Abstract
We tested a mixture of Tridax procumbens, known for its direct action against Leishmania mexicana, and Allium sativum, known for its immunomodulatory effect, as an alternative to treat cutaneous leishmaniasis. Acute oral toxicity was tested with the Up-and-Down Procedure (UDP) using a group of healthy mice administered with either T. procumbens or A. sativum extracts and compared with a control group. Liver injury and other parameters of toxicity were determined in mice at day 14. The in vivo assay was performed with mice infected with L. mexicana promastigotes and treated with either a mixture of T. procumbens and A. sativum or each extract separately. The thickness of the mice’s footpads was measured weekly. After the 12-week period of infection, blood samples were obtained by cardiac puncture to determine the total IgG, IgG1 and IgG2a immunoglobulins by a noncommercial indirect ELISA. We showed that the mixture of T. procumbens and A. sativum extracts was better at controlling L. mexicana infection while not being toxic when tested in the acute oral toxicity assay in mice. An increase in the ratio of IgG2a/IgG1 indicated a tendency to raise a Th1-type immune response in mice treated with the mixture. The mixture of T. procumbens and A. sativum extracts is a promising natural treatment for cutaneous leishmaniasis and its healing effects make it a good candidate for a possible new phytomedicine.
Keywords: Leishmanicidal activity, Tridax procumbens, Allium sativum, Acute oral toxicity, Phytomedicine
Abstract
Nous avons testé un mélange de Tridax procumbens, dont l’action directe contre le parasite Leishmania mexicana est connue, et d’Allium sativum, dont l’effet immunomodulateur est connu, comme une alternative pour traiter la leishmaniose cutanée. La toxicité orale aiguë a été testée avec la procédure Up and Down (UDP) sur un groupe de souris saines auxquelles ont été administrés soit des extraits de T. procumbens, soit des extraits de A. sativum, comparé à un groupe de contrôle. Les lésions hépatiques et d’autres paramètres de toxicité ont été déterminés au 14ème jour. L’essai in vivo a été réalisé avec des souris infectées avec L. mexicana et traitées soit avec un mélange de T. procumbens et A. sativum, soit avec les 2 extraits séparés. L’épaisseur des coussinets plantaires des souris a été mesurée une fois par semaine. Douze semaines après l’infection, un échantillon de sang a été prélevé par ponction cardiaque et les quantités totales d’immunoglobulines IgG, IgG1, et IgG2 ont été déterminées par ELISA indirect non commercial. Nous avons pu montrer que le mélange d’extraits de T. procumbens et A. sativum contrôlait mieux l’infection tout en n’étant pas toxique lors de l’essai de toxicité orale aiguë chez la souris. L’augmentation du ratio IgG2a/IgG1 a permis d’indiquer une tendance à augmenter une réponse immunitaire de type Th1 dans les souris traitées avec ce mélange. Le mélange des extraits de T. procumbens et A. sativum est un traitement naturel prometteur pour la leishmaniose cutanée et ses effets curatifs en font un bon candidat pour un possible nouveau phytomédicament.
Introduction
The disease caused by infection with Leishmania parasites has an important impact worldwide, with up to 200 million new cases per year [3]. In Mexico, the Yucatan peninsula is an endemic area for Leishmania mexicana, which causes localized cutaneous lesions, known popularly as “chiclero’s ulcer”, with an incidence of 5/1,000 inhabitants [4, 18], although it can also induce more severe forms of the disease such as the diffuse, mucocutaneous, or visceral forms [8]. Chemotherapy with antimonials is the first choice to treat leishmaniasis; however, the treatment is prolonged, expensive and not very effective, and may have severe side effects. The second-line drugs, such as amphotericin B and pentamidine, may even be more toxic. Besides the problems already mentioned, [28] reported increasing failures of miltefosine, currently used to treat visceral leishmaniasis, some of the possible causes being parasite resistance, discontinuity of treatment, and reinfection.
Currently, there is an urgent need for new leishmanicidal drugs and it is known that medicinal plants are an important source of new molecules with pharmacological activities [10, 24, 29]; several plants have been used as treatment for “chiclero’s ulcer” in Mayan traditional medicine. A number of them has been screened looking for in vitro leishmanicidal activity [15]. Tridax procumbens L. (Asteraceae), also known as bull’s herb in Guatemala and t’ulum (Maya language) in Yucatan, Mexico, was one of the most active [25, 26]. The whole plant of T. procumbens is used by the population in Guatemala for topical applications to treat chronic ulcers caused by leishmaniasis [7]. Based on its activity, T. procumbens was chosen for further chemical, in vitro and in vivo studies. Methanol extract had a damaging effect on L. mexicana by means of a mechanism of action independent of the production of nitric oxide (NO) in macrophages [19, 20], which suggests that its activity is not cell-mediated but toxic to the parasite. Accordingly, T. procumbens was selected for further in vivo studies.
Allium sativum (garlic) is another species that has shown activity against infection with L. mexicana, this time through an immunomodulatory effect that induces a Th1-type response, INF-γ increase, and stimulation of nitric oxide (NO) production in macrophages, which prevent the progress of the infection [12]. These results are consistent with other studies, in which A. sativum extract also showed an inhibitory effect against infection with other Leishmania species [16, 32].
Since our aim is related to the discovery of potential new pharmaceutical treatments from natural sources, we considered it imperative to perform an acute oral toxicity assay to confirm the antiprotozoal activity as opposed to general toxicity and to improve the selection process for promising extracts [31]. We have observed that a large number of reports on the in vitro antiprotozoal activities of plant extracts without reference to toxicity have been published elsewhere [5], thus it is not always clear if the observed activity could be related to a general toxicity. On this basis, in the present study we tested the acute oral toxicity of T. procumbens methanol and A. sativum aqueous extracts, before testing the in vivo activity of both, separated or combined, against a L. mexicana infection. On the other hand, we were also interested in knowing the kind of immune humoral response (total IgGs) and the IgG2a/IgG1 ratio (Th1-type response) of all treated mice whose L. mexicana lesions were controlled.
Materials and methods
Plant collection and extraction
1.5 kg of Tridax procumbens L. (Asteraceae) whole plant were collected in Merida, Yucatan, Mexico, in February 2004; a voucher specimen was authenticated by F. May-Pat and deposited at the herbarium of the CICY under the code number FMay-1955. The whole plant was first dried at room temperature and then in a desiccation stove at 50 °C, powdered, and then submitted to extraction with methanol (MeOH). The MeOH was evaporated to dryness in vacuo and the yield of the resultant crude extract was 150 g (yielding 10%). 1.5 kg of Allium sativum L. (Amaryllidaceae) (garlic) bulbs were peeled and dried in a desiccation stove at 100 °C, and after 48 h they were recovered at constant weight. The dried bulbs were homogenized with a blender in 1 L of water, filtered through Whatman No. 1 filter paper, and centrifuged. The filtrate was lyophilized, dosed and resuspended for treatment.
Parasites
Promastigotes of L. mexicana (strain Hd18-(MHET/MX/97/Hd18) were cultured in Tc 199 medium (Gibco, Invitrogen), supplemented with 10% fetal calf serum (Life Technologies), penicillin (100 U/mL), and streptomycin (100 g/mL). The parasites were harvested in the stationary phase after 8–10 days of culture, centrifuged (1000 g × 5 min), washed twice with Tc 199 medium, counted, and used to inoculate mice.
Mice
Either female BALB/c or CD-1 mice (6–8 weeks old) were fed with standard chow and water ad libitum and kept under standard temperature (26 °C and 60% humidity conditions) and a natural light-darkness cycle. All in vivo experiments with mice were performed according to the established bioethical standards of the CIR-UADY Bioethics in Research Committee.
Acute oral toxicity assay
Acute oral toxicity was tested using the Up-and-Down Procedure (UDP) recommended by the United States Environmental Protection Agency (EPA) and adopted by the Organization for Economic Cooperation and Development (OECD). This procedure significantly reduced the number of animals used in comparison with the traditional LD50 test [11].
Sixteen healthy, eight-week-old BALB/c mice were used to perform this test; six mice were used as a control group (saline solution) and ten were used as experimental groups (T. procumbens and A. sativum extracts). Animals were randomly selected and marked to allow individual identification. They were acclimatized and individually kept in their cages for at least five days, and fasted 24 h prior to dosing. Food and water were withheld for 3–4 h after dosing. First, two mice were dosed at 2000 mg/kg of body weight with either T. procumbens or A. sativum extracts using distilled water as a vehicle; as the mice survived, another eight were administered with the same dose of either T. procumbens or A. sativum. The control group was dosed only with the vehicle. Each mouse of the experimental groups was carefully observed in the first 3 h to look for toxicity parameters, such as tremors, convulsions, breathing changes, piloerection, loss of balance, hyperactivity, and lethargy, then animals were housed for 14 days counted from the dosage day. The parameters observed during these days were body weight, amount of food consumed, and any behavior that could be a sign of toxicity [6]. At day 14, the mice were sedated and blood was taken by heart puncture to determine liver injury. For toxicity assays in mice models, it is a good parameter to determine the increase in glutamate transaminase activities (GOT and GPT), which is a sign of liver injury, thus we determined the aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) using the IFCC Mod. LiquiUV Test. The mice were killed to remove the liver and spleen and evaluate their appearance and weight [22].
In vivo assay of T. procumbens and A. sativum extracts and their mixture
All mice were infected subcutaneously into the left hind footpad with 106 logarithmic phase promastigotes of L. mexicana to a final volume of 50 μL of physiological saline solution. The contralateral right footpad received an identical volume of saline solution without parasites as an internal control.
The in vivo assay was performed using four groups of six mice: (1) the control (saline solution), (2) treatment with T. procumbens, (3) treatment with A. sativum, and (4) treatment with a mixture of T. procumbens and A. sativum. The treatments with T. procumbens and A. sativum extracts were started two weeks after infection. The first group was used as infection control receiving saline solution injections with the same schedule as the groups treated with extracts. The other three groups were treated daily, for two weeks, by intraperitoneal (i.p.) injections of 20 mg/kg of either T. procumbens methanol extract or A. sativum aqueous extract, or 40 mg/kg of a mixture of T. procumbens and A. sativum extracts (1:1).
The thickness of infected and uninfected footpads was measured weekly with a vernier caliper, for 12 weeks, and the difference between the two measurements was considered as the size of the lesion. The mice were also regularly examined to detect cutaneous ulcers and secondary lesions. After the 12-week period of the experiment, the mice were anesthetized and a blood sample was obtained by cardiac puncture, and they were euthanized immediately after.
Immunoglobulin detection
The total IgG, IgG1 and IgG2a immunoglobulins were measured by a noncommercial indirect ELISA, using as an antigen a whole parasite lysate and goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase conjugate (Sigma) at 1:4000 or goat anti-mouse IgG1- or IgG2a-horseradish peroxidase-conjugated antibodies (Southern Biotechnology Associates, Birmingham, Ala.) in a 1:4000 dilution in blocking buffer, as previously described [2, 13].
Statistical analysis
Data are presented as mean and SD, and comparison among multiple groups was performed by an ANOVA test. Comparisons of footpad swelling between T. procumbens and A. sativum groups and saline were performed with Student’s t-test.
Results
Toxicity assay
In the acute oral toxicity study of T. procumbens methanol and A. sativum aqueous extracts, none of the animals died during the assay or showed signs and symptoms of toxicity up to a dose of 2000 mg/kg. These results indicated that the extracts have a high margin of safety. The loss of body weight as a toxicity indicator was within the normal parameters of the growth curve of BALB/c mice at day 14. Mice gained the same weight and there were no significant differences (p < 0.05) in weight gain (weight at the 14th day minus weight at the 1st day) in the comparison between the control group (2.6 ± 1.1) (mean ± SD) and the mice treated either with T. procumbens methanol extract (5 ± 2.5) or A. sativum aqueous extract (3 ± 0.70). All groups registered an equal amount of food consumed (data not shown). The weight of livers and spleens was similar among the control and experimental groups (p > 0.05) (data not shown), and no macroscopic signs of damage were observed in these organs for either extract.
GOT and GPT levels were measured and the data showed no significant differences in transaminases (p > 0.05) between the control group and the group treated with T. procumbens or A. sativum (Table 1).
Table 1.
Transaminase levels of GOT and GPT (UI) in mice treated with T. procumbens methanol, A. sativum aqueous or saline solution.
| Extracts | TGO | TGP |
|---|---|---|
| Control | 308.6 ± 168.9 | 58.9 ± 18.0 |
| T. procumbens | 346.1 ± 228.3 | 47.7 ± 15.8 |
| A. sativum | 289.7 ± 152.6 | 62. 6± 13.0 |
Each result is mean ± SD of TGO and TGP (UI) for three or six mice samples.
Effect of T. procumbens, Allium sativum, and mixed extracts on L. mexicana in vivo
The infection with L. mexicana induced a progressive increase in the size of the footpad of CD-1 mice. Both T. procumbens methanol extract and A. sativum aqueous extract in separate treatments showed a tendency to reduce the development of the cutaneous lesion in mice; however, four out of six mice treated with T. procumbens methanol extract presented minor lesions, while the other two did not respond to the treatment and developed severe lesions (Fig. 1 bottom). This variability can be explained by the genetic diversity of the CD-1 strain. The mixture of both extracts showed the best activity, significantly reducing the development of the lesion caused by L. mexicana (Fig. 1).
Figure 1.
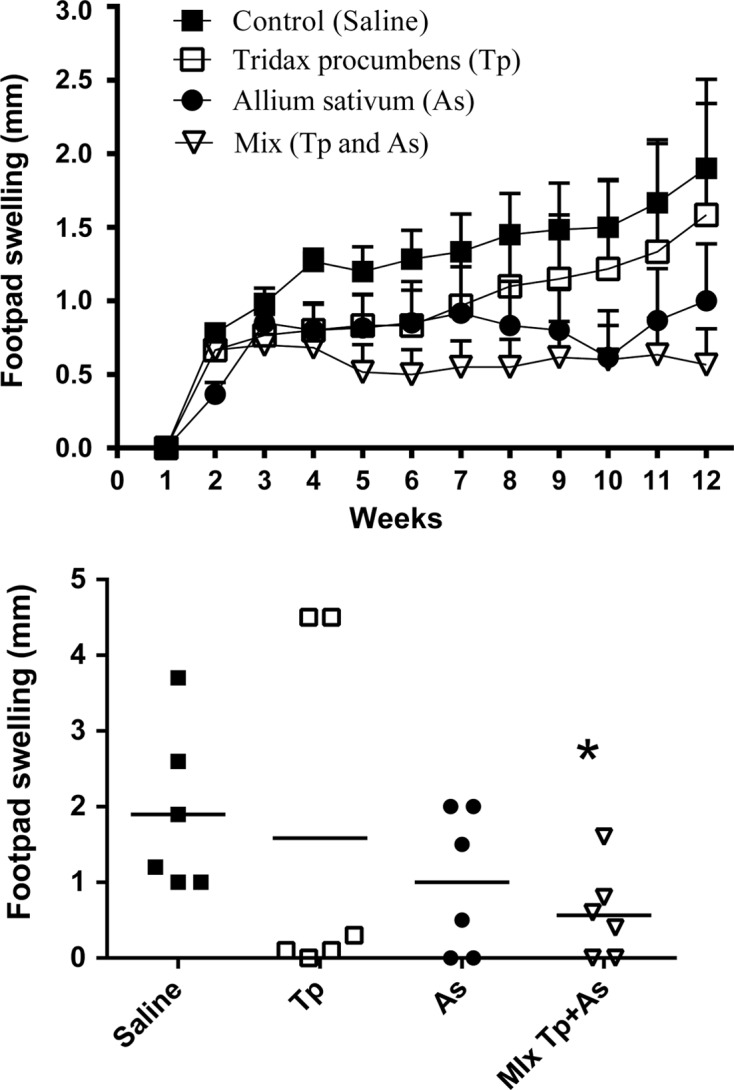
Footpad swelling in CD-1 mice after two weeks of infection with 1 × 106 L. mexicana promastigotes. Top: four groups of mice were treated either with saline, T. procumbens extract, A. sativum extract or the mixture of T. procumbens and A. sativum extracts. The treatments were applied i.p. daily for two weeks. Bottom: the footpad thickness was measured and compared to the noninoculated footpad for each mouse for 12 weeks. Each point represents the average increase of the infected footpad thickness, standard error (n = 6). Comparison of four experimental groups in 12 weeks after infection, each symbol represents each mouse of the group, the horizontal line represents the mean, and * indicates significant differences with the PBS-treatment control group (unpaired t-test, p < 0.05).
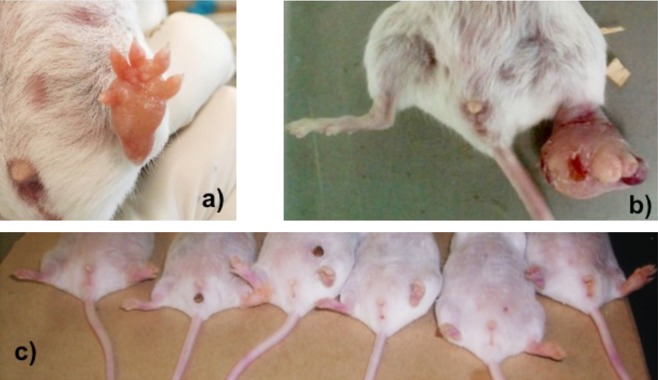
In general, lesions observed in mice treated with the mixture of T. procumbens and A. sativum did not present necrosis or cause damage to the skin compared with the control group treated with saline solution after day 12 (Fig. 2).
Figure 2.
Appearance of the lesions caused by Leishmania mexicana with and without the treatment (mixture of T. procumbens + A. sativum extracts) 12 weeks after infection. (a), (b) Deformed and ulcerated lesions on left foot of untreated mice. (c) Controlled and healed lesions on left foot of mice treated with the mixture.
In order to understand the function of the mixture in humoral immune responses to control the infection, we measured the level of total IgG, IgG1, and IgG2a against L. mexicana in serum of mice treated either with T. procumbens, A. sativum, or the mixture of both compared with mice without treatment (Fig. 3). The means of antibody levels of each group were compared. Previous studies have demonstrated that the immunomodulatory effect of A. sativum extract is due to an increase in IFN-γ and NO in infected mice [12]. In the present study it was shown that A. sativum extract is also able to promote the production of antibodies (Fig. 3A). The group treated with A. sativum showed the highest total IgG production compared with the control group; however, the highest ratio of IgG2a/IgG1 was observed in the mixture group (Fig. 3B).
Figure 3.
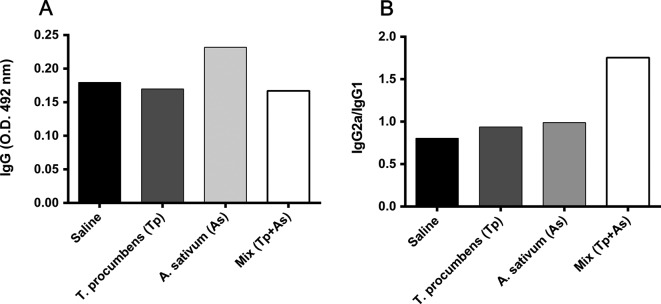
Total levels of IgGs, ratio IgG2a/IgG1 in mice with different treatments compared with PBS-treatment control group. Total IgGs relationship with general humoral immune response and IgG2a/IgG1 related to Th1-type immune response causing protection of cutaneous leishmaniasis. Control group (black bar), T. procumbens group (deep gray bar), A. sativum group (light gray bar) and Mix group (white bar).
Discussion
The results of the toxicity assay showed that the limit dose of 2000 mg/kg was innocuous to mice, and they were consistent with another toxicity study which demonstrated that T. procumbens methanol extract was not toxic at 5 g/kg body weight in rats, confirming a high margin of safety [23]. According to these results, T. procumbens LD50 is located over 2000 mg/kg body weight. Regarding the toxicity of A. sativum extract, previous studies have revealed its curative and protective effects in a nephrotoxicity model [27] and a protective effect in the kidney and liver of rats [17]. Accordingly, the present study shows no toxicity of A. sativum aqueous extract in livers of mice receiving an oral dose of 2000 mg/kg.
The simultaneous application of the mixed extract (T. procumbens and A. sativum extracts) avoided the development of a lesion; to our knowledge, there are no other studies reported using a mixture of two bioactive plant extracts against Leishmania spp. In a previous study, we demonstrated that A. sativum aqueous extract had a visible effect on the reduction of the lesion caused by L. mexicana [12]. This was confirmed by Wabwoba et al., who also concluded that the mechanism of action of A. sativum is apparently immunomodulatory, and that A. sativum compounds should be purified and tried as complementary medicine in the management of leishmaniasis [32]. However, we believe that these components perhaps do not need to be purified to obtain a good treatment, they just have to be standardized [14, 21].
The highest IgG2a/IgG1 ratio in mice treated with mixed extract, which controlled the progress of infection for L. mexicana, suggests that the simultaneous application of T. procumbens and A. sativum extracts redirects the immune response caused by the infection to a Th1-type immune response. This explains why the observed lesions in this group are less than those of the other experimental groups, as this type of response is considered most suitable for the control of infection [2].
There are reports on T. procumbens aqueous extract inducing an immunomodulatory response in mice [30] and immunomodulatory activity in T. procumbens methanol extract has been detected [1]. Phytochemical studies performed on T. procumbens against L. mexicana led to the isolation of an oxylipin, highly selective against promastigotes of L. mexicana [20]. This metabolite was tested in vitro against intracellular amastigotes, showing an IC50 = 0.48 μg/mL [19]. This suggests that T. procumbens methanol extract could have a direct effect not only on the amastigotes, but also an additive effect with A. sativum in modulating the immune response, so that the simultaneous application of both extracts has a more efficient therapeutic effect. Similar results were obtained in a study which demonstrated an in vivo immunomodulatory effect with direct and indirect actions with a flower extract of Tilia sp. to improve the antitumoral activity [9].
Our work suggests that T. procumbens and A. sativum extracts used together control localized cutaneous leishmaniasis caused by L. mexicana.
Acknowledgments
We are grateful to Lesly A. Mena-Chan for her collaboration with the in vivo assay. We are also grateful to Luis W. Torres-Tapia for his technical assistance in obtaining the T. procumbens and A. sativum extracts, and to Fernando J. Colli-Balam for his valuable help with the ELISA test procedure at CIR-UADY.
The authors declare that there is no conflict of interest.
Cite this article as: Gamboa-Leon R, Vera-Ku M, Peraza-Sanchez SR, Ku-Chulim C, Horta-Baas A & Rosado-Vallado M: Antileishmanial activity of a mixture of Tridax procumbens and Allium sativum in mice. Parasite, 2014, 21, 15.
References
- 1.Agarwal S, Khadase S, Talele G. 2010. Bioactive immunomodulatory fraction from Tridax procumbens. Asian Journal of Biological Sciences, 3, 120–127 [Google Scholar]
- 2.Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, García-Miss Mdel R, Palatnik de Sousa CB, Dumonteil E. 2005. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infection and Immunity, 73, 812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team 2012. Leishmaniasis worldwide and global estimates of its incidence. PloS One, 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade-Narváez FJ, Simmonds-Díaz E, Rico-Aguilar S, Andrade-Narváez M, Palomo-Cetina A, Canto-Lara SB, García-Miss MR, Madera-Sevilla M, Albertos-Alpuche N. 1990. Incidence of localized cutaneous leishmaniasis (chiclero’s ulcer) in Mexico. Transactions of the Royal Society of Tropical Medicine and Hygiene, 84, 219–220 [DOI] [PubMed] [Google Scholar]
- 5.Asres K, Bucar F, Knauder E, Yardley V, Kendrick H, Croft SL. 2001. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytotherapy Research, 15, 613–617 [DOI] [PubMed] [Google Scholar]
- 6.Bermúdez Toledo D, Monteagudo Jiménez E, Boffill Cárdenas M, Díaz Costa LE, Roca Simeón A, Betancourt Morgado E, Silveira Prado EA. 2007. Evaluación de la toxicidad aguda de extractos de plantas medicinales por un método alternativo. Revista Electrónica de Veterinaria, 8, 1–7 [Google Scholar]
- 7.Cáceres A, López B, González S, Berger I, Tada I, Maki J. 1998. Plants used in Guatemala for the treatment of protozoal infections. I. Screening of activity to bacteria, fungi and American trypanosomes of 13 natives plants. Journal of Ethnopharmacology, 62, 195–202 [DOI] [PubMed] [Google Scholar]
- 8.Castro-Grüber S, Zerpa-Rangel O, Rondón-Lugo A. 2003. Leishmaniasis en la infancia. Medicina Cutánea Ibero-Latino-Americana, 31, 351–361 [Google Scholar]
- 9.Davicino R, Zettler G, Brizi MR, Marrassini C, Ferraro G, Filip R, Anesini C. 2011. In vivo immunomodulatory effect of Tilia × viridis extracts on normal lymphocyte proliferation: a direct and an indirect action. Phytotherapy Research, 25, 1342–1347 [DOI] [PubMed] [Google Scholar]
- 10.Duran GR, Trejo-Torres JC, Ibarra-Manriquez G. 1998. Endemic phytotaxa of the Peninsula of Yucatan. Harvard Papers in Botany, 3, 263–314 [Google Scholar]
- 11.EPA (United States Environment Protection Agency) 2002. Health Effect Test Guidelines OPPTS 870.1100 Acute Oral Toxicity. Washington, DC: Government printing office; p. 1–8 [Google Scholar]
- 12.Gamboa-Leon MR, Aranda-González I, Mut-Martín M, García-Miss MR, Dumonteil E. 2007. In vivo and in vitro control of Leishmania mexicana due to garlic-induced NO production. Scandinavian Journal of Immunology, 66, 508–514 [DOI] [PubMed] [Google Scholar]
- 13.Gamboa-Leon R, Gonzalez-Ramirez C, Padilla-Raygoza N, Sosa-Estani S, Caamal-Kantun A, Buekens P, Dumonteil E. 2011. Do commercial serologic tests for Trypanosoma cruzi infection detect Mexican strains in women and newborns? Journal of Parasitology, 97, 338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Q, Li J, Cheung JKH, Duan J, Ding A, Cheung AWH, Zhao K, Li WZ, Dong TT, Tsim KWK. 2007. Verification of the formulation and efficacy of “Danggui Buxue Tang” (a decoction of Radix astragali and Radix angelicae sinensis): an exemplifying systematic approach to revealing the complexity of Chinese herbal medicine formulae. Chinese Medicine, 2, 12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getti G, Durgadoss P, Dominguez-Carmona D, Martin-Quintal Z, Peraza-Sanchez S, Peña-Rodriguez LM, Humber D. 2009. Leishmanicidal activity of Yucatecan medicinal plants on Leishmania species responsible for cutaneous leishmaniasis. Journal of Parasitology, 95, 456–460 [DOI] [PubMed] [Google Scholar]
- 16.Ghazanfari T, Hassan ZM, Khamsipour A. 2006. Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. Journal of Ethnopharmacology, 103, 333–337 [DOI] [PubMed] [Google Scholar]
- 17.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MHG, Ouhtit A. 2009. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. International Journal of Biological Sciences, 5, 249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaac-Marquez AP, Lezama-Davila CM. 2003. Detection of pathogenic bacteria in skin lesions of patients with chiclero’s ulcer. Reluctant response to antimonial treatment. Memórias do Instituto Oswaldo Cruz, 98, 1093–1095 [DOI] [PubMed] [Google Scholar]
- 19.Martin-Quintal Z, Garcia-Miss MR, Mut-Martín M, Matus-Moo A, Torres-Tapia LW, Peraza-Sánchez SR. 2010. The leishmanicidal effect of (3S)-16,17-didehydrofalcarinol, an oxylipin isolated from Tridax procumbens, is independent of NO production. Phytotherapy Research, 24, 1004–1008 [DOI] [PubMed] [Google Scholar]
- 20.Martin-Quintal Z, Moo-Puc R, González-Salazar F, Chan-Bacab MJ, Torres-Tapia LW, Peraza-Sánchez SR. 2009. In vitro activity of Tridax procumbens against promastigotes of Leishmania mexicana. Journal of Ethnopharmacology, 122, 463–467 [DOI] [PubMed] [Google Scholar]
- 21.Mok DKW, Chau FT. 2006. Chemical information of Chinese medicines: a challenge to chemist. Chemometrics and Intelligent Laboratory Systems, 82, 210–217 [Google Scholar]
- 22.Nagai H, Yakuo I, Yamada H, Shimazawa T, Koda A, Niu K, Asano K, Shimizu T, Kasahara M. 1988. Liver injury model in mice for immunopharmacological study. Japanese Journal of Pharmacology, 46, 247–254 [DOI] [PubMed] [Google Scholar]
- 23.Pareek H, Sharma S, Khajja BS, Jain K, Jain GC. 2009. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn). BMC Complementary and Alternative Medicine, 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peña-Rodríguez LM, Durán-García R, Vera-Ku BM, Fuentes-García AG, Domínguez-Carmona DB. 2010. El valor agregado de la biodiversidad, la flora nativa como fuente potencial de nuevos fármacos, in Biodiversidad y Desarrollo Humano en Yucatán. Durán R, Mendez M (Eds.). Merida, Mexico: CICY, PPD-FMAM, CONABIO, SEDUMA: p. 475–479 [Google Scholar]
- 25.Peraza-Sánchez SR, Poot-Kantún S, Torres-Tapia LW, May-Pat F, Simá-Polanco P, Cedillo-Rivera R. 2005. Screening of native plants from Yucatan for anti-Giardia lamblia activity. Pharmaceutical Biology, 43, 594–598 [Google Scholar]
- 26.Peraza-Sanchez SR, Cen-Pacheco F, Noh-Chimal A, May-Pat F, Sima-Polanco P, Dumonteil E. 2007. Leishmanicidal evaluation of extracts from native plants of the Yucatan peninsula. Fitoterapia, 78, 315–318 [DOI] [PubMed] [Google Scholar]
- 27.Rafieian-Kopaei M, Baradaran A, Merrikhi A, Nematbakhsh M, Madihi Y, Nasri H. 2013. Efficacy of co-administration of garlic extract and metformin for prevention of gentamicin-renal toxicity in Wistar rats: A biochemical study. International Journal of Preventive Medicine, 4, 258–264 [PMC free article] [PubMed] [Google Scholar]
- 28.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC. 2013. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clinical Infectious Diseases, 56, 1530–1538 [DOI] [PubMed] [Google Scholar]
- 29.Sülsen VP, Cazorla SI, Frank FM, Redko FC, Anesini CA, Coussio JD, Malchiodi EL, Martino VS, Muschietti LV. 2007. Trypanocidal and leishmanicidal activities of flavonoids from Argentine medicinal plants. American Journal of Tropical Medicine and Hygiene, 77, 654–659 [PubMed] [Google Scholar]
- 30.Tiwari U, Rastogi B, Singh P, Saraf DK, Vyas S. 2004. Immunomodulatory effects of aqueous extract of Tridax procumbens in experimental animals. Journal of Ethnopharmacology, 92, 113–119 [DOI] [PubMed] [Google Scholar]
- 31.Van Dyk S, Griffiths S, van Zyl RL, Malan SF. 2009. The importance of including toxicity assays when screening plant extracts for antimalarial activity. African Journal of Biotechnology, 8, 5595–5601 [Google Scholar]
- 32.Wabwoba BW, Anjili CO, Ngeiywa MM, Ngure PK, Kigondu EM, Ingonga J, Makwali J. 2010. Experimental chemotherapy with Allium sativum (Liliaceae) methanol extract in rodents infected with Leishmania major and Leishmania donovani. Journal of Vector Borne Diseases, 47, 160–167 [PubMed] [Google Scholar]