Abstract
Purpose of review
The diagnosis and care of bladder cancer (BC) represents a significant financial burden to the population in the United States. Therapeutic advances in BC care have come at a high cost to payers, providers, and patients. This study describes the principals of economic evaluation in health care and provides recommendations for more economic use of the resources in BC care.
Recent findings
While several studies have indicated that BC is a common disease associated with substantial economic burden for patients and society, the evidence of cost-effectiveness of BC interventions is limited and of insufficient quality. In addition, very little is known about quality of life, the preferred outcome measure for economic evaluations, associated with BC states and treatments. Moreover, current clinical guideline for BC care do not incorporate economic factor when evaluating most optimal clinical pathways.
Summary
While economic studies in BC could allow us to know how the money is being spent and assist in determining more effective ways to spend it, most of the currently used interventions have not undergone economic assessment.
Keywords: Bladder Cancer, Cost-effectiveness, Clinical guidelines, Evidence-based medicine, Health economics
INTRODUCTION
Bladder cancer (BC) care continues to represent a significant financial burden on the population and healthcare system of the United States with more than 68,800 new diagnoses and 14,000 deaths estimated for 2008.[1] Therapeutic advances have occurred in BC care, but these have come at an increased cost – to payers, providers, and patients. In 2006, the US spent over $2.1 trillion, or $7,026 per person, on providing health care to its citizens, and medical technology contributes a lot to these expenses.[2] Given the aging of the population and the continued technological advances likely to occur over the next decade, such as new urinary markers for BC, improved endoscopy, and the evolving role of minimally invasive surgery, managing patients with BC will likely become much more costly than it is today. At the same time, each patient’s out-of -pocket costs will likely increase. Thus, there will be growing pressures to contain costs and more efficiently manage care and societal resources. Economic studies in BC will allow us to know how the money is currently spent and help determine more effective ways to allocate resources.
BACKGROUND
Cost-Effectiveness Evaluation Design
Cost-effectiveness (CE) assessment is a comparative analysis of two or more alternative interventions in terms of both their health effects and cost.[3, 4] Hence, CE evaluations could help distinguish between the interventions that are less costly while provide more health benefit than the current standard (and, therefore, should be preferred over the standard treatment), from the treatment that is also more effective but also more costly than the currently used alternative (and hence, requires a more detailed analysis of additional cost per unit benefit).
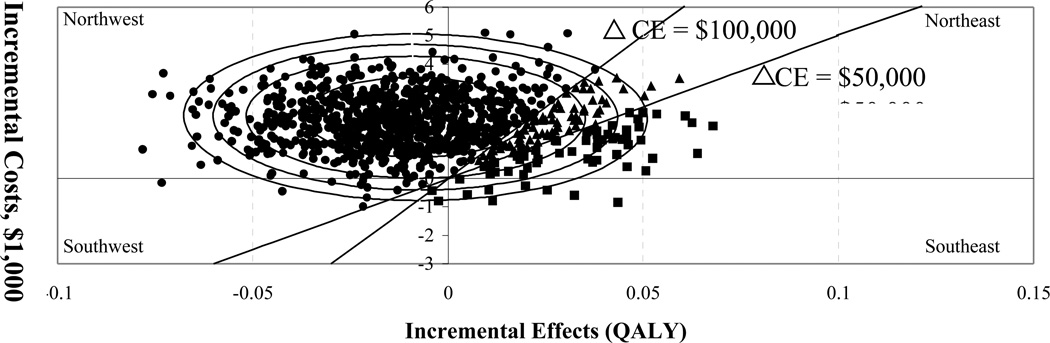
Costs and outcomes data may come from a single source (e.g., a clinical-economic trial) or be combined from multiple sources via a decision analytic model. Figure 1 is a graphical illustration of how to present CE of a new intervention (located at the center of the ellipse) compared to an old intervention (located at the intercept).
Figure 1. Cost-effectiveness plane, thresholds, and confidence ellipses.
Figure 1 is a graphical illustration of how to analyze cost-effectiveness of a new intervention compared to an old intervention (“located” at the intercept). The center of the ellipses represents the difference in average costs and effects between the two treatments. The 2-dimensional cost-effectiveness plane can be dissected into regions of cost-effectiveness based on the socially acceptable cost of one quality-adjusted life year (QALY) or societal “willing-to-pay” (WTP) thresholds.[4] Here we use thresholds of $50,000 per QALY or $100,000 per QALY. Hence, the CE regions are “cost-effective at WTP of $50,000/QALY” (squares); “cost-effective at WTP between $50K–100K/QALY” (triangles); and not cost-effective using societal WTP of $100K/QALY.
In addition, we demonstrate confidence ellipses defined as confidence regions for true incremental cost-effectiveness, analogous to commonly used confidence intervals. The largest ellipse represents the 95% confidence region. In addition, the 90%, 80%, and 50% confidence regions are presented. Each percentage indicates the probability that the true incremental cost-effectiveness of the new treatment is located somewhere within that region.
There are several key concepts that make designing and interpreting economic evaluations non-trivial and different from other practice-based evidence. These include: A) multiple possible study perspectives, such as that of the “patient”, “third-party payer”, “provider”, or “society” [5], B) difficulty collecting data on health care utilization that come from multiple sources [6], C) discrepancies between costs, charges, and reimbursements in health care [7, 8], D) lost patient productivity and other non-medical costs [3, 9], E) presenting the results of cost-effectiveness analyses [10–12], (Figures 1 & 2), and F) evaluating patient health preferences as one potential outcome measure. While most of these issues are explained well in the literature, we discuss the last point in a greater detail below because the trade-off between costs and outcomes is the essential concept in cost-effectiveness research and health preferences are the recommended outcome for economic evaluations.[3]
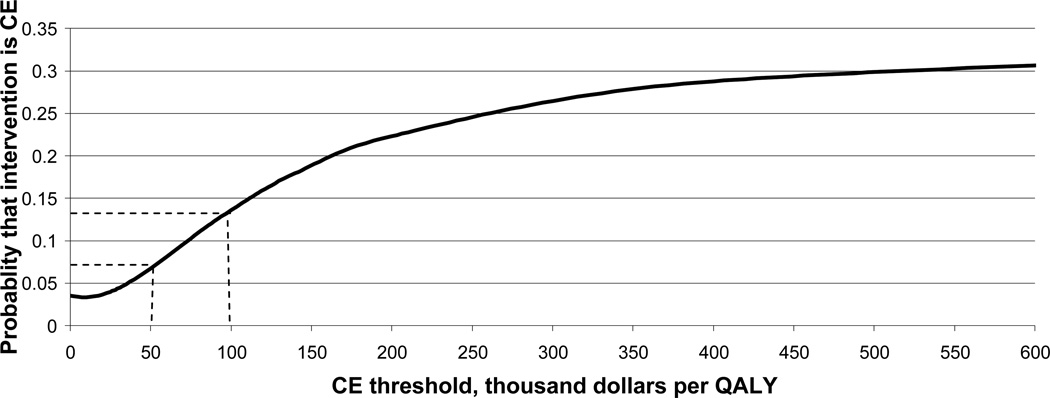
Figure 2. Cost-effectiveness acceptability curve (CEAC).
The CEAC represents the set of probabilities that the new intervention is cost-effective compared to the standard of care for a range of values of societal WTP for an incremental gain in health.[10–12]
Health Preference Measurement
In cost-effectiveness studies, the gold standard is to report results as cost per quality-adjusted life year (QALY) gained. QALYs can be perceived as a 2-dimensional outcome measure defined as a product of a time spent in a specific health state and the health preference (also called utility or health-related quality of life (HRQOL)) of this health state. Use of cost/QALY metric for economic evaluations and societal resource allocation allows for comparison of health interventions across multiple clinical and social areas (e.g., urology vs. neurology vs. public education) which is not possible using natural units (e.g., years of life saved vs change in Unified Parkinson Disease Ration Scale (UPDRS) score vs IQ score).
Health preferences are quantitative ratings of the desirability of health states, and should be distinguished from measures of health status.[13] Health status measures classify patients into specific health states; for example, pathologic stage Ta versus T3b BC. Health preferences reflect how individuals value the desirability of a given health state. The values of preferences are usually measured on a scale from 0 to 1, with death anchored at “0”, and the best imaginable health state anchored at “1”. In addition, some rating systems explicitly include health state valuations worse than death.[4, 14, 15]
Very little is known about HRQOL associated with BC states and treatment. The Tufts cost-effectiveness registry provides a comprehensive list of all studies addressing health utility or cost-effectiveness of individuals, groups, interventions, and programs.[16] For utility of bladder cancer, they reported one study [17] that used data on health preferences for health states associated with BC (0.89 for women, 0.91 for men) using the national data from the Netherlands [18, 19].
While earlier studies assumed that bladder preservation was associated with better HRQOL compared to radical cystectomy, recent improvements in surgical techniques have significantly improved HRQOL after cystectomy that reduced the incentives for performing extensive, morbid, and risky bladder-sparing procedures.[20–22]
Moreover, the comprehensive work done by Porter and colleagues to describe the landscape of HRQOL research in patients who have undergone cystectomy and urinary diversion demonstrated that although much work has been done on the subject, there is no evidence to suggest a differential impact on patient quality of life based on the type of urinary diversion performed.[23, 24] However, most of the publications they reviewed were hindered by methodological limitations, such as a heavy use of retrospective studies based at academic centers and the limited number of disease-specific validated survey instruments.
In summary, HRQOL provides an important complementary approach to the more frequently discussed endpoints of overall and disease-specific mortality and can play a significant role in a patient’s ability to make an informed choice about competing but effective courses of treatment.
STUDY FINDINGS
We conducted a systematic review of the literature on economics of BC using Medline, the UK National Health System (NHS) Economic Evaluation Database (http://www.york.ac.uk/inst/crd/crddatabases.htm) and the Tufts Cost Utility Registry (http://www.tufts-nemc.org/cearegistry/). The summary of the findings are presented in this section.
The most recent BC treatment guidelines published by the AUA (November 2007), EUA (March 2008) and NCCN (January 2008) display significant concordance in terms of the best practices they describe.[25–28] All agree on the need for meticulous attention to surgical technique during transurethral resection of bladder tumors (TURBT) since depth of tumor invasion is both a critical treatment and prognostic factor. Careful follow-up is required for high-grade non-muscle invasive tumors and carcinoma in situ due to their tendency for progression. Cystectomy is the gold-standard for muscle-invasive disease.[20] Despite these similarities, there are several areas that require further attention. Below we present current BC treatment, surveillance, and prevention interventions based on available evidence of their cost-effectiveness.
Cost-effective but underused
While several studies have indicated that BC is a common disease associated with substantial economic burden for patients and society, the evidence of cost-effectiveness of BC interventions is limited and of insufficient quality.[29] Below we review several BC interventions that have been established as cost-effective and should be, but are generally not, adopted for wider use.
The use of a single dose of intravesical chemotherapy post transurethral resection of bladder tumor (TURBT) has been shown to reduce the risk of BC recurrence. The AUA, EUA, and NCCN discuss the benefits and limitations of this practice in their respective guidelines, but differing economic arguments are put forward by the AUA and EUA to support their positions. The AUA recommends the practice in the setting of known low-grade, non-muscle invasive disease but made the use of single dose of intravesical chemotherapy optional if the presence of malignancy was suspected but not pathologically proven because of “potential cost issues, side effects, and patient preference.”[25] Unfortunately, the potential cost issues were not elaborated on nor were references cited. Presumably, these costs would include the price of the chemotherapy, increased time spent in the post-anesthesia recovery unit (or other ambulatory surgical care area), increased nursing care, and proper disposal of the intravesical agent. The EUA, in contrast, uses a cost-effectiveness analysis based on the number of patients needed to treat with intravesical chemotherapy to prevent a single BC recurrence (8.5 patients), and that the costs associated with 8.5 instillations of post-TURBT intravesical chemotherapy are cheaper than one repeat TURBT for recurrence, to support the wide use of this practice.[30] Moreover, Madeb and colleagues have demonstrated that use of a single dose of intravesical chemotherapy post-TURBT would result in a cost-savings of $689.39 (12%) per patient treated with TURBT and intravesical chemotherapy compared with those who did not receive same day instillation. Nationally, this would reflect a $24.8 million cost-saving per year.[31]
Among other ways of reducing costs associated with BC is to reduce the rate of post-cystectomy complications.[32, 33] The primary postoperative complications have the most significant impact on the health outcomes and cost of care. Furthermore, high risk inpatient BC procedures such as cystectomy, were shown to be more influenced by systems of care (e.g., availability of nursing staff, expensive equipment, multidisciplinary teams of providers) and in general were less expensive to perform in high volume centers.[34] At the same, lower risk procedures such as TURBT, which are not influenced by systems of care, may be more cost-effective when performed in ambulatory setting or at low volume centers.
Prevention of BC by reducing the rate of cigarette smoking in the population is another potentially highly cost efficient but underused strategy for reducing the cost associated with BC. Smoking has been long shown to be associated with increased risk for BC.[35–37] Numerous studies have demonstrated that the costs of smoking cessation interventions are relatively low compared with the resulting gains of avoided morbidity, mortality, and economic burden of smoking-related illnesses, including costs associated with BC.[17]
Potentially Overused and Not Cost-Effective
While all three BC guidelines discuss the importance of post-treatment surveillance across all disease stages, a single agreed upon strategy has not been established. Most components of follow-up visits are not very costly; office visit with interval history and physical exam, urinary cytology, flexible cystoscopy, blood chemistries, etc. Imaging, however, which is often done via computed tomography (CT), has both financial costs and a small but real risk of secondary malignancy from the ionizing radiation it uses. Brenner and colleagues outlined the seldom considered risks of CT scans in their recent review.[38] Increased risk of cancer due to the ionizing effects of radiation from CT scans occurs after a dose of 30–90mSv, which is accumulated in only two or three scans. To place this in context, scans performed to evaluate causes of hematura (CT urography) consist of at least 3 scans (noncontrast, contrast, delayed images) while post-cystectomy studies will image both the abdomen and pelvis and are performed at varying frequencies based on stage of disease, post-operative complications, and other clinical indicators. Since the majority of patients treated for BC are in their seventh decade and older, it is unlikely that they will live through the decades-long latency period of secondary tumors that may have been caused by exposure CT radiation. However, developing consensus guidelines about the use of imaging for BC surveillance is an attainable goal that could help curb the costs of imaging associated with BC and establish potentially safer patient care practices.
Need cost-effectiveness evaluation
The AUA, EUA, and NCCN guidelines all recognize pelvic lymphadenectomy as a fundamental component of radical cystectomy. Recent work by Dhar and colleagues comparing outcomes after radical cystectomy in patients who underwent pelvic lymph node dissection (PLND) with standard versus extended resection templates likely represents a paradigm shift for the surgical management of invasive bladder cancer.[39] The authors found that performing an extended PLND improved pathologic staging and increased survival for patients with node-negative, node-positive, and extravesical disease. While this study was limited by its retrospective nature and limited generalizability to non-academic centers, it demonstrates that the extent and thoroughness of PLND is a significant determinant of BC outcomes. This study did not comment on differences in cost or complication rates, but an extended lymphadenectomy is unlikely to drastically prolong operative times or increase morbidity (although approximately 1/3 to 1/4 of patients who undergo radical cystectomy experience some type of complication). The renewed importance of PLND as a prognostic and therapeutic tool raises the bar for laparoscopic and robotic approaches as they attempt to demonstrate oncologic and economic efficacy in the management of muscle-invasive BC.[40]
Several new BC diagnostic tests have been recently approved and many more are undergoing development and investigation.[41] However, because the FDA approval process does not require any proof of cost-effectiveness, new tests almost always mean higher costs of care.[41] To address this problem, Svatek and colleagues have demonstrated that to screen populations at high risk for BC could be substantially more cost-effective than universal screening.[42] Investigational technologies that have not yet been incorporated into treatment guidelines, such as fluorescence cystoscopy[43] and urine biomarkers[44], may facilitate the diagnosis, treatment and follow-up of patients with BC, but their economic impact will not be known until they receive FDA approval and fully enter the health care market.
CONCLUSIONS
Economic information can inform decisions about health care coverage, access, and cost, which can ultimately lead to improvements in quality of care. In addition, economic information can provide cost predictions so that providers can be paid appropriate rates for caring for groups of people including patients with BC. Patient advocacy groups, lobbyists, and researchers often use economic information (i.e., estimates of economic burden of disease) to support or further their cause of obtaining more funding for social programs and research.
One of the most common purposes for the economic evaluation of BC care is to help determine the relative value of new or expensive interventions compared to the standard of care and to improve the efficiency in the delivery of health care services. For example, bladder preservation for muscle-invasive BC using aggressive TURBT, chemotherapy, and external beam radiation therapy is a costly upfront investment in terms of time and money, and many would rightly ask if this investment is worth the long-term improvements in health gained.[20] Another example is robotic surgery, which requires a significant capital investment in equipment plus time to train operative and support staff while the procedure is reimbursed at the same rate as standard surgery. The methods of cost-effectiveness research provide the analytic framework to make such assessments.
In summary, Table 1 provides suggestions to improve the use of economic information in BC, by improving methodology for conducting economic assessments of BC interventions and by encouraging collaborative partnerships between urologists, health economists, quality of life researchers, and health policy researchers. While necessary for rational decision making, obtaining more information about economic values of BC interventions comes at a cost (e.g., costs of additional data collection, medical claims, patient interviews, designing and conducting new clinical-economic trials, data analysis, and information dissemination to providers and patients). Ideally, we would aim to evaluate the CE of all commonly used BC interventions and procedures aiming to only use the ones that are both clinically and economically advantageous; but conducting these evaluations also would contribute to the cost of health care.
Table 1.
How to Improve the Use of Economic Information in BC
|
In time, economic evaluations may play a greater role in the development and promulgation of BC treatment guidelines, such as those recently released by the AUA, EUA, and NCCN and similar to the approach used for developing clinical guidelines in other countries with national health systems (e.g., the UK, Canada, Australia, Israel etc).[45–47]
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA: a cancer journal for clinicians. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Catlin A, Cowan C, Hartman M, Heffler S. National health spending in 2006: a year of change for prescription drugs. Health affairs (Project Hope) 2008 Jan-Feb;27(1):14–29. doi: 10.1377/hlthaff.27.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Gold MR, Siegel JE, Russell LB, Weinstein MC. Panel on cost-effectiveness in health and medicine. 1st. ed. New York: Oxford University Press; 1996. [Google Scholar]
- 4.Drummond MF, O'Brien BJ, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press; 1997. [Google Scholar]
- 5.Davidoff AJ, Powe NR. The role of perspective in defining economic measures for the evaluation of medical technology. International journal of technology assessment in health care. 1996 Winter;12(1):9–21. doi: 10.1017/s026646230000934x. [DOI] [PubMed] [Google Scholar]
- 6.Goossens ME, Rutten-van Molken MP, Vlaeyen JW, van der Linden SM. The cost diary: a method to measure direct and indirect costs in cost-effectiveness research. Journal of clinical epidemiology. 2000 Jul;53(7):688–695. doi: 10.1016/s0895-4356(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 7.Mushlin AI, Hall WJ, Zwanziger J, Gajary E, Andrews M, Marron R, et al. The cost-effectiveness of automatic implantable cardiac defibrillators: results from MADIT. Multicenter Automatic Defibrillator Implantation Trial. Circulation. 1998 Jun 2;97(21):2129–2135. doi: 10.1161/01.cir.97.21.2129. [DOI] [PubMed] [Google Scholar]
- 8.Luce BR, Elixhauser A. Estimating costs in the economic evaluation of medical technologies. International journal of technology assessment in health care. 1990;6(1):57–75. doi: 10.1017/s026646230000893x. [DOI] [PubMed] [Google Scholar]
- 9.Russell LB. Opportunity costs in modern medicine. Health affairs (Project Hope) 1992 Summer;11(2):162–169. doi: 10.1377/hlthaff.11.2.162. [DOI] [PubMed] [Google Scholar]
- 10.van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health economics. 1994 Sep-Oct;3(5):309–319. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 11.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health economics. 2001 Dec;10(8):779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves--facts, fallacies and frequently asked questions. Health economics. 2004 May;13(5):405–415. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 13.Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annual review of public health. 2000;21:587–611. doi: 10.1146/annurev.publhealth.21.1.587. [DOI] [PubMed] [Google Scholar]
- 14.Furlong W, Feeney DH, Torrance GW, Barr R, Horsman J. Guide to design and development of health state utility instrumentation. Hamilton: McMaster University; 1990. [Google Scholar]
- 15.Torrance GW. Measurement of health state utilities for economic appraisal. Journal of health economics. 1986 Mar;5(1):1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 16. [cited 2008];Registry TCE. Available from: https://research.tufts-nemc.org/cear/default.aspx.
- 17.Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, Rutten-van Molken MP. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health. 2005 May-Jun;8(3):178–190. doi: 10.1111/j.1524-4733.2005.04008.x. [DOI] [PubMed] [Google Scholar]
- 18.Stouthard MEA, Essink-Bot ML, Bonsel GJ, et al. Disability weights for diseases in the Netherlands. Rotterdam: Erasmus University; 1997. [Google Scholar]
- 19.Van Oers JAM. Health on course? The 2002 Dutch public health status and forecasts report. Bilthoven: National Institute of Public Health and the Environment; 2002. [Google Scholar]
- 20.Merseburger AS, Kuczyk MA. The value of bladder-conserving strategies in muscle-invasive bladder carcinoma compared with radical surgery. Current opinion in urology. 2007 Sep;17(5):358–362. doi: 10.1097/MOU.0b013e3282c4afa0. [DOI] [PubMed] [Google Scholar]
- 21.Nagele U, Kuczyk M, Anastasiadis AG, Sievert KD, Seibold J, Stenzl A. Radical cystectomy and orthotopic bladder replacement in females. European urology. 2006 Aug;50(2):249–257. doi: 10.1016/j.eururo.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Stenzl A, Holtl L. Orthotopic bladder reconstruction in women--what we have learned over the last decade. Critical reviews in oncology/hematology. 2003 Aug;47(2):147–154. doi: 10.1016/s1040-8428(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 23.Porter MP, Penson DF. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. The Journal of urology. 2005 Apr;173(4):1318–1322. doi: 10.1097/01.ju.0000149080.82697.65. [DOI] [PubMed] [Google Scholar]
- 24.Porter MP, Wei JT, Penson DF. Quality of life issues in bladder cancer patients following cystectomy and urinary diversion. The Urologic clinics of North America. 2005 May;32(2):207–216. doi: 10.1016/j.ucl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta T1, and Tis): 2007 update. The Journal of urology. 2007 Dec;178(6):2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou J. Guidelines on TaT1 (non-muscle invasive) bladder cancer: European Association of Urology. 2008 doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 27.Stenzl A, Cowan C, De Santis M, Jakse G, Kuczyk M, Merseburger AS, et al. Guidelines on bladder cancer: Muscle invasive and metastatic: European Association of Urology. 2008 doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Panel NBC. NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer: National Comprehensive Cancer Network. 2008 [Google Scholar]
- 29.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003;21(18):1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 30.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. The Journal of urology. 2004 Jun;171(6 Pt 1):2186–2190. doi: 10.1097/01.ju.0000125486.92260.b2. quiz 435. [DOI] [PubMed] [Google Scholar]
- 31.Madeb RR, Golijanin DJ, Noyes K, Fisher S, Stephenson JJ, Long SR, et al. Treatment of superficial bladder cancer: Are we practicing evidence-based medicine? Cancer. 2008 In Press. [Google Scholar]
- 32.Konety BR, Allareddy V. Influence of post-cystectomy complications on cost and subsequent outcome. The Journal of urology. 2007 Jan;177(1):280–287. doi: 10.1016/j.juro.2006.08.074. discussion 7. [DOI] [PubMed] [Google Scholar]
- 33.Berrum-Svennung I, Hedelin H, Holmang S. Costs of radical cystectomy. Scandinavian journal of urology and nephrology. 2005;39(1):36–41. doi: 10.1080/00365590410002537. [DOI] [PubMed] [Google Scholar]
- 34.Konety BR, Dhawan V, Allareddy V, O'Donnell MA. Association between volume and charges for most frequently performed ambulatory and nonambulatory surgery for bladder cancer. Is more cheaper? The Journal of urology. 2004 Sep;172(3):1056–1061. doi: 10.1097/01.ju.0000136382.51688.21. [DOI] [PubMed] [Google Scholar]
- 35.Augustine A, Hebert JR, Kabat GC, Wynder EL. Bladder cancer in relation to cigarette smoking. Cancer research. 1988 Aug 1;48(15):4405–4408. [PubMed] [Google Scholar]
- 36.Howe GR, Burch JD, Miller AB, Cook GM, Esteve J, Morrison B, et al. Tobacco use, occupation, coffee, various nutrients, and bladder cancer. Journal of the National Cancer Institute. 1980 Apr;64(4):701–713. [PubMed] [Google Scholar]
- 37.Burch JD, Rohan TE, Howe GR, Risch HA, Hill GB, Steele R, et al. Risk of bladder cancer by source and type of tobacco exposure: a case-control study. International journal of cancer. 1989 Oct 15;44(4):622–628. doi: 10.1002/ijc.2910440411. [DOI] [PubMed] [Google Scholar]
- 38.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 39.Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. The Journal of urology. 2008 Mar;179(3):873–878. doi: 10.1016/j.juro.2007.10.076. discussion 8. [DOI] [PubMed] [Google Scholar]
- 40.Huang GJ, Stein JP. Open radical cystectomy with lymphadenectomy remains the treatment of choice for invasive bladder cancer. Current opinion in urology. 2007 Sep;17(5):369–375. doi: 10.1097/MOU.0b013e3282dc95b5. [DOI] [PubMed] [Google Scholar]
- 41.Simon MA, Lokeshwar VB, Soloway MS. Current bladder cancer tests: unnecessary or beneficial? Critical reviews in oncology/hematology. 2003 Aug;47(2):91–107. doi: 10.1016/s1040-8428(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 42.Svatek RS, Sagalowsky AI, Lotan Y. Economic impact of screening for bladder cancer using bladder tumor markers: a decision analysis. Urologic oncology. 2006 Jul-Aug;24(4):338–343. doi: 10.1016/j.urolonc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 43.Schmidbauer J, Marberger M. Recent developments in fluorescence cystoscopy: do novel agents bring a benefit? Current opinion in urology. 2007 Sep;17(5):347–351. doi: 10.1097/MOU.0b013e3282c8c73f. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez A, Lokeshwar VB. Bladder cancer biomarkers: current developments and future implementation. Current opinion in urology. 2007 Sep;17(5):341–346. doi: 10.1097/MOU.0b013e3282c8c72b. [DOI] [PubMed] [Google Scholar]
- 45.Australia. Australia. [cited, Available from: http://www.jstor.org/view/16187598/ap060005/06a00070/0.
- 46.PE. PE Guidelines. [cited; Available from: http://ispor.org/PEguidelines/COUNTRYSPECIFIC.asp.
- 47.NICE. NICE. cited; Available from: http://www.ispor.org/workingpaper/healthscience/QI.aspNICE.