Abstract
The ovary functions to chaperone the most precious cargo for female individuals, the oocyte, to allow the passage of genetic material to subsequent generations. Within the ovary, single oocytes are surrounded by a legion of granulosa cells inside each follicle. These two cell types depend upon one another to support follicle formation and oocyte survival. The infrastructure and events that work together to ultimately form these functional follicles within the ovary are unprecedented, given that the oocyte is fated like all other neighboring cells within the embryo prior to gastrulation. This review discusses the journey of the germ cell in the context of the developing female mouse embryo, with a focus on specific signaling events and cell-cell interactions that escort the primordial germ cell as it is specified into the germ-cell fate, migrates through the hindgut into the gonad, differentiates into an oocyte, and culminates upon formation of the primordial and then primary follicle.
Keywords: germ cell, primordial germ cell, oocyte, pre-granulosa cell, follicle, folliculogenesis
Introduction
Beginning at their inception and continuing throughout their lifespan, cells destined for a germ-cell fate are intimately dependent on neighboring cells. While several elegant reviews have been published that focus on distinct stages encompassing the life of the nascent germ cell, this review will focus on cell-cell interactions that define the path of germ cells within the female mouse from inception until just after birth. In both male and female mice, interactions between progenitor germ cells and distinct somatic-cell partners begin when primordial germ cell (PGC) fate is determined, and these interactions continue to facilitate the successful journey of PGCs through the hindgut to colonize the gonads. From then on, germ cells begin their sexually dimorphic pathways based on essential, neighborhood-specific interactions that mediate spermatogonia or oogonia maturation. At birth, the ovary presents a defined population of oocytes that reside in a specialized cocoon made up of supporting somatic cells called granulosa cells, which together comprise the functional unit of the ovary, the follicle. The oocyte continues to depend on neighboring granulosa cells along with other local and distant partners to grow and develop competency to be ovulated and ultimately fertilized by mature sperm so that the whole process can begin anew within the next generation.
Germ Cell-Somatic Cell Events Prior To Gonad Colonization
PGC Differentiation
Putative PGC Differentiation Markers
The founder of the gamete, the PGC, was first discovered as a cluster of cells positive for alkaline phosphatase (AP) within the posterior part of the mouse embryo around embryonic day 8.5 (E8.5) (Chiquoine, 1954). Other studies identified AP+ cells earlier, at E7-7.25, just posterior to the primitive streak, and like the original study, subsequently found them along the wall of the invaginating hind gut and ultimately in the genital ridges (Chiquoine, 1954; Ginsburg et al., 1990). The link between AP+ cells and PGC identity was strengthened upon phenotype characterization of mouse mutants Dominant white spotting (W) (Mintz and Russell, 1957) and Steel (Sl) (McCoshen and McCallion, 1975), each of which exhibited AP+ cells within the posterior primitive streak that failed to increase in number during migration and resulted in gonads devoid of germ cells as early as E12. More recently, other factors in addition to a special isoform of AP, tissue-nonspecific AP (TNAP) (Hahnel et al., 1990; MacGregor et al., 1995), have been associated with progenitor PGCs at earlier stages, within the proximal epiblast, and include BLIMP1 (Prdm1) (Ohinata et al., 2005), STELLA (Dppa3, Pgc7) (Saitou et al., 2002; Sato et al., 2002), fragilis (Ifitm) (Saitou et al., 2002), and OCT3/4 (Pou5f1) (Okamura et al., 2008). Progenitor PGCs are not intrinsically different from other cells within the epiblast. Indeed, this region, including the posterior primitive streak and extending into the base of the allantois, has been identified as the allantoic core domain, which houses a unique pool of precursor cells (Downs et al., 2009) (Figure 1). The allantoic core domain includes cells that co-express TNAP, OCT3/4, fragilis, STELLA, and BLIMP1. Combinatorial localization of these markers along with BLIMP1-mediated downregulation of regional homeobox genes have been used to delineate the putative PGC population (Downs and Harmann, 1997; Downs et al., 2009; MacGregor et al., 1995; Mikedis and Downs, 2012, 2013; Nichols et al., 1998; Ohinata et al., 2005; Saitou et al., 2002; Yeom et al., 1996). It is important to note, however, that these marked cells give rise to putative PGCs in addition to cells within extraembryonic lineages and all three embryonic layers of the posterior mouse embryo, thus bringing into question the timing and definition of the true PGC identity (Mikedis and Downs, 2012; 2013). If we assume that PGCs eventually emerge from this population of cells, it stands to reason that certain epiblast cells eventually acquire competence to differentiate towards the germ-cell lineage by signals emanating from neighboring cells.
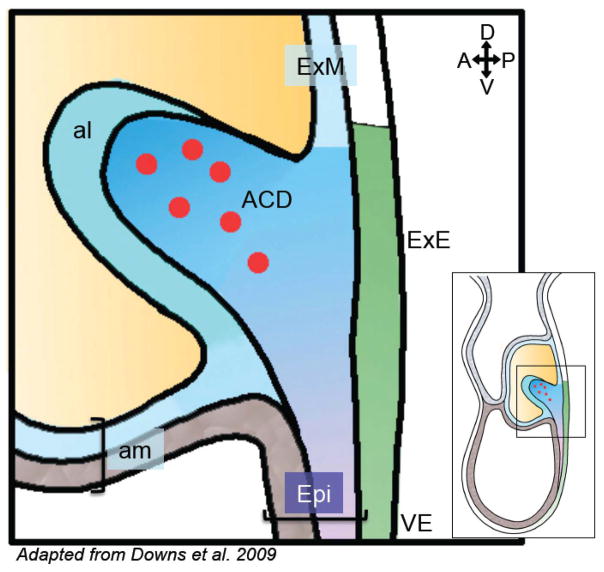
Figure 1. The allantoic core domain houses putative precursor PGCs.
Schematic sagittal view of the embryo’s posterior region illustrating that precursor PGCs (red circles) are specified within the allantoic core domain (ACD; royal blue), which incorporates the primitive streak/epiblast. The inset shows an illustration of an entire embryo (~E7.25) to give context to the location of the mesodermal bulge that houses the ACD; the boxed area corresponds to magnified view. al, allantois; am, amnion; Epi, epiblast; ExE, extraembryonic endoderm; VE, visceral endoderm; D, dorsal; V, ventral; A, anterior; P, posterior.
Defining The Neighborhood That Determines Putative PGC Fate
Transplantation experiments showed that epiblast cells were not predetermined to become PGCs. Epiblast cells from any portion of the region could be induced into the PGC lineage, as long as they were injected into and juxtaposed to extraembryonic ectoderm at the appropriate time, suggesting that extracellular signals and/or cell-cell communication promoted PGC fate (Tam and Zhou, 1996; Yoshimizu et al., 2001). Additional transplantation and lineage tracing studies established that PGC ancestors were localized to proximal epiblast cells immediately adjacent to the extraembryonic ectoderm (Gardner and Rossant, 1979; Lawson and Hage, 1994). As it became clear that cell-cell interactions were critical, the search was on for candidate mediators whose signals originated from extraembryonic cells juxtaposed to the proximal epiblast (Table 1).
Table 1.
PGC Fate Initiation, E6.25-7.5
| Cell-cell contact
| |||
|---|---|---|---|
| Factor | Site | Onset of Expression | References |
| CDH1 | ExM, ACD | E6.25 | Okamura et al. 2003 |
| Local Signals
| |||
|---|---|---|---|
| Factor | Site | Onset of Expression | References |
| Bmp4 | ExE, ExM | E6.25, 7.0 | Lawson et al. 1999 |
| Bmp8b | ExE | E6.0 | Ying et al. 2000 |
| Bmp2 | VE | E6.0 | Ying et al. 2001 |
| Wnt3 | Epi | E5.5 | Kemp et al. 2005; Ohinata et al. 2009 |
| Unknown Inhibitory Signals | VE | E6.0 | Pfister et.al. 2005; Ohinata et al. 2009; Tam et al. 2009 |
| Transcription Factors
| |||
|---|---|---|---|
| Factor | Site | Onset of Expression | References |
| SMAD1 | VE, (Epi) | E6.5 | Tremblay et al. 2001 |
| SMAD5 | Epi | E6.5 | Chang et al. 1999, 2001 |
| HOX genes decreased | Epi | E6.0 Decreased |
Saitou et al. 2002 |
| BLIMP1 | VE, Epi | E5.5, E6.25 | Ohinata et al. 2005, 2009; Magnúsdóttir et al. 2013 |
| PRDM14 | Epi | E6.25 | Yamaji et al. 2008; Magnúsdóttir et al. 2013 |
| AP2γ | Epi | E7.25 | Weber et al. 2010; Magnúsdóttir et al. 2013 |
ACD, allantoic core domain; ExE, extraembryonic endoderm; ExM, extracellular matrix; Epi, epiblast; VE, visceral endoderm.
Because putative PGCs were identified as a cluster, it was hypothesized that direct cell-cell contact contributed to their specification. Hence, the first of two roles for E-cadherin (CDH1) in PGC fate and maturation was discovered. E-cadherin expression was restricted to cells within the extraembryonic mesoderm that were also eventually positive for OCT4 and STELLA; however, this progression was disrupted in the presence of an antibody that blocked E-cadherin activity (ECCD1) (Okamura et al., 2003). While these studies implicated E-cadherin-mediated signals in determination of germ-cell fate, specific pathways remain to be identified. Other studies focused on local morphogens, and showed that prior to and at the beginning of gastrulation, transforming growth factor β (TGFβ) family members bone morphogenic protein 4 (BMP4), BMP8b, and BMP2 molecules emanated from the extraembryonic ectoderm (BMP4, BMP8b) and visceral endoderm (BMP2), and ultimately converged on proximal epiblast cells to promote PGC specification (Lawson et al., 1999; Ying et al., 2000; Ying and Zhao, 2001). In support of these findings, downstream mediators of BMP signaling, Smad1 and Smad5, were localized to proximal epiblast cells, among others, while deletion of either factor resulted in defective PGC differentiation (Chang et al., 1999; Chang and Matzuk, 2001; Tremblay et al., 2001). Finally, recent studies implicated a tripartite transcription factor network that includes the products of two genes stimulated by BMP4, BLIMP1 and PRDM14, along with AP2γ, in differentiating PGCs from neighboring somatic cells and resetting their epigenetic profile towards a basal state (Magnúsdóttir et al., 2013; Weber et al., 2010; Yamaji et al., 2008).
WNT signaling was also notable for its contributions during gastrulation, suggesting another potential role in PGC specification (Liu et al., 1999). Indeed, studies indicated that WNT3 signals originating from the posterior visceral endoderm and epiblast conferred a receptive environment for proximal epiblast cells to interpret BMP4 activity (Ohinata et al., 2009). Yet, BMP signals alone could not explain why only a subset of proximal epiblast cells was induced to a germ-cell fate. Thus, it was hypothesized that both positive and negative signals directed PGC fate. Subsequently, an interesting model emerged, based on a sophisticated combination of in vivo and in vitro experiments, that portrayed an intricate collaboration between yet-unidentified inhibitory signals originating from the anterior visceral endoderm and positive influences from BMP4, BMP8b, and WNT3 factors on epiblast cells to ensure only a small population of cells proceeded into the PGC fate (Ohinata et al., 2009; Pfister et al., 2007; Tam and Loebel, 2009). Altogether, these data highlight the importance of the immediate neighborhood, local signals, and cell-cell interactions on determining and maintaining PGC fate.
PGC Migration
Although it is not clear exactly when PGC specification is complete, epiblast cells, including those destined to become PGCs, take up temporary residence within the allantoic core domain before being incorporated into the hind gut and continuing on towards the developing gonad (Chiquoine, 1954; Ginsburg et al., 1990; Lawson and Hage, 1994; Mikedis and Downs, 2012). Interactions with new somatic-cell partners during this journey are likely necessary for definitive PGC differentiation (Fujiwara et al., 2001; Mikedis and Downs, 2012). While several cell types migrate during development, the length and varied terrain that PGCs traverse is unrivaled. Even so, reported literature to date suggests that basic principles of migratory cells hold true for PGCs during migration. This includes their dependence on receptor tyrosine kinases for inherent motility and a combination of chemokine signaling via G protein coupled receptors and cell-cell/cell-extracellular matrix adhesion interactions for directionality (Table 2) (Richardson and Lehmann, 2010).
Table 2.
PGC Migration, E7.5-11.5
| Cell-cell contact
| ||||
|---|---|---|---|---|
| Factor | Site | Expression | Function | References |
| IFITM | Epi | E5.5, 7.5 | Repulsive/guiding actions during initiation of PGC migration | Lange et al. 2003; Tanaka et al. 2005 |
| CDH1 | Hindgut | E8.5 | Expression transitions from PGC in epiblast to hindgut epithelium to facilitate cell-cell Interactions | Bendel-Stenzel et al. 2000 |
| CDH1 | PGC | E9.5 | Expression transitions back to PGC upon exit from hindgut; promotes aggregation in gonad | Bendel-Stenzel et al. 2000; DiCarlo et al. 2000 |
| Integrin β1 | PGC, somatic cell | E10.5 | Extracellular matrix interactions, PGC homing to gonad | Anderson et al. 1999 |
| Local Signals
| ||||
|---|---|---|---|---|
| Factor | Site | Expression | Function | References |
| KIT/KITL | PGC/hindgut somatic cells | E6.25, 7.0 | PGC survival; proliferation; migratory function without directionality | McCoshen et al. 1975; Buehr et al. 1993; Mahakali Zama et al. 2005; Runyan et al. 2006; Gu et al. 2009 |
| ROR2/WNT5A | PGC/hindgut, gonad soma, PGC | E10.5 | Migratory function: PGC polarization, elongation | Laird et al. 2011; Chawengsaksophak et al. 2012 |
| CXCR4/SDF1 | PGC/dorsal mesenchyme, gonad | E9.5-10.5 | PGC survival; directional migration | Ara et al. 2003; Molyneaux et al. 2003 |
| Transcription Factors
| ||||
|---|---|---|---|---|
| Factor | Site | Expression | Function | References |
| Brachyury (T) | PGC/somatic cells | E7.0 | Regulates cell rearrangements | Saitou et al. 2002; Viebahn et al. 2002; Kwan et al. 2003 |
Epi, epiblast
The road to the genital ridges has been segregated into six stages, depending on PGC activity (Figure 2) (Molyneaux and Wylie, 2004). Once putative PGCs have been specified, they actively invade the primitive streak to take residence within the allantois/posterior embryo (Step 1). Next, the PGCs are incorporated into the hindgut by either an active or passive process (Step 2). Shortly thereafter, the hindgut elongates and forms a tubular structure; at this time, the PGCs migrate within the gut epithelium, but in no particular direction (Step 3). Next, chemokine signals promote their directional migration within hindgut epithelium, through the dorsal body wall (Step 4), and finally into the gonadal ridges (Step 5). Those putative PGCs left behind may undergo apoptotic death or may not be PGCs at all, instead representing cells of different lineages that mature into other somatic cell types and become incorporated into other tissues (Step 6) (Molyneaux and Wylie, 2004; Molyneaux et al., 2001; Stallock et al., 2003; Mikedis and Downs, 2012; 2013).
Figure 2. Path for PGC migration into developing gonads.
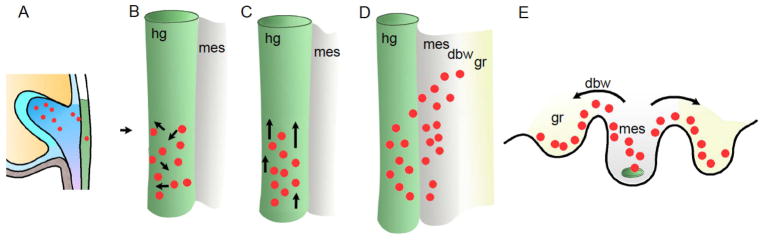
PGCs travel from the epiblast, through the hindgut epithelium, to invade the developing gonadal ridge. (A) Steps 1–2 (E7.5-8.5): PGCs from the allantoic core domain are incorporated into the hindgut. (B) Step 3 (E9.0): upon entering the hindgut, PGCs begin moving in response to KIT/KITL signals, but in no particular direction. (C) Step 4 (E9.0-9.5): Signals, including ROR2/WNT5 and CXCR4/SDF1, work with KIT/KITL to promote directional migration through the hindgut. (D) Steps 4–5 (E10.0-10.5): CXCR4/SDF1 signals attract PGCs to the gonadal ridge. (E) Steps 5–6 (E10.5-11.5): PGCs also use extracellular matrix and cell-cell interactions to invade gonadal ridges via the dorsal body wall. dbw, dorsal body wall; hg, hindgut; mes, mesentery.
Initiation of PGC Migration
The initiation of PGC migration occurs between E7.5-8.5, and may be incorporated as part of the specification process or may be a byproduct of gastrulation. Time-lapse photography documented that OCT4-GFP/TNAP+ cells extended leading edges and actively migrated into the allantois/posterior embryo (Anderson et al., 2000). Before putative PGCs begin the first leg of their journey, however, expression of factors associated with adhesion were localized to these cells, implicating that their role in determining PGC fate includes restricting their movement while they receive critical signals from neighboring cells. Such factors include Interferon-inducing transmembrane protein (IFITM, from fragilis) family members, Ifitm1, -2 and -3; E-cadherin (Cdh1); and Brachyury (Lange et al., 2003; Okamura et al., 2003; Saitou et al., 2002; Tanaka et al., 2005). IFITM family members are known to coordinate homotypic cell-cell adhesion, suggesting a role in migration (Evans et al., 1993; Evans et al., 1990). Immotile proximal epiblast cells corresponding to putative PGCs were found to express Ifitm genes, and ectopic expression studies showed that Ifitm2 and -3 worked together to properly home these cells into mouse endoderm (Lange et al., 2003; Tanaka et al., 2005). Other molecules must contribute to this activity, however, because deletion of all Ifitm family members had no consequence on PGC lineage specification or migration (Lange et al., 2008). Cadherins are responsible for cell adhesion through calcium-dependent, homotypic interactions, and contribute to many different cell functions, including both non-motile and motile phenotypes. It was mentioned above that blockage of E-cadherin-mediated adhesions disrupted PGC specification (Okamura et al., 2003). It is interesting to contemplate if blocking E-cadherin activity affects PGC specification because adhesion blockage would allow precursor cells to move away from each other, and disruption of this cluster of cells could impair their reception of other critical signals. Finally, Brachyury (T), a T-box transcription factor, was found in nascent PGCs and neighboring somatic cells, where it may regulate cell rearrangements during gastrulation (Kwan and Kirschner, 2003; Saitou et al., 2002; Viebahn et al., 2002). In sum, much remains to be learned regarding the initiation of putative PGC migration and their uptake into the endoderm.
PGC Migration Within the Hindgut
Shortly after PGCs arrive within the hindgut, they receive signals that first promote random motility by E9.0, followed by new signals that promote directional migration toward the developing genital ridge between E9.0-9.5. E-cadherin expression wanes within PGCs that have colonized the hindgut, whereas hindgut epithelium expresses high levels (Bendel-Stenzel et al., 2000). Together with decreased E-cadherin expression and consequential reduction in adhesion, receptor tyrosine kinase activity via KIT/KITL interactions promotes PGC motility. This receptor/ligand pair has long been appreciated for their role in PGC migration, proliferation, and survival (Buehr et al., 1993; Mahakali Zama et al., 2005; McCoshen and McCallion, 1975; Runyan et al., 2006). Recent studies showed that PGCs harboring KIT tyrosine kinase receptors were continuously surrounded by somatic cells expressing KITL throughout their migratory journey in the hindgut, which provided a niche friendly towards migratory activity, but not directionality (Gu et al., 2009). In collaboration with KIT/KITL activity, another receptor tyrosine kinase receptor/ligand pair, ROR2/WNT5A, was found to promote polarization and elongation of migrating PGCs; disruption of either component caused PGCs to round up and cease migration (Chawengsaksophak et al., 2012; Laird et al., 2011). The source of directional activity, however, has been associated with the chemokine signal, stromal cell-derived factor 1 (SDF1), which emanates from neighboring mesenchyme and the developing genital ridge to interact with PGCs via its G-protein coupled receptor, CXCR4 (Ara et al., 2003; Molyneaux et al., 2003).
PGC Migration Into The Gonad
The final leg of the journey occurs between E10.5-11.5, and requires PGCs to migrate from the hindgut through the dorsal body wall to the genital ridge. SDF/CXCR4 interactions provide the homing mechanism (Ara et al., 2003; Molyneaux et al., 2003), but cell-cell and cell-extracellular matrix interactions are critical. It was hypothesized that PGC-extracellular matrix interactions were required to maintain PGCs on the narrow path towards the gonad in the context of diffuse SDF signals. Interactions between PGCs and extracellular matrix glycoproteins integrin β1, laminin, fibronectin, and collagen IV were found, but were dependent on the stage of PGC migration (Anderson et al., 1999; Garcia-Castro et al., 1997). Specific to PGC homing, integrin β1-deficient PGCs failed to colonize the gonads (Anderson et al., 1999). After PGCs emigrated from the hindgut, they were also found to extend long cellular processes that interacted with each other and their surroundings while finding their way to the gonad (Gomperts et al., 1994). Subsequent analysis revealed that E-cadherin expression was re-established once PGCs left the hindgut, and was concentrated with β-catenin at sites of PGC-PGC interactions (Bendel-Stenzel et al., 2000). In this case, treatment with E-cadherin blocking antibodies prevented PGCs from directional migration in both in vitro and in vivo experiments, suggesting its role in mediating cell-cell interactions and promoting migration to the gonad (Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000). Finally, E-cadherin was also found to play an important role as PGCs settle within the gonad, stop migration, and take up a rounded appearance (Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000). Once PGCs arrive at their final destination, new somatic-cell partners take over to initiate the next stage of their journey: sex-specific gamete development.
Germ Cell-Somatic Cell Maturation During Ovary Development
PGC Invasion Into the Gonad And Ovigerous Cord Formation
Prior to PGC arrival, the bipotential gonad forms as a result of active proliferation of somatic cells from the gonad-mesonephros border, mesenchyme, and coelomic epithelium (Merchant, 1975). At this nascent stage, distinct boundaries marked by basal lamina separate surface epithelial cells from proliferating mesonephric cells. Once PGCs invade, the basal lamina disintegrates and the tissue begins to reorganize as cord-like structures that gradually emerge as clusters of PGCs surrounded by somatic cells (Figure 3) (Merchant, 1975); these cords emerge in the gonads of both sexes, albeit the newly delineated structures are more subtle in ovaries. This cord-like structure provides the basic and critical infrastructure needed to support homotypic and heterotypic communication between germ and somatic cells (Table 3). The importance of this cord structure was revealed after it was discovered that follicle formation failed and oocytes died after ovaries containing premeiotic germ cells were disrupted, re-aggregated, and transplanted (Nicholas et al., 2010).
Figure 3. Germ cell differentiation and maturation within the developing ovary.
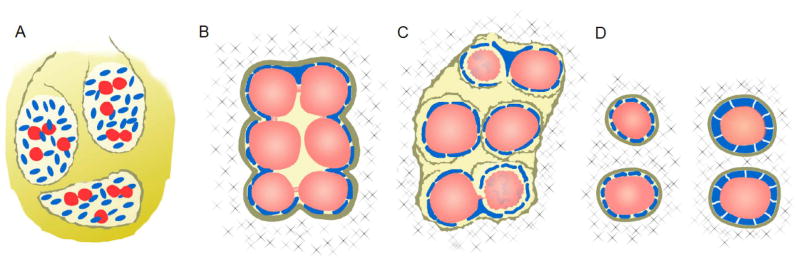
Germ cells undergo synchronous divisions and may aggregate with other dividing germ cells to form germ-line cysts. Thereafter, they differentiate into oocytes by initiating the first meiotic division. Around the time of birth, germ-line cysts break down, some oocytes die, and primordial follicles containing single oocytes form. (A) PGC arrival and organization into ovigerous cords (germ-line cysts) (E11.5-13.5): the basal lamina (brown lines) break down and then reform around proliferating somatic cells (blue cells) to surround invading PGCs (red cells) that aggregate and initiate synchronous divisions to form germ-line cysts. Synchronous division results in germ cell-germ cell syncytia with intercellular bridges. (B) Germ-line cysts coordinate oocyte specification (E13.5-17.5): each germ-line cyst includes clusters of germ cells that are completely surrounded by pre-granulosa cells (blue), a new basal lamina (brown line), and extracellular matrix (X). Several germ-line cysts grow within the developing ovary. Germ cells within the cyst begin to differentiate by initiating meiotic division (germ cell color changes from red to light coral) in response to retinoic acid signaling. Somatic cells remain intimately associated with oocytes and send projections deep into the syncytia. (C) Germ-line cyst breakdown initiates shortly before birth (E17.5-P2): Somatic cells begin to send long, invasive projections between oocytes, the basal lamina break down, and many oocytes die (wrinkled coral/blue germ cells) as germ-line cysts break down and follicles begin to form. The earliest follicles begin to form in the medulla as early as E17.5; the last follicles form at the cortex (P0-P2). (D) Primordial follicle formation and maturation to primary follicle (P0-P4): Interactions between the extracellular matrix (X), basal lamina (brown lines), somatic cells (blue), and oocytes (coral) facilitate organization into primordial follicles. Primordial follicles are characterized by squamous somatic cells (granulosa cells), which transition to a cuboidal shape upon maturation to primary follicles.
Table 3.
Germ Cell Differentiation & Maturation In Developing Ovary, E11.5-P4
| Cell-cell contact
| ||||
|---|---|---|---|---|
| Factor | Site | Expression | Function | References |
| CDH1 | Germ cell | E11.5 | Movement within gonad; germ cell aggregation and proliferation | DiCarlo et al. 2000; Donovan et al. 1986 |
| CDH1/βcat | Germ cell | E11.5 | Adherens junctions | Fleming et al. 2012; Liu et al. 2009; Naillat et al. 2010 |
| CDH2/βcat | Somatic cell | E11.5 | Adherens junctions | Fleming et al. 2012; Liu et al. 2009; Merchant et al. 1975; Mitchell et al. 1986 |
| Gja1 | Germ/Somatic cell | E12.5 | Heterocellular gap junctions | DeFelici et al. 1989; Mitchell et al. 1986; Perez-Armenderiz et al. 2003 |
| Local Signals
| ||||
|---|---|---|---|---|
| Factor | Site | Expression | Function | References |
| Wnt4/Rspo1/βcat | Somatic cells | XX specific E12.5 | Germ cell survival; inhibit male vasculature and ductal system; prevent steroidogenic cell differentiation | Chang et al. 2008; Chassot et al. 2008; Liu et al. 2009; Manuylov et al. 2009; Tomizuka et al. 2008; Vainio et al. 1999 |
| Jag1/Notch2/Lfng | Germ/Somatic/Somatic cell | E15.5 | Oocyte health; primordial follicle formation | Hahn et al. 2005; Manosalva et al. 2013; Trombly et al. 2009; Xu et al. 2013 |
| Transcription Factors
| ||||
|---|---|---|---|---|
| Factor | Site | Expression | Function | References |
| FOXL2 | Somatic cell | E12.5 | Maintenance of female somatic cell phenotype | Schmidt et al. 2004; Uda et al. 2004; Ottolenghi et al. 2007; Uhlenhaut et al. 2009 |
| IRX3, 5 | Somatic cell; Germ cell late | E12.5; P0 | Maintenance of germ cell-somatic cell communication | Jorgensen et al. 2005; Kim et al. 2011; Herein |
| FIGLA | Germ cell | E12.5 | Germ cell survival; primordial follicle formation suppression of male genes; synchronize germ-somatic cells | Hu et al. 2010; Joshi et al. 2007; Lei et al. 2006; Liang et al. 1997; Soyal et al. 2000 |
| LHX8 | Germ cell | E13.5 | Oocyte health; primordial-primary follicle transition | Choi et al. 2008; Pangas et al. 2006 |
| NOBOX | Germ cell | E15.5 | Oocyte health; primordial-primary follicle transition; germ-somatic cell interactions | Lechowska et al. 2012; Rajkovic et al. 2004 |
| SOHLH1, 2 | Germ cell | E12.5 | Oocyte health; primordial-primary follicle transition | Ballow et al. 2006a; Ballow et al. 2006b; Choi et al. 2008; Pangas et al. 2008 |
Germ Cell Interactions
Communication between germ cells is clearly evident as PGCs take up residence in gonads. There is something about their arrival into the gonad neighborhood that fosters new PGC activity, whereby PGC locomotor activity ceases while E-cadherin interactions promote movement within the gonad and facilitate aggregation and continued division (Di Carlo and De Felici, 2000; Donovan et al., 1986). Germ-line clusters form nests or germ-line cysts made up of a collection of aggregated, individual PGCs that undergo synchronous divisions (Figure 3) (Mork et al., 2012b; Pepling and Spradling, 1998). Recently, cell-lineage tracking experiments were used to monitor the progeny of single PGCs and showed evidence that growing cysts fragmented into smaller cysts, which then associated with other, unrelated mini-cysts to form a germ-line nest (Lei and Spradling, 2013). A characteristic feature of incomplete division is the presence of intercellular bridges between synchronously dividing PGCs (De Felici et al., 1989; Pepling and Spradling, 1998). Although significant cellular machinery, including mitochondria and microtubules, have been visualized within these intercellular bridges, their presence, as evidenced by elimination of the integral TEX14 protein, is not required for ovary and follicle formation or for female fertility (Gondos, 1987; Gondos and Conner, 1973; Greenbaum et al., 2009; Pepling and Spradling, 1998; Ruby et al., 1969). Besides intercellular bridges, what may facilitate intercellular communication to promote cord formation and facilitate synchronous oogonia maturation? Germ cells do not express gap junctions (De Felici et al., 1989; Merchant, 1975); however, there is colocalization of E-cadherin and β catenin on germ-cell membranes (Fleming et al., 2012; Liu et al., 2009; Naillat et al., 2010). The importance of adherens junction complex activity will be considered in more detail below.
Somatic Cell Interactions
In conjunction with invading PGCs, ovigerous cords require somatic cells to surround and isolate the developing germ-line cyst structure. In contrast to PGCs, somatic cells that surround germ-line cysts do communicate with each other via gap junctions along with N-cadherin/βcatenin adherens junction activity (Fleming et al., 2012; Liu et al., 2009; Merchant, 1975; Mitchell and Burghardt, 1986). These presumptive pre-granulosa cells could arise from several founding populations of somatic cells in the bipotential gonad: the gonad-mesonephros border (presumptive rete-ovarii), mesenchyme, and coelomic or surface epithelial cells (Albrecht and Eicher, 2001; McLaren, 1992; Merchant, 1975; Mitchell and Burghardt, 1986). One model implicates the same population of cells within the bipotential gonad as precursors for support cells of germ cells of both sexes in developing gonads, namely Sertoli and granulosa cells in the testis and ovary, respectively. This model is based on evidence that granulosa and Sertoli cells can each transdifferentiate into the complementary cell type in certain situations (Behringer et al., 1990; Britt et al., 2002; Couse et al., 1999; Dupont et al., 2000; Matson et al., 2011; McLaren, 1991; Ottolenghi et al., 2007; Uhlenhaut et al., 2009), and transgenic mice expressing EGFP under control of a partial Sry promoter (Sry-EGFP) express EGFP within both Sertoli and pre-granulosa cells in developing gonads (Albrecht and Eicher, 2001). Recent studies, however, identified FOXL2 expression as a pre-granulosa cell marker, and discovered what appears to be two waves of granulosa cell differentiation that contribute to two discrete populations of follicles (Mork et al., 2012a). The source of both populations of granulosa cells arises from the ovarian surface epithelium and upregulate FOXL2 as they exit the cell cycle and surround germ cells. The first wave of differentiation follows the model that supports bipotential somatic cell differentiation; these pre-granulosa cells surround germ-line cysts and eventually form the earliest follicles that develop within the medulla, which subsequently undergo rapid maturation and die. The second wave of differentiation occurs at the ovarian cortex, where follicles are formed and arrest until puberty and beyond (Mork et al., 2012a).
A somewhat different interpretation of somatic cell differentiation was presented based on studies using bovine ovaries. Close examination of bovine ovaries at different stages of development uncovered a distinct population of cells named GREL (Gonadal Ridge Epithelial-Like) cells that originated from the surface epithelium of the mesonephros (Hummitzsch et al., 2013). Following breakdown of mesonephric basal lamina, proliferating GREL cells, along with invading PGCs and mesonephros-derived stromal cells, facilitated growth of the developing gonad. It was not until ovigerous cords were formed that some GREL cells differentiated into either pre-granulosa (FOXL2-expressing) cells within cords or ovarian surface epithelia. Pre-granulosa cells differentiated from the GREL cell population as follicles formed, commencing with the first population of follicles in the medulla, which were the earliest cells to be exposed to invading stroma (Hummitzsch et al., 2013). Nuances in delineating progenitor granulosa cells may be explained by differences in ovarian development among mammalian species (Fridmacher et al., 1992; Gondos, 1975; Motta and Makabe, 1982; Sawyer et al., 2002; Wilhelm et al., 2007). Together, these studies resonate a common theme regarding active proliferation of surface epithelial cells that eventually express FOXL2 upon cessation of proliferation and contact with germ cells (Hummitzsch et al., 2013; Mork et al., 2012a).
Germ Cell-Somatic Cell Interactions
Molecular machinery within somatic cells, but not germ cells, determines gonad sex (Albrecht and Eicher, 2001; Burgoyne et al., 1988; Maatouk et al., 2012; Merchant, 1975; Merchant-Larios and Centeno, 1981). That said, however, germ cell presence is critical for follicle formation and maturation (Eppig, 1991, 2001). Even as PGCs first invade and ovarian cords assemble, there are intimate contacts between germ cells and the selected somatic cells that outline germ-line cysts. Long cytoplasmic processes of pre-granulosa cells extend between germ cells within the same cyst, often making direct contact with intercellular bridges (Lechowska et al., 2011; Pepling and Spradling, 1998). Hetero-cellular gap junctions are formed between pre-granulosa and germ cells, composed of at least Gap junction 1 protein (GJA1, Connexin 43) (De Felici et al., 1989; Mitchell and Burghardt, 1986; Perez-Armendariz et al., 2003). Although the physical presence of somatic cell-germ cell communication is clear, the molecular components and mechanisms by which germ and somatic cells communicate during the transformation from oogonia to oocyte within germ-line cysts, and then from germ-line cysts into follicles remain undetermined.
Several studies proposed two criteria for successful transformation from germ-line cysts to follicles within developing ovaries: the age of germ cells and somatic cells must be synchronized throughout germ-line cyst and cord formation, and oogonia must commit to an oocyte fate (Byskov et al., 1997; Lei et al., 2006; Nicholas et al., 2010; Qing et al., 2008). Studies showed that culture of germ cells and somatic cells from ovaries at the same stage successfully formed follicles, but co-culture of early-stage germ cells with late-stage somatic cells, or vice versa, failed to support follicle formation (Lei et al., 2006; Qing et al., 2008). Notch signaling has emerged as an important mediator of primordial follicle development with the Notch ligand, Jagged 1 (Jag1), and receptor, Notch2, respectively expressed in the oocyte and somatic cell (Hahn et al., 2005; Johnson et al., 2001; Trombly et al., 2009b; Xu and Gridley, 2013). The Notch receptor modulator, Lunatic fringe (Lfng), and downstream mediator of Notch signaling, Hes1, are also expressed in somatic cells beginning at E15.5 (Hahn et al., 2005; Manosalva et al., 2013). Disruption of Notch signaling by pharmacological inhibition, global deletion of Notch2 or Hes1, or conditional knockout of Lfng or Hes1 in somatic cells each resulted in abnormal oocyte maturation and multi-oocyte follicles (Hahn et al., 2005; Manosalva et al., 2013; Trombly et al., 2009b; Xu and Gridley, 2013). Together, these data suggest that Notch signaling between juxtaposition of germ cells and somatic cells initiates after oocyte transition, around E15.5, heralding a new stage of signaling between the two cell types that facilitates maturation of both cell types, culminating in single oocyte-containing primordial follicles.
Germ cell growth and differentiation and the onset of meiosis are considered the fundamental events that must occur to achieve the oogonia-to-oocyte transition, or oogenesis. The extent to which somatic cells promote oogenesis is unclear. Disaggregation and reaggregation of ovaries at stages ranging from E11.5-13.5 demonstrated that intact germ-line cysts were not necessary for the onset of meiosis or oocyte growth, and that somatic cells derived from the lung were sufficient to support oocyte differentiation (McLaren and Southee, 1997; Nicholas et al., 2010; Ohkubo et al., 1996). Prolonged culture of re-aggregated ovaries in vitro or within a kidney capsule transplant, however, failed to support follicle formation, suggesting a role for intact cord structure to facilitate signals mediating somatic cell organization around single oocytes (McLaren and Southee, 1997; Nicholas et al., 2010). Recently, discovery of meiosis-incompetent germ cells enabled the evaluation of a meiotic division requirement for follicle formation. Retinoic acid stimulates expression of Stra8 (stimulated by retinoic acid 8), which promotes replication of germ cell chromatin and the transition of oogonia into meiotic division (Koubova et al., 2006(Anderson et al., 2008; Bowles et al., 2006; Bowles and Koopman, 2007; Griswold et al., 2012; Koubova et al., 2006). Although Stra8−/− oogonia failed to initiate meiosis, they grew and differentiated into oocyte-like cells (Dokshin et al., 2013). Importantly, most Stra8−/− oocyte-like cells died; however, those that survived organized surrounding somatic cells into follicles, and were successfully ovulated and fertilized (Dokshin et al., 2013). Together, these studies suggest that meiotic germ cells are not required for follicle formation, but that the immediate surroundings of germ cells within the ovary promote an oocyte-like fate and support follicle formation.
Ovarian Differentiation Factors And Their Roles In Cell-Cell Communication
Two independent pathways derived from somatic cells are generally accepted as the major drivers of ovarian development: canonical β catenin signaling and FOXL2 activity. β catenin has two distinct modes of activity, distinguished by location and molecular partners: cell-cell communication via adherens junction complexes at the cell membrane and co-activation of gene transcription within the nucleus (Heuberger and Birchmeier, 2010; Nelson and Nusse, 2004). Most ovarian events have been associated with nuclear β catenin activity, either through Rspondin (RSPO1)-mediated pathways or by canonical WNT (WNT4) signaling (Chassot et al., 2008; Maatouk et al., 2008; Manuylov et al., 2008). Loss of β catenin activity in XX gonads or stabilization of β catenin in XY gonads led to at least partial sex reversal, whereas loss of β catenin in XY gonads had no apparent effect (Chang et al., 2008; Liu et al., 2009; Maatouk et al., 2008; Manuylov et al., 2008). Membrane-associated β catenin activity cannot be ruled out as WNT signaling has been shown to stabilize β catenin and to promote activity at both the cell membrane and the nucleus (Heuberger and Birchmeier, 2010; Hinck et al., 1994; Nelson and Nusse, 2004). Careful evaluation of β catenin and its associated cadherins at cell membranes in both somatic and germ cells have highlighted the importance of adherens junctions in defining cords and patterning of developing gonads in both sexes (Fleming et al., 2012). Further, loss of WNT4 signals altered β catenin, cadherin, and GJA1 expression, and caused physical disruption of ovarian structure with loss of germ cell-germ cell and germ cell-somatic cell membrane contacts (Fleming et al., 2012; Naillat et al., 2010). One critical phenotype corresponding to the loss of somatic cell-derived β catenin is massive germ cell death by E16.5, illustrating the importance of somatic cell-derived factors in germ cell survival (Chassot et al., 2008; Liu et al., 2009; Liu et al., 2010b; Manuylov et al., 2008; Tomizuka et al., 2008). Further studies implicated elevated expression of somatic cell-derived Inhbb in germ cell death within Wnt4−/− ovaries, which was rescued upon further removal of Inhbb (Liu et al., 2010b). Ultimately, ovaries from Rspo1−/−, Wnt4−/−, and somatic cell-specific loss of β catenin (SF1Cre; CtnnbF/F) mice all share a similar masculinized phenotype, supporting a role for somatic cell-germ cell interactions in maintaining the female phenotype (Chang et al., 2008; Chassot et al., 2008; Liu et al., 2009; Manuylov et al., 2008; Tomizuka et al., 2008; Vainio et al., 1999).
FOXL2 is a winged helix/forkhead domain transcription factor that exhibits sexually dimorphic expression within somatic cells of the XX gonad as early as E12.5 (Schmidt et al., 2004). While a naturally occurring Foxl2 mutation within goats results in a phenotype of complete sex reversal (Pailhoux et al., 2001), targeted deletion within mice results in a milder ovarian phenotype that more closely resembles ovarian defects in humans with FOXL2 mutations(Crisponi et al., 2001; De Baere et al., 2001; Schmidt et al., 2004; Uda et al., 2004). Ovaries form within Foxl2−/− mice, but signals emanating from abnormal pre-granulosa cells allowed oocytes to undergo premature activation within primordial follicles that failed to progress, which eventually lead to the demise of both oocyte and granulosa cells (Schmidt et al., 2004; Uda et al., 2004). Additional studies highlighted the somatic-cell role of FOXL2 in maintaining the granulosa-cell phenotype, as the loss of FOXL2 facilitated transdifferentiation into a Sertoli-cell phenotype (Ottolenghi et al., 2007; Uhlenhaut et al., 2009). Altogether, the phenotype of Foxl2−/− ovaries included pervasive disruption of all compartments, including stromal, somatic, and germ cells, supporting the long-held view that the communicative networks established within the early ovary are critical for formation of functional follicles (Byskov, 1986; Byskov et al., 1997; Eppig, 1991; Peters, 1969; Rajah et al., 1992; Uda et al., 2004). Thus, while Rspo1/Wnt4/β catenin activity worked to maintain female identity of the early ovary, FOXL2 on its own proved more critical later, in folliculogenesis, and both depended on somatic cell-oocyte communication. Their roles as gatekeepers to the ovarian phenotype and FOXL2 activity in the early ovary were cemented upon the discovery that disruption of Wnt4 and Foxl2 together or Rspo1 and Foxl2 together resulted in sex reversal, even in the absence of Sry (Auguste et al., 2011; Ottolenghi et al., 2007).
Germ Cell-Somatic Cell Events During Folliculogenesis
Germ-line cysts begin to breakdown right around the time of birth in the mouse, culminating in the formation of primordial follicles that consist of a single oocyte surrounded by squamous granulosa cells (Figure 3) (Hirshfield, 1991; Pepling and Spradling, 2001). Events leading to germ-line cyst breakdown and primordial follicle formation include the invasion of somatic cell processes between connected germ cells and massive germ cell death by apoptosis and other means (Pepling and Spradling, 2001) (Wartenberg et al., 2001). The intimate somatic cell-germ cell interactions that existed in germ-line cysts prior to breakdown reform within new primordial follicles, and include classical interactions such as desmosomes, zona adherens, and gap junctions (De Felici et al., 1989; Lechowska et al., 2011; Mitchell and Burghardt, 1986; Motta et al., 1994; Pepling and Spradling, 1998; Perez-Armendariz et al., 2003).
As follicles transition from primordial to primary stages, squamous, flattened granulosa cells morph into polyhedral and then cuboidal shapes while surrounding a growing oocyte. During this transition, scanning electron microscopy imaging of human follicles illuminated the presence of cytoplasmic projections originating from both the oocyte and granulosa cells. Oocytes projected mostly short microvilli, but follicular cells launched long, penetrating extensions through deep folds within the ooplasma; these included tortuous microvilli that were often closely associated with several organelles on their way to almost touching the nucleus. The deep microvilli mostly disappeared as follicles matured (Motta et al., 1994). What do these oocyte-somatic cell contacts mean for ovarian and follicle development? Is the function of these contacts the same within germ-line cysts and nascent follicles? If so, it is essential to identify the molecular networks that are present at both stages of ovarian development and understand their roles in follicle formation and survival.
Factors that Define the Neighborhood Infrastructure
While there are elegant reviews of germ-line cyst breakdown, primordial follicle formation, the onset of follicle activation, and cell-cell communication during antral follicle development (for example, see Albertini et al., 2001; Binelli and Murphy, 2010; Edson et al., 2009; Jagarlamudi and Rajkovic, 2012; Liu et al., 2010a; Pepling, 2006, 2012; Sanchez and Smitz, 2012; Su et al., 2009; Trombly et al., 2009a), the remainder of this review will focus on a specific cadre of factors that have the unique properties of being present throughout the duration of germ-line cyst formation to establishment of primordial follicles, in addition to clearly playing a role in survival of early stage follicles. Their known activities support the hypothesis that the foundation of folliculogenesis begins at the onset of ovarian cord formation, coinciding with transition to the oocyte fate, and requires cooperation between both germ cells and somatic cells (Byskov et al., 1997; Eppig, 1991; Lei et al., 2006; Nicholas et al., 2010; Qing et al., 2008). Further, their presence during both stages of ovarian development could help to frame the molecular events leading to follicle formation and survival. Their known and potential roles in cell-cell interactions during ovarian and follicle development will be discussed (Table 3).
Mechanisms by which germ cell-somatic cell interactions facilitate the progression of oocytes from germ-line cyst to primordial follicle cannot be discussed without first recognizing the seminal discoveries of two oocyte-derived factors, Growth Differentiation Factor 9 (GDF9) and BMP15, both TGFβ superfamily members that are secreted from oocytes to communicate with surrounding granulosa cells, and thereby promote folliculogenesis beyond the primary follicle stage (Dong et al., 1996; Yan et al., 2001). Although these factors are expressed by oocytes of primary follicles, a time beyond the scope of this review, characterization of their activities provided the springboard for subsequent studies that exposed each of the factors that will be highlighted here. An obvious example of a factor whose expression profile spans germ-line cyst to primordial follicle stage is somatic cell-derived FOXL2. Although Foxl2 is expressed at high levels just after sex determination and the onset of cord formation (E12.5), no clear deficit is observed in Foxl2−/− mice until well after birth, when follicles fall apart. Other factors, both somatic cell- and oocyte-derived, share Foxl2 characteristics, namely the onset of sexually dimorphic expression that coincides with oocyte specification and whose disruption causes no obvious deficiency during ovary development, but instead derails later formative events, including folliculogenesis or follicle maturation. Besides Foxl2, examples include germ cell-derived factors Sohlh1 and -2 (spermatogenesis and oogenesis helix-loop-helix 1 and 2), Lhx8 (LIM homeobox 8), Figla (Factor in germ line alpha), and Nobox (newborn ovary homeobox), and other somatic cell-derived factors such as Irx3 and -5 (Iroquois homeobox transcription factors 3 and 5). It is intriguing that ovary development initially appears to proceed uneventfully in mice with null mutations of these factors; however, it is important to consider that none of them work in isolation, with each contributing to the somatic cell-germ cell neighborhood that builds critical networks that persist through folliculogenesis.
Ovaries in mouse lines lacking germ cell-derived Figla, a basic helix-loop-helix transcription factor, failed to form primordial follicles and exhibited massive germ-cell death (Soyal et al., 2000). Figla is expressed at low levels starting at E12.5, and increases until it peaks at post-natal day 2 (P2) with protein first detected by E19 (Lei et al., 2006; Millar et al., 1993; Soyal et al., 2000). Normal numbers of oocytes were observed at E18.5, but were depleted by P2 after failed primordial follicle formation. This devastating phenotype was attributed to FIGLA’s role in regulating zona pellucida genes (Zp1, Zp2, and Zp3), which produce the glycoprotein coating of the oocyte, and its suppression of male-specific genes (Hu et al., 2010; Joshi et al., 2007; Liang et al., 1997; Soyal et al., 2000). Additional studies highlighted the importance of coordinated expression of Figla along with Zp1-3, Bax, and Gja1 in synchronizing somatic cell-germ cell interactions during the oogonia-to-oocyte transition, oocyte differentiation, and primordial follicle formation (Lei et al., 2006).
FIGLA is an important node within a regulatory pathway that includes other germ cell-derived transcription factors, whose disruption allows primordial follicle formation, but failure of follicle maturation. SOHLH1 stimulates expression of Lhx8, which in turn directly regulates Nobox and contributes to the presence of FIGLA and the zona pellucida proteins, among others (Ballow et al., 2006a; Choi et al., 2008a; Choi et al., 2008b; Pangas et al., 2006). Sohlh1-2 and Lhx8 are expressed beginning E12.5-13.5 and Nobox is first detected by E15.5. Disruption of each caused a similar ovarian phenotype that included failure in primordial to primary follicle transition and concomitant oocyte loss (Ballow et al., 2006b; Choi et al., 2008a; Choi et al., 2008b; Pangas et al., 2006; Rajkovic et al., 2004). Additional analysis of Nobox−/− ovaries illuminated severe defects in somatic cell-oocyte interactions that originated from faulty oocyte communication early within the germ-line cyst. Somatic cells must be able to send long projections between germ cells to facilitate germ-line cyst breakdown. Nobox−/− somatic cells, however, have very few short projections that fail to project between oocytes (Lechowska et al., 2011). Furthermore, while adherens junctions normally exist between somatic cells and between somatic cells and germ cells, they have never been noted between germ cells, except in the case of the Nobox−/− ovary (Bogard et al., 2007; Cerda et al., 1999; De Felici et al., 1989; Lechowska et al., 2011; Merchant, 1975). These results strengthen the hypothesis that germ cell-derived factors are important contributors in developing intercellular networks within germ-line cysts that also affect follicle formation and maturation.
It is unclear whether or not different somatic cells have unique roles in ovarian development, although distinct populations have been identified (Chen et al., 2012). In contrast to distinct somatic cell-specific FOXL2 expression patterns, IRX3 and IRX5 are detected in somatic cells throughout the developing ovary (Jorgensen and Gao, 2005; Kim et al., 2011b; Nef et al., 2005). The onset of somatic cell-specific IRX3 and -5 (herein referred to as IRX3/5) expression coincided with establishment of the germ-line cyst, and evidence suggested they were upregulated by WNT4 signals (Kim et al., 2011b) (Coveney et al., 2007; Manuylov et al., 2008; Naillat et al., 2010). Later, upon germ-line cyst breakdown, a burst of IRX3/5 expression was also detected within oocytes while somatic cell expression remained only in cells at the ovarian surface, surrounding germ-line cysts, and encapsulating new primordial follicles (Li et al., 2011; Jorgensen, unpublished data). Once primordial follicles were formed, expression in somatic cells diminished, and as they progressed to primary follicles, IRX3/5 expression was gone. Based on these data, it is hypothesized that IRX3/5 re-establish somatic cell-germ cell communication after germ-line cyst breakdown within nascent primordial follicles; once the follicle is established, IRX3/5 relinquish control to other yet-unknown factors as their expression diminishes (Jorgensen, unpublished data).
Fused toes (Ft) mutant mice that harbor a 1.5-Mbp deletion within chromosome 8 were used to demonstrate an essential role of Irx3/5 in follicle maturation. Ft mutants lack six genes, including the entire IrxB locus (Irx3, -5, and -6), and die by E13.5 (Peters et al., 2002). To bypass early lethality, ovaries from wild-type and mutant embryos were harvested at E12.5 and transplanted under the kidney capsule of nude-mouse hosts (Kim et al., 2011a). Wild-type ovaries developed primary, secondary, and pre-antral follicles 2 and 3 weeks after transplantation. In contrast, maturation of the ovarian follicles derived from Ft-mutant embryos was stunted at the primary stage. Asymmetrical follicles contained oocytes that failed to grow, incomplete layers of granulosa cells, and a disrupted basement membrane (Kim et al., 2011a). Focusing solely on Irx factors, Irx3−/− and Irx5G/G single-knockout mice were viable and fertile, suggesting that each factor compensated for the other (Kim et al., 2011b; Zhang et al., 2011). Like Ft mice, however, Irx3−/−;Irx5G/G double-knockout embryos die early (E13.5), and histology of kidney capsule-transplanted ovaries was similar after 2–3 weeks:.compared to transplanted wild-type ovaries, Irx3−/−;Irx5G/G ovaries exhibited disjointed connections between granulosa cells and the oocyte, incomplete layers of granulosa cells, and gaps between the two cell types. Several misshapen follicles containing zona pellucida remnants were also detected, suggesting that the defective granulosa cell-oocyte interactions culminated in follicle death (Figure 4). Transmission electron microscopy images from wild-type ovaries after 2 weeks kidney capsule transplantation illustrated plentiful cell-cell contacts between oocytes and granulosa cells and between granulosa cells. In contrast, images from Irx3−/−;Irx5G/G follicles demonstrated significant gaps between oocytes and abnormal granulosa cells (Figure 4). Adherens junctions and microvilli projections were fewer in number, and were clustered in the few areas where granulosa cells were in close proximity to the oocyte. Preliminary studies suggest that defective cell-cell signaling is caused, at least in part, by decreased Gja1 expression within Irx3−/−;Irx5G/G ovaries (Jorgensen, unpublished data). These findings are supported by molecular analyses that showed that IRX3 regulated transcription of both GJA1 and GJA5 in cardiac ventricular cells (Zhang et al., 2011). While roles for Irx3/5 in somatic and germ cells are currently under investigation, these data suggest clear somatic cell-germ cell interactions that are initiated within the germ-line cyst and are maintained through early folliculogenesis.
Figure 4. Irx3−/−;Irx5G/G ovaries contain follicles with disrupted granulosa cell-oocyte contacts.
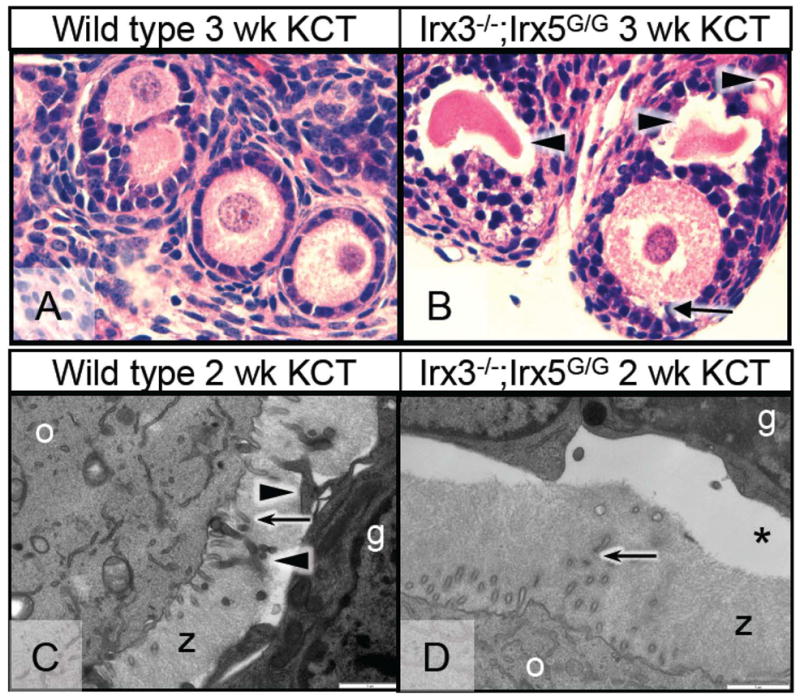
Ovaries were harvested at E12.5 from wild-type and Irx3−/−;Irx5G/G embryos 2 or 3 weeks after kidney capsule transplantation (KCT). (A, B) Hematoxylin and eosin staining of ovaries 3 weeks after transplantation. (A) Primary and secondary follicles within a wild-type ovary. (B) Follicles within an Irx3−/−;Irx5G/G ovary. Severe disruption of contact between oocyte and granulosa cells was detected (arrow), forewarning oocyte death (arrowheads, zona pellucida remnants). (C, D) Transmission electron microscopy of ovaries harvested 2 weeks after transplantation. Scale bar, 1 μm. (C) Wild-type granulosa cells (g) and an oocyte (o) are in close proximity with the zona pellucida matrix (z) evident between them. Granulosa cell trans-zonal processes (arrowheads) and oocyte-derived microvilli (arrows) are plentiful, exemplifying substantial cell-cell communication. (D) A follicle from an Irx3−/−;Irx5G/G ovary displays a considerable gap between the granulosa cells (g) and oocyte (o) (asterisk). Microvilli originating from the oocyte are fewer, but present (arrow); however, trans-zonal projections from granulosa cells are completely absent.
Summary
This review followed somatic cell-germ cell interactions from the onset of germ cell differentiation until the earliest stages of folliculogenesis within the mouse ovary. Ovarian development clearly depends on factors that determine and maintain the ovarian fate. Here, the focus is on the road towards formation of the functional follicle. Studies suggest that a distinct cohort of factors contribute to the integral relationship between somatic and germ cells that begin at the onset of germ-line cyst formation, encompassing the oogonia-to-oocyte transition, and is maintained and required for successful primordial and primary follicle development. Undoubtedly, more factors and their relationships will and must be discovered. Without the foundation laid by these somatic and germ cell-derived factors, follicles may form, but none will be structurally sound enough to proceed through follicle maturation and ultimately release a viable oocyte that can contribute to the next generation.
Acknowledgments
Grant support: Ovary development studies in the Jorgensen Laboratory have been supported by the National Institute of Health Grant R01HD075079
The author would like to acknowledge Drs. Karen Downs, Sakhila Banu, Humphrey Yao, and Ms. Maria Mikedis for critical review of the manuscript, and Gail Loughridge for her artistic talents displayed in Figures 1–3.
Abbreviations
- BMP
bone morphogenic protein
- E#
embryonic day #
- Gja
gap junction protein alpha
- P#
post-natal day #
- PGC
primordial germ cell
- TGFβ
transforming growth factor β
REFERENCE LIST
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–68. doi: 10.1016/s0925-4773(99)00271-3. [DOI] [PubMed] [Google Scholar]
- Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie C. Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999;126:1655–1664. doi: 10.1242/dev.126.8.1655. [DOI] [PubMed] [Google Scholar]
- Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1) Proc Natl Acad Sci U S A. 2003;100:5319–5323. doi: 10.1073/pnas.0730719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste A, Chassot AA, Gregoire EP, Renault L, Pannetier M, Treier M, Pailhoux E, Chaboissier MC. Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal in XX mice. Sex Dev. 2011;5:304–317. doi: 10.1159/000334517. [DOI] [PubMed] [Google Scholar]
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006a;294:161–167. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A. Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns. 2006b;6:1014–1018. doi: 10.1016/j.modgep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature. 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- Bendel-Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C. The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev. 2000;91:143–152. doi: 10.1016/s0925-4773(99)00287-7. [DOI] [PubMed] [Google Scholar]
- Binelli M, Murphy BD. Coordinated regulation of follicle development by germ and somatic cells. Reprod Fertil Dev. 2010;22:1–12. doi: 10.1071/RD09218. [DOI] [PubMed] [Google Scholar]
- Bogard N, Lan L, Xu J, Cohen RS. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development. 2007;134:3413–3418. doi: 10.1242/dev.008466. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134:3401–3411. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- Britt KL, Kerr J, O’Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J. 2002;16:1389–1397. doi: 10.1096/fj.01-0992com. [DOI] [PubMed] [Google Scholar]
- Buehr M, McLaren A, Bartley A, Darling S. Proliferation and migration of primordial germ cells in We/We mouse embryos. Dev Dyn. 1993;198:182–189. doi: 10.1002/aja.1001980304. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX----XY chimaeric mouse testes. Development. 1988;102:443–450. doi: 10.1242/dev.102.2.443. [DOI] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Guoliang X, Andersen CY. The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol Hum Reprod. 1997;3:795–800. doi: 10.1093/molehr/3.9.795. [DOI] [PubMed] [Google Scholar]
- Cerda J, Reidenbach S, Pratzel S, Franke WW. Cadherin-catenin complexes during zebrafish oogenesis: heterotypic junctions between oocytes and follicle cells. Biol Reprod. 1999;61:692–704. doi: 10.1095/biolreprod61.3.692. [DOI] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- Chang H, Matzuk MM. Smad5 is required for mouse primordial germ cell development. Mech Dev. 2001;104:61–67. doi: 10.1016/s0925-4773(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, Svingen T, Ng ET, Epp T, Spiller CM, Clark C, Cooper H, Koopman P. Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol Reprod. 2012;86:1–12. doi: 10.1095/biolreprod.111.095232. [DOI] [PubMed] [Google Scholar]
- Chen H, Palmer JS, Thiagarajan RD, Dinger ME, Lesieur E, Chiu H, Schulz A, Spiller C, Grimmond SM, Little MH, et al. Identification of novel markers of mouse fetal ovary development. PLoS One. 2012;7:e41683. doi: 10.1371/journal.pone.0041683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquoine AD. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954;118:135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod. 2008a;79:442–449. doi: 10.1095/biolreprod.108.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Yuan D, Rajkovic A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol Reprod. 2008b;79:1176–1182. doi: 10.1095/biolreprod.108.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Coveney D, Ross AJ, Slone JD, Capel B. A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation. Gene expression patterns: GEP. 2007;7:82–92. doi: 10.1016/j.modgep.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- De Baere E, Dixon MJ, Small KW, Jabs EW, Leroy BP, Devriendt K, Gillerot Y, Mortier G, Meire F, Van Maldergem L, et al. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype--phenotype correlation. Hum Mol Genet. 2001;10:1591–1600. doi: 10.1093/hmg/10.15.1591. [DOI] [PubMed] [Google Scholar]
- De Felici M, Dolci S, Siracusa G. Fetal germ cells establish cell coupling with follicle cells in vitro. Cell differentiation and development: the official journal of the International Society of Developmental Biologists. 1989;28:65–69. doi: 10.1016/0922-3371(89)90024-5. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, De Felici M. A role for E-cadherin in mouse primordial germ cell development. Dev Biol. 2000;226:209–219. doi: 10.1006/dbio.2000.9861. [DOI] [PubMed] [Google Scholar]
- Dokshin GA, Baltus AE, Eppig JJ, Page DC. Oocyte differentiation is genetically dissociable from meiosis in mice. Nat Genet. 2013;45:877–883. doi: 10.1038/ng.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie CC. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell. 1986;44:831–838. doi: 10.1016/0092-8674(86)90005-x. [DOI] [PubMed] [Google Scholar]
- Downs KM, Harmann C. Developmental potency of the murine allantois. Development. 1997;124:2769–2780. doi: 10.1242/dev.124.14.2769. [DOI] [PubMed] [Google Scholar]
- Downs KM, Inman KE, Jin DX, Enders AC. The Allantoic Core Domain: new insights into development of the murine allantois and its relation to the primitive streak. Dev Dyn. 2009;238:532–553. doi: 10.1002/dvdy.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Evans SS, Collea RP, Leasure JA, Lee DB. IFN-alpha induces homotypic adhesion and Leu-13 expression in human B lymphoid cells. J Immunol. 1993;150:736–747. [PubMed] [Google Scholar]
- Evans SS, Lee DB, Han T, Tomasi TB, Evans RL. Monoclonal antibody to the interferon-inducible protein Leu-13 triggers aggregation and inhibits proliferation of leukemic B cells. Blood. 1990;76:2583–2593. [PubMed] [Google Scholar]
- Fleming A, Ghahramani N, Zhu MX, Delot EC, Vilain E. Membrane beta-catenin and adherens junctions in early gonadal patterning. Dev Dyn. 2012;241:1782–1798. doi: 10.1002/dvdy.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridmacher V, Locquet O, Magre S. Differential expression of acidic cytokeratins 18 and 19 during sexual differentiation of the rat gonad. Development. 1992;115:503–517. doi: 10.1242/dev.115.2.503. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Dunn NR, Hogan BL. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc Natl Acad Sci U S A. 2001;98:13739–13744. doi: 10.1073/pnas.241508898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro MI, Anderson R, Heasman J, Wylie C. Interactions between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo. J Cell Biol. 1997;138:471–480. doi: 10.1083/jcb.138.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL, Rossant J. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol. 1979;52:141–152. [PubMed] [Google Scholar]
- Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gomperts M, Garcia-Castro M, Wylie C, Heasman J. Interactions between primordial germ cells play a role in their migration in mouse embryos. Development. 1994;120:135–141. doi: 10.1242/dev.120.1.135. [DOI] [PubMed] [Google Scholar]
- Gondos B. Surface epithelium of the developing ovary. Possible correlation with ovarian neoplasia. Am J Pathol. 1975;81:303–321. [PMC free article] [PubMed] [Google Scholar]
- Gondos B. Comparative studies of normal and neoplastic ovarian germ cells: 1. Ultrastructure of oogonia and intercellular bridges in the fetal ovary. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists. 1987;6:114–123. doi: 10.1097/00004347-198706000-00003. [DOI] [PubMed] [Google Scholar]
- Gondos B, Conner LA. Ultrastructure of developing germ cells in the fetal rabbit testis. Am J Anat. 1973;136:23–42. doi: 10.1002/aja.1001360104. [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori N, Agno JE, Matzuk MM. Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol Reprod. 2009;80:449–457. doi: 10.1095/biolreprod.108.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2012;86:35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development. 2009;136:1295–1303. doi: 10.1242/dev.030619. [DOI] [PubMed] [Google Scholar]
- Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- Hahnel AC, Rappolee DA, Millan JL, Manes T, Ziomek CA, Theodosiou NG, Werb Z, Pedersen RA, Schultz GA. Two alkaline phosphatase genes are expressed during early development in the mouse embryo. Development. 1990;110:555–564. doi: 10.1242/dev.110.2.555. [DOI] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harbor perspectives in biology. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hu W, Gauthier L, Baibakov B, Jimenez-Movilla M, Dean J. FIGLA, a basic helix-loop-helix transcription factor, balances sexually dimorphic gene expression in postnatal oocytes. Mol Cell Biol. 2010;30:3661–3671. doi: 10.1128/MCB.00201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ. A new model of development of the mammalian ovary and follicles. PLoS One. 2013;8:e55578. doi: 10.1371/journal.pone.0055578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagarlamudi K, Rajkovic A. Oogenesis: transcriptional regulators and mouse models. Mol Cell Endocrinol. 2012;356:31–39. doi: 10.1016/j.mce.2011.07.049. [DOI] [PubMed] [Google Scholar]
- Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J. Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev. 2001;109:355–361. doi: 10.1016/s0925-4773(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns. 2005;5:756–762. doi: 10.1016/j.modgep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davies H, Sims LP, Levy SE, Dean J. Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol. 2007;7:67. doi: 10.1186/1471-213X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kim Y, Cooke PS, Rüther U, Jorgensen JS. The Fused Toes locus is essential for somatic-germ cell interactions that foster germ cell maturation in developing gonads in mice. Biol Reprod. 2011a;84:1024–1032. doi: 10.1095/biolreprod.110.088559. [DOI] [PubMed] [Google Scholar]
- Kim B, Kim Y, Sakuma R, Hui CC, Ruther U, Jorgensen JS. Primordial germ cell proliferation is impaired in Fused Toes mutant embryos. Dev Biol. 2011b;349:417–426. doi: 10.1016/j.ydbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. Xbra functions as a switch between cell migration and convergent extension in the Xenopus gastrula. Development. 2003;130:1961–1972. doi: 10.1242/dev.00412. [DOI] [PubMed] [Google Scholar]
- Laird DJ, Altshuler-Keylin S, Kissner MD, Zhou X, Anderson KV. Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet. 2011;7:e1002428. doi: 10.1371/journal.pgen.1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange UC, Adams DJ, Lee C, Barton S, Schneider R, Bradley A, Surani MA. Normal germ line establishment in mice carrying a deletion of the Ifitm/Fragilis gene family cluster. Mol Cell Biol. 2008;28:4688–4696. doi: 10.1128/MCB.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange UC, Saitou M, Western PS, Barton SC, Surani MA. The fragilis interferon-inducible gene family of transmembrane proteins is associated with germ cell specification in mice. BMC Dev Biol. 2003;3:1. doi: 10.1186/1471-213X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Hage WJ. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Foundation symposium. 1994;182:68–84. doi: 10.1002/9780470514573.ch5. discussion 84–91. [DOI] [PubMed] [Google Scholar]
- Lechowska A, Bilinski S, Choi Y, Shin Y, Kloc M, Rajkovic A. Premature ovarian failure in nobox-deficient mice is caused by defects in somatic cell invasion and germ cell cyst breakdown. Journal of assisted reproduction and genetics. 2011;28:583–589. doi: 10.1007/s10815-011-9553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development. 2013;140:2075–2081. doi: 10.1242/dev.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Zhang H, Jin S, Wang F, Fu M, Wang H, Xia G. Stage-specific germ-somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J Cell Physiol. 2006;208:640–647. doi: 10.1002/jcp.20702. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang H, Wang Y, Wen J, Teng Z, Mao G, Wang J, Guo M, Mu X, Xia G. Stage-specific mice ovarian somatic cell is involved in primordial folliculogenesis. Front Biosci (Elite Ed) 2011;3:1025–1033. doi: 10.2741/E308. [DOI] [PubMed] [Google Scholar]
- Liang L, Soyal SM, Dean J. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development. 1997;124:4939–4947. doi: 10.1242/dev.124.24.4939. [DOI] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Liu C, Yao HH. Building pathways for ovary organogenesis in the mouse embryo. Curr Top Dev Biol. 2010a;90:263–290. doi: 10.1016/S0070-2153(10)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Parker K, Yao HH. WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS One. 2010b;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, Mork L, Hinson A, Kobayashi A, McMahon AP, Capel B. Germ cells are not required to establish the female pathway in mouse fetal gonads. PLoS One. 2012;7:e47238. doi: 10.1371/journal.pone.0047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor GR, Zambrowicz BP, Soriano P. Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development. 1995;121:1487–1496. doi: 10.1242/dev.121.5.1487. [DOI] [PubMed] [Google Scholar]
- Magnúsdóttir E, Dietmann S, Murakami K, Gunesdogan U, Tang F, Bao S, Diamanti E, Lao K, Gottgens B, Azim Surani M. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol. 2013;15:905–915. doi: 10.1038/ncb2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahakali Zama A, Hudson FP, 3rd, Bedell MA. Analysis of hypomorphic KitlSl mutants suggests different requirements for KITL in proliferation and migration of mouse primordial germ cells. Biol Reprod. 2005;73:639–647. doi: 10.1095/biolreprod.105.042846. [DOI] [PubMed] [Google Scholar]
- Manosalva I, Gonzalez A, Kageyama R. Hes1 in the somatic cells of the murine ovary is necessary for oocyte survival and maturation. Dev Biol. 2013;375:140–151. doi: 10.1016/j.ydbio.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoshen JA, McCallion DJ. A study of the primordial germ cells during their migratory phase in Steel mutant mice. Experientia. 1975;31:589–590. doi: 10.1007/BF01932475. [DOI] [PubMed] [Google Scholar]
- McLaren A. Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays. 1991;13:151–156. doi: 10.1002/bies.950130402. [DOI] [PubMed] [Google Scholar]
- McLaren A. Development of primordial germ cells in the mouse. Andrologia. 1992;24:243–247. doi: 10.1111/j.1439-0272.1992.tb02647.x. [DOI] [PubMed] [Google Scholar]
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- Merchant H. Rat gonadal and ovarian organogenesis with and without germ cells. An ultrastructural study. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Centeno B. Morphogenesis of the ovary from the sterile W/Wv mouse. Prog Clin Biol Res. 1981;59B:383–392. [PubMed] [Google Scholar]
- Mikedis MM, Downs KM. STELLA-positive subregions of the primitive streak contribute to posterior tissues of the mouse gastrula. Dev Biol. 2012;363:201–218. doi: 10.1016/j.ydbio.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikedis MM, Downs KM. Widespread but tissue-specific patterns of interferon-induced transmembrane protein 3 (IFITM3, FRAGILIS, MIL-1) in the mouse gastrula. Gene Expr Patterns. 2013 doi: 10.1016/j.gep.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE, Lader ES, Dean J. ZAP-1 DNA binding activity is first detected at the onset of zona pellucida gene expression in embryonic mouse oocytes. Dev Biol. 1993;158:410–413. doi: 10.1006/dbio.1993.1199. [DOI] [PubMed] [Google Scholar]
- Mintz B, Russell ES. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957;134:207–237. [Google Scholar]
- Mitchell PA, Burghardt RC. The ontogeny of nexuses (gap junctions) in the ovary of the fetal mouse. The Anatomical record. 1986;214:283–288. doi: 10.1002/ar.1092140307. [DOI] [PubMed] [Google Scholar]
- Molyneaux K, Wylie C. Primordial germ cell migration. Int J Dev Biol. 2004;48:537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O’Brien W, Raz E, Littman D, Wylie C, et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod. 2012a;86:37. doi: 10.1095/biolreprod.111.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mork L, Tang H, Batchvarov I, Capel B. Mouse germ cell clusters form by aggregation as well as clonal divisions. Mech Dev. 2012b;128:591–596. doi: 10.1016/j.mod.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PM, Makabe S. Development of the ovarian surface and associated germ cells in the human fetus. A correlated study by scanning and transmission electron microscopy. Cell Tissue Res. 1982;226:493–510. doi: 10.1007/BF00214779. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S, Naguro T, Correr S. Oocyte follicle cells association during development of human ovarian follicle. A study by high resolution scanning and transmission electron microscopy. Arch Histol Cytol. 1994;57:369–394. doi: 10.1679/aohc.57.369. [DOI] [PubMed] [Google Scholar]
- Naillat F, Prunskaite-Hyyrylainen R, Pietila I, Sormunen R, Jokela T, Shan J, Vainio SJ. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum Mol Genet. 2010;19:1539–1550. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Haston KM, Pera RA. Intact fetal ovarian cord formation promotes mouse oocyte survival and development. BMC Dev Biol. 2010;10:2. doi: 10.1186/1471-213X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571–584. doi: 10.1016/j.cell.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O’Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Shirayoshi Y, Nakatsuji N. Autonomous regulation of proliferation and growth arrest in mouse primordial germ cells studied by mixed and clonal cultures. Exp Cell Res. 1996;222:291–297. doi: 10.1006/excr.1996.0037. [DOI] [PubMed] [Google Scholar]
- Okamura D, Kimura T, Nakano T, Matsui Y. Cadherin-mediated cell interaction regulates germ cell determination in mice. Development. 2003;130:6423–6430. doi: 10.1242/dev.00870. [DOI] [PubMed] [Google Scholar]
- Okamura D, Tokitake Y, Niwa H, Matsui Y. Requirement of Oct3/4 function for germ cell specification. Dev Biol. 2008;317:576–584. doi: 10.1016/j.ydbio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Vigier B, Chaffaux S, Servel N, Taourit S, Furet JP, Fellous M, Grosclaude F, Cribiu EP, Cotinot C, et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet. 2001;29:453–458. doi: 10.1038/ng769. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8090–8095. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143:139–149. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Perez-Armendariz EM, Saez JC, Bravo-Moreno JF, Lopez-Olmos V, Enders GC, Villalpando I. Connexin43 is expressed in mouse fetal ovary. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;271:360–367. doi: 10.1002/ar.a.10040. [DOI] [PubMed] [Google Scholar]
- Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol (Copenh) 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- Peters T, Ausmeier K, Dildrop R, Ruther U. The mouse Fused toes (Ft) mutation is the result of a 1.6-Mb deletion including the entire Iroquois B gene cluster. Mamm Genome. 2002;13:186–188. doi: 10.1007/s00335-001-2142-7. [DOI] [PubMed] [Google Scholar]
- Pfister S, Steiner KA, Tam PP. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr Patterns. 2007;7:558–573. doi: 10.1016/j.modgep.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Qing T, Liu H, Wei W, Ye X, Shen W, Zhang D, Song Z, Yang W, Ding M, Deng H. Mature oocytes derived from purified mouse fetal germ cells. Hum Reprod. 2008;23:54–61. doi: 10.1093/humrep/dem334. [DOI] [PubMed] [Google Scholar]
- Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn. 1992;194:177–192. doi: 10.1002/aja.1001940303. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby JR, Dyer RF, Skalko RG. The occurrence of intercellular bridges during oogenesis in the mouse. J Morphol. 1969;127:307–339. doi: 10.1002/jmor.1051270304. [DOI] [PubMed] [Google Scholar]
- Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development. 2006;133:4861–4869. doi: 10.1242/dev.02688. [DOI] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- Sanchez F, Smitz J. Molecular control of oogenesis. Biochimica et biophysica acta. 2012;1822:1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Sato M, Kimura T, Kurokawa K, Fujita Y, Abe K, Masuhara M, Yasunaga T, Ryo A, Yamamoto M, Nakano T. Identification of PGC7, a new gene expressed specifically in preimplantation embryos and germ cells. Mech Dev. 2002;113:91–94. doi: 10.1016/s0925-4773(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Stallock J, Molyneaux K, Schaible K, Knudson CM, Wylie C. The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development. 2003;130:6589–6597. doi: 10.1242/dev.00898. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Specifying mouse embryonic germ cells. Cell. 2009;137:398–400. doi: 10.1016/j.cell.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H, Tam PP. IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev Cell. 2005;9:745–756. doi: 10.1016/j.devcel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development. 2001;128:3609–3621. doi: 10.1242/dev.128.18.3609. [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009a;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology. 2009b;150:1014–1024. doi: 10.1210/en.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Stortz C, Mitchell SA, Blum M. Low proliferative and high migratory activity in the area of Brachyury expressing mesoderm progenitor cells in the gastrulating rabbit embryo. Development. 2002;129:2355–2365. doi: 10.1242/dev.129.10.2355. [DOI] [PubMed] [Google Scholar]
- Wartenberg H, Ihmer A, Schwarz S, Miething A, Viebahn C. Mitotic arrest of female germ cells during prenatal oogenesis. A colcemid-like, non-apoptotic cell death. Anatomy and embryology. 2001;204:421–435. doi: 10.1007/s00429-001-0216-7. [DOI] [PubMed] [Google Scholar]
- Weber S, Eckert D, Nettersheim D, Gillis AJ, Schafer S, Kuckenberg P, Ehlermann J, Werling U, Biermann K, Looijenga LH, et al. Critical function of AP-2 gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biol Reprod. 2010;82:214–223. doi: 10.1095/biolreprod.109.078717. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Xu J, Gridley T. Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol. 2013;11:13. doi: 10.1186/1741-7007-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet. 2008;40:1016–1022. doi: 10.1038/ng.186. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol. 2000;14:1053–1063. doi: 10.1210/mend.14.7.0479. [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–492. doi: 10.1006/dbio.2001.0173. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Obinata M, Matsui Y. Stage-specific tissue and cell interactions play key roles in mouse germ cell specification. Development. 2001;128:481–490. doi: 10.1242/dev.128.4.481. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Kim KH, Rosen A, Smyth JW, Sakuma R, Delgado-Olguin P, Davis M, Chi NC, Puviindran V, Gaborit N, et al. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His-Purkinje network. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13576–13581. doi: 10.1073/pnas.1106911108. [DOI] [PMC free article] [PubMed] [Google Scholar]