J., Budhram-Mahadeo V.S. Hsp-27 induction requires POU4F2/Brn-3b TF in doxorubicin-treated breast cancer cells, whereas phosphorylation alters its cellular localisation following drug treatment. Cell Stress Chaperones. 2011;16:427–439. doi: 10.1007/s12192-011-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ounzain S., Bowen S., Patel C., Fujita R., Heads R.J., Budhram-Mahadeo V.S. Proliferation-associated POU4F2/Brn-3b transcription factor expression is regulated by oestrogen through ERalpha and growth factors via MAPK pathway. Breast Cancer Res. 2011;13:R5. doi: 10.1186/bcr2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oesterreich S., Schunck H., Benndorf R., Bielka H. Cisplatin induces the small heat shock protein hsp25 and thermotolerance in Ehrlich ascites tumor cells. Biochem. Biophys. Res. Commun. 1991;180:243–248. doi: 10.1016/S0006-291X(05)81283-5. [DOI] [PubMed] [Google Scholar]
- 47.Schafer C., Seeliger H., Bader D.C., Assmann G., Buchner D., Guo Y., Ziesch A., Palagyi A., Ochs S., Laubender R.P., et al. Heat shock protein 27 as a prognostic and predictive biomarker in pancreatic ductal adenocarcinoma. J. Cell Mol. Med. 2012;16:1776–1791. doi: 10.1111/j.1582-4934.2011.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori-Iwamoto S., Kuramitsu Y., Ryozawa S., Mikuria K., Fujimoto M., Maehara S., Maehara Y., Okita K., Nakamura K., Sakaida I. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int. J. Oncol. 2007;31:1345–1350. [PubMed] [Google Scholar]
- 49.Nakashima M., Adachi S., Yasuda I., Yamauchi T., Kawaguchi J., Itani M., Yoshioka T., Matsushima-Nishiwaki R., Hirose Y., Kozawa O., et al. Phosphorylation status of heat shock protein 27 plays a key role in gemcitabine-induced apoptosis of pancreatic cancer cells. Cancer Lett. 2011;313:218–225. doi: 10.1016/j.canlet.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D., Wong L.L., Koay E.S. Phosphorylation of Ser78 of Hsp27 correlated with HER-2/neu status and lymph node positivity in breast cancer. Mol. Cancer. 2007;6:52. doi: 10.1186/1476-4598-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul C., Simon S., Gibert B., Virot S., Manero F., Arrigo A.P. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27) Exp. Cell Res. 2010;316:1535–1552. doi: 10.1016/j.yexcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Tweedle E.M., Khattak I., Ang C.W., Nedjadi T., Jenkins R., Park B.K., Kalirai H., Dodson A., Azadeh B., Terlizzo M., et al. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut. 2010;59:1501–1510. doi: 10.1136/gut.2009.196626. [DOI] [PubMed] [Google Scholar]
- 53.Moyano J.V., Evans J.R., Chen F., Lu M., Werner M.E., Yehiely F., Diaz L.K., Turbin D., Karaca G., Wiley E., et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J. Clin. Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen P., Ji W., Liu F.Y., Tang H.Z., Fu S., Zhang X., Liu M., Gong L., Deng M., Hu W.F., et al. Alpha-crystallins and tumorigenesis. Curr. Mol. Med. 2012;12:1164–1173. doi: 10.2174/156652412803306747. [DOI] [PubMed] [Google Scholar]
- 55.Chelouche-Lev D., Kluger H.M., Berger A.J., Rimm D.L., Price J.E. alphaB-crystallin as a marker of lymph node involvement in breast carcinoma. Cancer. 2004;100:2543–2548. doi: 10.1002/cncr.20304. [DOI] [PubMed] [Google Scholar]
- 56.Kim H.S., Lee Y., Lim Y.A., Kang H.J., Kim L.S. AlphaB-crystallin is a novel oncoprotein associated with poor prognosis in breast cancer. J. Breast Cancer. 2011;14:14–19. doi: 10.4048/jbc.2011.14.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sitterding S.M., Wiseman W.R., Schiller C.L., Luan C., Chen F., Moyano J.V., Watkin W.G., Wiley E.L., Cryns V.L., Diaz L.K. AlphaB-crystallin: A novel marker of invasive basal-like and metaplastic breast carcinomas. Ann. Diagn. Pathol. 2008;12:33–40. doi: 10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Ivanov O., Chen F., Wiley E.L., Keswani A., Diaz L.K., Memmel H.C., Rademaker A., Gradishar W.J., Morrow M., Khan S.A., et al. alphaB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2008;111:411–417. doi: 10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- 59.Aoyama A., Steiger R., Frohli E., Schafer R., von Deimling A., Wiestler O., Klemenz R. Expression of alpha B-crystallin in human brain tumors. Int. J. Cancer. 1993;55:760–764. doi: 10.1002/ijc.2910550511. [DOI] [PubMed] [Google Scholar]
- 60.Khalil A.A. Biomarker discovery: A proteomic approach for brain cancer profiling. Cancer Sci. 2007;98:201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Q., Liu Y.F., Zhu X.J., Li Y.H., Zhu J., Zhang J.P., Feng Z.Q., Guan X.H. Expression and prognostic significance of the alpha B-crystallin gene in human hepatocellular carcinoma. Hum. Pathol. 2009;40:300–305. doi: 10.1016/j.humpath.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Liu X.L., Guo K.P., Ma F., Xie G.Y., He Y., Hu C.H. Expression profile of heat shock proteins in tissues and cells of lung adenocarcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:660–664. [PubMed] [Google Scholar]
- 63.Takashi M., Katsuno S., Sakata T., Ohshima S., Kato K. Different concentrations of two small stress proteins, alphaB crystallin and HSP27 in human urological tumor tissues. Urol. Res. 1998;26:395–399. doi: 10.1007/s002400050075. [DOI] [PubMed] [Google Scholar]
- 64.Bai F., Xi J.H., Wawrousek E.F., Fleming T.P., Andley U.P. Hyperproliferation and p53 status of lens epithelial cells derived from alphaB-crystallin knockout mice. J. Biol. Chem. 2003;278:36876–36886. doi: 10.1074/jbc.M304010200. [DOI] [PubMed] [Google Scholar]
- 65.Shi T., Dong F., Liou L.S., Duan Z.H., Novick A.C., DiDonato J.A. Differential protein profiling in renal-cell carcinoma. Mol. Carcinog. 2004;40:47–61. doi: 10.1002/mc.20015. [DOI] [PubMed] [Google Scholar]
- 66.Mineva I., Gartner W., Hauser P., Kainz A., Loffler M., Wolf G., Oberbauer R., Weissel M., Wagner L. Differential expression of alphaB-crystallin and Hsp27–1 in anaplastic thyroid carcinomas because of tumor-specific alphaB-crystallin gene (CRYAB) silencing. Cell Stress Chaperones. 2005;10:171–184. doi: 10.1379/CSC-107R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lung H.L., Lo C.C., Wong C.C., Cheung A.K., Cheong K.F., Wong N., Kwong F.M., Chan K.C., Law E.W., Tsao S.W., et al. Identification of tumor suppressive activity by irradiation microcell-mediated chromosome transfer and involvement of alpha B-crystallin in nasopharyngeal carcinoma. Int. J. Cancer. 2008;122:1288–1296. doi: 10.1002/ijc.23259. [DOI] [PubMed] [Google Scholar]
- 68.Solares C.A., Boyle G.M., Brown I., Parsons P.G., Panizza B. Reduced alphaB-crystallin staining in perineural invasion of head and neck cutaneous squamous cell carcinoma. Otolaryngol. Head. Neck. Surg. 2010;142:S15–S19. doi: 10.1016/j.otohns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Chen J., He Q.Y., Yuen A.P., Chiu J.F. Proteomics of buccal squamous cell carcinoma: The involvement of multiple pathways in tumorigenesis. Proteomics. 2004;4:2465–2475. doi: 10.1002/pmic.200300762. [DOI] [PubMed] [Google Scholar]
- 70.He Q.Y., Chen J., Kung H.F., Yuen A.P., Chiu J.F. Identification of tumor-associated proteins in oral tongue squamous cell carcinoma by proteomics. Proteomics. 2004;4:271–278. doi: 10.1002/pmic.200300550. [DOI] [PubMed] [Google Scholar]
- 71.Stronach E.A., Sellar G.C., Blenkiron C., Rabiasz G.J., Taylor K.J., Miller E.P., Massie C.E., Al-Nafussi A., Smyth J.F., Porteous D.J., et al. Identification of clinically relevant genes on chromosome 11 in a functional model of ovarian cancer tumor suppression. Cancer Res. 2003;63:8648–8655. [PubMed] [Google Scholar]
- 72.Bosman J.D., Yehiely F., Evans J.R., Cryns V.L. Regulation of alphaB-crystallin gene expression by the transcription factor Ets1 in breast cancer. Breast Cancer Res Treat. 2010;119:63–70. doi: 10.1007/s10549-009-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng M., Chen P.C., Xie S., Zhao J., Gong L., Liu J., Zhang L., Sun S., Liu J., Ma H., et al. The small heat shock protein alphaA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim. Biophys. Acta. 2010;1802:621–631. doi: 10.1016/j.bbadis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Mahon K.A., Chepelinsky A.B., Khillan J.S., Overbeek P.A., Piatigorsky J., Westphal H. Oncogenesis of the lens in transgenic mice. Science. 1987;235:1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- 75.Rigas P.K., Kase S., Rao N.A. Expression of alpha-crystallins in human sebaceous carcinoma of the eyelid. Eur. J. Ophthalmol. 2009;19:702–707. doi: 10.1177/112067210901900503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basha E., O’Neill H., Vierling E. Small heat shock proteins and alpha-crystallins: Dynamic proteins with flexible functions. Trends Biochem. Sci. 2011;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arrigo A.P. Structure-functions of HspB1 (Hsp27) Methods Mol. Biol. 2011;787:105–119. doi: 10.1007/978-1-61779-295-3_9. [DOI] [PubMed] [Google Scholar]
- 78.Simon S., Dimitrova V., Gibert B., Virot S., Mounier N., Nivon M., Kretz-Remy C., Corset V., Mehlen P., Arrigo A.P. Analysis of the dominant effects mediated by wild type or R120G mutant of alphaB-crystallin (HspB5) towards Hsp27 (HspB1) PLoS One. 2013;8:e70545. doi: 10.1371/journal.pone.0070545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arrigo A.-P., Suhan J.P., Welch W.J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol. Cell. Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehlen P., Arrigo A.-P. The serum-induced phosphorylation of mammalian hsp27 correlates with changes in its intracellular localization and levels of oligomerization. Eur. J. Biochem. 1994;221:327–334. doi: 10.1111/j.1432-1033.1994.tb18744.x. [DOI] [PubMed] [Google Scholar]
- 81.Mehlen P., Hickey E., Weber L., Arrigo A.-P. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem. Biophys. Res. Comm. 1997;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- 82.Garrido C. Size matters: Of the small HSP27 and its large oligomers. Cell Death Differ. 2002;9:483–485. doi: 10.1038/sj.cdd.4401005. [DOI] [PubMed] [Google Scholar]
- 83.Arrigo A.P. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv. Exp. Med. Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- 84.Stengel F., Baldwin A.J., Painter A.J., Jaya N., Basha E., Kay L.E., Vierling E., Robinson C.V., Benesch J.L. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc. Natl. Acad. Sci. USA. 2010;107:2007–2012. doi: 10.1073/pnas.0910126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ciocca D.R., Luque E.H. Immunological evidence for the identity between the hsp27 estrogen- regulated heat shock protein and the p29 estrogen receptor-associated protein in breast and endometrial cancer. Breast. Cancer Res. Treat. 1991;20:33–42. doi: 10.1007/BF01833355. [DOI] [PubMed] [Google Scholar]
- 86.Al-Madhoun A.S., Chen Y.X., Haidari L., Rayner K., Gerthoffer W., McBride H., O’Brien E.R. The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol. Cell Endocrinol. 2007;270:33–42. doi: 10.1016/j.mce.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Kerr B.A., Byzova T.V. alphaB-crystallin: A novel VEGF chaperone. Blood. 2010;115:3181–3183. doi: 10.1182/blood-2010-01-262766. [DOI] [PubMed] [Google Scholar]
- 88.Ghosh J.G., Shenoy A.K., Jr., Clark J.I. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry. 2007;46:6308–6317. doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- 89.Zoubeidi A., Zardan A., Beraldi E., Fazli L., Sowery R., Rennie P., Nelson C., Gleave M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67:10455–10465. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 90.Adhikari A.S., Singh B.N., Rao K.S., Rao Ch M. alphaB-crystallin, a small heat shock protein, modulates NF-kappaB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim. Biophys. Acta. 2011;1813:1532–1542. doi: 10.1016/j.bbamcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y., Liu J., Zhang Z., Huang H., Shen J., Zhang S., Jiang Y., Luo L., Yin Z. HSP27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cell. Signal. 2009;21:143–150. doi: 10.1016/j.cellsig.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Charette S.J., Landry J. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann. NY Acad. Sci. 2000;926:126–131. doi: 10.1111/j.1749-6632.2000.tb05606.x. [DOI] [PubMed] [Google Scholar]
- 93.Lee H.J., Lee Y.S. Repeated-dose toxicity of HSP27-binding heptapeptide in mice. Drug Chem. Toxicol. 2010;33:284–290. doi: 10.3109/01480540903483425. [DOI] [PubMed] [Google Scholar]
- 94.Patil S.B., Pawar M.D., Bitar K.N. Direct association and translocation of PKC-alpha with calponin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G954–G963. doi: 10.1152/ajpgi.00477.2003. [DOI] [PubMed] [Google Scholar]
- 95.Wu R., Kausar H., Johnson P., Montoya-Durango D.E., Merchant M., Rane M.J. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J. Biol. Chem. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 96.Liu J.P., Schlosser R., Ma W.Y., Dong Z., Feng H., Lui L., Huang X.Q., Liu Y., Li D.W. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp. Eye Res. 2004;79:393–403. doi: 10.1016/j.exer.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 97.Chebotareva N.A., Makeeva V.F., Bazhina S.G., Eronina T.B., Gusev N.B., Kurganov B.I. Interaction of Hsp27 with native phosphorylase kinase under crowding conditions. Macromol. Biosci. 2010;10:783–789. doi: 10.1002/mabi.200900397. [DOI] [PubMed] [Google Scholar]
- 98.Zoubeidi A., Zardan A., Wiedmann R.M., Locke J., Beraldi E., Fazli L., Gleave M.E. Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res. 2010;70:2307–2317. doi: 10.1158/0008-5472.CAN-09-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cayado-Gutierrez N., Moncalero V.L., Rosales E.M., Beron W., Salvatierra E.E., Alvarez-Olmedo D., Radrizzani M., Ciocca D.R. Downregulation of Hsp27 (HSPB1) in MCF-7 human breast cancer cells induces upregulation of PTEN. Cell Stress Chaperones. 2012;18:243–249. doi: 10.1007/s12192-012-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doppler H., Storz P., Li J., Comb M.J., Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J. Biol. Chem. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 101.Wang J., Huo K., Ma L., Tang L., Li D., Huang X., Yuan Y., Li C., Wang W., Guan W., et al. Toward an understanding of the protein interaction network of the human liver. Mol. Syst. Biol. 2011;7:536. doi: 10.1038/msb.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li D.W., Liu J.P., Mao Y.W., Xiang H., Wang J., Ma W.Y., Dong Z., Pike H.M., Brown R.E., Reed J.C. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol. Biol. Cell. 2005;16:4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dall’Era M.A., Oudes A., Martin D.B., Liu A.Y. HSP27 and HSP70 interact with CD10 in C4–2 prostate cancer cells. Prostate. 2007;67:714–721. doi: 10.1002/pros.20558. [DOI] [PubMed] [Google Scholar]
- 104.Van Noort J.M., Bsibsi M., Nacken P.J., Gerritsen W.H., Amor S., Holtman I.R., Boddeke E., van Ark I., Leusink-Muis T., Folkerts G., et al. Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials. 2013;34:831–840. doi: 10.1016/j.biomaterials.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 105.Thuringer D., Jego G., Wettstein G., Terrier O., Cronier L., Yousfi N., Hebrard S., Bouchot A., Hazoume A., Joly A.L., et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- 106.Pandey P., Farber R., Nakazawa A., Kumar S., Bharti A., Nalin C., Weichselbaum R., Kufe D., Kharbanda S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- 107.Hu W.F., Gong L., Cao Z., Ma H., Ji W., Deng M., Liu M., Hu X.H., Chen P., Yan Q., et al. alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr. Mol. Med. 2012;12:177–187. doi: 10.2174/156652412798889036. [DOI] [PubMed] [Google Scholar]
- 108.Bruey J.M., Ducasse C., Bonniaud P., Ravagnan L., Susin S.A., Diaz-Latoud C., Gurbuxani S., Arrigo A.P., Kroemer G., Solary E., et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell. Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 109.Padival A.K., Crabb J.W., Nagaraj R.H. Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells. FEBS Lett. 2003;551:113–118. doi: 10.1016/S0014-5793(03)00874-3. [DOI] [PubMed] [Google Scholar]
- 110.Mao Y.W., Liu J.P., Xiang H., Li D.W. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 111.Liu S., Li J., Tao Y., Xiao X. Small heat shock protein alphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem. Biophys. Res. Commun. 2007;354:109–114. doi: 10.1016/j.bbrc.2006.12.152. [DOI] [PubMed] [Google Scholar]
- 112.Beresford P.J., Jaju M., Friedman R.S., Yoon M.J., Lieberman J. A role for heat shock protein 27 in CTL-mediated cell death. J. Immunol. 1998;161:161–167. [PubMed] [Google Scholar]
- 113.Lee J.S., Kim H.Y., Jeong N.Y., Lee S.Y., Yoon Y.G., Choi Y.H., Yan C., Chu I.S., Koh H., Park H.T., et al. Expression of alphaB-crystallin overrides the anti-apoptotic activity of XIAP. Neuro. Oncol. 2012;14:1332–1345. doi: 10.1093/neuonc/nos247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arany I., Clark J.S., Reed D.K., Ember I., Juncos L.A. Cisplatin enhances interaction between p66Shc and HSP27: Its role in reorganization of the actin cytoskeleton in renal proximal tubule cells. Anticancer Res. 2012;32:4759–4763. [PubMed] [Google Scholar]
- 115.Preville X., Salvemini F., Giraud S., Chaufour S., Paul C., Stepien G., Ursini M.V., Arrigo A.P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 1999;247:61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- 116.Cosentino C., Grieco D., Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perng M.D., Cairns L., van den I.P., Prescott A., Hutcheson A.M., Quinlan R.A. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J. Cell Sci. 1999;112:2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- 118.Mounier N., Arrigo A.P. Actin cytoskeleton and small heat shock proteins: How do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ke L., Meijering R.A., Hoogstra-Berends F., Mackovicova K., Vos M.J., van Gelder I.C., Henning R.H., Kampinga H.H., Brundel B.J. HSPB1, HSPB6, HSPB7 and HSPB8 protect against RhoA GTPase-induced remodeling in tachypaced atrial myocytes. PLoS One. 2011;6:e20395. doi: 10.1371/journal.pone.0020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seit-Nebi A.S., Datskevich P., Gusev N.B. Commentary on paper: Small heat shock proteins and the cytoskeleton: An essential interplay for cell integrity? (Wettstein et al.) Int. J. Biochem. Cell Biol. 2013;45:344–346. doi: 10.1016/j.biocel.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 121.Del Vecchio P.J., MacElroy K.S., Rosser M.P., Church R.L. Association of alpha-crystallin with actin in cultured lens cells. Curr. Eye Res. 1984;3:1213–1219. doi: 10.3109/02713688409000824. [DOI] [PubMed] [Google Scholar]
- 122.Wang K., Spector A. alpha-Crystallin stabilizes actin filaments and prevents cytochalasin- induced depolymerization in a phosphorylation-dependent manner. Eur. J. Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- 123.Singh B.N., Rao K.S., Ramakrishna T., Rangaraj N., Rao Ch M. Association of alphab-crystallin, a small heat shock protein, with actin: Role in modulating actin filament dynamics in vivo. J. Mol. Biol. 2007;366:756–767. doi: 10.1016/j.jmb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 124.Ghosh J.G., Houck S.A., Clark J.I. Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int. J. Biochem. Cell Biol. 2007;39:1804–1815. doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hino M., Kurogi K., Okubo M.A., Murata-Hori M., Hosoya H. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem. Biophys. Res. Commun. 2000;271:164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- 126.Ohto-Fujita E., Fujita Y., Atomi Y. Analysis of the alphaB-crystallin domain responsible for inhibiting tubulin aggregation. Cell Stress Chaperones. 2007;12:163–171. doi: 10.1379/CSC-255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghosh J.G., Houck S.A., Clark J.I. Interactive domains in the molecular chaperone human alphaB crystallin modulate microtubule assembly and disassembly. PLoS One. 2007;2:e498. doi: 10.1371/journal.pone.0000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xi J.H., Bai F., McGaha R., Andley U.P. Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. FASEB J. 2006;20:846–857. doi: 10.1096/fj.05-5532com. [DOI] [PubMed] [Google Scholar]
- 129.Djabali K., de Nechaud B., Landon F., Portier M.M. AlphaB-crystallin interacts with intermediate filaments in response to stress. J. Cell Sci. 1997;110:2759–2769. doi: 10.1242/jcs.110.21.2759. [DOI] [PubMed] [Google Scholar]
- 130.Djabali K., Piron G., de Nechaud B., Portier M.M. alphaB-crystallin interacts with cytoplasmic intermediate filament bundles during mitosis. Exp. Cell Res. 1999;253:649–662. doi: 10.1006/excr.1999.4679. [DOI] [PubMed] [Google Scholar]
- 131.Fanelli M.A., Montt-Guevara M., Diblasi A.M., Gago F.E., Tello O., Cuello-Carrion F.D., Callegari E., Bausero M.A., Ciocca D.R. P-cadherin and beta-catenin are useful prognostic markers in breast cancer patients; beta-catenin interacts with heat shock protein Hsp27. Cell Stress Chaperones. 2008;13:207–220. doi: 10.1007/s12192-007-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thedieck C., Kalbacher H., Kratzer U., Lammers R., Stevanovic S., Klein G. alpha B-crystallin is a cytoplasmic interaction partner of the kidney-specific cadherin-16. J. Mol. Biol. 2008;378:145–153. doi: 10.1016/j.jmb.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 133.Muchowski P.J., Valdez M.M., Clark J.I. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest. Ophthalmol. Vis. Sci. 1999;40:951–958. [PubMed] [Google Scholar]
- 134.Barton K.A., Hsu C.D., Petrash J.M. Interactions between small heat shock protein alpha-crystallin and galectin-related interfiber protein (GRIFIN) in the ocular lens. Biochemistry. 2009;48:3956–3966. doi: 10.1021/bi802203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rocchi P., Beraldi E., Ettinger S., Fazli L., Vessella R.L., Nelson C., Gleave M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65:11083–11093. doi: 10.1158/0008-5472.CAN-05-1840. [DOI] [PubMed] [Google Scholar]
- 136.Brunet Simioni M., de Thonel A., Hammann A., Joly A.L., Bossis G., Fourmaux E., Bouchot A., Landry J., Piechaczyk M., Garrido C. Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene. 2009;28:3332–3344. doi: 10.1038/onc.2009.188. [DOI] [PubMed] [Google Scholar]
- 137.De Thonel A., Vandekerckhove J., Lanneau D., Selvakumar S., Courtois G., Hazoume A., Brunet M., Maurel S., Hammann A., Ribeil J.A., et al. HSP27 controls GATA-1 protein level during erythroid cell differentiation. Blood. 2010;116:85–96. doi: 10.1182/blood-2009-09-241778. [DOI] [PubMed] [Google Scholar]
- 138.Cuesta R., Laroia G., Schneider R.J. Chaperone Hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 2000;14:1460–1470. [PMC free article] [PubMed] [Google Scholar]
- 139.Andrieu C., Taieb D., Baylot V., Ettinger S., Soubeyran P., De-Thonel A., Nelson C., Garrido C., So A., Fazli L., et al. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene. 2010;29:1883–1896. doi: 10.1038/onc.2009.479. [DOI] [PubMed] [Google Scholar]
- 140.Sinsimer K.S., Gratacos F.M., Knapinska A.M., Lu J., Krause C.D., Wierzbowski A.V., Maher L.R., Scrudato S., Rivera Y.M., Gupta S., et al. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol. Cell. Biol. 2008;28:5223–5237. doi: 10.1128/MCB.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Knapinska A.M., Gratacos F.M., Krause C.D., Hernandez K., Jensen A.G., Bradley J.J., Wu X., Pestka S., Brewer G. Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol. Cell. Biol. 2011;31:1419–1431. doi: 10.1128/MCB.00907-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun Y., Zhou M., Fu D., Xu B., Fang T., Ma Y., Chen J., Zhang J. Ubiquitination of heat shock protein 27 is mediated by its interaction with Smad ubiquitination regulatory factor 2 in A549 cells. Exp. Lung Res. 2011;37:568–573. doi: 10.3109/01902148.2011.619627. [DOI] [PubMed] [Google Scholar]
- 143.Parcellier A., Brunet M., Schmitt E., Col E., Didelot C., Hammann A., Nakayama K., Nakayama K.I., Khochbin S., Solary E., et al. HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. FASEB J. 2006;20:1179–1181. doi: 10.1096/fj.05-4184fje. [DOI] [PubMed] [Google Scholar]
- 144.Parcellier A., Schmitt E., Gurbuxani S., Seigneurin-Berny D., Pance A., Chantome A., Plenchette S., Khochbin S., Solary E., Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol. Cell. Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin D.I., Barbash O., Kumar K.G., Weber J.D., Harper J.W., Klein-Szanto A.J., Rustgi A., Fuchs S.Y., Diehl J.A. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol. Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boelens W.C., Croes Y., de Jong W.W. Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7. Biochim. Biophys. Acta. 2001;1544:311–319. doi: 10.1016/s0167-4838(00)00243-0. [DOI] [PubMed] [Google Scholar]
- 147.Mehlen P., Mehlen A., Godet J., Arrigo A.-P. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J. Biol. Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- 148.Arrigo A.P. Editorial: Heat shock proteins in cancer. Curr. Mol. Med. 2012;12:1099–1101. doi: 10.2174/156652412803306738. [DOI] [PubMed] [Google Scholar]
- 149.Paul C., Manero F., Gonin S., Kretz-Remy C., Virot S., Arrigo A.P. Hsp27 as a negative regulator of cytochrome C release. Mol. Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chauhan D., Li G., Hideshima T., Podar K., Mitsiades C., Mitsiades N., Catley L., Tai Y.T., Hayashi T., Shringarpure R., et al. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- 151.Garrido C., Bruey J.M., Fromentin A., Hammann A., Arrigo A.P., Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 152.Mehlen P., Préville X., Chareyron P., Briolay J., Klemenz R., Arrigo A.-P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J. Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- 153.Charette S.J., Lavoie J.N., Lambert H., Landry J. Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 2000;20:7602–7612. doi: 10.1128/MCB.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rane M.J., Pan Y., Singh S., Powell D.W., Wu R., Cummins T., Chen Q., McLeish K.R., Klein J.B. Heat shock protein 27 controls apoptosis by regulating Akt activation. J. Biol. Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 155.Golembieski W.A., Thomas S.L., Schultz C.R., Yunker C.K., McClung H.M., Lemke N., Cazacu S., Barker T., Sage E.H., Brodie C., et al. HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia. 2008;56:1061–1075. doi: 10.1002/glia.20679. [DOI] [PubMed] [Google Scholar]
- 156.McClung H.M., Golembieski W.A., Schultz C.R., Jankowski M., Schultz L.R., Rempel S.A. Deletion of the SPARC acidic domain or EGF-like module reduces SPARC-induced migration and signaling through p38 MAPK/HSP27 in glioma. Carcinogenesis. 2012;33:275–284. doi: 10.1093/carcin/bgr276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Andley U.P., Song Z., Wawrousek E.F., Fleming T.P., Bassnett S. Differential protective activity of {alpha}A- and {alpha}B-crystallin in lens epithelial cells. J. Biol. Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 158.Launay N., Tarze A., Vicart P., Lilienbaum A. Serine 59 phosphorylation of {alpha}B-crystallin down-regulates its anti-apoptotic function by binding and sequestering Bcl-2 in breast cancer cells. J. Biol. Chem. 2010;285:37324–37332. doi: 10.1074/jbc.M110.124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee Y.S., Lim K.H., Guo X., Kawaguchi Y., Gao Y., Barrientos T., Ordentlich P., Wang X.F., Counter C.M., Yao T.P. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res. 2008;68:7561–7569. doi: 10.1158/0008-5472.CAN-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ghosh A., Lai C., McDonald S., Suraweera N., Sengupta N., Propper D., Dorudi S., Silver A. HSP27 expression in primary colorectal cancers is dependent on mutation of KRAS and PI3K/AKT activation status and is independent of TP53. Exp. Mol. Pathol. 2013;94:103–108. doi: 10.1016/j.yexmp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 161.Yang Y., Ludwig R.L., Jensen J.P., Pierre S.A., Medaglia M.V., Davydov I.V., Safiran Y.J., Oberoi P., Kenten J.H., Phillips A.C., et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547–559. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 162.Bausero M.A., Bharti A., Page D.T., Perez K.D., Eng J.W., Ordonez S.L., Asea E.E., Jantschitsch C., Kindas-Muegge I., Ciocca D., et al. Silencing the hsp25 gene eliminates migration capability of the highly metastatic murine 4T1 breast adenocarcinoma cell. Tumour Biol. 2006;27:17–26. doi: 10.1159/000090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ciocca D.R., Fanelli M.A., Cuello-Carrion F.D., Castro G.N. Heat shock proteins in prostate cancer: From tumorigenesis to the clinic. Int. J. Hyperth. 2010;26:737–747. doi: 10.3109/02656731003776968. [DOI] [PubMed] [Google Scholar]
- 164.Lavoie J.N., Hickey E., Weber L.A., Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of Heat Shock Protein 27. J. Biol. Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 165.Huot J., Houle F., Spitz D.R., Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 166.Dalle-Donne I., Rossi R., Milzani A., di Simplicio P., Colombo R. The actin cytoskeleton response to oxidants: From small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 2001;31:1624–1632. doi: 10.1016/S0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- 167.Wettstein G., Bellaye P.S., Micheau O., Bonniaud P. Small heat shock proteins and the cytoskeleton: An essential interplay for cell integrity? Int. J. Biochem. Cell Biol. 2012;44:1680–1686. doi: 10.1016/j.biocel.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 168.Xu L., Chen S., Bergan R.C. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25:2987–2998. doi: 10.1038/sj.onc.1209337. [DOI] [PubMed] [Google Scholar]
- 169.Yoshimura T., Nagahara M., Kuo C., Turner R.R., Soon-Shiong P., Hoon D.S. Lymphovascular invasion of colorectal cancer is correlated to SPARC expression in the tumor stromal microenvironment. Epigenetics. 2011;6:1001–1011. doi: 10.4161/epi.6.8.16063. [DOI] [PubMed] [Google Scholar]
- 170.Schultz C.R., Golembieski W.A., King D.A., Brown S.L., Brodie C., Rempel S.A. Inhibition of HSP27 alone or in combination with pAKT inhibition as therapeutic approaches to target SPARC-induced glioma cell survival. Mol. Cancer. 2012;11:20. doi: 10.1186/1476-4598-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nagaraja G.M., Kaur P., Neumann W., Asea E.E., Bausero M.A., Multhoff G., Asea A. Silencing Hsp25/Hsp27 gene expression augments proteasome activity and increases CD8+ T-cell-mediated tumor killing and memory responses. Cancer Prev. Res. Phila. 2012;5:122–137. doi: 10.1158/1940-6207.CAPR-11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gruvberger-Saal S.K., Parsons R. Is the small heat shock protein alphaB-crystallin an oncogene? J. Clin. Invest. 2006;116:30–32. doi: 10.1172/JCI27462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tsvetkova N.M., Horvath I., Torok Z., Wolkers W.F., Balogi Z., Shigapova N., Crowe L.M., Tablin F., Vierling E., Crowe J.H., et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chowdary T.K., Bakthisaran R., Tangirala R., Rao M.C. Interaction of mammalian Hsp22 with lipid membranes. Biochem. J. 2006;401:437–445. doi: 10.1042/BJ20061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rayner K., Chen Y.X., McNulty M., Simard T., Zhao X., Wells D.J., de Belleroche J., O’Brien E.R. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ. Res. 2008;103:133–141. doi: 10.1161/CIRCRESAHA.108.172155. [DOI] [PubMed] [Google Scholar]
- 177.Delneste Y., Magistrelli G., Gauchat J., Haeuw J., Aubry J., Nakamura K., Kawakami-Honda N., Goetsch L., Sawamura T., Bonnefoy J., et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/S1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 178.Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J.P., Boireau W., Rouleau A., Simon B., Lanneau D., et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salari S., Seibert T., Chen Y.X., Hu T., Shi C., Zhao X., Cuerrier C.M., Raizman J.E., O’Brien E.R. Extracellular HSP27 acts as a signaling molecule to activate NF-kappaB in macrophages. Cell Stress Chaperones. 2012;18:53–63. doi: 10.1007/s12192-012-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gruden G., Bruno G., Chaturvedi N., Burt D., Schalkwijk C., Pinach S., Stehouwer C.D., Witte D.R., Fuller J.H., Perin P.C. Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: A novel circulating marker for diabetic neuropathy. Diabetes. 2008;57:1966–1970. doi: 10.2337/db08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Joachim S.C., Bruns K., Lackner K.J., Pfeiffer N., Grus F.H. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr. Eye Res. 2007;32:501–509. doi: 10.1080/02713680701375183. [DOI] [PubMed] [Google Scholar]
- 182.Tezel G., Wax M.B. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J. Neurosci. 2000;20:3552–3562. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 184.Alford K.A., Glennie S., Turrell B.R., Rawlinson L., Saklatvala J., Dean J.L. HSP27 functions in inflammatory gene expression and TAK1-mediated signalling. J. Biol. Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 185.Rothbard J.B., Kurnellas M.P., Brownell S., Adams C.M., Su L., Axtell R.C., Chen R., Fathman C.G., Robinson W.H., Steinman L. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J. Biol. Chem. 2012;287:9708–9721. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mehlen P., Préville X., Kretz-Remy C., Arrigo A.-P. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these protein against TNFα-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 187.Arrigo A.P. Small stress proteins: Chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- 188.Park Y.M., Han M.Y., Blackburn R.V., Lee Y.J. Overexpression of HSP25 reduces the level of TNF alpha-induced oxidative DNA damage biomarker, 8-hydroxy-2’-deoxyguanosine, in L929 cells. J. Cell. Physiol. 1998;174:27–34. doi: 10.1002/(SICI)1097-4652(199801)174:1<27::AID-JCP4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 189.Rogalla T., Ehrnsperger M., Preville X., Kotlyarov A., Lutsch G., Ducasse C., Paul C., Wieske M., Arrigo A.P., Buchner J., et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 190.Arrigo A.P. Hsp27: Novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 191.Merendino A.M., Paul C., Vignola A.M., Costa M.A., Melis M., Chiappara G., Izzo V., Bousquet J., Arrigo A.P. Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: Possible implication in asthma. Cell Stress Chaperones. 2002;7:269–280. doi: 10.1379/1466-1268(2002)007<0269:HSPPHB>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wyttenbach A., Sauvageot O., Carmichael J., Diaz-Latoud C., Arrigo A.P., Rubinsztein D.C. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 193.Yan L.J., Christians E.S., Liu L., Xiao X., Sohal R.S., Benjamin I.J. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Arrigo A.P., Virot S., Chaufour S., Firdaus W., Kretz-Remy C., Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid. Redox Signal. 2005;7:414–422. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- 195.Firdaus W.J., Wyttenbach A., Diaz-Latoud C., Currie R.W., Arrigo A.P. Analysis of oxidative events induced by expanded polyglutamine huntingtin exon 1 that are differentially restored by expression of heat shock proteins or treatment with an antioxidant. FEBS J. 2006;273:3076–3093. doi: 10.1111/j.1742-4658.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- 196.Aloy M.T., Hadchity E., Bionda C., Diaz-Latoud C., Claude L., Rousson R., Arrigo A.P., Rodriguez-Lafrasse C. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:543–553. doi: 10.1016/j.ijrobp.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 197.Christians E.S., Ishiwata T., Benjamin I.J. Small heat shock proteins in redox metabolism: Implications for cardiovascular diseases. Int. J. Biochem. Cell Biol. 2012;44:1632–1645. doi: 10.1016/j.biocel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen H., Zheng C., Zhang Y., Chang Y.Z., Qian Z.M., Shen X. Heat shock protein 27 downregulates the transferrin receptor 1-mediated iron uptake. Int. J. Biochem. Cell Biol. 2006;38:1402–1416. doi: 10.1016/j.biocel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 199.Preville X., Gaestel M., Arrigo A.P. Phosphorylation is not essential for protection of L929 cells by Hsp25 against H2O2-mediated disruption actin cytoskeleton, a protection which appears related to the redox change mediated by Hsp25. Cell Stress Chaperones. 1998;3:177–187. doi: 10.1379/1466-1268(1998)003<0177:PINEFP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim E.H., Lee H.J., Lee D.H., Bae S., Soh J.W., Jeoung D., Kim J., Cho C.K., Lee Y.J., Lee Y.S. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res. 2007;67:6333–6341. doi: 10.1158/0008-5472.CAN-06-4344. [DOI] [PubMed] [Google Scholar]
- 201.Kuo W.Y., Tang T.K. Effects of G6PD overexpression in NIH3T3 cells treated with tert-butyl hydroperoxide or paraquat. Free Radic. Biol. Med. 1998;24:1130–1138. doi: 10.1016/S0891-5849(97)00413-9. [DOI] [PubMed] [Google Scholar]
- 202.Salvemini F., Franze A., Iervolino A., Filosa S., Salzano S., Ursini M.V. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J. Biol. Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- 203.McCollum A.K., Teneyck C.J., Sauer B.M., Toft D.O., Erlichman C. Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res. 2006;66:10967–10975. doi: 10.1158/0008-5472.CAN-06-1629. [DOI] [PubMed] [Google Scholar]
- 204.Aoki Y., Feldman G.M., Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 205.Brierley M.M., Fish E.N. Stats: Multifaceted regulators of transcription. J. Interferon Cytokine Res. 2005;25:733–744. doi: 10.1089/jir.2005.25.733. [DOI] [PubMed] [Google Scholar]
- 206.Gritsko T., Williams A., Turkson J., Kaneko S., Bowman T., Huang M., Nam S., Eweis I., Diaz N., Sullivan D., et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin. Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 207.Diaz N., Minton S., Cox C., Bowman T., Gritsko T., Garcia R., Eweis I., Wloch M., Livingston S., Seijo E., et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin. Cancer Res. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 208.Song H., Ethier S.P., Dziubinski M.L., Lin J. Stat3 modulates heat shock 27 kDa protein expression in breast epithelial cells. Biochem. Biophys. Res. Commun. 2004;314:143–150. doi: 10.1016/j.bbrc.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 209.Silva C.M. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017–8023. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 210.Yuan Z.L., Guan Y.J., Wang L., Wei W., Kane A.B., Chin Y.E. Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol. Cell Biol. 2004;24:9390–9400. doi: 10.1128/MCB.24.21.9390-9400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.De la Iglesia N., Konopka G., Puram S.V., Chan J.A., Bachoo R.M., You M.J., Levy D.E., Depinho R.A., Bonni A. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev. 2008;22:449–462. doi: 10.1101/gad.1606508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee J., Kim J.C., Lee S.E., Quinley C., Kim H., Herdman S., Corr M., Raz E. Signal transducer and activator of transcription 3 (STAT3) protein suppresses adenoma-to-carcinoma transition in Apcmin/+ mice via regulation of Snail-1 (SNAI) protein stability. J. Biol. Chem. 2012;287:18182–18189. doi: 10.1074/jbc.M111.328831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zheng R., Blobel G.A. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–1188. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Diaz-Latoud C., Buache E., Javouhey E., Arrigo A.P. Substitution of the unique cysteine residue of murine hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid. Redox. Signal. 2005;7:436–445. doi: 10.1089/ars.2005.7.436. [DOI] [PubMed] [Google Scholar]
- 215.Ghosh J.G., Houck S.A., Clark J.I. Interactive sequences in the molecular chaperone, human alphaB crystallin modulate the fibrillation of amyloidogenic proteins. Int. J. Biochem. Cell Biol. 2008;40:954–967. doi: 10.1016/j.biocel.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Colas P., Cohen B., Jessen T., Grishina I., McCoy J., Brent R. Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature. 1996;380:548–550. doi: 10.1038/380548a0. [DOI] [PubMed] [Google Scholar]
- 217.De Chassey B., Mikaelian I., Mathieu A.L., Bickle M.B., Olivier D., Negre D., Cosset F.L., Rudkin B.B., Colas P. An antiproliferative genetic screening identifies a peptide aptamer that targets calcineurin and upregulates its activity. Mol. Cell. Proteomics. 2006;6:551–549. doi: 10.1074/mcp.M600102-MCP200. [DOI] [PubMed] [Google Scholar]
- 218.Bickle M.B., Dusserre E., Moncorge O., Bottin H., Colas P. Selection and characterization of large collections of peptide aptamers through optimized yeast two-hybrid procedures. Nat. Protoc. 2006;1:1066–1091. doi: 10.1038/nprot.2006.32. [DOI] [PubMed] [Google Scholar]
- 219.Nouvion A.L., Thibaut J., Lohez O.D., Venet S., Colas P., Gillet G., Lalle P. Modulation of Nr-13 antideath activity by peptide aptamers. Oncogene. 2007;26:701–710. doi: 10.1038/sj.onc.1209832. [DOI] [PubMed] [Google Scholar]
- 220.Rerole A.L., Gobbo J., de Thonel A., Schmitt E., Pais de Barros J.P., Hammann A., Lanneau D., Fourmaux E., Deminov O., Micheau O., et al. Peptides and aptamers targeting HSP70: A novel approach for anticancer chemotherapy. Cancer Res. 2011;71:484–495. doi: 10.1158/0008-5472.CAN-10-1443. [DOI] [PubMed] [Google Scholar]
- 221.Gibert B., Simon S., Dimitrova V., Diaz-Latoud C., Arrigo A.P. Peptide aptamers: Tools to negatively or positively modulate HSPB1(27) function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120075. doi: 10.1098/rstb.2012.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rocchi P., Jugpal P., So A., Sinneman S., Ettinger S., Fazli L., Nelson C., Gleave M. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006;28:28. doi: 10.1111/j.1464-410X.2006.06425.x. [DOI] [PubMed] [Google Scholar]
- 223.Morino M., Tsuzuki T., Ishikawa Y., Shirakami T., Yoshimura M., Kiyosuke Y., Matsunaga K., Yoshikumi C., Saijo N. Specific regulation of HSPs in human tumor cell lines by flavonoids. In Vivo. 1997;11:265–270. [PubMed] [Google Scholar]
- 224.Tanaka Y., Fujiwara K., Tanaka H., Maehata K., Kohno I. Paclitaxel inhibits expression of heat shock protein 27 in ovarian and uterine cancer cells. Int. J. Gynecol. Cancer. 2004;14:616–620. doi: 10.1111/j.1048-891X.2004.14409.x. [DOI] [PubMed] [Google Scholar]
- 225.Taba K., Kuramitsu Y., Ryozawa S., Yoshida K., Tanaka T., Mori-Iwamoto S., Maehara S., Maehara Y., Sakaida I., Nakamura K. KNK437 downregulates heat shock protein 27 of pancreatic cancer cells and enhances the cytotoxic effect of gemcitabine. Chemotherapy. 2011;57:12–16. doi: 10.1159/000321019. [DOI] [PubMed] [Google Scholar]
- 226.Oba M., Yano S., Shuto T., Suico M.A., Eguma A., Kai H. IFN-gamma down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int. J. Oncol. 2008;32:1317–1324. doi: 10.3892/ijo_32_6_1317. [DOI] [PubMed] [Google Scholar]
- 227.Kuramitsu Y., Wang Y., Taba K., Suenaga S., Ryozawa S., Kaino S., Sakaida I., Nakamura K. Heat-shock protein 27 plays the key role in gemcitabine-resistance of pancreatic cancer cells. Anticancer Res. 2012;32:2295–2299. [PubMed] [Google Scholar]
- 228.Shin K.D., Lee M.Y., Shin D.S., Lee S., Son K.H., Koh S., Paik Y.K., Kwon B.M., Han D.C. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J. Biol. Chem. 2005;280:41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 229.Heinrich J.C., Tuukkanen A., Schroeder M., Fahrig T., Fahrig R. RP101 (brivudine) binds to heat shock protein HSP27 (HSPB1) and enhances survival in animals and pancreatic cancer patients. J. Cancer Res. Clin. Oncol. 2011;137:1349–1361. doi: 10.1007/s00432-011-1005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Resprotect—Prevention of Chemoresistance. [(Accessed on 16 November 2013)]. Available online: http://www.resprotect.de/Pipeline-Summary/Pipeline-Summary.html/
- 231.Faiella L., Piaz F.D., Bisio A., Tosco A., de Tommasi N. A chemical proteomics approach reveals Hsp27 as a target for proapoptotic clerodane diterpenes. Mol. Biosyst. 2012;8:2637–2644. doi: 10.1039/c2mb25171j. [DOI] [PubMed] [Google Scholar]
- 232.Simon S., Michiel M., Skouri-Panet F., Lechaire J.P., Vicart P., Tardieu A. Residue R120 is essential for the quaternary structure and functional integrity of human alphaB-crystallin. Biochemistry. 2007;46:9605–9614. doi: 10.1021/bi7003125. [DOI] [PubMed] [Google Scholar]
- 233.Bagneris C., Bateman O.A., Naylor C.E., Cronin N., Boelens W.C., Keep N.H., Slingsby C. Crystal structures of alpha-crystallin domain dimers of alphab-crystallin and hsp20. J. Mol. Biol. 2009;392:1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 234.Mymrikov E.V., Seit-Nebi A.S., Gusev N.B. Large potentials of small heat shock proteins. Physiol. Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 235.Fragoso M.A., Patel A.K., Nakamura R.E., Yi H., Surapaneni K., Hackam A.S. The Wnt/beta-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells. PLoS One. 2012;7:e46892. doi: 10.1371/journal.pone.0046892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zantema A., Vries M.V.-D., Maasdam D., Bol S., Eb A.V.D. Heat shock protein 27 and αB-cristallin can form a complex, which dissociates by heat shock. J. Biol. Chem. 1992;267:12936–12941. [PubMed] [Google Scholar]
- 237.Mymrikov E.V., Seit-Nebi A.S., Gusev N.B. Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones. 2012;17:157–169. doi: 10.1007/s12192-011-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]