Abstract
Relatively little is known about the Campylobacter genotypes colonizing extensively reared broiler flocks and their survival through the slaughter process, despite the increasing demand for free-range and organic products by the consumer. Campylobacter isolates from a free-range boiler flock, sampled before and after slaughter, were genotyped by MLST (multilocus sequence typing) and sequence analysis of the flaA short variable region (SVR). The Campylobacter genotypes isolated before and after slaughter were diverse, with up to five sequence types (STs) (seven-locus allelic profiles resulting from MLST) identified per live bird, up to eight STs identified per carcass and 31 STs identified in all. The majority (72.0%) of isolates sampled from carcasses post-slaughter were indistinguishable from those isolated from the live flock before slaughter by ST and flaA SVR type, however, sampling ‘on-farm’ failed to capture all of the diversity seen post-slaughter. There were statistically significant increases in the genetic diversity of Campylobacter (p=0.005) and the proportion of C. coli (p=0.002), with some evidence for differential survival of genotypes contaminating the end product. C. coli genotypes isolated after slaughter were more similar to those from free-range and organic meat products sampled nationally, than from the live flock sampled previously. This study demonstrated the utility of MLST in detecting genetic diversity before and after the slaughter process.
Keywords: Campylobacter, MLST, Chicken, Free-range, Slaughter
1. Introduction
Campylobacter is the most common bacterial cause of gastroenteritis worldwide, causing an estimated 2.5 million cases a year in the United States, and representing a substantial economic impact when taking into account lost work hours and health care costs (Allos, 2001; Roberts et al., 2003; Withington and Chambers, 1997). In industrialized countries infection is typically self-limiting but occasionally patients develop severe secondary conditions such as Guillain Barré Syndrome, or Miller-Fisher Syndrome (Allos, 1997). In a UK study Campylobacter jejuni caused 93% of human disease with Campylobacter coli accounting for most of the remainder (Gillespie et al., 2002).
Campylobacter can be isolated from many environmental samples as well as from the intestines of many animals (Frost, 2001). Contaminated chicken meat is a common source of human infection, with the Campylobacter genotypes from both sources showing a high degree of similarity (Baker et al., 2006; Colles et al., 2008; Wilson et al., 2008). The automation of the slaughter process is thought to be a factor leading to cross-contamination between flocks, including those that were negative for Campylobacter prior to slaughter (Johnsen et al., 2006). Many Campylobacter genotypes appear to survive processing and they can often be isolated from cloacal or faecal samples from live birds within the flock before slaughter (Allen et al., 2007; Berndtson et al., 1996a; Herman et al., 2003; Klein et al., 2007; Lienau et al., 2007; Lindmark et al., 2006). Cleaning and disinfection between flocks appears to have limited effectiveness (Wedderkopp et al., 2001), with some Campylobacter strains able to survive overnight (Peyrat et al., 2008). Potential sources of contamination, which may be air or water borne, include flocks that are slaughtered previously, machinery and transport crates (Allen et al., 2007; Miwa et al., 2003; Newell et al., 2001; Posch et al., 2006; Rivoal et al., 1999; VanWorth et al., 2006).
Compared to intensively reared flocks, there have been relatively few studies on free-range and organic chickens, which are becoming increasingly popular with the consumer as a consequence of health and welfare considerations (Heuer et al., 2001). Typically, a large proportion of flocks are colonized by multiple Campylobacter genotypes, presumably since there are more opportunities for infection to occur (El-Shibiny et al., 2005). C. coli has been frequently isolated from free-range and organic chickens as well as parent flocks (Colles et al., 2008; El-Shibiny et al., 2005; Petersen et al., 2001) and may represent part of the mature natural gut microbiota. In contrast to housed birds, free-range and organic flocks are raised over a longer time period using a slower growing breed of bird and lower stocking density than is used conventionally (Heuer et al., 2001).
Campylobacter isolates obtained from a free-range broiler flock before and after slaughter were characterized using multilocus sequence typing (MLST) and nucleotide sequencing typing of the flaA short variable region (SVR) in order to investigate the relative survival of different Campylobacter genotypes through the slaughter process. Nucleotide sequence-based typing provides results that are unambiguous, directly comparable with those from other sources, and amenable to further statistical and genetic analyses (Allen et al., 2007; Maiden, 2006). The data from the intensively sampled free-range flock on one farm were compared with those from a national retail poultry meat survey to investigate the extent to which Campylobacter genotypes may be potentially circulating within the industry. It is essential to understand the population genetics of the organism at the various stages of food preparation, from farm to fork, in order to design intervention strategies.
2. Materials and methods
2.1. Sampling of free-range broiler chickens before and after slaughter
A free-range flock of 2400 Hubbard crossbreed birds, occupying four houses and reared to 81 days of age, was sampled for Campylobacter before and after slaughter. Twenty-five live chickens were sampled for Campylobacter in different areas of the four houses on day 80 (15.3.2005) using swabs of the anal area, which were then transported immediately to the laboratory in charcoal transport media. The flock was transported 25 miles to a small abattoir specializing in organic and local produce on the following day (16.3.2005), and 25 chicken carcasses from the same flock were sampled immediately after slaughter for Campylobacter. The flock that was tested was the first to be slaughtered in the abattoir that day, with standard meat hygiene service regulations followed for disinfection between flocks, and at the end of the previous day. At the time of the study, the abattoir processed flocks from only four closely regulated farms located in three different counties.
2.2. Bacterial culture and identification
The anal swabs were cultured directly onto Campylobacter selective blood free agar (mCCDA) plates (PO0119A, Oxoid Ltd., Basingstoke, UK) and incubated at 42 °C for 48 h in a microaerobic atmosphere generated using the GenBox microaer system (96126 and 96128, bioMérieux UK Ltd., Basingstoke, UK). The chicken carcasses were placed whole into large plastic bags, to which 200 ml of Maximum Recovery Diluent (CM0733, Oxoid Ltd., Basingstoke, UK) was added. The carcasses were massaged for 1 min, after which 100 μl of the diluent was spread onto mCCDA plates and incubated at 42 °C for 48 h in a microaerobic atmosphere. Presumptive Campylobacter colonies were identified by their typical appearance, gram negative curved rod morphology, and positive catalase and oxidase reactions. Up to ten colonies were sub-cultured from the mCCDA plates from all of the samples, onto Columbia blood agar (PB0122A, Oxoid Ltd., Basingstoke, UK) and incubated at 42 °C for a further 48 h in microaerobic conditions. Chromosomal DNA was extracted by preparing a thick cell suspension in Phosphate Buffered Saline which was boiled at 100 °C for 10 min on a heat block. The supernatant was retained after the boiled suspension was centrifuged at 13,200 rpm for 5 min.
2.3. Nucleotide sequence typing
The published protocol and reaction conditions for MLST were used to sequence portions of seven housekeeping genes (aspA, aspartase A, glnA, glutamine synthetase, gltA, citrate synthase, glyA, serine hydroxymethyl-transferase, pgm, phosphoglucomutase, tkt, transketolase and uncA, ATP synthase α subunit), although the nucleotide extension reactions were modified to 1/32 size reactions and a combination of published primers were used (Dingle et al., 2001; Miller et al., 2005). In addition the flaA SVR was sequenced using primers and reaction conditions described previously (Dingle et al., 2002; Meinersmann et al., 1997). The nucleotide extension reaction products were detected on an ABI Prism 3730 automated DNA analyzer and assembled using methods described previously (Dingle et al., 2001). Allele numbers, STs, clonal complexes and flaA SVR alleles were assigned using the Campylobacter MLST database (http://pubmlst.org/campylobacter/).
2.4. Analysis of genetic differentiation
Nucleotide sequence data from 734 C. jejuni isolates (185 STs) to 250 C. coli (77 STs) isolates from nationally surveyed retail chicken meat, both fresh and frozen, in the UK in 2001 and 2004/2005 were compared with data from this study (Food Standards Agency, 2003; Meldrum et al., 2006). A further 19 isolates (14 STs) from the study that contained a mixture of alleles from both C. jejuni and C. coli were discounted from the analyses. The sample set comprised isolates from housed flocks (708 C. jejuni isolates, 180 STs and 221 C. coli isolates, 72 STs) and free-range and organic flocks (26 C. jejuni isolates, 18 STs and 29 C. coli isolates, 21 STs). The pairwise Fisher statistic (FST) used to measure genetic differentiation, and the associated test of significance calculations, were performed using Arlequin software, version 3.0 (Schneider et al., 2000; Wright, 1951). Data input files were prepared from concatenated sequence from the seven housekeeping loci using DnaSP software, version 4.50 (Rozas et al., 2003). A value of 0 indicates that two populations are indistinguishable, whilst a value of 1 indicates maximum genetic differentiation between two populations. The prediction of host origin analyses was performed using Structure software, with data input as allelic profiles (Falush et al., 2003; McCarthy et al., 2007; Pritchard et al., 2000). The population origin was given for the live chicken and national retail poultry meat profiles forming the training sets, but not for the processed chicken profiles. The no-admixture model with lambda=1 and independent allele frequency parameters were used, with 1000 burn-in cycles and 10,000 further replications for each analysis and the number of populations was assumed to be two.
2.5. Analysis of genetic diversity
A modified version of Simpson’s diversity index, together with confidence intervals, was used to compare the diversity of STs taking into account their frequency, within the Campylobacter populations isolated from the chickens before and after slaughter (Grundmann et al., 2001; Hunter, 1990). A D value of 1 indicates that each member of a population can be distinguished from every other and a D value of 0 indicates that all members of a population are identical. The unpaired Student’s t test was used to compare the number and diversity of STs isolated from individual chickens before and after slaughter. The Fisher’s exact test was used to test the distribution of STs before and after slaughter where an observation was less than five, and the Chi-squared test was used to test the distribution of C. coli before and after slaughter where all observations were greater than five. The Student’s t test, Fisher’s exact tests and Chi-squared analysis were performed using Stata (StataCorp LP, Texas, U.S.).
3. Results
3.1. Prevalance of Campylobacter
A total of 222 Campylobacter isolates were obtained from 24 of the 25 live birds sampled. Of these 179 (80.6%) were C. jejuni and 43 were (19.4%) C. coli.
Campylobacter was isolated from each of the 25 chicken carcasses sampled after slaughter, giving a total of 250 Campylobacter isolates. Of these 155 (62.0%) were C. jejuni, 93 (37.2%) were C. coli and two isolates (0.8%) contained alleles from both species and were omitted from further analysis.
3.2. Genetic diversity of Campylobacter
A total of 31 STs, of which 13 were C. jejuni and 18 were C. coli were identified amongst the 472 isolates from the live and processed chickens (Table 1). Nineteen STs belonged to one of eight C. jejuni and two C. coli clonal complexes, leaving 12 STs that could not be assigned to a clonal complex. The STs most commonly isolated from the live chickens were ST-1489 accounting for 64/222 (28.8%) isolates and ST-51 accounting for 40/222 (18.0%) isolates. These STs were also the most commonly isolated from the processed chickens, with ST-51 accounting for 42/250 (16.8%) isolates and ST-1489 accounting for 40/250 (16.0%) isolates.
Table 1. The Campylobacter genotypes isolated from free-range chickens within the flock before and after slaughter.
| Clonal complex | ST | Live chickens |
Processed chickens |
||||
|---|---|---|---|---|---|---|---|
| Frequency (%) |
No. chickens colonized | flaA SVR allele-peptide | Frequency (%) |
No. carcases contaminated | flaA SVR allele- peptide | ||
| 257 | 257 | 1(0.5) | 1 | 16-12 | 0 | 0 | - |
| 354 | 1489a | 64 (28.8) | 16 | 222-33 | 40 (16.0) | 15 | 222-33 |
| 443 | 51 | 40 (l8.0) | 11 | 21-2 | 42 (16.8) | 12 | 21-2 |
| 433 | 433 | 0 | 0 | - | 1 (0.4) | 1 | 49-1 |
| 460 | 606 | 0 | 0 | - | 2 (0.8) | 2 | 34-1 |
| 573 | 573b | 5 (2.3) | 2 | 314-8(4), 66-1(1) | 27 (10.8) | 12 | 314-8(26), 222-33(1) |
| 607 | 607a | 23 (10.4) | 5 | 14-11 | 2 (0.8) | 2 | 14-11 |
| 661 | 814 | 1 (0.5) | 1 | 191-33 | 2 (0.8) | 1 | 191-33 |
| 1496a | 27 (12.2) | 9 | 191-33 | 0 | 0 | - | |
| 828c | 825c | 4 (1.8) | 1 | 30-11 | 6 (2.4) | 5 | 30-11(2), 16-12(3) |
| 829c | 3 (1.4) | 2 | 18-20 | 1 (0.4) | 1 | 293-8 | |
| 855bc | 0 | 0 | - | 23 (9.2) | 11 | 314-8 | |
| 867c | 0 | 0 | - | 1 (0.4) | 1 | 17-11 | |
| 1084c | 0 | 0 | - | 1 (0.4) | 1 | 66-1 | |
| 1089c | 1 (0.5) | 0 | 415-1 | 5 (2.4) | 2 | 415-1 | |
| 1497c | 1 (0.5) | 1 | 18-20 | 0 | 0 | - | |
| 1498ac | 7 (3.2) | 1 | 467-1 | 0 | 0 | - | |
| 1541c | 0 | 0 | - | 1 (0.4) | 1 | 295-8 | |
| 1150c | 1487ac | 19 (8.6) | 8 | 351-111 | 3 (1.2) | 2 | 351-111 |
| Unassigned | 1090bc | 5 (2.3) | 4 | 66-1 | 35 (14.0) | 11 | 66-1 |
| 1488bc | 0 | 0 | - | 13 (5.2) | 5 | 66-1 | |
| 1490c | 0 | 0 | - | 2 (0.8) | 2 | 17-11 | |
| 1491 | 0 | 0 | - | 3 (1.2) | 3 | 314-8(2), 66-1(1) | |
| 1492b | 4 (1.8) | 1 | 117-8 | 23 (9.2) | 15 | 117-8 | |
| 1493c | 0 | 0 | - | 2 (0.8) | 2 | 316-1 | |
| 1494c | 0 | 0 | - | 2 (0.8) | 2 | 415-1 | |
| 1495 | 14 (6.3) | 5 | 117-8(11), 222-33(2) | 8 (3.2) | 7 | 117-8(7), 100-33(1) | |
| 1499c | 0 | 0 | - | 2 (0.8) | 2 | 17-11 | |
| 1531c | 1 (0.5) | 1 | 466-1 | 1 (0.4) | 1 | 17-11 | |
| 1532 | 1 (0.5) | 1 | 21-2 | 0 | 0 | - | |
| 4239c | 1 (0.5) | 1 | 17-11 | 0 | 0 | - | |
Key:
Significant decrease, and
significant increase in frequency following slaughter,
C. coli.
Between one and five STs were detected from individual live chickens, giving an average of 3.1 STs per chicken. Between two and eight STs were identified from individual chicken carcasses, giving an average of 4.8 STs per processed chicken. The difference was significant, t=−4.72, 46 d.f. p=<0.00001.
Most STs, accounting for 151/222, 68.0% isolates from the live chickens and 177/250, 70.8% isolates from the carcasses were uniform with respect to flaA SVR. There were exceptions however, with five STs (1495, 825, 573, 829 and 1491) that were associated with up to three different flaA SVR types. In addition a particular flaA SVR type was often shared by more than one ST.
3.3. Campylobacter genotypes before and after slaughter
A total of 19 STs, ten C. jejuni and nine C. coli were isolated from the live chickens, and 25 STs, ten C. jejuni and 15 C. coli were isolated from the processed chickens (Table 1 and Fig. 1). The live and processed chickens had 13 STs in common, seven C. jejuni and six C. coli, accounting for 184/222 (82.8%) isolates in live chickens and 195/250 (78.0%) isolates in processed chickens. Of these, eight STs were associated with the same flaA SVR allele before and after slaughter, two STs were associated with different flaA SVR alleles before and after slaughter and three STs were associated with mixed flaA SVR alleles before and after slaughter. Using a combination of ST and flaA SVR type to give higher resolution, 175/222 (78.8%) of isolates from the live chickens were indistinguishable from 180/250 (72.0%) of isolates after slaughter.
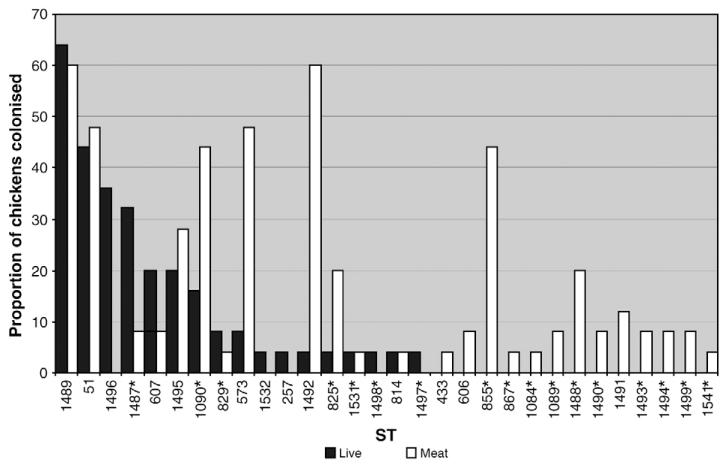
Fig. 1. The proportion of 25 live chickens colonized before slaughter and 25 carcasses contaminated after slaughter by Campylobacter STs.
Key: *C. coli.
Six STs, three C. jejuni (257, 1496 and 1532) and three C. coli (1497,1498 and 4239), accounting for 38/222 (17.1%) isolates, were detected in live chickens but not in processed chickens. Twelve STs, three C. jejuni and nine C. coli, accounting for 53/250 (21.2%) isolates, were isolated from the processed chickens but not from the live chickens. Using FST as a measure, the C. jejuni isolates sampled before and after slaughter were 7.2% differentiated and the C. coli isolates 32.7% differentiated, both values were significant (p<0.000001) (Table 2). The diversity of Campylobacter genotypes calculated using Simpson’s Index of Diversity showed a small but significant increase after slaughter from D=0.84 (CI 0.83–0.86) to D=0.89 (CI 0.89–0.90) when the populations were considered as a whole, and a significant increase from an average of D=0.50 to D=0.69, t=−2.9 p=0.005 when the populations colonizing birds individually were considered.
Table 2. Measure of genetic differentiation (FST) amongst Campylobacter isolated from the live and processed chickens and a national survey of retail poultry meat, using concatenated sequence.
All values were significant with a p value <0.000001.
| Campylobacter species | Host source |
Fst (number of STs in common) |
|
|---|---|---|---|
| Live chickens | Processed chickens | ||
| C. jejuni | Processed chickens | 0.072 (7) | – |
| National retail poultry meat: | 0.160 (5) | 0.200 (4) | |
| housed | |||
| : free-range/organic | 0.275 (3) | 0.338 (2) | |
| : combined | 0.161 (5) | 0.201 (4) | |
| C. coli | Processed chickens | 0.327 (5) | – |
| National retail poultry meat: | 0.656 (3) | 0.402 (8) | |
| housed | |||
| : free-range/organic | 0.486 (3) | 0.288 (5) | |
| : combined | 0.667 (3) | 0.411 (8) | |
The proportion of C. coli increased significantly after slaughter, χ2=24.68, p<0.0001. The distribution of ten STs (five C. jejuni and five C. coli) differed significantly with p values <0.005 before and after slaughter using Fisher’s Exact test when the overall frequency was considered. Of these, only ST-1496 showed a significant difference (p=0.002) in the number of birds colonized before slaughter (nine) and the number of carcasses contaminated after slaughter (zero), although the statistical power for the other STs was low. Survival of the Campylobacter genotypes through to the slaughtering process was not limited to only central genotypes, with many single, double and triple locus variants, particularly from the large C. coli ST-828 complex, being isolated from the chicken carcasses.
3.4. Genetic differentiation of Campylobacter isolates from national samples of retail chicken meat
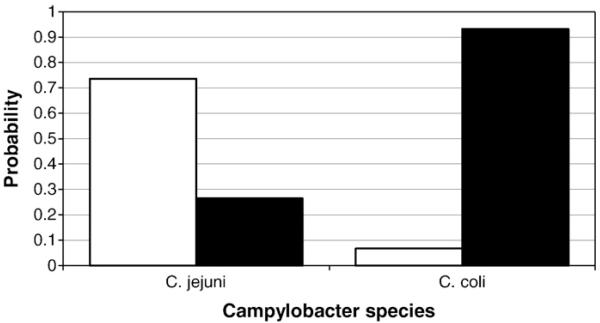
The FST values derived from concatenated nucleotide sequence data, together with the number of shared STs, indicated that the C. jejuni isolates from the processed chickens were more similar to those from the live flock than from the retail poultry meat survey (Table 2). In addition the C. jejuni isolates from both the live and processed chickens better matched those from housed birds rather than other free-range and organic birds. Structure analysis using allelic profile data for C. jejuni and predefined data sets from the live flock and retail poultry meat predicted that isolates from the processed birds shared ancestry with those from the live flock with a probability of 0.735 (Fig. 2).
Fig. 2. The predicted ancestry of Campylobacter isolated from the free-range flock after slaughter, given the populations isolated from the live flock before slaughter (white) and from a national survey of retail poultry meat (black).
The analysis was performed using Structure and allelic profile data.
The C. coli isolates from the processed chickens were more similar to those isolated from free-range and organic birds in the retail poultry meat survey than from the live flock previously (Table 2). C. coli isolates from the live birds were highly differentiated from those isolated from the poultry meat survey (0.486–0.667). Given the two populations of live chickens and retail poultry meat, Structure analysis predicted a shared ancestry of C. coli isolates from the processed chickens and retail poultry meat with a probability of 0.933 (Fig. 2).
4. Discussion
A high prevalence of Campylobacter was detected in the flock before and after slaughter, in common with other studies of free-range and organic chickens (Heuer et al., 2001; Van Overbeke et al., 2006; Wittwer et al., 2005). The Campylobacter genotypes identified by MLST were diverse, with 19 STs isolated from the flock before slaughter and 25 STs after slaughter. Between one and five STs were isolated from a single live bird and between two and eight STs were isolated from a single carcass. Some studies report limited co-colonization of up to three strains amongst housed flocks (Bull et al., 2006; Lindmark et al., 2006; Newell and Fearnley, 2003; Petersen et al., 2001) and it may be that the greater number of STs seen here are typical of free-range and organically raised birds (El-Shibiny et al., 2005). The results emphasize the need to take sufficiently large numbers of samples to determine the full extent of diversity within a flock.
In this study, 72.0% of the Campylobacter isolates from the carcasses were indistinguishable from 78.8% of those isolated from the live flock before slaughter by ST and flaA SVR type. Evidence from FST, Student’s t tests and the fact that ST-1489 and ST-51 were the most common before and after slaughter support the findings from previous studies that the source of the majority of Campylobacter genotypes contaminating the flock through the slaughter process is likely to be the live flock (Allen et al., 2007; Berndtson et al., 1996b; Herman et al., 2003; Klein et al., 2007; Lienau et al., 2007; Rosenquist et al., 2003). The C. jejuni populations being 92.8% similar before and after slaughter by FST were more stable than the C. coli populations that were 67.3% similar before and after slaughter.
There was some differential survival of STs through the slaughter process, as noted in previous studies, with 5/19, 26.3% of STs from the live chickens showing a significant drop in overall frequency post-slaughter (Johnsen et al., 2006; Newell et al., 2001). In particular, ST-1496 was common amongst the live birds sampled before slaughter, and indeed was frequently isolated from other live flocks on the same farm, but was not recovered after slaughter. ST-607, present also in retail poultry meat, has been isolated from human disease cases in different continents on the Campylobacter MLST database (http://pubmlst.org/campylobacter/) implying relative success in reaching the human consumer, but in this study it decreased significantly in frequency post-slaughter. The prevalence of five STs (two C. jejuni and three C. coli), however, significantly increased after slaughter, with three, STs 573, 855 and 1090 being common amongst the national retail poultry meat survey also. The reasons for the differences are not clear. It is possible that the carcass rinse samples taken post-slaughter diluted the frequency of some STs, and that the birds chosen after slaughter were colonized with different genotypes when they were alive compared to those selected before slaughter. Even with these caveats, it remains that the carcasses were not contaminated by Campylobacter genotypes equally after slaughter, and the frequencies of some STs were unaffected by sampling. The abattoir in this study specialized in organic and free-range poultry which could have lead to an increased prevalence and contamination by associated genotypes, particularly C. coli. The distribution of C. jejuni and C. coli was found to vary amongst processing plants in a previous study (Logue et al., 2003), although others using strain identification by PCR based methods report no differences (Miraglia et al., 2007; Wittwer et al., 2005).
There was evidence that the slaughter process increased the diversity of the Campylobacter genotypes isolated from the flock and, in particular, from the individual carcasses compared to the live birds. The results demonstrate that the full diversity of Campylobacter genotypes at slaughter was not captured with the on-farm sampling which could have implications for future studies. The aerosols and intestinal leakage created during processing provides the ideal environment for mixing and dissemination of all genotypes that were present in the live flock (Jozwiak et al., 2006; Miwa et al., 2003; Rivoal et al., 1999; Rosenquist et al., 2003). Other possible sources of contamination include flocks from the previous day, or transport crates (Allen et al., 2007; Bull et al., 2006; Peyrat et al., 2008; VanWorth et al., 2006; Wedderkopp et al., 2001). Nearly half the STs (12/25, 48.0%), accounting for just over a fifth of the isolates (53/250, 21.2%) isolated from the carcasses in this study had not been detected in the live flock prior to slaughter. In particular, the incidence of C. coli increased significantly from 43/222, 19.4% of isolates before slaughter, to 93/250, 37.2% isolates post-slaughter. Nine new C. coli STs were identified after processing although the incidence of many was low and they may not have been detectable in the live flock.
Comparison with isolates from a national survey of poultry meat using FST values calculated from concatenated nucleotide sequence demonstrated 79.9% similarity between C. jejuni isolates from the processed free-range flock and nationally sampled retail meat. Using allelic profile data from the live flock and the national retail meat survey as training sets, analysis using Structure predicted that 26.5% of STs isolated from the processed chickens were more similar to those isolated on a national scale than from the live flock beforehand. These results were surprising given the different sampling frames in relation to time, location and the number of flocks tested, and suggest that a small subset of Campylobacter genotypes, for example ST-51, ST-573, ST-607 and ST-814 in this study, are widely distributed within the industry and are stable over time. Greater overlap was seen between isolates from the free-range flock and meat from housed birds rather than extensively reared birds indicating that some genotypes are successful in both systems, but this may also be a sampling effect since housed birds form the majority of the market. Analyses using FST and Structure predicted 59.8–93.3% similarity between C. coli isolated from the processed chickens and the retail meat, with greatest overlap amongst isolates from free-range flocks, but only 6.7-33.3% similarity between isolates from the live flock and retail meat. The different results for C. jejuni and C. coli reflect the significant increase in prevalence of C. coli post-slaughter, and also the fact that nine of the C. coli STs isolated from the free-range flock are unique to the study at the present time and could be farm or abattoir specific genotypes. There could also be a sampling effect with the majority of retail poultry meat isolates coming from housed flocks.
In conclusion, this study demonstrates the utility of high resolution, high throughput genotyping in the analysis of Campylobacter colonizing a chicken flock before and after slaughter. These data provide considerable information not available from other typing systems or from culture alone, and support the idea that the chicken production industry forms an agricultural niche for particular Campylobacter genotypes that have national distribution and are stable over a number of years.
Acknowledgements
The work and internet accessible databases were supported by contract number OZ0611 awarded by the Department for Environment, Food and Rural Affairs (DEFRA), UK. We also thank Julian Howe from the Zoology Department, Oxford University, UK for help with sampling and Lynne Richardson and Rebecca Busby of the Oxford University Zoology Department Sequencing Facility.
References
- Allen VM, Bull SA, Corry JE, Domingue G, Jorgensen F, Frost JA, Whyte R, Gonzalez A, Elviss N, Humphrey TJ. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. International Journal of Food Microbiology. 2007;113:54–61. doi: 10.1016/j.ijfoodmicro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Allos BM. Association between Campylobacter infection and Guillain Barré syndrome. Journal of Infectious Diseases. 1997;176(Suppl.2):S125–S128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- Allos B. Campylobacter jejuni infections: update on emerging issues and trends. Clinical Infectious Diseases. 2001;32(8):1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- Baker M, Wilson N, Ikram R, Chambers S, Shoemack P, Cook G. Regulation of chicken contamination is urgently needed to control New Zealand’s serious campylobacteriosis epidemic. New Zealand Medical Journal. 2006;119:U2264. [PubMed] [Google Scholar]
- Berndtson E, Danielsson-Tham ML, Engvall A. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. International Journal of Food Microbiology. 1996a;32:35–47. doi: 10.1016/0168-1605(96)01102-6. [DOI] [PubMed] [Google Scholar]
- Berndtson E, Emanuelson U, Engvall A, Danielsson-Tham M-L. A 1-year epidemiological study of Campylobacters in 18 Swedish chicken farms. Preventative Veterinary Medicine. 1996b;26:167–185. [Google Scholar]
- Bull SA, Allen VM, Domingue G, Jorgensen F, Frost JA, Ure R, Whyte R, Tinker D, Corry JE, Gillard-King J, Humphrey TJ. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Applied and Environmental Microbiology. 2006;72:645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles FM, Jones TA, McCarthy ND, Sheppard SK, Cody AJ, Dingle KE, Dawkins MS, Maiden MC. Campylobacter infection of broiler chickens in a free-range environment. Environmental Microbiology. 2008;10:2042–2050. doi: 10.1111/j.1462-2920.2008.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle KE, Colles FM, Wareing DRA, Ure R, Fox AJ, Bolton FJ, Bootsma HJ, Willems RJL, Urwin R, Maiden MCJ. Multilocus sequence typing system for Campylobacter jejuni. Journal of Clinical Microbiology. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle KE, Colles FM, Ure R, Wagenaar J, Duim B, Bolton FJ, Fox AJ, Wareing DRA, Maiden MCJ. Molecular characterisation of Campylobacter jejuni clones: a rational basis for epidemiological investigations. Emerging Infectious Diseases. 2002;8:949–955. doi: 10.3201/eid0809.02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shibiny A, Connerton PL, Connerton IF. Enumeration and diversity of campylobacters and bacteriophages isolated during the rearing cycles of free-range and organic chickens. Applied and Environmental Microbiology. 2005;71:1259–1266. doi: 10.1128/AEM.71.3.1259-1266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food Standards Agency . UK-wide Survey of Salmonella and Campylobacter Contamination of Fresh and Frozen Chicken on Retail Sale. Food Standards Agency; 2003. [Google Scholar]
- Frost JA. Current epidemiological issues in human campylobacteriosis. Symp Ser Soc Appl Microbiol. 2001:85S–95S. doi: 10.1046/j.1365-2672.2001.01357.x. [DOI] [PubMed] [Google Scholar]
- Gillespie IA, O’Brien SJ, Frost JA, Adak GK, Horby P, Swan AV, Painter MJ, Neal KR, C. S. S. S. Collaborators A case–case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerging Infectious Diseases. 2002;8:937–942. doi: 10.3201/eid0809.10.3201/eid0809.010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann H, Hori S, Tanner G. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. Journal of Clinical Microbiology. 2001;39:4190–4192. doi: 10.1128/JCM.39.11.4190-4192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I, De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiology and Infection. 2003;131:1169–1180. doi: 10.1017/s0950268803001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer OE, Pedersen K, Andersen JS, Madsen M. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Letters in Applied Microbiology. 2001;33:269–274. doi: 10.1046/j.1472-765x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Hunter PR. Reproducibility and indices of discriminatory power of microbial typing methods. Journal of Clinical Microbiology. 1990;28:1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen G, Kruse H, Hofshagen M. Genotyping of Campylobacter jejuni from broiler carcasses and slaughterhouse environment by amplified fragment length polymorphism. Poultry Science. 2006;85:2278–2284. doi: 10.1093/ps/85.12.2278. [DOI] [PubMed] [Google Scholar]
- Jozwiak A, Reichart O, Laczay P. The occurrence of Campylobacter species in Hungarian broiler chickens from farm to slaughter. Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health. 2006;53:291–294. doi: 10.1111/j.1439-0450.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- Klein G, Beckmann L, Vollmer HM, Bartelt E. Predominant strains of thermophilic Campylobacter spp. in a German poultry slaughterhouse. International Journal of Food Microbiology. 2007;117:324–328. doi: 10.1016/j.ijfoodmicro.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lienau JA, Ellerbroek L, Klein G. Tracing flock-related Campylobacter clones from broiler farms through slaughter to retail products by pulsed-field gel electrophoresis. Journal of Food Protection. 2007;70:536–542. doi: 10.4315/0362-028x-70.3.536. [DOI] [PubMed] [Google Scholar]
- Lindmark H, Diedrich IC, Andersson L, Lindqvist R, Engvall EO. Distribution of Campylobacter genotypes on broilers during slaughter. Journal of Food Protection. 2006;69:2902–2907. doi: 10.4315/0362-028x-69.12.2902. [DOI] [PubMed] [Google Scholar]
- Logue CM, Sherwood JS, Elijah LM, Olah PA, Dockter MR. The incidence of Campylobacter spp. on processed turkey from processing plants in the midwestern United States. Journal of Applied Microbiology. 2003;95:234–241. doi: 10.1046/j.1365-2672.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Maiden MC. Multilocus sequence typing of bacteria. Annual Review of Microbiology. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- McCarthy ND, Colles FM, Dingle KE, Bagnall MC, Manning G, Maiden MC, Falush D. Host-associated genetic import in Campylobacter jejuni. Emerging Infectious Diseases. 2007;13:267–272. doi: 10.3201/eid1302.060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinersmann RJ, Helsel LO, Fields PI, Hiett KL. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. Journal of Clinical Microbiology. 1997;35:2810–2814. doi: 10.1128/jcm.35.11.2810-2814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum RJ, Smith RM, Wilson IG. Three-year surveillance program examining the prevalence of Campylobacter and Salmonella in whole retail raw chicken. Journal of Food Protection. 2006;69:928–931. doi: 10.4315/0362-028x-69.4.928. [DOI] [PubMed] [Google Scholar]
- Miller WG, On SL, Wang G, Fontanoz S, Lastovica AJ, Mandrell RE. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. Journal of Clinical Microbiology. 2005;43:2315–2329. doi: 10.1128/JCM.43.5.2315-2329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia D, Ranucci D, Branciari R, Cioffi A, Mammoli R, Cenci Goga BT, Avellini P. Prevalence of Campylobacter jejuni and Campylobacter coli in chicken hybrids with different growth rates, reared according to conventional and “free-range” production methods. Veterinary Research Communications. 2007;31(Suppl 1):381–384. doi: 10.1007/s11259-007-0042-3. [DOI] [PubMed] [Google Scholar]
- Miwa N, Takegahara Y, Terai K, Kato H, Takeuchi T. Campylobacter jejuni contamination on broiler carcasses of C. jejuni-negative flocks during processing in a Japanese slaughterhouse. International Journal of Food Microbiology. 2003;84:105–109. doi: 10.1016/s0168-1605(02)00398-7. [DOI] [PubMed] [Google Scholar]
- Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Applied and Environmental Microbiology. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell DG, Shreeve JE, Toszeghy M, Domingue G, Bull S, Humphrey T, Mead G. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Applied and Environmental Microbiology. 2001;67:2636–2640. doi: 10.1128/AEM.67.6.2636-2640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Nielsen EM, On SL. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Veterinary Microbiology. 2001;82:141–154. doi: 10.1016/s0378-1135(01)00382-0. [DOI] [PubMed] [Google Scholar]
- Peyrat MB, Soumet C, Maris P, Sanders P. Recovery of Campylobacter jejuni from surfaces of poultry slaughterhouses after cleaning and disinfection procedures: analysis of a potential source of carcass contamination. International Journal of Food Microbiology. 2008;124:188–194. doi: 10.1016/j.ijfoodmicro.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Posch J, Feierl G, Wuest G, Sixl W, Schmidt S, Haas D, Reinthaler FF, Marth E. Transmission of Campylobacter spp. in a poultry slaughterhouse and genetic characterisation of the isolates by pulsed-field gel electrophoresis. British Poultry Science. 2006;47:286–293. doi: 10.1080/00071660600753763. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoal K, Denis M, Salvat G, Colin P, Ermel G. Molecular characterization of the diversity of Campylobacter spp. isolates collected from a poultry slaughterhouse: analysis of cross-contamination. Letters in Applied Microbiology. 1999;29:370–374. doi: 10.1046/j.1472-765x.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Cumberland P, Sockett PN, Wheeler J, Rodrigues LC, Sethi D, Roderick PJ. The study of infectious intestinal disease in England: socioeconomic impact. Epidemiology and Infection. 2003;130:1–11. doi: 10.1017/s0950268802007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist H, Nielsen NL, Sommer HM, Norrung B, Christensen BB. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. International Journal of Food Microbiology. 2003;83:87–103. doi: 10.1016/s0168-1605(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- Schneider S, Roessli D, Excoffier L. Arlequin Version 2.000: a Software for Population Genetic Data Analysis. University of Geneva; Geneva: 2000. [Google Scholar]
- Van Overbeke I, Duchateau L, De Zutter L, Albers G, Ducatelle R. A comparison survey of organic and conventional broiler chickens for infectious agents affecting health and food safety. Avian Diseases. 2006;50:196–200. doi: 10.1637/7448-093005R.1. [DOI] [PubMed] [Google Scholar]
- VanWorth C, McCrea BA, Tonooka KH, Boggs CL, Schrader JS. Diversity of flaA genotypes among Campylobacter jejuni isolated from six niche-market poultry species at farm and processing. Journal of Food Protection. 2006;69:299–307. doi: 10.4315/0362-028x-69.2.299. [DOI] [PubMed] [Google Scholar]
- Wedderkopp A, Gradel KO, Jorgensen JC, Madsen M. Pre-harvest surveillance of Campylobacter and Salmonella in Danish broiler flocks: a 2-year study. International Journal of Food Microbiology. 2001;68:53–59. doi: 10.1016/s0168-1605(01)00463-9. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Gabriel E, Leatherbarrow AJH, Cheesbrough J, Gee S, Bolton E, Fox A, Fearnhead P, Hart A, Diggle PJ. Tracing the source of campylobacteriosis. PLoS Genetics. 2008;4:e1000203. doi: 10.1371/journal.pgen.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington SG, Chambers ST. The cost of campylobacteriosis in New Zealand in 1995. New Zealand Medical Journal. 1997;110:222–224. [PubMed] [Google Scholar]
- Wittwer M, Keller J, Wassenaar TM, Stephan R, Howald D, Regula G, Bissig-Choisat B. Genetic diversity and antibiotic resistance patterns in a Campylobacter population isolated from poultry farms in Switzerland. Applied and Environmental Microbiology. 2005;71:2840–2847. doi: 10.1128/AEM.71.6.2840-2847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]