Abstract
The majority of patients with differentiated thyroid cancer are cured with standard primary treatments including surgery, radioactive iodine and TSH suppression. A small proportion of patients who develop radioactive iodine-refractory metastatic disease have few treatment options. Recent discovery of the molecular mechanisms that contribute to thyroid cancer tumorigenesis and its progression have revealed key targets that are currently being evaluated in clinical trials. In the last decade several novel targeted therapies have shown encouraging results and have brought hope to patients with advanced disease. However, identifying the subpopulation of patients who may benefit from systemic therapies remains a challenge as the use of these therapeutic modalities is associated with high toxicity rates and most patients have a long indolent phase where the tumor is stable or slowly progressive and asymptomatic. The objective of this review is to summarize the management of patients with metastatic, radioactive iodine refractory differentiated thyroid cancer.
Keywords: Papillary, follicular, systemic therapy, tyrosine kinase inhibitors, chemotherapy
INTRODUCTION
Thyroid cancer is the most common endocrine cancer. The estimated number of new cases for 2012 is 56,400 (13,250 males and 43,210 females) and the number of deaths from thyroid cancer is 1,780 (780 male and 1,000 females). In women, thyroid cancer is the 5th most common cancer, accounting for 5% of all new cancers diagnosed in 20121.
The differentiated thyroid carcinomas (DTC) include papillary (PTC), follicular, and Hurthle cell carcinoma and they account for more than 90% of thyroid cancer cases. Approximately 85% of patients with DTC are cured with surgery, radioactive iodine (RAI), and TSH suppression and the majority of patients who undergo such thyroid cancer treatment have a good prognosis. In patients with localized DTC, the 5-year relative survival rate is 98%. However, patients with widely metastatic DTC can pose a challenge to oncologists and endocrinologists, especially those who see few cases of this relatively rare presentation. While the 10-year median survival after the discovery of metastatic disease is 42%, the prognosis can be vastly different among patients and depends on the age of patient, histology, location and size of the distant disease, as well as whether the disease takes up and responds to RAI2. For example, younger patients (<40 years) with micronodular (<1cm) lung disease have an excellent overall survival of 95% at 10 years, while older patients with macronodular lung metastases or multiple bone metastases have a 14% overall survival at 10 years2.
Metastatic DTC tends to be indolent in the majority of patients whereas a minority of others have metastatic disease that grows rapidly from the outset. Also, patients who initially had indolent disease may later demonstrate a more aggressive course. An understanding of the natural history and appropriate management of this disease is important so that patients are not under or over treated. Because metastatic DTC is often slow growing and asymptomatic, a more restrained approach, compared with other solid tumors, is needed. On the other hand, in some cases, patients with rapidly progressive and/or symptomatic disease or disease in areas that are life-threatening or have the potential to become life-threatening require a more aggressive approach.
Until recently, the standard of care for DTC patients was limited to surgery and RAI and TSH suppression. However, the recent explosion of knowledge in tumor biology and the identification of the biologic targets in thyroid cancer have led to several clinical trials with multi-kinase inhibitors. The aim of this paper is to discuss the treatment options for patients with metastatic, RAI-refractory DTC.
MANAGEMENT OF METASTATIC DTC
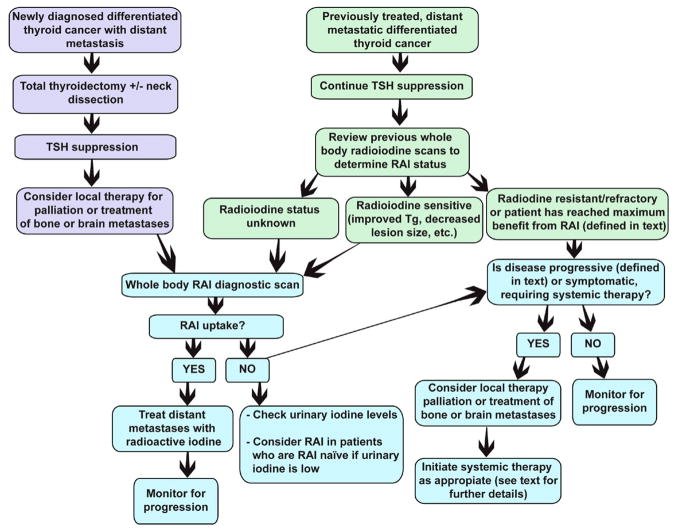
Patients who present with newly diagnosed DTC with distant metastases should be treated with first line, standard therapy, which consists of surgery, subsequent RAI and thyroid hormone therapy to suppress the TSH. The thyroid and any regional disease are removed prior to RAI in order to remove a source of large iodine uptake. Once the thyroid and involved cervical lymph nodes are removed, RAI can be highly effective to target distant metastatic disease. However, if the distant disease is RAI-refractory (defined in Table 1), monitoring for pace of progression in most cases is warranted. The “watch and wait” approach is appropriate in patients who are asymptomatic, have a low tumor burden, and/or have a slow pace of disease progression. Consideration of local therapy or systemic treatment with the newer tyrosine kinase inhibitors (TKIs) or cytotoxic chemotherapy is appropriate once significant progression is documented or the patient develops symptoms related to disease burden. Figure 1 shows the algorithm for treatment of patients who present with metastatic disease and those who later develop metastatic disease after first line therapy.
Table 1.
Definition of RAI-refractory
| Table 1: Definition of RAI-refractory (any of the following): |
|---|
| The disease does not take up iodine at known sites of metastatic disease |
| Continued growth of disease despite RAI treatment and confirmed uptake |
| Distant disease grows over a 1 year period after RAI |
| Total cumulative dose of RAI of ≥600 mCi |
Figure 1.
Algorithm for management of DTC patients presenting with metastatic disease and DTC patients who develop metastatic disease
The management of metastatic DTC can be divided into 3 major categories: 1) watch and wait approach, 2) local therapy, and 3) systemic therapy.
1) Watch and Wait
Dormancy of the metastatic disease is common in DTC. The events which lead to progression likely are due to genetic and epigenetic events in the tumor which cause the disease to become more aggressive3. The rationale for the ‘watch and wait’ approach is based mainly on the fact that most patients with widely metastatic, RAI-refractory DTC have a long indolent phase where the tumor is stable or slowly progressive and asymptomatic. These patients can enjoy a good quality of life for many years before requiring systemic therapy. Because systemic therapies have many side effects, some of which can be fatal, delaying therapy until the disease accelerates is prudent. TKIs, which are cytostatic drugs, are considered chronic therapies, as stopping these drugs usually results in progression of disease. Furthermore, the TKIs have not cured any patient thus far and it is not clear whether they will prolong overall survival.
Distant disease limited to the mediastinum and lung that is asymptomatic and does not threaten vital structures can usually be followed with serial imaging. Metastatic, RAI-refractory disease to the brain, liver, and bone may indicate that the disease is more aggressive and warrant local treatment or very close follow-up for progression and subsequent initiation of local and/or systemic therapy. Patients should have full staging exams to determine extent of disease and pace of progression. The association of an elevated thyroglobulin(Tg) or thyroglobulin antibody(TgAb) level and a negative radioactive iodine scan is an indication of non-radioiodine avid residual or recurrent disease. The imaging modalities used to detect the non-radioiodine avid disease include: ultrasound of the neck, thin spiral CT of the chest, and when indicated by clinical scenario MRI of the brain, spine, pelvis, bone scan, and fluorodeoxyglucose positron-emission tomography (18FDG-PET/CT scan).
18FDG-PET/CT is an increasingly useful imaging study used in the detection of RAI negative, thyroglobulin positive thyroid cancer as it provides anatomical as well as prognostic information. Patients with DTC with evidence of RAI-refractory disease usually have higher glucose metabolism and positive FDG-PET scans. Patients with larger volumes of FDG avid disease or higher SUVs are less likely to respond to RAI and have a higher mortality over a 3-year follow-up compared with the patients with no FDG uptake4,5. A recent retrospective study was conducted to compare empiric 131I WBS (whole body scan) and FDG PET/CT in patients with normal post-ablation WBS and elevated Tg or persistent TgAb. The patients included in this study were older with fairly aggressive histologies. The sensitivities for the detection of individual lesions and for the diagnosis of metastatic organs were 88% and 97% for PET/CT and 16% and 22% for WBS, respectively6. The malignant nature of the disease should be confirmed prior to further treatment as false positive PET scans due to granulomatous, infectious or postoperative changes are not uncommon.
Monitoring for progressive disease
Serial cross-sectional imaging with the same modality is the most accurate way of monitoring for progression of disease. While RECIST guidelines (Response Evaluation Criteria In Solid Tumors) 7, 8 has its limitations9 (ex. necrosis and edema in target lesions may result in increase in tumor size), this is a standardized method of determining progression in patients with solid tumors, including thyroid cancer. In general, a 20% increase in target lesion tumor measurements is considered progressive disease (PD), 30% decrease is considered a partial response (PR), and anything in between (−29 to +19%) is stable disease (SD). RECIST versions 1.07 and 1.18 are summarized in Table 2. One major problem with RECIST is that radiologists do not issue their reports with this information. Therefore, endocrinologists and oncologists should not depend solely on the radiology report. Progression can be overestimated if a standardized method of selecting and following lesion growth over time is not implemented. For example, a lesion that is 3 mm would not be considered a target lesion under the RECIST definition because it cannot be accurately measured. If this lesion were to be selected and subsequently reported as 6 mm, one may believe that this patient should start systemic treatment due to a 50% increase in tumor size. This may lead to treating a patient with systemic therapy too soon.
Table 2.
Summary of RECIST criteria versions 1.0 and 1.1 (adapted from Wahl et al91)
| RECIST 1.0 | RECIST 1.1 | |
|---|---|---|
| Measurability and selection of target lesions at baseline |
|
|
|
|
||
| Selection of nontarget lesions | Nontarget lesions: lesions < 1 cm, cystic lesions, bone lesions without soft tissue component, pleural and pericardial effusions, ascites, leptomeningeal disease, lesions in an irradiated area. | Nontarget lesions: Lymph nodes: 10 to 15 mm nodes. Non-lymph nodes: lesions < 1 cm, bone lesions without soft tissue component, pleural and pericardial effusions, ascites, leptomeningeal disease. |
| Objective response |
|
|
|
|
||
| Overall Response |
|
|
2) Local Therapies for Metastatic Disease
Patients with DTC develop distant metastases during their disease course, and distant metastases are present at the time of diagnosis in 7–23% and 1–4%, respectively. The most common site of metastasis is lung, followed by bone, brain, liver, and skin. The reported 10-year survival rates after the discovery of distant metastases range from 25% to 42%2,10,11.
Treatment options for patients with RAI-refractory, metastatic disease depends on the site of disease and tumor burden. Consideration should be given to use of local therapy when the disease burden is localized to one area or the disease is in a potentially threatening location such as the spinal cord.
2.1. Neck Disease
Recurrent locoregional disease in the setting of distant metastases should be treated surgically if there is impending airway or other vital structural compromise. Otherwise, if systemic therapy is a consideration, surgery may be delayed and the neck can be monitored closely. Surgical wound healing is impaired by antiangiogenic therapy so sufficient time for wound healing must be given prior to initiation of these types of drugs.
The lack of prospective studies to assess the role of external beam radiation therapy (EBRT) in patients with DTC who do not have other distant disease makes the recommendation for its use very challenging. Most clinicians do not recommend EBRT for gross locoregional residual disease control in young patients (less than 45 years of age), with microscopic disease. EBRT is generally avoided in patients less than 45 years of age because of their good prognosis, the potential late side effects of therapy, and further need for surgery in the future if the tumor recurs. Although it is controversial12, EBRT may improve locoregional control in high risk patients in the setting of unresectable gross residual disease, which is RAI refractory. EBRT may be also used as adjuvant therapy for older patients who had a complete resection of all visible non-RAI avid tumor in the setting of gross extrathyroidal extension into surrounding major structures, especially if the tumor has aggressive features13–16.
EBRT to gross disease in the neck in the setting of other progressive, distant disease (and consequently, need for systemic therapy) is not recommended in most cases. First of all, EBRT may delay systemic therapy due to common side effects such as esophagitis. Furthermore, there is a theoretical risk of upper tracheo-esohpageal and tracheo-tumor fistula formation in the setting of EBRT to the neck and antiangiogenic therapy. Airway fistulas have been described in lung cancer patients treated with bevacizumab and antiangiogenic therapy in the setting of EBRT. This is likely due to the poor wound healing associated with antingiogenic drugs and chronic esophagitis from the EBRT17. It is important to balance the risk of EBRT related complications and delay of initiation of systemic therapy with the potential benefit in terms of local control without a clear overall survival benefit.
2.2. Lung metastases
Lung metastases are the most common distant metastases from thyroid cancer. Distant disease limited to the lung that is indolent and asymptomatic and does not threaten vital structures can usually be followed with serial imaging. Pulmonary metastases can occasionally cause symptoms such as dyspnea, obstructive pneumonia, and hemoptysis. In selected patients with isolated or localized pulmonary metastases, surgical resection can be considered. Radiation may be another possible treatment method however EBRT to the mediastinum, in the setting of use of antiangiogenic drugs introduces the potential for tracheo-esophageal fistula formation and bleeding17.
2.3. Bone metastases
Bone metastases are the second most common site for distant metastases after lung. They occur in 2–13% of all patients with DTC and in 44% of patients with metastatic DTC18. The presence of bone metastases is a sign of poor prognosis in patients with DTC, and effective treatment is challenging2,19–21. In contrast to lung metastases, bone metastases are often symptomatic. Searching for bone metastases in patients with musculoskeletal pain and elevated thyroglobulin should be considered, because skeletal related events (SREs) account for increased morbidity and mortality. SREs are defined as spinal cord compression, pathological fracture, and need for external beam irradiation or surgery to control pain or prevent facture. In a recent retrospective review of 245 DTC patients with bone metastases, 78% of patients either presented with or developed at least one SRE after the diagnosis of metastatic bone disease. The median time from identification of bone metastasis to development of first SRE was 5 months. Sixty-five percent of those patients who sustained an initial SRE developed a second SRE at a median of 10.7 months 22. In a previous study of 146 thyroid cancer patients with bone metastases, 27% of patients suffered a pathological fracture and 14% developed cord compression19.
The treatment of metastatic bone disease in DTC has been limited to RAI, surgical excision, and/or EBRT. Although bone lesions tend to concentrate radioiodine well, there is complete resolution in less than 10% of the patients. Local therapy with EBRT is often considered in two scenarios: to palliate painful metastases and to provide local control of disease when RAI is no longer effective or rapid disease control is imperative (such as treating bony metastatic disease in the vertebral spine). Embolization of a site of bony metastatic disease is useful in some cases and can lead to tumor shrinkage and palliation of pain. Metastatectomy is sometimes performed if there is only one site of disease. 131I treatment can be used after metastatectomy if the tumor takes up RAI. A retrospective study reported that patients with a solitary bony metastasis treated with 131I and surgery had a better prognosis than those who did not23. Agents that inhibit osteoclast activity, such as bisphosphonates and the RANK ligand inhibitor, denosumab, are used in patients with osteolytic bone metastases from several neoplasms (lung, breast, prostate, kidney, and multiple myeloma), but their use in patients with DTC were not extensively studied to date. Zoledronic acid is the most potent bisphosphonate currently available and is the first drug in its class approved for use in all solid tumor patients with bone metastases as well as in multiple myeloma. The recommended dose is 4 mg infused over 15 minutes every 3–4 weeks. Based on favorable experience in other cancers with bone metastases, the use of bisphosphonates has become an encouraging treatment option for pain and disease control although little data in thyroid cancer exists to support the latter. A retrospective study in patients with bony metastases from DTC reported fewer SREs in patients receiving zoledronic acid when compared to those without treatment24.
Denosumab is indicated for prevention of SREs in patients with bone metastases from solid tumors. The FDA-approved dose for this indication is 120 mg subcutaneously every 4 weeks. Several phase II studies in patients with bone metastasis and advanced cancer showed that treatment with denosumab was associated with rapid and sustained suppression of bone turnover markers and delay of SREs25,26. The role of denosumab for the prevention of SREs in patients with bone metastasis from solid tumors has been established in three large phase III clinical trials. In one phase III study, the efficacy and safety of denosumab compared with zoledronic acid in patients with solid tumors and bone metastases (excluding breast and prostate) or with osteolytic lesions from myeloma was evaluated27. Although, overall survival and disease progression were similar between groups, patients treated with denosumab experienced a greater suppression of bone turnover markers compared with zoledronic acid, demonstrating its more potent antiresorptive effects. With the every 4 weeks dosing schedule, a higher proportion of patients achieved sustained suppression of bone turnover markers and this supports the use of the every 4 weeks regimen in phase III studies. In a second randomized phase III trial comparing denosumab with zoledronic acid it was found that denosumab significantly delayed time to first on-study SRE by 18% and reduced the risk of developing multiple SREs (time to first and subsequent SREs) by 23% compared with zoledronic acid. Reduction in bone turnover markers was greater with denosumab28. Clinical trials of these agents in patients with DTC and bone metastases are needed to evaluate which agents are effective and what frequency of dosing and duration of therapy is appropriate.
2.4. Brain metastases
Brain metastases are rare, occurring in about 0.15–1.3% of thyroid carcinomas. Eighteen percent of patients with distant metastases from papillary thyroid cancer develop brain metastases during their disease course11. Brain metastases are associated with poor prognosis. The reported survival after diagnosis of brain metastases from thyroid carcinoma is typically less than 1 year. It is not uncommon for brain metastases to be asymptomatic. Twenty-three percent of brain metastases were discovered at postmortem examination. Therefore, imaging of the brain should be considered prior to proceeding with systemic therapy and should be treated with local therapy. The optimal treatment for brain metastases is unclear. 131I is rarely used. If radioiodine imaging is positive, consideration for radioiodine treatment with steroid prophylaxis should be taken (to minimize tumor swelling). For solitary lesions, either neurosurgical resection or stereotactic radiosurgery is preferred. Radiosurgery should be utilized in patients with high risk for surgery or if surgery is not possible, and may be considered as initial therapy with later surgical rescue if needed29. A retrospective review of 47 cases of thyroid cancer with brain metastases found that patients treated with surgical resection had significantly longer survival than those who did not undergo surgery for their brain metastases30. The median disease-specific survival from diagnosis of brain metastases was 16.7 months for patients who underwent local excision of one or more brain metastases, compared with 3.4 months for those who did not. In a multi-institutional retrospective study of patients diagnosed with brain metastases from thyroid cancer, patients had an overall median survival time of 20.8 months31. Patients undergoing stereotactic radiosurgery had an overall median survival time of 37.4 months in comparison to 12.3 months for those patients who did not. Radiosurgery provided high local control rate of 96% and prolonged progression-free periods with a mean of 14.6 months32. Whole brain irradiation is considered in patients with extensive multifocal metastatic disease.
2.5. Liver metastases
Liver metastases from DTC are rare, with a reported frequency of 0.5%33. The presence of liver metastases is often a sign of widespread disease thus most patients are treated with systemic therapy when this is the case. There are little data to support local therapy to liver metastases from DTC. Transarterial chemoembolization is the preferred local treatment option for patients with medullary thyroid cancer (MTC)34 but little is known about its efficacy in DTC. There are data, albeit few, to support radiofrequency ablation35,36 in DTC. Surgical resection of liver lesions has been reported to prolong survival37. However the risk benefit ratio must be weighed carefully in patients being considered for surgery as these patients likely have widely metastatic disease.
3) Systemic Therapy
RAI-refractory patients with progression within 12 months, particularly those with symptomatic or life-threatening disease, should be considered for systemic therapy. Most clinical trials in thyroid cancer require progression within 1 year in order to qualify for enrollment in a trial38 and therefore this is the standard most centers use for initiating systemic therapy in asymptomatic patients. Symptomatic patients or patients with bulky disease that compromises organ function and who cannot be managed with local therapies alone should initiate systemic therapy. High FDG uptake on PET scan has been considered as a criterion for initiating systemic therapy because these patients exhibit a more aggressive course and are usually RAI-refractory5,39. However, it is important to prove RAI-refractoriness prior to recommending systemic therapy. Although doxorubicin is the only FDA approved drug for DTC, response rates are very low and short-lived and this drug has become obsolete in thyroid cancer. Other cytotoxic agents also have failed to show significant efficacy and therefore use of these drugs should be a last resort and limited to patients who are not able to receive the newer systemic targeted therapies. TKIs should be considered first because these have been shown to be efficacious in DTC. While there are no TKIs approved for DTC, the ATA40 and NCCN guidelines41 recommend use of TKIs on a clinical trial or “off-label” use of the commercially available anti-angiogenic TKIs. Sorafenib, sunitinib, and pazopanib, are oral antiangiogenics approved for other indications and are commercially available. These drugs have been used in phase II DTC trials and are promising agents for patients with progressive, RAI-refractory disease. Although small phase II clinical trials have reported favorable responses, to date, there are no published results of large phase III trials in DTC. Entry into phase 2 and 3 clinical trials is usually limited to patients with RAI-refractory, progressive disease. Phase 1 trials can be considered for patients who do not meet entry criteria. For example, a patient with very rapidly progressive disease who has an unresectable primary tumor in the thyroid would likely not benefit from RAI due to the large tumor burden in the neck which is likely to take up most of the RAI. Rather than administer a treatment known to be ineffective, this patient could be offered a phase 1 trial. Table 3 shows the list of drugs that have been studied in DTC, their targets, the reported responses, and progression-free survival.
Table 3.
| Drug | VEGFR1 (IC50) | VEGFR2 (IC50) | VEGFR3 (IC50) | RET (IC50) | Other (IC50) | PR | SD | PFS | Citations |
|---|---|---|---|---|---|---|---|---|---|
| Motesanib | X (2) | X (3) | X (6) | X (59) | c-KIT (8) | 14% | 35% | 40 weeks | Sherman et al73 |
|
| |||||||||
| Axitinib | X (1.2) | X (0.25) | X (0.29) | c-KIT (1.7) | 30% | 38% | 18.1 months | Cohen et al71 | |
|
| |||||||||
| Sorafenib | X (90) | X (20) | X (49) | BRAF (22) c-KIT (68) |
15% | 56% | 15 months | Kloos et al57 | |
| 23% | 53% | 84 weeks | Gupta-Abramson et al56 | ||||||
| 25% | 34% | 58 weeks | Hoftijzer et al58 | ||||||
| 31% | 42% | 18 months | Schneider et al59 | ||||||
| 18% | 79% | n/a | Ahmed et al60 | ||||||
| 20% | 60% | 19 months | Cabanillas et al61 | ||||||
|
| |||||||||
| Sunitinib | X (2) | X (9) | X (17) | X (41) | c-KIT (7) | 28% | 46% | TTP 12.8 months | Carr et al62 |
|
| |||||||||
| Pazopanib | X (10) | X (30) | X (47) | X (2800) | c-KIT (74)95 | 49% | n/a | 11.7 months | Bible et al66 |
|
| |||||||||
| Lenvatinib | X (22) | X (4) | X (5.2) | X (35) | FGFR (46) 96 c-KIT (100)97 |
50% | n/a | 12.6 months | Sherman et al77 |
|
| |||||||||
| Cabozantinib | X (0.035) | X (5.2) | c-MET (1.8) c-KIT (4.6)98 |
53% | 40% | n/a | Cabanillas et al76 | ||
|
| |||||||||
| Vandetanib | X (1600) | X (40) | X (110) | X (100) | EGFR (500) | ≤8% | 56% | 11.1 months | Leboulleux et al68 |
|
| |||||||||
| Selumetenib | MEK (14)99 | 3% | 54% | 8 months | Hayes et al75 | ||||
VEGFR=vascular endothelial growth factor receptor; FGFR= fibroblast growth factor receptor; EGFR= epidermal growth factor receptor; PFS=progression-free survival; PR=partial response; CR=complete response; TTP=time to progression; IC50=half maximal inhibitory concentration in nmol.
3. 1. Signaling pathways
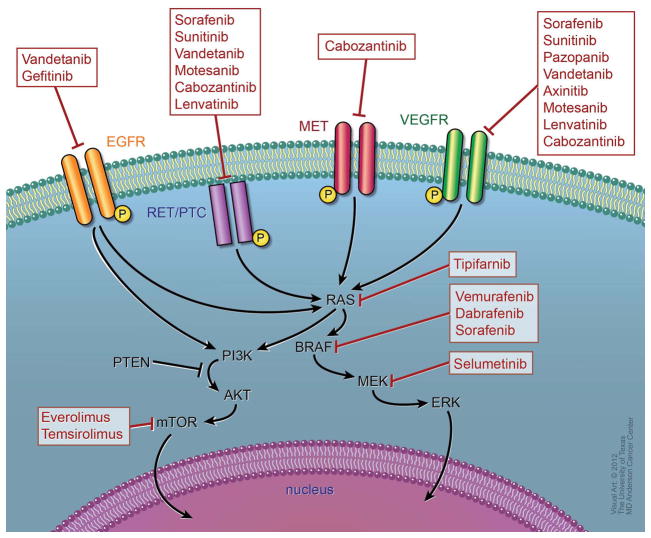
In the last decade, many studies focused on identifying the molecular mechanisms that contribute to thyroid cancer tumorigenesis and its progression. The recognition of the presence of oncogenic mutations in BRAF, RAS, and RET/PTC rearrangements has prognostic implications and represents an important step towards the development of molecularly targeted therapy in DTC. As the vascular endothelial growth factor receptor (VEGFR) is also upregulated in patients with DTC, drugs that target VEGFR and other important targets are currently under investigation. Figure 1 describes the molecular pathways involved in tumorigenesis and the drugs being studied in DTC which inhibit these pathways.
The Ras-Raf-MEK-MAP-ERK (MAPK) kinase signaling pathway plays a key role in development of DTC. The BRAF mutation is the most common genetic alteration in papillary thyroid cancer and is the most potent activator of the MAPK pathway. Kinase-activating mutations in the BRAF proto-oncogene are found in a to 40% of primary PTC tumors and up to 70% of recurrent PTC42,43. BRAF V600E is the most common mutation found in the BRAF gene, accounting for more than 90%. The presence of BRAF V600E is associated with more aggressive features44–47. High incidence of BRAF and BRAF plus RET/PTC rearrangements has been reported in recurrent thyroid cancer42. RET/PTC genetic alterations have been found in 40% and 60% of PTCs in adults and children. RAS mutations have also been found in well differentiated, and more commonly, in poorly differentiated DTCs. The presence of RAS mutations may confer a worse prognosis48. Activating mutations of RAS oncogenes are more common in follicular thyroid cancer and PTC follicular variant occurring in approximately 30% of cases49.
Researchers’ attention has focused on the BRAF mutation as a prognostic marker. Many studies have shown that BRAF mutations correlate with adverse clinicopathological features and earlier recurrences. In a retrospective study with 67 PTC patients, a significant association was seen between BRAF V600E and extrathyroidal extension, advanced stage, and cancer recurrence during a median postoperative follow-up period of 3 years. The majority of these recurrences had no avidity for RAI. It was concluded that BRAFV600E may be a prognostic factor in PTC that correlates with a high risk of recurrences and less differentiated tumors due to the loss of Na+/I− symporter-mediated 131I uptake50. In another study, BRAF-positive status was associated with PTC that was tall cell variant, had extrathyroidal extension, lymph node involvement, advanced stage, and disease recurrence. In short-term follow-up (18 months), persistent or recurrent PTC occurred more likely in patients who were both BRAF-positive and elderly (22%)51. The presence of BRAF V600E mutation showed an independent correlation with the worst outcome in a retrospective study of 102 PTC patients with a median follow-up of 15 years. There were a lower percentage of survivors in the BRAF V600E-mutated group. The age at diagnosis older than 60 years, stage, and vascular invasion were significantly correlated with the BRAF V600E mutation43. Combined positivity for BRAF and extra-nodal extension has been shown to have additive prognostic value in predicting disease specific survival (79% at 10 years versus 100% at 10 years)52.
Angiogenesis is important for tumor cell growth, progression, and development of metastases53. Vascular endothelial growth factor (VEGF), an important pro-angiogenic factor, binds to VEGF receptors that in turn can activate MAP kinase signaling and promote tumor growth. Thyroid tumors are highly vascular and overexpress VEGF which plays an important role in the development and progression of thyroid cancer. VEGF expression is associated with higher risk of recurrence and shorter disease-free survival54,55.
3.2. Systemic targeted therapies
3.2.1. Commercially available drugs
Sorafenib (Nexavar™) is currently approved in the United States (U.S.) and Europe for advanced renal cell carcinoma and unresectable hepatocellular carcinoma. It is an orally active multi–tyrosine kinase inhibitor with multiple targets, including BRAF, VEGFR1, and VEGFR2. This drug affects tumor cell proliferation and angiogenesis. The recommended daily dose is 400 mg twice daily. The most common adverse reactions are fatigue, weight loss, rash/desquamation, hand-foot skin reaction, alopecia, diarrhea, anorexia, nausea and abdominal pain.
Several phase II clinical trials have been performed in patients with DTC that have shown favorable results. An open-label phase II trial was conducted to determine the efficacy of sorafenib in patients with advanced thyroid carcinoma56. Thirty patients were enrolled in this study and treated with sorafenib for a minimum of 16 weeks. All patients had evidence of measurable disease by RECIST, progressive disease within 1 year before initiation of treatment, and almost all patients (93%) had uptake on PET. Five patients had received prior chemotherapy, all were TKI naive, and 11 patients had prior EBRT. The patient population consisted mostly of patients with DTC; 27 of 30 patients had either papillary (18 patients) or follicular (9 patients) subtypes. One patient had MTC and 2 patients had poorly differentiated/anaplastic carcinoma. Seven patients (23%) had a PR lasting 18 to 84 weeks. Sixteen patients (53%) had SD lasting 14 to 89 weeks. Median progression free survival (PFS) time was estimated to be 18 months. Treatment with sorafenib provided a clinical benefit (PR plus SD) rate of 77% in patients with RAI-refractory, metastatic thyroid cancer. Six patients (20%) discontinued treatment due to adverse events. A dose reduction was needed in 47% of patients to control toxicities. Drug holidays were required as a result of adverse events in 63% of patients. One patient developed liver function test elevations after 8 weeks of treatment and, despite cessation of sorafenib, experienced progression and ultimately died of liver failure 3 months later.
In another, larger phase II trial a total of 58 patients were enrolled57. The majority of patients had PTC (41 patients, 73%); Thirty patients had classic PTC, 5 had follicular variant, 4 had tall cell variant, 2 had poorly differentiated PTC. All patients had experienced 131I therapy failure or were not candidates to receive 131I. Of 41 PTC patients, 38 patients had lymph node metastasis, 40 had lung metastases, 5 bone metastases, and 5 liver, kidney or adrenal metastases. At study entry, 5 PTC patients had symptomatic progression in the preceding 6 months, 18 had RECIST progression in preceding 12 months, 14 had stable disease and 4 unknown status. Of 41 PTC patients, 6 patients had a PR (15%) and 23 patients (56%) had SD longer than 6 months. Median PFS was 15 months. Dose reduction due to toxicity was necessary in 52% of patients. The most common grade 3 adverse events were hand or foot pain (12%), arthralgia (11%), fatigue (16%), hand foot skin reaction (7%), musculoskeletal chest pain (7%), and asymptomatic hyponatremia (5%). BRAF mutation was detected in 17 (77%) of 22 PTCs, which were analyzed. Four of 10 paired tumor biopsies from PTCs showed a reduction in levels of VEGFR phosphorylation, ERK phosphorylation, and in VEGF expression during sorafenib therapy.
A phase II study was performed in The Netherlands to assess the efficacy of sorafenib in patients with iodine refractory metastatic DTC, focusing on the re-induction of RAI uptake and the efficacy in patients with bone metastases58. Thirty-two patients were included in the intention-to-treat analysis, 31 patients started sorafenib, but only a total of 22 patients completed 26 weeks of treatment. Of 32 patients, 13 were PTC, 3 follicular variant PTC, 15 follicular, and 1 mixed papillary follicular. Tumor extent at study entry was as follows: thyroid bed only 3%, lungs only 25%, lungs and bones only 22%, locally advanced and distant metastases 33%, other 22%. At 26 weeks of sorafenib therapy, no re-induction of RAI uptake at metastatic sites was observed in 20 evaluable patients. The response rates were as follows: PR 25%, SD 34%, clinical beneficial response (PR plus SD) 59%, and PD 22%. Patients who had bone metastases responded significantly worse. Although this study was not designed to assess PFS, estimation of median PFS was 58 weeks. PFS was 69 weeks in patients without bone metastases and 47 weeks in patients with bone metastases. The long term results of the above study were recently published and showed favorable results59. All 31 patients were included in this long term outcomes study. Eight patients (31%) achieved a PR and 11 patients (42%) showed SD after a median follow-up of 25 months. Median PFS was 18 months, which was influenced by the presence of bone metastases (20 versus 12 months). The median OS was 34.5 months, but the presence of bone metastases resulted in worse OS (23 months). At the time of data analysis, median OS was not reached for patients without bone metastases.
Another phase II trial was conducted in the UK60. Thirty-four patients (15 with MTC and 19 with DTC) were enrolled. In the DTC group, PR was seen in 18% of patients. Median PFS was not reached at 19 months. Toxicity included hand–foot syndrome, diarrhea and alopecia, and dose reductions were required in 79% of patients.
A retrospective review of 15 patients with metastatic, progressive DTC treated with off-label sorafenib and sunitinib (13 sorafenib and 2 sunitinib) found a partial remission rate of 20%, durable response rate of 66%, and a clinical benefit rate of 80%61. In this study lack of response in bony metastatic disease was also reported.
A multicenter, randomized phase III trial has finished recruitment and results are expected to be reported soon.
Sunitinib (Sutent™) is approved in the U.S. and Europe for the treatment of metastatic renal cell carcinoma, gastrointestinal stromal tumor (GIST) and unresectable, metastatic, or locally advanced pancreatic neuroendocrine tumors. It is a multi-targeted agent, with antiangiogenic and antitumor properties, acting as a selective inhibitor of VEGFR 1, 2, and 3, platelet-derived growth factor (PDGFR), cKIT, and RET. The recommended dose for GIST and advanced renal cell carcinoma is 50 mg taken once a day for 4 weeks followed by a 2 week break before beginning the next dosing cycle. The dose for pancreatic neuroendocrine tumors is 37.5 mg daily continuously. Important safety information includes hepatotoxicity, which has been observed in clinical trials and post-marketing experience. This hepatotoxicity may be severe, and deaths have been reported.
Sunitinib has been tested in phase II clinical trials in metastatic iodine-refractory DTC, showing promising results. To date, the largest phase II trial was performed by Carr et al62. In this study a total of 35 patients with metastatic PET-positive, RAI-refractory recurrent or metastatic DTC or MTC were treated with sunitinib. Seven patients had MTC, 28 patient had DTC (18 papillary, 4 follicular, 5 Hurthle cell, 1 insular subtypes). Eight of 29 patients with DTC and 3 of 6 patients with MTC achieved a response using RECIST criteria (response rate 28% for DTC and 50% for MTC). There was 1 CR in target lesions (3%) and 10 PRs (28%). The patient with CR continued to have visible bony metastases63. Sixteen patients (46%) had SD. Seventy seven percent did not have disease progression at the first evaluation (3 months), whereas progressive disease was seen in 17%. The median time to progression was 12.8 months and the median OS had not been reached. The median percent change in average SUVs in 22 patients who had a repeat FDG-PET done, was −11.7%, −13.9%, and 8.6% for patients with PR, SD, and PD, respectively. Dose reductions were necessary in 21 of 35 patients (60%) secondary to non-hematologic adverse events. The most common toxicities included fatigue (11%), neutropenia (34%), hand/foot syndrome (17%), diarrhea (17%), and leukopenia (31%). The most serious complication was bleeding, with one treatment-related death.
Two other smaller trials were previously presented at the American Society of Clinical Oncology (ASCO) in 2008. The first trial included 17 patients (8 papillary, 4 medullary, 1 anaplastic and 4 miscellaneous)64. Fifteen patients were evaluable for response: 1 patient had a PR, 12 patients had a SD disease65. The second trial included patients with progressive metastatic DTC or MTC, refractory to curative treatment or RAI. Forty-three patients (37 DTC, 6 MTC) were enrolled. Best response in 31 evaluable DTC patients who completed 2 cycles was PR 13%, SD 68%, PD 10%, and NE 13%.
Pazopanib (Votrient™) is approved in the U.S. and Europe for the treatment of advanced renal cell carcinoma and soft tissue sarcoma. It is a tyrosine-kinase inhibitor targeting VEGF receptors, PDGFR, and c-KIT. Pazopanib is taken on a continuous cycle at a dose of 800 mg daily. This drug carries a black box warning due to severe and fatal hepatotoxicity observed in a renal cell carcinoma patient. The recommendation is to monitor hepatic function and interrupt, reduce, or discontinue the drug in case transaminitis occurs.
Pazopanib was studied in a phase II trial on 39 patients with progressive, radioiodine-resistant DTC66. Clinical outcomes could be assessed in 37 patients (one patient was found to be ineligible, having had no previous treatment with RAI, and one patient withdrew consent before treatment initiation). PR was seen in 18 patients (49%), and the median duration of PFS was 11.7 months. PRs were seen in 8 (73%) of 11 patients with follicular, 5 (45%) of 11 patients with Hürthle cell, and 5 (33%) of 15 patients with papillary tumors. Median OS at 1 year was 81%. The maximum plasma pazopanib concentration during treatment cycle 1 correlated with the maximum change in tumor size. Sixteen (43%) patients required dose reductions due to adverse events. The most frequent toxicities observed were fatigue, skin and hair hypopigmentation, diarrhea, and nausea. Two patients discontinued treatment due to serious hemorrhagic events (one grade 3 lower-gastrointestinal hemorrhage and one grade 4 intracranial hemorrhage).
Vandetanib (Caprelsa™) is approved in the U.S. and Europe for the treatment of MTC in patients with unresectable, locally advanced or metastatic disease. The approval was based on a phase III trial showing PFS survival prolongation when compared to placebo67. It is an oral TKI that selectively targets RET, VEGFR, and EGFR tyrosine kinases. The recommended dose is 300 mg daily. Important safety information includes a black box warning for QT interval prolongation, torsades de pointes, ventricular tachycardia, and sudden death. Treatment should not be started in patients whose QTcF interval (corrected QT interval, Fridericia) is greater than 450 ms. Hypocalcemia, hypokalemia and/or hypomagnesemia must be corrected prior to its administration and should be periodically monitored. ECGs should be obtained to monitor the QT interval at baseline, at 2–4 weeks and 8–12 weeks after starting treatment and every 3 months thereafter. Because of this risk, vandetanib is only available through the Risk Evaluation and Mitigation Strategy (REMS) Program. Under the CAPRELSA REMS Program, only prescribers and pharmacies enrolled in the restricted distribution program can prescribe and dispense the drug.
A recent randomized, double-blind, phase II trial aiming to assess the efficacy and safety of vandetanib compared to placebo in patients with locally advanced or metastatic DTC included 145 patients (72 patients randomized to receive vandetanib and 73 placebo)68. The randomized phase of study ended after patients had disease progression or 12 months of SD; patients were then given the option to either discontinue study treatment or begin open-label treatment with vandetanib. Patients treated with vandetanib were allowed to continue treatment at the end of the randomized phase if the investigator believed that they were obtaining a clinical benefit or until they received another anticancer treatment. Twenty-eight (39%) of patients randomly allocated to vandetanib and 59 (81%) of patients randomly allocated to placebo received open label vandetanib. Seventy-two percent of patients in the vandetanib group compared with 84% in the placebo group had disease progression at data cutoff. Median PFS for patients in the vandetanib group and the placebo group was 11.1 months and 5.9 months, respectively. For patients with PTC, median PFS was 16.2 months and for patients with follicular or poorly differentiated carcinoma, median PFS was 7.7 months in the vandetanib group, however this difference was not statistically significant. Although PFS was statistically significant favoring the treatment group, response rates were surprisingly low. PRs occurred in only 8% of patients in the treatment arm (site review) and this number decreased to 1% upon central review. There was no difference between treatment groups in the proportion of patients who achieved an objective response. There was also no difference in OS between the vandetanib and placebo groups, however this may have been due to cross-over. The most common grade 3 or worse adverse events were QTc prolongation (14%), diarrhea (10%), asthenia (7%), and fatigue (5%).
Vemurafenib (Zelboraf™) is approved in the U.S. and Europe for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation. It is a potent inhibitor of oncogenic BRAF kinases with specific antitumor effects against the mutated BRAF V600E gene. The recommended dose is 960 mg twice daily. Vemurafenib has no contraindications or black box warnings. The most common adverse reactions of any grade include arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus and skin papilloma. Serious adverse events associated with vemurafenib include cutaneous squamous cell carcinoma, new primary malignant melanoma, hypersensitivity reactions, severe dermatologic reactions, QT prolongation, uveitis, and liver enzyme elevation.
In a phase I trial using vemurafenib, 3 patients with PTC that carried the V600E BRAF mutation were enrolled69. One of 3 patients with PTC had a PR and 2 had SD, with the response lasting 8 months in 1 patient (who was progression-free for 12 months) and SD lasting 11 and 13 months in each of the other 2 patients. Vemurafenib is currently being studied in a phase II trial in patients with BRAF-mutated papillary thyroid cancer.
Axitinib (Inlyta™) is approved in the U.S. for the treatment of advanced renal cell carcinoma after the failure of one prior systemic therapy. It is an oral, potent, and selective inhibitor of VEGFRs 1, 2, and 3. There are no contraindications listed within the manufacturer’s labeling. The most common adverse reactions in patients treated with axitinib are diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar-plantar erythrodysesthesia (hand-foot) syndrome, decreased weight, vomiting, asthenia, and constipation. Other severe adverse reactions reported in axitinib-treated patients include hypertensive crisis, arterial and venous thrombotic events, hemorrhage, gastrointestinal perforation and fistula formation, and reversible posterior leukoencephalopathy syndrome.
The phase I trial with axitinib included 36 patients with advanced solid tumors, 5 of whom had thyroid cancer70. Decrease in tumor burden was noted in 1 patient with thyroid cancer, but this did not qualify as a response by RECIST criteria. In the axitinib phase II trial, 60 patients were enrolled (30 with PTC, 15 with follicular, 11 with MTC, 2 with anaplastic, and 2 with other)71. Almost all patients (93%) had received previous treatment, including chemotherapy (15%). PRs were observed in 18 patients (31%). SD lasting 16 weeks was reported in 23 patients (38%) and 4 patients (7%) had PD. There was no apparent association between response rate and histology. Fourteen out of 45 patients (31%) with DTC had PR and 19 out of 45 (42%) had SD. Median PFS was 18.1 months. Eight patients (13%) discontinued treatment because of adverse events and axitinib dose was reduced in 23 patients (38%) because of adverse events, most commonly fatigue, hematuria, and diarrhea.
Gefitinib (Iressa™) is approved in Europe for locally advanced or metastatic non-small cell lung cancer. In the U.S. it was initially approved for the same indication but in 2005, the U.S. Food and Drug Administration approved new labeling for gefitinib that limits the indication to cancer patients who, in the opinion of their treating physician, are currently benefiting, or have previously benefited, from gefitinib treatment. It is a small molecule inhibitor of the EGFR tyrosine kinase that, in the presence of activating mutations of the EGFR gene, has been shown to be effective in the treatment of NSCLC patients. A phase II study of gefitinib enrolled 27 patients with RAI-refractory, locally advanced, or metastatic thyroid cancer72. Histologic subtypes included papillary (41%), follicular (22%), anaplastic (19%), medullary (15%), and Hurthle cell carcinomas (4%). There were no PRs or CRs seen in the 25 patients available for evaluation. Eight patients (32%) had objective reductions in measurable disease, but did not reach the threshold for a PR by RECIST criteria. After 3, 6, and 12 months of treatment, 48%, 24%, and 12% of patients had SD, respectively. Median PFS and OS were 3.7 and 17.5 months, respectively. Because of these disappointing results, gefitinib is not recommended for treatment of metastatic DTC.
Everolimus (RAD001; Afinitor™) is an mTOR inhibitor. It is approved in the U.S. and Europe for postmenopausal women with advanced hormone receptor-positive, HER2- negative breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole, progressive neuroendocrine tumors of pancreatic origin that are unresectable, locally advanced or metastatic, advanced renal cell carcinoma after failure of treatment with sunitinib or sorafenib. In addition, the drug has received approval for certain tumors associated with the tuberous sclerosis. A phase II trial using everolimus for patients with RAI-refractory thyroid cancer is currently underway. The goal of this clinical study is to assess the efficacy of everolimus in controlling locally-advanced or metastatic thyroid cancer.
3.2.2. Investigational drugs (not commercially available)
Motesanib (AMG706) is currently not commercially available. It is an oral tyrosine kinase inhibitor that inhibits VEGFRs 1-3, PDGFR, and KIT. In a phase 1 study, treatment with motesanib resulted in antitumor activity in patients with advanced solid cancers, including 5 patients with DTC (2 papillary, 1 follicular, 1 follicular and papillary, 1 Hurthle cell), 1 MTC and 1 anaplastic carcinoma. Three patients with DTC (1 Hurthle cell and 2 papillary) showed PR. The favorable responses in thyroid cancer led to interest from the pharmaceutical company to pursue a phase II trial in thyroid cancer. An open-label phase II study was conducted to evaluate the efficacy and tolerability of motesanib in patients with progressive, locally advanced or metastatic, RAI-resistant DTC73. Of the 93 patients enrolled in this trial, 57 (61%) had PTC. Confirmed objective response was achieved in 14% of the patients, all of whom had PR. SD was achieved in 67% of the patients, with durable SD (disease that was stable for 24 weeks or more) in 35% of the patients. Eight percent of patinets had PD. The median PFS was 40 weeks. There was no association between BRAF V600E mutation and the clinical outcome. Twelve patients (13%) discontinued treatment owing to adverse events and Grade 3 events were reported in 51 patients (55%). Eighty seven patients (94%) had at least one treatment-related adverse event during the course of the study, the most commonly reported events being diarrhea, hypertension, fatigue, and weight loss.
A planned subanalysis of the above motesanib thyroid cancer study investigated whether baseline levels and/or changes in specific biomarkers were associated with tumor response and/or PFS74. The changes in the serum placental growth factor (PlGF) and soluble VEGFR 2 levels after initiation of therapy predicted response to motesanib in patients with advanced DTC or metastatic MTC. The change from baseline in PlGF level after 1 week of treatment correlated with best tumor response. The response rate in patients with a greater than 4.7-fold increase in PlGF was 30% as compare with 3% below this threshold. Lower baseline VEGF levels were associated with longer PFS.
Selumetenib (AZD6244) is a potent, selective inhibitor of the MAPK kinases, MEK 1/2. A multicenter, open-label, phase II trial was conducted to evaluate the efficacy, safety, and tolerability of selumetinib in iodine-refractory papillary thyroid cancer75. Thirty-nine patients were enrolled, out of which 32 were evaluable patients for objective response. Best responses were 1 PR (3%), 21 SD (54%), and 11 PD (28%). Median PFS was 32 weeks. The most common drug-related adverse events included rash (77%), fatigue (49%), diarrhea (49%), and peripheral edema (36%). Fourteen patients required dose delays,12 patients required dose reductions and 6 patients discontinued treatment due to toxicity.
Cabozantinib (XL184) is an oral multi- kinase receptor inhibitor. Its targets include VEGFR-2, c-MET, RET, c-Kit, FLT3, and Tie-2; thus, it targets angiogenesis, and overexpression of MET. A phase I trial designed to investigate potential drug-drug interactions restricted enrollment to renal cell and DTC patients. Results of the DTC cohort presented at the 2012 ASCO meeting are encouraging76. Fifteen DTC patients were enrolled. Fifty-three percent of the patients had a confirmed PR and 40% had best response of SD. Disease control rate (PR plus SD) was 80% at 16 weeks. Median PFS and OS were not reached. Grade 3 and 4 adverse events included diarrhea (20%), lipase increased (20%), hypertension (13%) and palmar-plantar erythrodyesthesia (13%). A phase 2 trial in DTC patients is planned. Cabozantinib is currently under review by the FDA as a treatment for patients with progressive, unresectable, locally advanced, or metastatic MTC.
Lenvatinib (E7080) is an oral tyrosine kinase inhibitor targeting VEGFR1-3, FGFR1-4, RET, KIT and PDGFR. In the phase I study, partial responses were observed in thyroid as well as melanoma, endometrial, and renal cancers. A phase II trial studied the effect of lenvatinib in 58 patients with progressive, radioiodine-resistant DTC77. PRs were observed in 50% of patients, and median PFS was 12.6 months. Thirty-five percent of patients required dose reduction, and 23% were withdrawn from therapy due to toxicity. The most common adverse events were hypertension (64%), fatigue (55%), diarrhea (45%), decreased appetite (44%), weight loss (43%) and proteinuria (39%). A multicenter, randomized, double-blind, placebo-controlled, phase 3 trial of lenvatinib in RAI-refractory DTC is currently underway.
Dabrafenib (GSK2118436) is an inhibitor of BRAF kinase that is selective for mutant BRAF. A recent phase I dose-escalation trial aimed to investigate the safety, tolerability, and phase 2 recommended dose of dabrafenib, was conducted in patients with incurable solid tumors, enriching for patients with BRAF-mutant cancers78. A total of 184 patients were enrolled (28 patients with non-melanoma tumors and 156 with metastatic melanoma). In 28 patients with BRAF-mutant non-melanoma solid tumors, antitumor activity was noted in a gastrointestinal stromal tumor, PTC, non-small-cell lung cancer, ovarian cancer, and colorectal cancer. Of 14 patients with BRAF-mutant PTC, 4 could not be assessed because the first restaging scan was not available, and one patient had received previous treatment with MEK inhibitor and had PD as best response. Three of the nine (33%) remaining patients achieved PR, 2 of which were confirmed PRs. The most common adverse events of grade 2 or worse were cutaneous squamous-cell carcinoma (11%), fatigue (8%), and pyrexia (6%). Dose reductions were necessary in 7% of the patients.
3.2.3. Combination therapy
The combination of sorafenib with tipifarnib has been reported in a phase I study. In this trial were included 22 patients with metastatic DTC (16 PTC, 5 FTC, and 1 poorly differentiated) and 13 patients with MTC. All DTC patients were RAI refractory79. PR rates were 38% and durable SD (more than 6 months) 31% in patients with MTC. In patients with DTC, the PR rate was 4.5% and durable SD was 36%. The median PFS in 22 DTC patients was 20 months. The median OS had not been reached, but at 24 months, OS in the DTC patients was 79%.
The preliminary results of a phase II study of the combination of sorafenib and temsirolimus shows promising results in patients with RAI-refractory thyroid carcinoma80. The data were presented at the 2012 ASCO Annual Meeting. Of the 37 eligible patients, 23 were papillary, 1 follicular, 5 Hürthle cell, 6 poorly differentiated and 2 anaplastic. PRs were observed in 8 patients, including 3 with anaplastic/poorly differentiated cancer; SD in 21 patients; PD in 1 patient. Seven patients were inevaluable. The PR rate was 38% in the cohort of patients that previously did not receive any systemic treatment. There was no correlation of response to either BRAF or RAS mutational status.
A phase II trial of everolimus with sorafenib for patients with DTC is currently underway.
3.2.4. Sequential administration
There is minimal evidence regarding sequential administration of the TKIs in DTC, and is not understood if a patient who had progressive disease with one TKI may still respond to the next one. In a cohort of metastatic renal cell carcinoma treated with sunitinib after progression through sorafenib, the response rate seen with second line sunitinib after sorafenib was similar to that of first line81.
A small, retrospective review of metastatic, progressive DTC treated with off-label sorafenib and sunitinib showed that patients had a clinical response to sunitinib despite progression on sorafenib. Sorafenib was given to all 15 patients initially. Two patients discontinued sorafenib and resumed therapy with sunitinib (one due to PD and the other due to toxicity). The sunitinib patient previously refractory to sorafenib had a 38% reduction in tumor size61.
3.2.5. Agents to Restore Radioactive Iodine Uptake
Finding drugs to restore the ability to concentrate radioiodine would be of great value in treating patients with advanced thyroid cancer. Retinoic acid82, bexarotene83, desipeptide84, rosiglitazone85, and sorafenib58 have been studied as re-differentiating agents but results are not encouraging. The most encouraging of the drugs studied to date is selumetenib. Preclinical data showed that in thyroid tumors carrying BRAF V600E mutations treatment with selective MEK or BRAF inhibitors rendered the tumor cells susceptible to a therapeutic dose of RAI86. Clinical data presented at the 2012 ASCO Annual Meeting showed that selumetenib can enhance iodine uptake in a subset of RAI-refractory metastatic thyroid cancer. This may be particularly effective for RAS mutated tumors87. Twenty-four patients were enrolled in this study, of which 20 were evaluable. Nineteen patients had tumors analyzed for BRAF and N-, K- RAS mutations (8 were BRAF mutant and 11 were BRAF wild-type). Selumetenib increased 124I uptake in 12 of the 20 patients (4 of 8 BRAF mutant; 8 of the 12 remaining patients). Eight of those 12 patients achieved sufficient iodine avidity to warrant RAI therapy, including all 5 patients known to be NRAS mutated and 1 BRAF mutated patient. Of the 7 patients who had received RAI 5 had PRs, and 2 had SD.
3.3. Treatment challenges
Patients receiving kinase inhibitors should be carefully monitored for serious adverse events (AEs). Common side effects of sorafenib, sunitinib, and pazopanib include hypertension, skin toxicity (rash and hand-foot syndrome), diarrhea, weight loss and fatigue. Less common but potentially fatal AEs include congestive heart failure, slow wound healing or wound dehiscence, bleeding, and upper airway fistula formation. The toxicity profile of most commonly used TKIs is summarized in Table 4.
Table 4.
Major adverse events associated with commercially available TKIs used for thyroid cancer (adapted from 90)
| Adverse event | Sorafenib (%) | Sunitinib (%) | Pazopanib(%) | |||
|---|---|---|---|---|---|---|
| All-grade | ≥grade 3 | All-grade | ≥grade 3 | All-grade | ≥grade 3 | |
| Hypertension | 17 | 4 | 30 | 12 | 40 | 4 |
| CHF or LVEF decline | 1.7 | NR | 13 | 3 | <1% | NR |
| Proteinuria | NR | NR | NR | NR | 9 | <1 |
| Hand-foot skin reaction | 30 | 6 | 29 | 6 | 6 | NR |
| Stomatitis | NR | NR | 30 | 1 | 4 | NR |
| Anorexia | 16 | <1 | 34 | 2 | 22 | 2 |
| Weight loss | 10 | <1 | 12 | <1 | 52 | 3.5 |
| Diarrhea | 43 | 2 | 61 | 9 | 52 | 3.5 |
| AST elevation | NR | NR | 56 | 2 | 53 | 7.5 |
| ALT elevation | NR | NR | 51 | 2.5 | 53 | 12 |
| Fatigue | 37 | 5 | 54 | 11 | 19 | 2 |
| Hypothyroidism | NR | NR | 14 | 2 | 7 | NR |
| Arterial thromoembolism | 2.9 | NR | NR | NR | 3 | 2 |
| Hemorrhage/bleeding | 15 | 3 | 30 | 3 | 13 | 2 |
CHF: congestive heart failure; LVEF: left ventricular ejection fraction; AST: aspartate aminotransferase; ALT: alanine aminotransferase; NR: not reported.
FUTURE DIRECTIONS
There remain several unmet needs in the area of thyroid cancer therapeutics. Understanding the resistance pathways that develop with treatment will help us to better understand which combinations of therapies should be studied. A potential mode of thyroid cancer resistance to targeted therapy is the diminished immune response to tumor. Recent studies suggest that the immune response associated with both primary and metastatic PTC may be dysfunctional88,89. The functional capacity of the patient’s immune system should be assessed in cancer therapy trials to determine whether the therapeutic drug has an effect on the immune response thus indirectly contributing to the observed response or resistance. Several drugs which target the tumor by enhancing the immune system are being used in other tumor types.
Another unmet need is the management of mixed responses. An example of this is a patient who has bony metastastic disease which is progressing on systemic therapy but has other areas of disease which are responding. We have published one case of a patient treated effectively with external beam radiation to the bony site of progression while on a TKI90 but more data are needed to make such recommendations. Many patients present with only bone metastases and are not eligible for clinical trials due to lack of RECIST target lesions. Thus, there is little known about the best therapy for bony metastatic disease in RAI-refractory patients. Furthermore, studies examining how non-anti-angiogenic drugs can be used in the neoadjuvant setting could improve surgical outcomes and possibly extend survival.
SUMMARY
Recent advances in the understanding of the important signaling pathways and molecular biology in cancer have paved the way to development of modern chemotherapeutics. Development of targeted therapies has revolutionized therapy for many tumor types such as gastrointestinal stromal tumors, renal cell carcinoma, chronic myelogenous leukemia, and melanoma. TKIs hold great promise for RAI-refractory, progressive DTC but none to date are approved for this disease. Since few DTC patients require systemic therapy, it is important to be able to identify them so that they may initiate treatment when appropriate. In general, only patients with progressive and/or symptomatic, RAI-refractory disease should be started on a TKI since these treatments are chronic therapies, not curative and can have serious side effects. Furthermore, it is unknown if the TKIs will prolong overall survival or improve quality of life. Thus, other treatment options such as close observation for patients with indolent disease and local therapies for patients with few sites of metastatic disease should be considered.
Figure 2.
Molecular signaling pathways and drug targets in DTC: The main signaling pathways in thyroid carcinogenesis are the Ras/Raf/MAPK and P13K-AKT pathways. Extracellular signals activate tyrosine kinase receptors leading to activation of Ras, which in turn activates Raf (mainly BRAF in DTC). Activated BRAF phosphorylates and activates the MEK, which in turn phosphorylates and activates ERK. Activated ERK translocates into the nucleus, where it regulates transcription of the genes involved in cell differentiation, proliferation, and survival. PI3K-Akt indirectly activates mTOR and it is a key regulator of cell proliferation and inhibitor of apoptosis. Signaling cascade is blocked with newer targeted therapies. The key targets that are currently under evaluation in phase II and III clinical trials in DTC are shown. *Although, no information is available to date, inhibitors of RET should theoretically inhibit RET/PTC similarly
Abbreviations: EGFR= epidermal growth factor receptor, VEGFR=vascular endothelial growth factor receptor, MAPK= mitogen-activated protein kinases; MEK= MAPK kinase; ERK= extracellular signal-regulated kinase; PI3K=phosphatidylinositol 3-kinase; mTOR= mammalian target of rapamycin.
Footnotes
Disclosures: MEC has received research funding from Eisai, Exelixis, and Roche. RD has nothing to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 3.Ringel MD. Metastatic dormancy and progression in thyroid cancer: targeting cells in the metastatic frontier. Thyroid. 21(5):487–92. doi: 10.1089/thy.2011.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr Relat Cancer. 18(1):159–69. doi: 10.1677/ERC-10-0233. [DOI] [PubMed] [Google Scholar]
- 5.Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91(2):498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 6.Leboulleux S, El Bez I, Borget I, Elleuch M, Deandreis D, Al Ghuzlan A, et al. Post-Radioiodine Treatment Whole Body Scan in the Era of Fluorodesoxyglucose Positron Emission Tomography for Differentiated Thyroid Carcinoma with elevated serumb thyroglobulin levels. Thyroid. 2012 doi: 10.1089/thy.2012.0081. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25(13):1760–4. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 10.Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(1):313–9. doi: 10.1210/jc.2005-1322. [DOI] [PubMed] [Google Scholar]
- 11.Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80(7):2041–5. doi: 10.1210/jcem.80.7.7608252. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz DL, Lobo MJ, Ang KK, Morrison WH, Rosenthal DI, Ahamad A, et al. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys. 2009;74(4):1083–91. doi: 10.1016/j.ijrobp.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle RM, Rondeau G, Lee NY. A risk-adapted approach to the use of radioactive iodine and external beam radiation in the treatment of well-differentiated thyroid cancer. Cancer Control. 2011;18(2):89–95. doi: 10.1177/107327481101800203. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Tuttle M. The role of external beam radiotherapy in the treatment of papillary thyroid cancer. Endocr Relat Cancer. 2006;13(4):971–7. doi: 10.1677/ERC-06-0039. [DOI] [PubMed] [Google Scholar]
- 15.Brierley JD, Tsang RW. External beam radiation therapy for thyroid cancer. Endocrinol Metab Clin North Am. 2008;37(2):497–509. xi. doi: 10.1016/j.ecl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Strasser JF, Raben A, Koprowski C. The role of radiation therapy in the management of thyroid cancer. Surg Oncol Clin N Am. 2008;17(1):219–32. x. doi: 10.1016/j.soc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Spigel DR, Hainsworth JD, Yardley DA, Raefsky E, Patton J, Peacock N, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28(1):43–8. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 18.Muresan MM, Olivier P, Leclere J, Sirveaux F, Brunaud L, Klein M, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15(1):37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 19.Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10(3):261–8. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 20.Orita Y, Sugitani I, Matsuura M, Ushijima M, Tsukahara K, Fujimoto Y, et al. Prognostic factors and the therapeutic strategy for patients with bone metastasis from differentiated thyroid carcinoma. Surgery. 2010;147(3):424–31. doi: 10.1016/j.surg.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Sugitani I, Fujimoto Y, Yamamoto N. Papillary thyroid carcinoma with distant metastases: survival predictors and the importance of local control. Surgery. 2008;143(1):35–42. doi: 10.1016/j.surg.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Farooki A, Leung V, Tala H, Tuttle RM. Skeletal-Related Events due to Bone Metastases from Differentiated Thyroid Cancer. J Clin Endocrinol Metab. 2012;97(7):2433–9. doi: 10.1210/jc.2012-1169. [DOI] [PubMed] [Google Scholar]
- 23.Qiu ZL, Song HJ, Xu YH, Luo QY. Efficacy and survival analysis of 131I therapy for bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96(10):3078–86. doi: 10.1210/jc.2011-0093. [DOI] [PubMed] [Google Scholar]
- 24.Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21(1):31–5. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 25.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 26.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25(28):4431–7. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 27.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 28.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 29.McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer. 2003;98(2):356–62. doi: 10.1002/cncr.11488. [DOI] [PubMed] [Google Scholar]
- 30.Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997;82(11):3637–42. doi: 10.1210/jcem.82.11.4386. [DOI] [PubMed] [Google Scholar]
- 31.Bernad DM, Sperduto PW, Souhami L, Jensen AW, Roberge D. Stereotactic radiosurgery in the management of brain metastases from primary thyroid cancers. J Neurooncol. 2010;98(2):249–52. doi: 10.1007/s11060-010-0175-z. [DOI] [PubMed] [Google Scholar]
- 32.Kim IY, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for metastatic brain tumors from thyroid cancer. J Neurooncol. 2009;93(3):355–9. doi: 10.1007/s11060-008-9783-2. [DOI] [PubMed] [Google Scholar]
- 33.Salvatori M, Perotti G, Rufini V, Maussier ML, Summaria V, Fadda G, et al. Solitary liver metastasis from Hurthle cell thyroid cancer: a case report and review of the literature. J Endocrinol Invest. 2004;27(1):52–6. doi: 10.1007/BF03350911. [DOI] [PubMed] [Google Scholar]
- 34.Fromigue J, De Baere T, Baudin E, Dromain C, Leboulleux S, Schlumberger M. Chemoembolization for liver metastases from medullary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(7):2496–9. doi: 10.1210/jc.2005-2401. [DOI] [PubMed] [Google Scholar]
- 35.Wertenbroek MW, Links TP, Prins TR, Plukker JT, van der Jagt EJ, de Jong KP. Radiofrequency ablation of hepatic metastases from thyroid carcinoma. Thyroid. 2008;18(10):1105–10. doi: 10.1089/thy.2008.0080. [DOI] [PubMed] [Google Scholar]
- 36.Berber E, Ari E, Herceg N, Siperstein A. Laparoscopic radiofrequency thermal ablation for unusual hepatic tumors: operative indications and outcomes. Surg Endosc. 2005;19(12):1613–7. doi: 10.1007/s00464-005-0236-0. [DOI] [PubMed] [Google Scholar]
- 37.Andreou A, Brouquet A, Bharathy KG, Perrier ND, Abdalla EK, Curley SA, et al. Liver resection for liver metastases from nondigestive endocrine cancer: extrahepatic disease burden defines outcome. Surgery. 2012;151(6):851–9. doi: 10.1016/j.surg.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlumberger M, Sherman SI. Clinical trials for progressive differentiated thyroid cancer: patient selection, study design, and recent advances. Thyroid. 2009;19(12):1393–400. doi: 10.1089/thy.2009.1603. [DOI] [PubMed] [Google Scholar]
- 39.Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocrine-related cancer. 2011;18(1):159–69. doi: 10.1677/ERC-10-0233. [DOI] [PubMed] [Google Scholar]
- 40.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 41.Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8(11):1228–74. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 42.Henderson YC, Shellenberger TD, Williams MD, El-Naggar AK, Fredrick MJ, Cieply KM, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15(2):485–91. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–9. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 44.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 45.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 46.Ugolini C, Giannini R, Lupi C, Salvatore G, Miccoli P, Proietti A, et al. Presence of BRAF V600E in very early stages of papillary thyroid carcinoma. Thyroid. 2007;17(5):381–8. doi: 10.1089/thy.2006.0305. [DOI] [PubMed] [Google Scholar]
- 47.Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65(3):364–8. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21(17):3226–35. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 49.Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW, 2nd, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88(5):2318–26. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 50.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13(1):257–69. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 51.Howell GM, Carty SE, Armstrong MJ, Lebeau SO, Hodak SP, Coyne C, et al. Both BRAF V600E mutation and older age (>/= 65 years) are associated with recurrent papillary thyroid cancer. Ann Surg Oncol. 2011;18(13):3566–71. doi: 10.1245/s10434-011-1781-5. [DOI] [PubMed] [Google Scholar]
- 52.Ricarte-Filho J, Ganly I, Rivera M, Katabi N, Fu W, Shaha A, et al. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extra-nodal extension. Thyroid. 2012;22(6):575–84. doi: 10.1089/thy.2011.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 54.Lennard CM, Patel A, Wilson J, Reinhardt B, Tuman C, Fenton C, et al. Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery. 2001;129(5):552–8. doi: 10.1067/msy.2001.112592. [DOI] [PubMed] [Google Scholar]
- 55.Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plenat F, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86(2):656–8. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 56.Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26(29):4714–9. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27(10):1675–84. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161(6):923–31. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 59.Schneider T, Abdulrahman R, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. 2012 doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed M, Barbachano Y, Riddell A, Hickey J, Newbold KL, Viros A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165(2):315–22. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 61.Cabanillas ME, Waguespack SG, Bronstein Y, Williams MD, Feng L, Hernandez M, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab. 2010;95(6):2588–95. doi: 10.1210/jc.2009-1923. [DOI] [PubMed] [Google Scholar]
- 62.Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 16(21):5260–8. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martins Renato., editor. Personal communication. 2010.
- 64.Ravaud A, De la Fouchardiere C, Courbon F, Asselineau J, Klein M, Nicoli-Sire P, et al. Sunitinib in patients with refractory advanced thyroid cancer: the THYSU phase II trial. J Clin Oncol. 2008;26(suppl) abstr 6058. [Google Scholar]
- 65.Ravaud A, de la Fouchardiere C, Asselineau J, Delord JP, Do Cao C, Niccoli P, et al. Efficacy of sunitinib in advanced medullary thyroid carcinoma: intermediate results of phase II THYSU. Oncologist. 2010;15(2):212–3. doi: 10.1634/theoncologist.2009-0303. author reply 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 11(10):962–72. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 69.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rugo HS, Herbst RS, Liu G, Park JW, Kies MS, Steinfeldt HM, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23(24):5474–83. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 71.Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pennell NA, Daniels GH, Haddad RI, Ross DS, Evans T, Wirth LJ, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18(3):317–23. doi: 10.1089/thy.2007.0120. [DOI] [PubMed] [Google Scholar]
- 73.Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 74.Bass MB, Sherman SI, Schlumberger MJ, Davis MT, Kivman L, Khoo HM, et al. Biomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancer. J Clin Endocrinol Metab. 2010;95(11):5018–27. doi: 10.1210/jc.2010-0947. [DOI] [PubMed] [Google Scholar]
- 75.Hayes DN, Lucas AS, Tanvetyanon T, Krzyzanowska MK, Chung CH, Murphy BA, et al. Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18(7):2056–65. doi: 10.1158/1078-0432.CCR-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cabanillas ME, Brose MS, Lee Y, Miles D, Sherman SI. Antitumor activity of cabozantinib (XL184) in a cohort of patients (pts) with differentiated thyroid cancer (DTC) J Clin Oncol. 2012;30(suppl) abstr 5547. [Google Scholar]
- 77.Sherman SI, Jarzab B, Cabanillas ME, Licitra L, Pacini F, Martins R, et al. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC) J Clin Oncol. 2011;29(suppl) abstr 5503. [Google Scholar]
- 78.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong DS, Cabanillas ME, Wheler J, Naing A, Tsimberidou AM, Ye L, et al. Inhibition of the Ras/Raf/MEK/ERK and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. J Clin Endocrinol Metab. 2011;96(4):997–1005. doi: 10.1210/jc.2010-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sherman E, Ho AL, Fury M, Baxi SS, Haque S, Korte SH, et al. A phase II study of temsirolimus/sorafenib in patients with radioactive iodine (RAI)-refractory thyroid carcinoma. J Clin Oncol. 2012;30(suppl) abstr 5514. [Google Scholar]
- 81.Zimmermann K, Schmittel A, Steiner U, Asemissen AM, Knoedler M, Thiel E, et al. Sunitinib treatment for patients with advanced clear-cell renal-cell carcinoma after progression on sorafenib. Oncology. 2009;76(5):350–4. doi: 10.1159/000209961. [DOI] [PubMed] [Google Scholar]
- 82.Gruning T, Tiepolt C, Zophel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer--does it hold its promise? Eur J Endocrinol. 2003;148(4):395–402. doi: 10.1530/eje.0.1480395. [DOI] [PubMed] [Google Scholar]
- 83.Liu YY, Stokkel MP, Pereira AM, Corssmit EP, Morreau HA, Romijn JA, et al. Bexarotene increases uptake of radioiodide in metastases of differentiated thyroid carcinoma. Eur J Endocrinol. 2006;154(4):525–31. doi: 10.1530/eje.1.02123. [DOI] [PubMed] [Google Scholar]
- 84.Su YB, Tuttle RM, Fury M, Ghossein R, Singh B, Herman K, et al. A phase II study of single agent depsipeptide (DEP) in patients (pts) with radioactive iodine (RAI)-refractory, metastatic, thyroid carcinoma: Preliminary toxicity and efficacy experience. J Clin Oncol. 2006;24(suppl) abstr 5554. [Google Scholar]
- 85.Kebebew E, Peng M, Reiff E, Treseler P, Woeber KA, Clark OH, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140(6):960–6. doi: 10.1016/j.surg.2006.07.038. discussion 66–7. [DOI] [PubMed] [Google Scholar]
- 86.Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–11. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho L, Leboeuf R, Grewal RK, Sherman EJ, Deandreis D, Pentlow K, et al. Reacquisition of RAI uptake in RAI-refractory metastatic thyroid cancers by pretreatment with the MEK inhibitor selumetinib. J Clin Oncol. 2012;30(suppl) abstr 5509. [Google Scholar]
- 88.French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC, Jr, Klopper JP, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97(6):E934–43. doi: 10.1210/jc.2011-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95(5):2325–33. doi: 10.1210/jc.2009-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res. 2011;2011:985780. doi: 10.4061/2011/985780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J Thyroid Res. 2012:618985. doi: 10.1155/2012/618985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sherman SI. Molecularly targeted therapies for thyroid cancers. Endocr Pract. 2009;15(6):605–11. doi: 10.4158/EP09131.RAR. [DOI] [PubMed] [Google Scholar]
- 94.Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J Thyroid Res. 2012;2012:618985. doi: 10.1155/2012/618985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonpavde G, Hutson TE, Sternberg CN. Pazopanib, a potent orally administered small-molecule multitargeted tyrosine kinase inhibitor for renal cell carcinoma. Expert Opin Investig Drugs. 2008;17(2):253–61. doi: 10.1517/13543784.17.2.253. [DOI] [PubMed] [Google Scholar]
- 96.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459–65. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 97.Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664–71. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 98.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 99.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13(5):1576–83. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]