Abstract
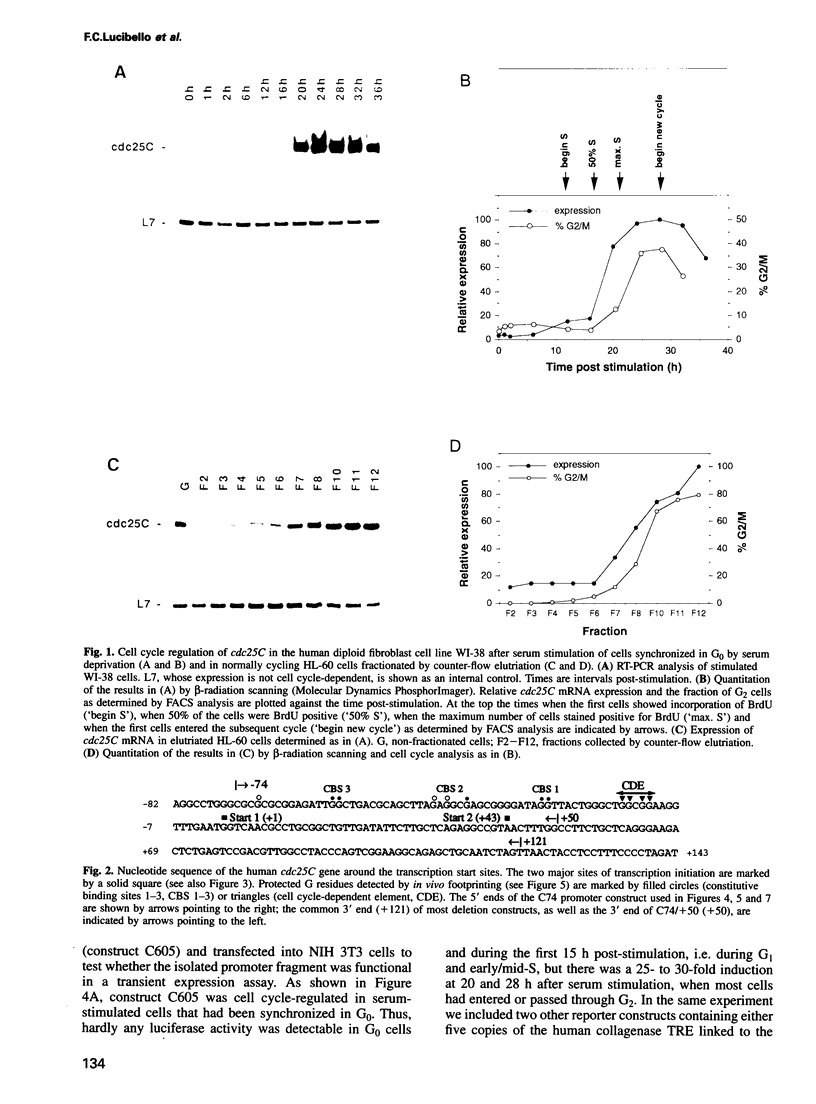
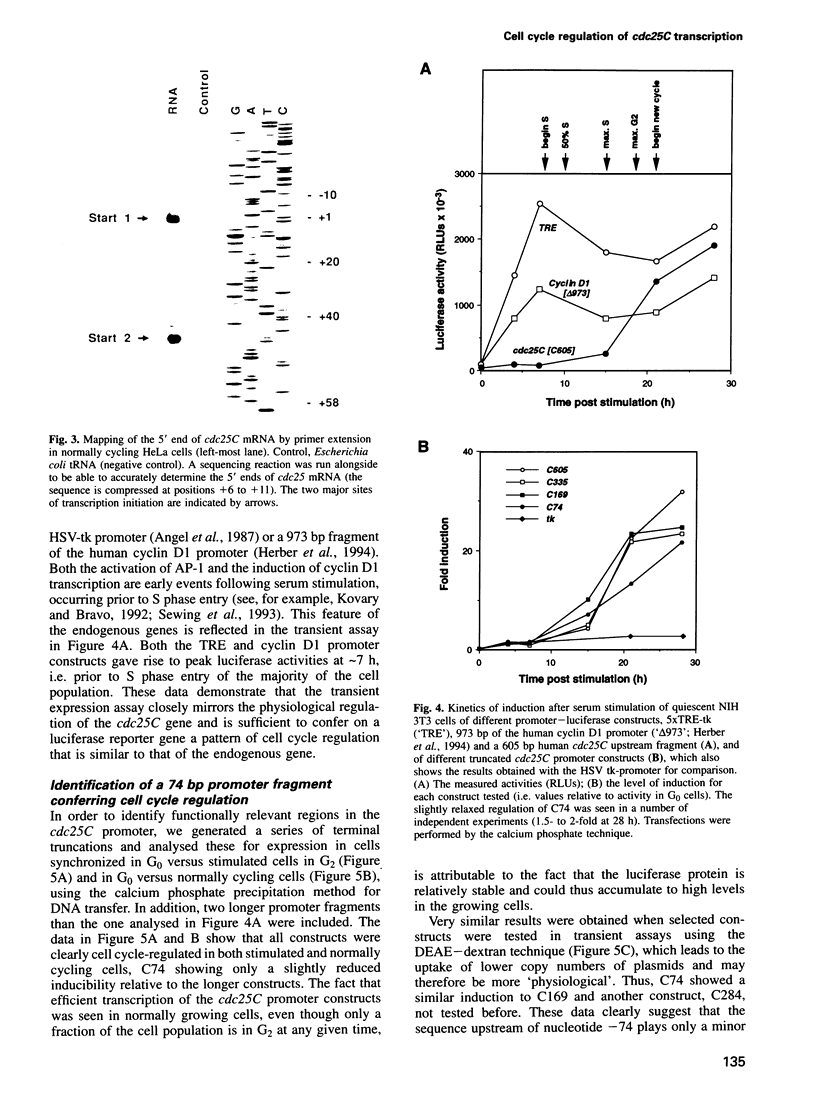
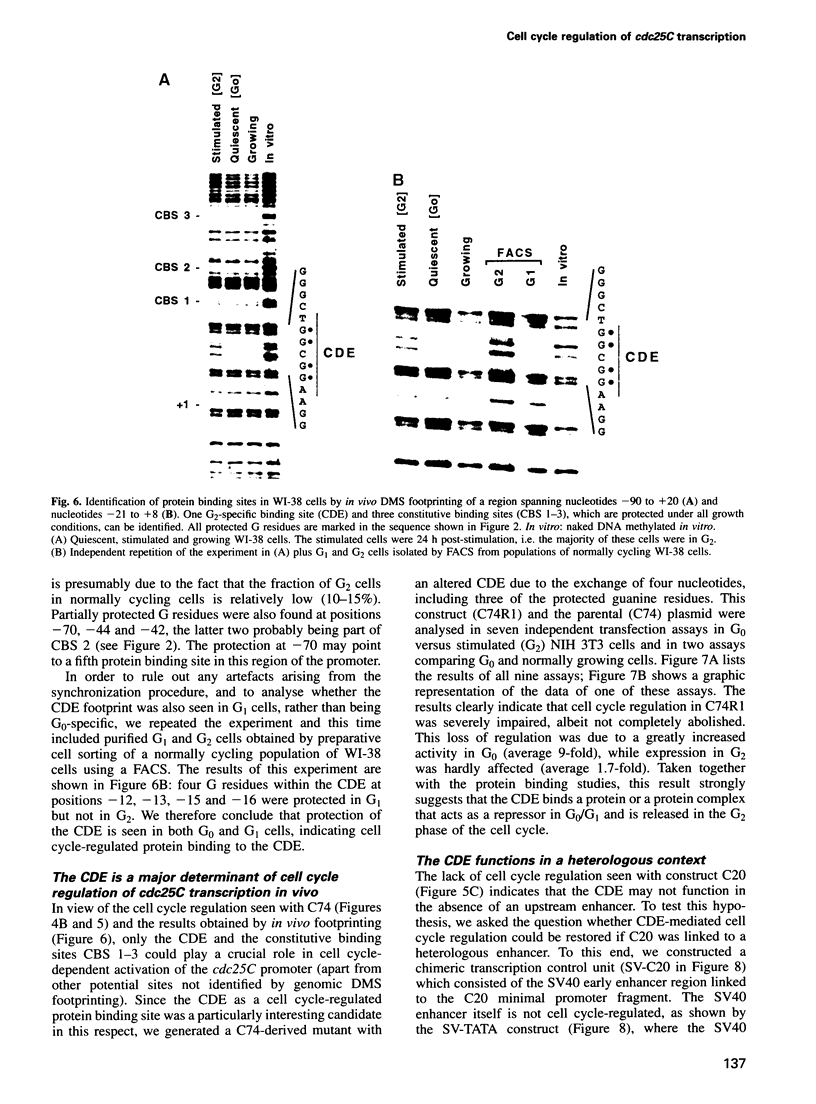
We show that the cell cycle-regulated transcription of the TATA-less cdc25C gene in late S/G2 is largely mediated by a novel promoter element (CDE) located directly 5' to one of the two major transcription initiation sites. Genomic dimethylsulfate footprinting experiments, using either synchronized or sorted normally cycling cells, show the formation in vivo of a CDE-protein complex in both G0 and G1 cells and its dissociation in G2. Mutation of the CDE severely impairs cell cycle regulation of the cdc25C promoter and results in high expression in G0/G1, indicating that the CDE functions as a cell cycle-regulated cis-acting repressor element. Cell cycle regulation is also lost upon removal of the enhancer region located immediately upstream of the CDE, but is largely restored when this enhancerless minimal cdc25C promoter fragment is linked to the constitutive SV40 early enhancer. This indicates that the CDE is dependent on the presence of a transcriptional enhancer to effect cell cycle regulation. Our observations suggest that the periodic activation of the cdc25C gene in late S/G2 is brought about, at least in part, by a unique regulatory mechanism involving the cell cycle-regulated dissociation of a repressor from the CDE.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abken H., Reifenrath B. A procedure to standardize CAT reporter gene assay. Nucleic Acids Res. 1992 Jul 11;20(13):3527–3527. doi: 10.1093/nar/20.13.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Belyavsky A., Vinogradova T., Rajewsky K. PCR-based cDNA library construction: general cDNA libraries at the level of a few cells. Nucleic Acids Res. 1989 Apr 25;17(8):2919–2932. doi: 10.1093/nar/17.8.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D., Bartlett R., Crawford K., Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991 Jan 31;349(6308):388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- Bürger C., Wick M., Brüsselbach S., Müller R. Differential induction of 'metabolic genes' after mitogen stimulation and during normal cell cycle progression. J Cell Sci. 1994 Jan;107(Pt 1):241–252. doi: 10.1242/jcs.107.1.241. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Cowell I. G. Repression versus activation in the control of gene transcription. Trends Biochem Sci. 1994 Jan;19(1):38–42. doi: 10.1016/0968-0004(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992 May;11(5):1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989 Apr 7;57(1):177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone C., Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991 Feb 28;349(6312):808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. J., Mavrothalassitis G., Kondoh A., Papas T. S. High-affinity DNA-protein interactions of the cellular ETS1 protein: the determination of the ETS binding motif. Oncogene. 1991 Dec;6(12):2249–2254. [PubMed] [Google Scholar]
- Galaktionov K., Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991 Dec 20;67(6):1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Good L., Nazar R. N. An improved thermal cycle for two-step PCR-based targeted mutagenesis. Nucleic Acids Res. 1992 Sep 25;20(18):4934–4934. doi: 10.1093/nar/20.18.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Hass R., Gunji H., Hirano M., Weichselbaum R., Kufe D. Phorbol ester-induced monocytic differentiation is associated with G2 delay and down-regulation of cdc25 expression. Cell Growth Differ. 1993 Mar;4(3):159–166. [PubMed] [Google Scholar]
- Herber B., Truss M., Beato M., Müller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994 Apr;9(4):1295–1304. [PubMed] [Google Scholar]
- Hisatake K., Hasegawa S., Takada R., Nakatani Y., Horikoshi M., Roeder R. G. The p250 subunit of native TATA box-binding factor TFIID is the cell-cycle regulatory protein CCG1. Nature. 1993 Mar 11;362(6416):179–181. doi: 10.1038/362179a0. [DOI] [PubMed] [Google Scholar]
- Jessus C., Beach D. Oscillation of MPF is accompanied by periodic association between cdc25 and cdc2-cyclin B. Cell. 1992 Jan 24;68(2):323–332. doi: 10.1016/0092-8674(92)90473-p. [DOI] [PubMed] [Google Scholar]
- Jinno S., Suto K., Nagata A., Igarashi M., Kanaoka Y., Nojima H., Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994 Apr 1;13(7):1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizuka A., Sebastian B., Borgmeyer U., Hermans-Borgmeyer I., Bolado J., Hunter T., Hoekstra M. F., Evans R. M. A mouse cdc25 homolog is differentially and developmentally expressed. Genes Dev. 1992 Apr;6(4):578–590. doi: 10.1101/gad.6.4.578. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992 Nov;12(11):5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991 Mar 8;64(5):903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B. DP and E2F proteins: components of a heterodimeric transcription factor implicated in cell cycle control. Curr Opin Cell Biol. 1994 Jun;6(3):443–450. doi: 10.1016/0955-0674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Lam E. W., Watson R. J. An E2F-binding site mediates cell-cycle regulated repression of mouse B-myb transcription. EMBO J. 1993 Jul;12(7):2705–2713. doi: 10.1002/j.1460-2075.1993.tb05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello F. C., Sewing A., Brüsselbach S., Bürger C., Müller R. Deregulation of cyclins D1 and E and suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell Sci. 1993 May;105(Pt 1):123–133. doi: 10.1242/jcs.105.1.123. [DOI] [PubMed] [Google Scholar]
- Means A. L., Slansky J. E., McMahon S. L., Knuth M. W., Farnham P. J. The HIP1 binding site is required for growth regulation of the dihydrofolate reductase gene promoter. Mol Cell Biol. 1992 Mar;12(3):1054–1063. doi: 10.1128/mcb.12.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., Blevitt J., Gerace L., Sadhu K., Featherstone C., Russell P. p55CDC25 is a nuclear protein required for the initiation of mitosis in human cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10500–10504. doi: 10.1073/pnas.88.23.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991 Dec;10(13):4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., Russell P. The cdc25 M-phase inducer: an unconventional protein phosphatase. Cell. 1992 Feb 7;68(3):407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P., Russell P. Regulation of mitosis by cyclic accumulation of p80cdc25 mitotic inducer in fission yeast. Nature. 1990 Apr 5;344(6266):549–552. doi: 10.1038/344549a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Mumberg D., Lucibello F. C. Signals and genes in the control of cell-cycle progression. Biochim Biophys Acta. 1993 Aug 23;1155(2):151–179. doi: 10.1016/0304-419x(93)90003-u. [DOI] [PubMed] [Google Scholar]
- Nagata A., Igarashi M., Jinno S., Suto K., Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol. 1991 Oct;3(10):959–968. [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer G. P., Steigerwald S. D., Mueller P. R., Wold B., Riggs A. D. Genomic sequencing and methylation analysis by ligation mediated PCR. Science. 1989 Nov 10;246(4931):810–813. doi: 10.1126/science.2814502. [DOI] [PubMed] [Google Scholar]
- Pollock R., Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991 Dec;5(12A):2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Wang E. H., Tjian R. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell-cycle regulation. Nature. 1993 Mar 11;362(6416):175–179. doi: 10.1038/362175a0. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Sadhu K., Reed S. I., Richardson H., Russell P. Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5139–5143. doi: 10.1073/pnas.87.13.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sebastian B., Kakizuka A., Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3521–3524. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewing A., Bürger C., Brüsselbach S., Schalk C., Lucibello F. C., Müller R. Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J Cell Sci. 1993 Feb;104(Pt 2):545–555. doi: 10.1242/jcs.104.2.545. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld U., Labbé J. C., Fesquet D., Cavadore J. C., Picard A., Sadhu K., Russell P., Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991 May 16;351(6323):242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994 Feb 11;263(5148):811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- Wick M., Bürger C., Brüsselbach S., Lucibello F. C., Müller R. Identification of serum-inducible genes: different patterns of gene regulation during G0-->S and G1-->S progression. J Cell Sci. 1994 Jan;107(Pt 1):227–239. doi: 10.1242/jcs.107.1.227. [DOI] [PubMed] [Google Scholar]
- Woods D. B., Ghysdael J., Owen M. J. Identification of nucleotide preferences in DNA sequences recognised specifically by c-Ets-1 protein. Nucleic Acids Res. 1992 Feb 25;20(4):699–704. doi: 10.1093/nar/20.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]







