Abstract
C–H oxidation has a long history and an ongoing presence in research at the forefront of chemistry and interrelated fields. As such, numerous highly useful texts and reviews have been written on this subject. Logically, these are generally written from the perspective of the scope and limitations of the reagents employed. This minireview instead attempts to emphasize chemoselectivity imposed by the nature of the substrate. Consequently many landmark discoveries in the field of C–H oxidation are not discussed, but hopefully the perspective taken herein will allow for the more ready incorporation of C–H oxidation reactions into synthetic planning.
Keywords: C–H Activation, Synthetic Methods, Synthesis Design, Natural Products
It has been a widely recognized fact that Nature’s use of C–H activation reactions and strategies have contributed to the “ideality” of biosynthesis.[1] Not surprisingly, there are fundamental advantages to incorporating such logic in chemical synthesis, specifically from the vantage point of synthesis economy.[2] In order to plan a retrosynthetic analysis of a complex natural product that strategically employs a C–H oxidation step,[3] the innate reactivity of such bonds must be intimately understood. Over the past century, an enormous body of literature has provided a foundation for peering into the “eyes” of C–H bonds.[4–8] If C–H bonds could talk, what stories would they tell? The purpose of this review is to compile some of those stories, inferred from the literature. From these stories, the factors that dictate the inherent preferences of sp2 and sp3 C–H bonds in diverse settings are illuminated: inductive effects, conjugation, hyperconjugation, steric hindrance, and strain release
1. Inductive Effects
Through-bond effects are a major factor in determining the electronic character of C–H bonds, which largely dominates their propensity to be oxidized. Since the vast majority of non-metal C–H activation reagents are electrophilic, the more electron rich a C–H bond is, the more easily it is oxidized. One of the most dominant factors that affects the rate of C–H bond oxidation is the presence of electron-withdrawing groups (EWGs) on a substrate. Both the extent of electron withdrawal and distance from the EWG can modulate its influence on the selectivity of an oxidation reaction.
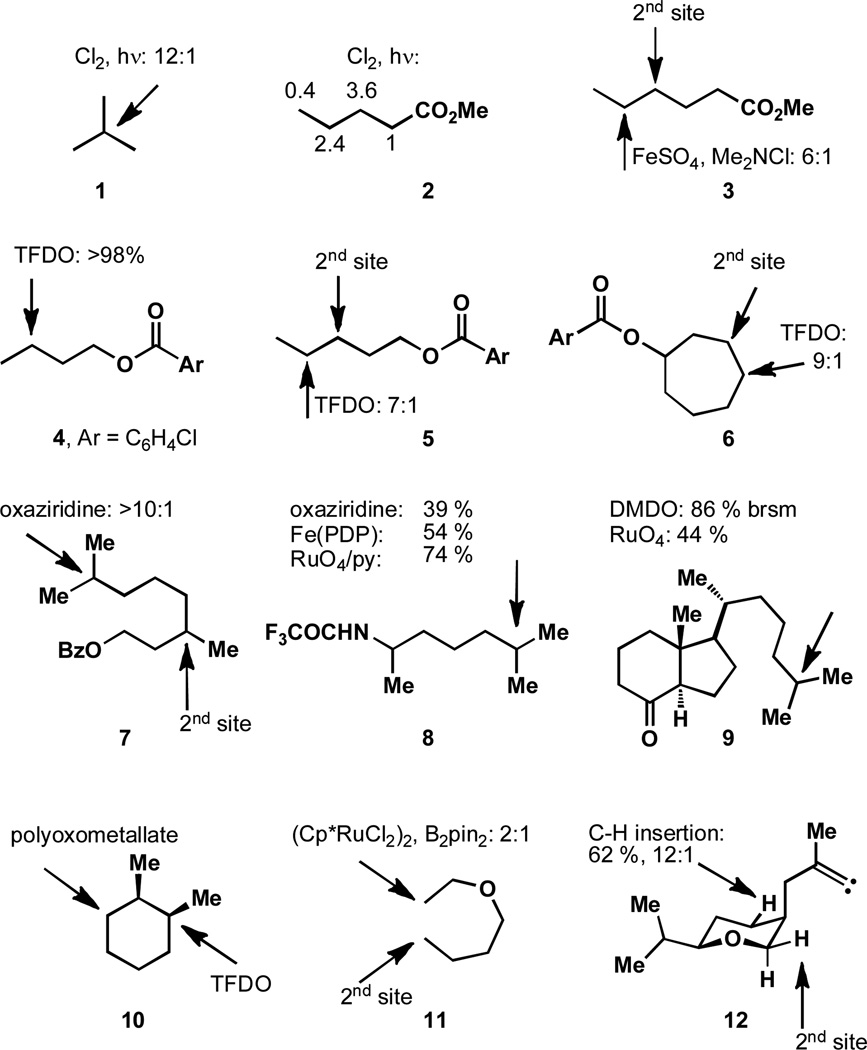
Figure 1 illustrates the effects of electron donating and withdrawing groups through a number of examples. In compound 1 it is shown that the most electron rich position is oxidized (i.e. the tertiary position). For non-metal insertion pathways, the reactivity trend is typically: tertiary > secondary > primary and has been understood as such for some time.[9] The effect of electron-withdrawing groups is also a well-studied phenomenon and in the radical chlorination of 2, the effect of the ester functionality is shown.[10] More selective radical halogenation reactions have subsequently been developed, as is the case for the oxidation of compound 3.[11]
Figure 1.
Electron-Withdrawing groups influence the site of C–H activation.
Similarly, compound 4 has three methylene units and the least electron deficient position is the one that is oxidized.[12] While the methyl group is more distal to the EWG, it has one fewer electron donating alkyl groups and is therefore left unscathed. As with compounds 5 and 6, oxidation occurs selectively at the most distal site, but a decreasing sensitivity to the EWG is observed when the reactive site is four instead of three bonds away (i.e. oxidation of a δ position rather than a γ position as in 4). Likewise compounds 7 and 8 each have two tertiary sites and the one that is more distal is preferentially oxidized.[13–15] Discrimination between two electronically dissimilar tertiary sites can also be seen in 9,[16–17] but it should be stated that the difference in steric environment may also synergistically contribute to the observed selectivity in this case.
The oxidation of alkane substrates is well studied and here we present the specific case of cis-1,2-dimethylcyclohexane, 10. Depending on the oxidation system employed, selectivity for one of the methines or one of the methylenes can be obtained. While tertiary positions are the most electron rich, the secondary positions are less sterically hindered and selectivity can be influenced by reagent selection. For example, oxidation by small reagents occurs selectively at tertiary positions (e.g. TFDO),[18] while large reagents exhibit reduced rates of oxidation at tertiary sites (e.g. a polyoxo metallate)[19] and selectivity for methylenes can be obtained. Similar trends have been observed on other substrates with other reagent systems.
Certain metal-mediated C–H activation reactions (wherein the metal is bound to the substrate) show distinct reactivity from other methods such as metal carbenoid and nitrenoid insertions. For these mechanistic pathways, the reactivity order of oxidation is, with considerable exception, the inverse of what is seen in non-metal mediated oxidation reactions: primary > secondary >> tertiary.[5d] This trend is largely reflective of the dominance of steric interactions in determining C–H bond selectivity. Additionally, in some instances this trend may be the result of an increased lability of a C–H bond that has a geminal C–H bond participating in an agostic interaction – an impossible situation in methine oxidation.[20] This is highlighted with the ruthenium-mediated oxidation of compound 11, wherin the primary positions are the ones that are oxidized. Furthermore, it is shown that the more electron deficient site (the one closer to the heteroatom) is the one that is oxidized first.[21]
This reactivity contrasts with metal nitrenoid and carbenoid insertion reactions, which proceed with the following order of reactivity: tertiary > secondary >> primary. Since these reactive intermediates proceed as electrophilic reagents, stabilization of partial positive charge in the transition state at the reacting site (by electron-donating groups) results in an increased rate of insertion. Although it should be stated that the judicious choice of ligands is of paramount importance in determining chemoselectivity, as it is sometimes possible to invert selectivity trends.[22a–d] A discrete vinyl carbene intermediate, 12, responds to electronic stimuli similarly; the inductive effect of oxygen functionality has been uniquely demonstrated in this instance.[22e] While geometric considerations limit the positions that can be oxidized, the electronegative oxygen functionality drives the oxidation event to the less deactivated C–H bond. Remarkably, since the axial C–H bond vicinal to the oxygen atom is inaccessible, this highly activated position is not oxidized. The selectivity factor that activates such positions is discussed in the next section.
2. Conjugation and Hyperconjugation
Hyperconjugative effects are another form of electronic activation or deactivation of alkane oxidation. While this force has a less pronounced effect than inductive effects, hyperconjugation can impart significant biases in certain systems. For example, it has been found that cyclopropanes bias the site of oxidation to their vicinal position in a marked way.
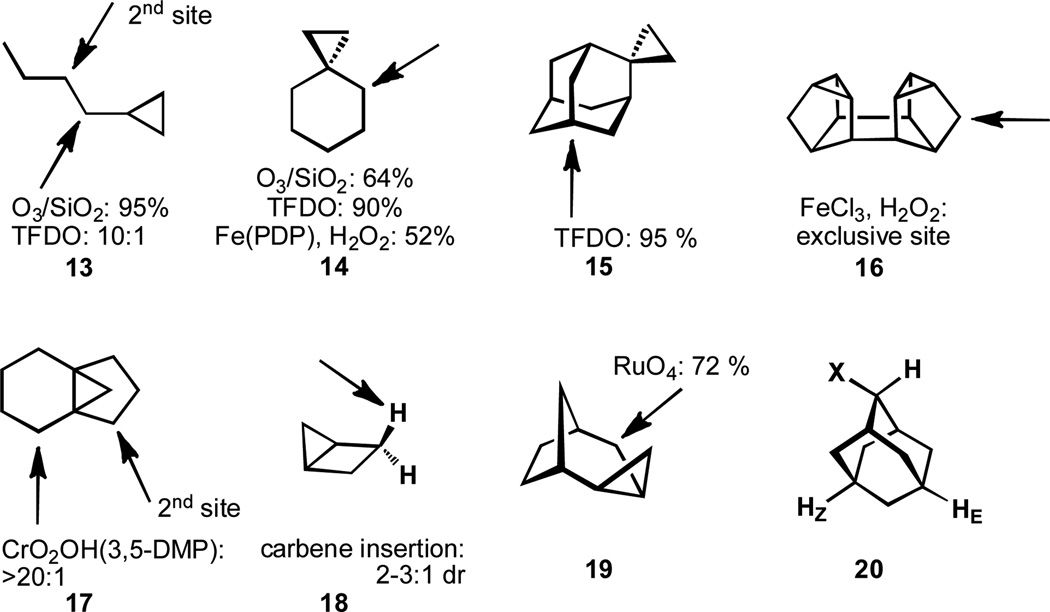
Although most non-metal electrophilic reagents, such as TFDO, typically prefer oxidation of tertiary sites, compound 13 (similar to other cyclopropanes) is oxidized at the position vicinal to the cyclopropane rather than on the cyclopropane itself (Figure 2).[23a–b] Oxidation of 14 also occurs at the position vicinal to the activating cyclopropane ring.[23]
Figure 2.
Hyperconjugation by cyclopropanes activates neighboring C–H bonds
This activating effect requires orbital overlap between the cyclopropane C–C σ bonding orbital of the cyclopropane and the neighboring C–H σ antibonding orbital.[24] In instances wherein this geometric configuration occurs, delocalization of electron density from the bent bonds of the cyclopropane to the neighboring C–H antibonding orbital renders an oxidation event with an electrophilic reagent more facile. Cyclopropanes are well-suited to this mode of activation due to their high-lying highest occupied molecular orbital (the C–C σ bonding orbital of the cyclopropane has considerable π character and is approximately sp5 in nature), rendering cyclopropanes good electron donors.[24] These same geometric requirements also hold true for other activating groups, such as the nonbonding electrons on an ethereal oxygen atom (21 in Figure 3).
Figure 3.
Hyperconjugation by heteroatoms activates neighboring C–H bonds.
The strict orbital requirements of hyperconjugation are clarified by the oxidation of 15, wherein the proper orbital overlap is lacking at the positions vicinal to the cyclopropane.[23b] This position is now deactivated likely as a result of the steric hinderance imposed.[25] An interesting exception to this general trend is the oxidation of Binor S (16), by one of the Gif systems which occurs exclusively at one of the methylene positions in 8% yield at 10% conversion.[26–28] A subtle example of the orbital requirements of this activating effect can be seen in 17; wherein the oxidation of the cyclohexane ring is preferred relative to that of the cyclopentane. This selectivity may be the result of multiple factors; however, it is possible that the C–C σ bond of the cyclopropane is better situated to donate into the C–H σ antibonding orbital of the cyclohexane, resulting in a high degree of selectivity.[29] The stereoselective oxidation of 18 further illustrates this stereoelectronic effect that exists for cyclopropanes to selectively activate vicinal C–H bonds. Stereoselective activation of C–H bonds vicinal to cyclopropanes can be observed in a substrate such as 18 (diastereoselectivities of 2:1 to 3:1, depending on the carbene employed[30] and a more complicated substrate 19 shows a similar preference for the C–H bond activated by the cyclopropane.[31]
The degree to which hyperconjugation can influence the course of C–H oxidation depends on how electron-withdrawing or electron-donating a substituent is. This trend has been quantified by comparing the relative ratios of oxidation products for 1-substituted adamantanes, 20.[32] Due to orbital considerations, the long-range hyperconjugative effects from the carbon-X bond are more pronounced for the carbon-hydrogen-E bond than for the carbon-hydrogen-Z bond. Thus, the electronic influence at HE varies with the substituent, whereas HZ remains relatively unchanged. This effect follows a linear relationship with the substituent parameters of the substituents X (ρ = −2.3).[32]
Although oxygen is an electronegative atom that can exert a withdrawing inductive effect, the nonbonding electrons on oxygen may also donate electron density into neighboring C–H bonds through hyperconjugation. The nonbonding electrons on oxygen are highly activating to positions with the proper orbital alignment and the overall result is the selective oxidation of these positions.
Numerous oxidation systems can affect oxidation of tetrahydrofuran or tetrahydropyran (21) and a few examples are shown in Figure 3.[23c,33–34] Selectivity for the position adjacent to oxygen is easily obtained. Interestingly, carbenoid insertion by 23 onto 22 readily occurs with Rh2(S-DOSP)4 for the position α to the oxygen atom, rather than the benzylic position of 22.[35] In the case of 24, nitrenoid insertion occurs at the benzylic position, in part due to delocalization of the oxygen atom’s nonbonding electrons into the aromatic ring decreasing the ability of this lone pair to activate the vicinal C–H bond.[36] While the oxidation of carbinols is a notoriously low-energy process, enantioselective processes remain challenging: a notable example is the oxidation of racemic 25 by palladium-sparteine or manganese salen catalysis and can occur with high enantioselectivity.[37] It is noteworthy that the selective oxidation of this position is also the result of prior coordination of the alcohol functional group to the transition metal center. In a similar vein, nitrogen atom lone pairs may also influence the course of site-selectivity in C–H oxidation. The oxidation of nicotine (26) to cotinine can be affected by a number of reagents as a result of hyperconjugation from the pyrrolidine nitrogen atom’s lone pair to the neighboring C–H bond.[38–39]
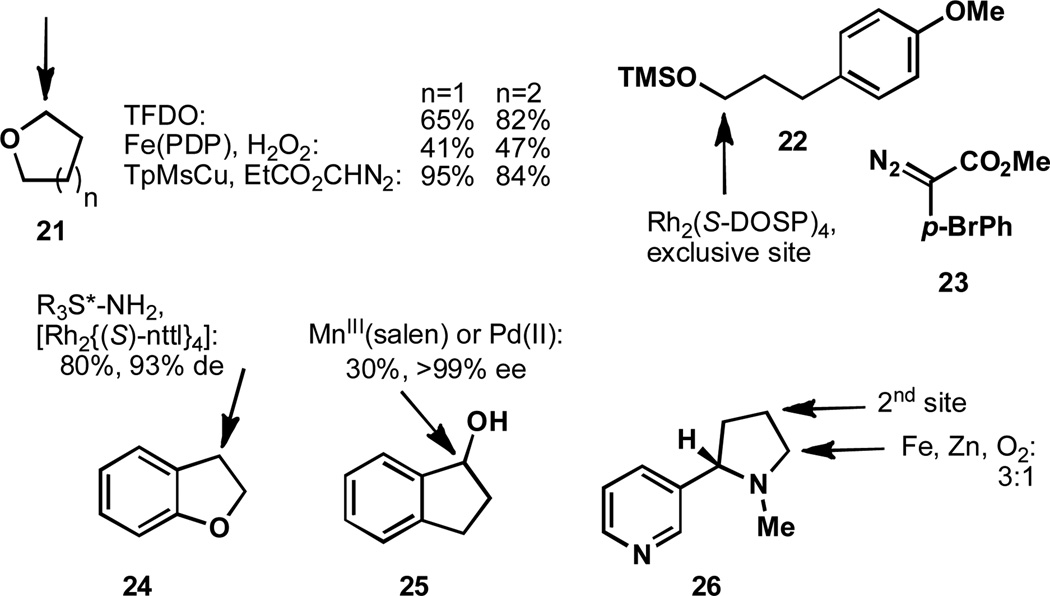
Much as cyclopropanes and heteroatoms can serve as activating groups through hyperconjugation, the oxidation of unsaturated systems typically exhibits increased rates of oxidation relative to their saturated counterparts (Figure 4).[40] An illustration of this point is that singlet oxygen readily oxidizes olefinic substrates via an ene process, but sp3 C–H bonds are relatively unreactive towards singlet oxygen.[40b] The oxidation of geranyl acetate, 27, is selective for the more electron rich olefin in the selenium dioxide mediated oxidation.[41] The copper-catalyzed peroxide oxidation of olefins to yield peresters is known as the Kharasch reaction and one such example is shown with compound 28.[42] In this case the positional selectivity is governed by the steric bulk of the tert-butyldiphenyl silyl protecting group, evidenced by the lack of selectivity for the less sterically demanding case of the corresponding tert-butyldimethyl silyl β-lactam. Further examples of sterics influencing the course of C–H oxidation will be shown in the next section. It has also been shown that palladium and rhodium catalysts may activate enones, as in 29.[43] A creative use of the Kharasch reaction can be seen by the cyclopropane opening of 30. It can be imagined that after an allylic radical is formed at C11; this species isomerizes to open the vicinal cyclopropane and the primary radical is then trapped to give an oxidized product.[44]
Figure 4.
Oxidation of allylic and benzylic systems.
The formation of π-allyl palladium complexes from olefins has allowed for allylic amination of these substrates, such as 31 (with olefin transposition).[45] The interception of meso π-allyl palladium complexes (derived from substrates such as 32) with nitronates has allowed for the enantioselective synthesis of enones.[46] The oxidative cyclization of 34,[47] is another example of allylic oxidation, here forming a C–C bond. For transition metal-mediated formation of π-allyl complexes, the metal center first coordinates to the olefin, rendering C–H insertion more facile.
The ready oxidation of benzylic systems is a well-studied phenomenon and as a representative case, the oxidation of 8-methylquinoline, 35, is shown. This may be affected by treatment of 35 with selenium dioxide[48a] or by metal-mediated pathways in either a stoichiometric[48b–c] or catalytic[49] manifold. It is notable that the transition metal-mediated oxidations are enabled by initial coordination to the quinoline nitrogen and should be considered directed oxidations (See Section 5 for additional examples).[50] Despite the many advances in C–H activation, new systems that can oxidize substrates while avoiding hyperconjugatively activated functionalities is a remaining challenge in the field and would greatly benefit the synthesis of complex molecules.
3. Sterics
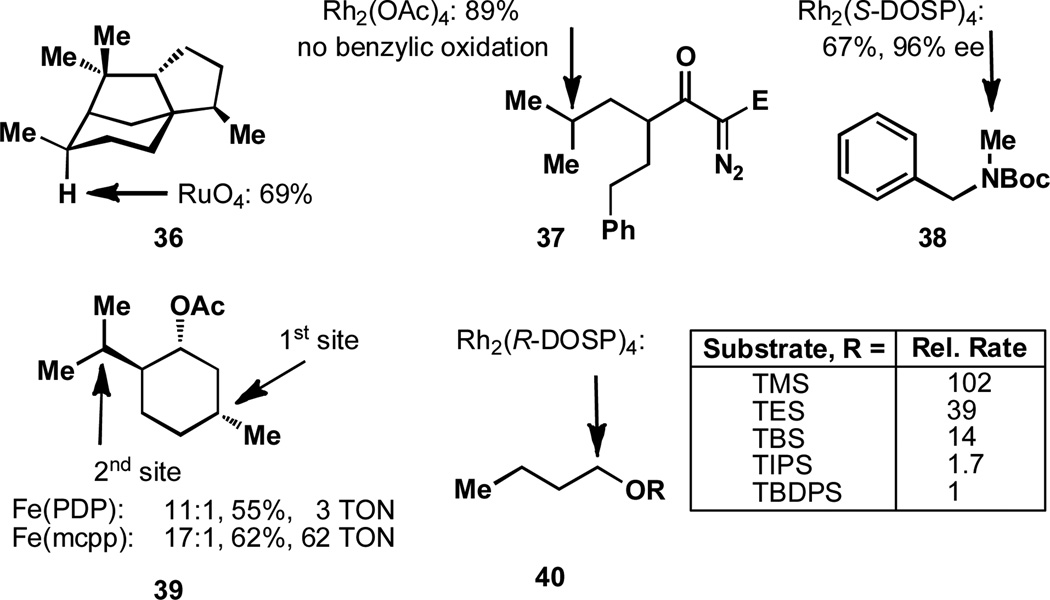
A reduction of rate of C–H oxidation is commonly observed for hindered C–H bonds. An excellent example of how hindered sites are not oxidized comes from the oxidation of cedrane, (36), with a ruthenium system (Figure 5).[51] While there are three tertiary sites present in this molecule, a high yield for only one of the sites is observed. This is attributed to the fact that the other two positions are neopentyl and bis-neopentyl. A number of other examples where steric effects play a significant role in determining selectivity can be found in the case studies (Section 7).
Figure 5.
Steric hindrance effects the rate of C–H oxidation.
While steric hindrance to reagent approach does exert a significant bias, other factors can overcome this influence. For example, in the intramolecular C–H oxidation of substrate 37, C–H insertion occurs selectively at the tertiary site, rather than the benzylic position.[52–53] Although the benzylic position is secondary and less hindered, the methine is oxidized, possibly as a result of the more electron rich nature of this position. The authors speculate that delocalization of the C–H σ bond into the aromatic system results in a reduced availability of electron density. Furthermore, it could be said that the tertiary position is more electron rich as a result of the greater ability of the two methyl groups to donate electron density relative to a hydrogen and an aromatic ring.[52] Another important factor in this cyclization reaction is that a favorable Thorpe-Ingold effect may bias the C–H insertion to the tertiary position. In an intermolecular case of rhodium catalyzed C–H insertion, the activated methyl group in 38 is oxidized in preference to the benzylic position. In this case the authors believe that the selectivity observed is primarily the result of the less hindered environment at the methyl group.[54]
The iron-catalyzed oxidation of the menthol derivative, 39, has demonstrated the importance of sterics in dictating positional selectivity,[14,55] which is feasible due to a discrete number of mechanistic scenarios.[7m] It was shown that the tertiary position on the cyclohexyl ring is oxidized in preference to the isopropyl group. The rationalization for this selectivity is that the cyclohexyl tertiary site is the less hindered one, since both tertiary positions are in nearly identical electronic environments. It should be stated that the rigidity of the cyclohexane ring relative to the isopropyl group may also contribute in this instance. As will be seen in Section 5, such inherent selectivity can be completely reversed using directing groups.
One of the most dramatic examples of the effect of the steric environment on the rate of oxidation comes from the Rh2(R-DOSP)4 oxidation of 40.[35] A number of different silyl ethers are oxidized and it is clearly observed that the more hindered the C–H bond is the slowest. In fact, the TMS-protected alcohol is oxidized 102 times faster than the TBDPS derivative. Clearly, sterically hindered positions are oxidized more slowly than their less hindered counterparts. Future challenges in this area are the development of reagents and catalysts that can more generally take advantage, or perhaps override, subtle nuances in steric effects.
4. Strain Release
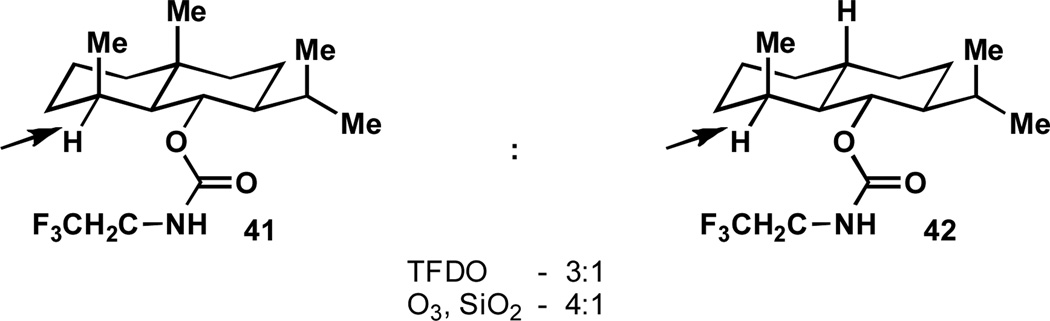
In the middle of the twentieth century, a theory to explain the relative rates of oxidation of axial and equatorial alcohols was put forward to understand why axial alcohols were oxidized with greater rates. Eschenmoser hypothesized that the elimination of A1,3 strain in the transition state for the case of axial alcohols results in an increased rate of oxidation.[56] This theory of strain release has also been recently advanced to the realm of C–H activation.[57]
This theory was supported by the relative rates of oxidation of the substrates 41 and 42 (Figure 6). While substrate 41 has a 1,3-syndiaxial interaction between two methyl groups, substrate 42 has a hydrogen atom in the place of a methyl group. Thus, the A1,3 strain in 42 is considerably lower than that of 41, resulting in a slower rate of oxidation of 42 (by a factor of 3 to 4). More commonly recognized factors do not account for this difference in selectivity, including inductive effects, hyperconjugation, or sterics. This example clearly shows the effect of strain release on the rate of oxidation and selectivities observed in other instances may also be attributed to this phenomenon.
Figure 6.
Strain release may be an important factor in controling site-selectivity in C–H activation reactions.
5. Directed Oxidations
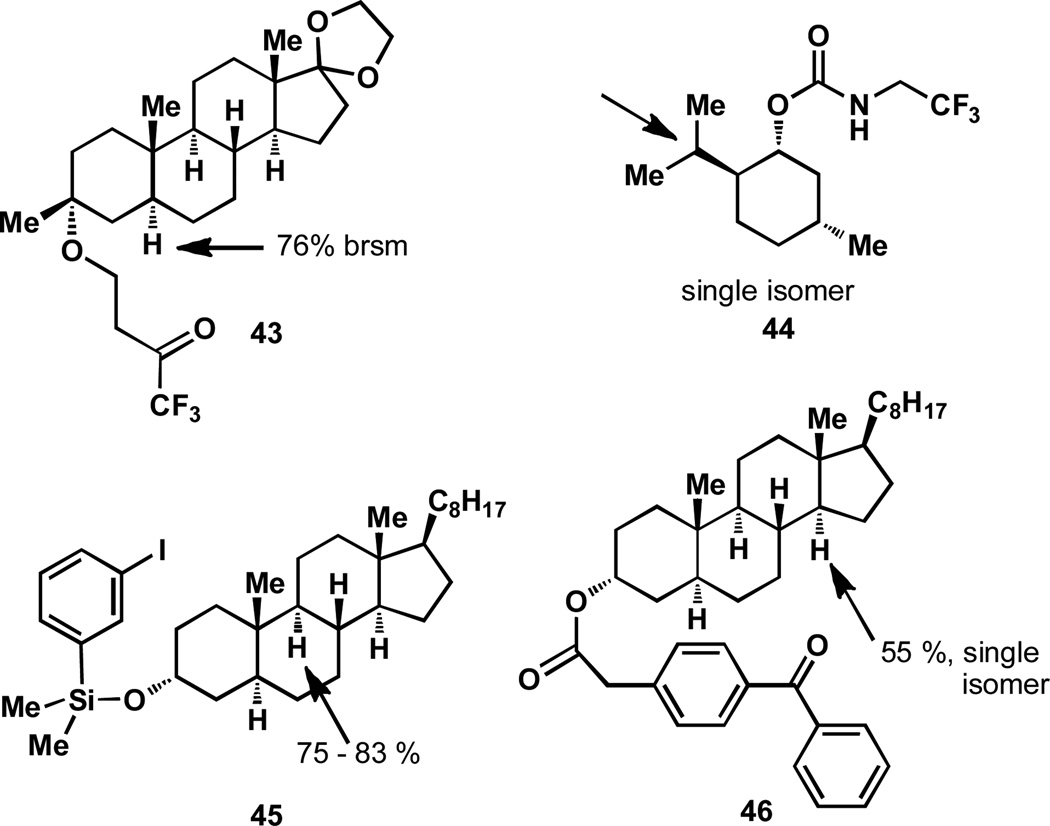
In a complex system there will likely be functionality that is prone to oxidation even though a specific C–H activation event is desired. For example, substrate 43 contains a ketal group that is prone to oxidation[33b] as a result of hyperconjugative activation by the oxygen atoms (see section 2 for further explanation), yet this substrate may be oxidized elsewhere, likely as a result of the intramolecular nature of this oxidation reaction.[58]
Directed oxidations represent an excellent opportunity to functionalize a particular C–H bond in the presence of opposing steric and electronic factors. In essense, directing groups are an easy way to override inherent substrate preferences. However, these strategies often require functional group manipulations to both install and remove the directing group. In the case of 43, a tethered electron deficient ketone is used to direct the oxidation event away from the ketal with remarkable selectivity (Figure 7).[58] An in situ dioxirane formation and subsequent intramolecular oxidation results in the observed selectivity.
Figure 7.
Directed oxidations offer an opportunity to effect positional selectivity.
Another example of inverting the selectivity of a C–H activation is the use of a trifluoroethyl carbamate directing group on menthol, (44). By employing the Hofmann–LöffleRFreytag reaction with this carbamate, the selective functionalization of the isopropyl group, rather than oxidation of the tertiary position ipso to the methyl group (compare to 39, Figure 5),[59] can be achieved.
The concept of “designer” directing groups for C–H activation was pioneered by Breslow and an early example is shown with substrate 45.[60] In this case, a silyl ether is used as a tether, a functional group that is convenient for removal at a later point. The positional selectivity toward the B/C ring junction is the result of geometric considerations of the tether, rather than the inherent reactivities of the C–H bonds present in the oxidized substrate. This point is corroborated by a reversal of selectivity in the oxidation of 46, wherein a longer tether results in selective oxidation of the C/D ring junction.[61] Breslow has reported a number of other strategies to impart chemoselectivity with a wide array of directing groups.
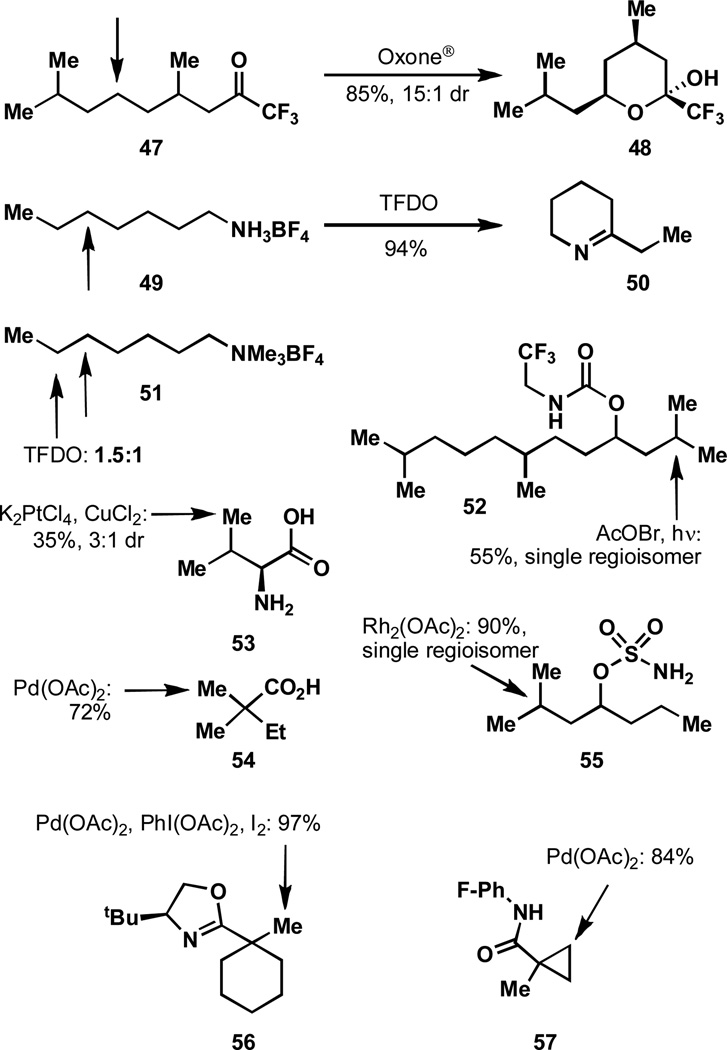
As was shown with compound 43 (see Figure 7), trifluoromethylketones may act as directing groups for intramolecular dioxirane oxidations. In the case of 47 (Figure 8), geometric considerations result in oxidation of a methylene, rather than a methine, thereby oxidizing the electronically disfavored position.[62]
Figure 8.
A number of directing groups may be employed in C–H activation.
Dioxirane mediated C–H activations may also be directed by the force of hydrogen-bonding, as illustrated by the selectivities observed for the oxidations of 49 and 51 in Figure 7.[63] The tetrafluoroborate salt of a protonated amine is an EWG and thus oxidation is directed away from this functionality. However, in the TFDO mediated oxidation of substrate 49, selectivity is not observed for the most distal methylene position. Rather oxidation is directed five positions away and after the intermediate ketone condenses with the pendant amine the didehydropiperidine 50 is formed. It is believed that hydrogen bonding of the dioxirane to the protonated amine results in the high degree of selectivity for a position that is otherwise not electronically or sterically favored. To support this hypothesis, oxidation of analog 51, which lacks the ability to donate a hydrogen bond, was performed. It was found that a mixture of oxidation products disfavoring the methylene five positions away was obtained (selectivity for the ζ versus ε position is 1.5 to 1).
Carbamates may also direct oxidation by homolytic fragmentation of an intermediate N-halogen bond, as was seen in compound 44 (see Figure 7). In this case, the selectivity for a seven-, rather than eight-membered transition state is observed, as demonstrated by the selective oxidation of 52.[59] Thus, site selectivity for the more electron deficient site is obtained as a result of the trifluoroethylcarbamate directing group. This was demonstrated to be a beneficial strategy in the total synthesis of the eudesmane sesquiterpenes.[64]
The oxidation of the methyl group in substrate 53 is the result of a number of different factors. It is postulated that the most dominant factor in this oxidative cyclization concerns initial bidentate chelation of platinum to the amino acid functionality. This results in a geometrically imposed constraint on which position may be oxidized, reflected by the preferential formation of products containing five-membered rings.[65a] The diastereoselectivity of the lactone formation (by desymmetrization of the isopropyl group) may be the result of preorganization to a lower energy transition state, corresponding to the observed anti diastereoselectivity that occurs in the carboxylic acid directed C–H activation. Similar trends in five-membered ring formation during metal-mediated carbenoid insertion events have been observed.[65b]
While many directing groups for C–H activation suffer from less than ideal synthetic utility (because these must be installed and later removed), the employment of carboxylic acids is a rapidly developing and synthetically useful area.[66] An excellent example of the ability to use carboxylic acids as directing groups for aliphatic C–H bonds is demonstrated in the acid substrate 54.[66a] A protocol was developed to access the Heck and Suzuki coupling pathways to execute olefination and arylation reactions. The chemoselectivity of the C–H cleavage step in this case may be the result of a kinetic preference for the formation of an intermediate five-membered palladacycle. Additionally, the selectivity for a methyl group, rather than a methylene, may arise as a result of the greater steric accessibility of primary over secondary positions.
Sulfamate esters, including 55 in Figure 8, can also direct oxidation events, likely via intermediate iminoiodinanes in the presence of a rhodium catalyst. Interestingly, sulfamate esters generally prefer the formation of six-membered ring products, while analogous reactions to form cyclic carbamates prefer the formation of five-membered rings. The utility of these types of directing groups has been demonstrated in a number of complex settings for the purpose of total synthesis.[67]
Another example of a directed oxidation can be found in the palladium-catalyzed iodination of 56.[68] In this case the directing group allows for C–H insertion under mild conditions and situations where this strategy could be used to influence site-selectivity can easily be imagined. Considerable research has further advanced the utility of 2-oxazolines for directed C–H activation.[69]
In an olefination of 57 catalyzed by palladium, selectivity is observed for the cyclopropane rather than the methyl group.[70] This contrasts with the selectivity between a methyl group and a cyclohexyl ring, as in 56. Many other directed oxidations are known and these shown here are only a sampling of what is possible.[71] The many challenges in this area leave much to be discovered.
6. Activation of sp2 Centers
Although distinctly different in nature, the chemoselectivity observed in C–H activation reactions of sp2 centers nevertheless follows similar trends as their sp3 counterparts. The absence of three-dimensionality reduces the factors that may affect positional selectivity to steric hindrance, inductive effects or resonance stabilization. In the case of transition metal-mediated directed oxidations, the conformational effects resulting from the intermediate metallacycles have a critical impact on chemoselectivity.[5] While the oxidation of benzene derivatives has a rich history dating back to early studies on electrophilic aromatic substitution and the Heck reaction,[72] research centered on metal-mediated catalytic methods is a vibrant area of investigation.
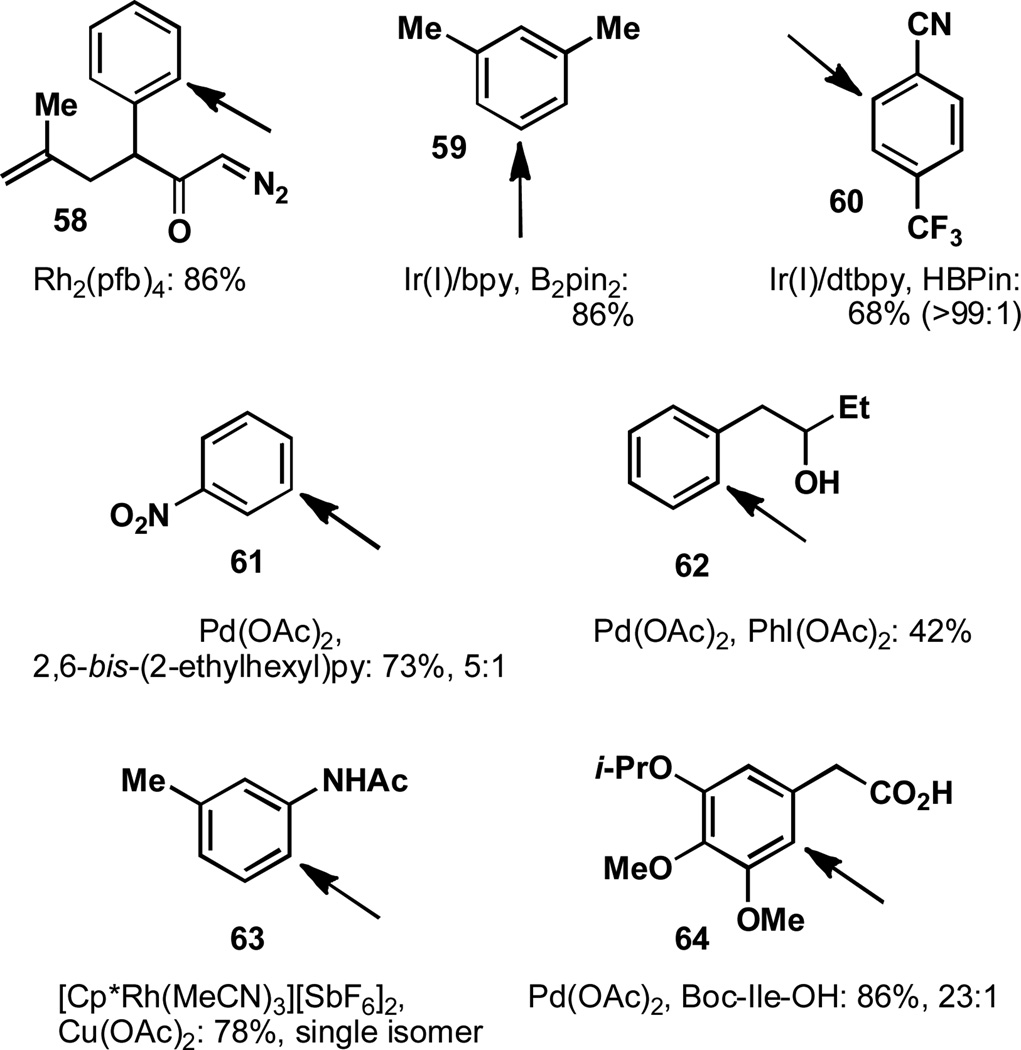
The decomposition of 58 in the presence of Rh2(pfb)4 is one example of obtaining selectivity for aromatic C–H insertion (over cyclopropanation) and catalyst selection was critical in this case (Figure 9).[73] Positional selectivity about the aromatic ring is dictated by the tethered diazoketone. In some intermolecular cases, selectivity for the least sterically-hindered position on an aromatic ring has been obtained by the use of iridium (I) catalysts. In the case of 59, this results in meta borylation,[74] while in the case of 60 borylation occurs extremely selectively ortho to the nitrile, rather than ortho to the bulkier trifluoromethyl group.[75–77]
Figure 9.
C–H activation of sp2 centers presents unique challenges.
Olefination in a meta-selective fashion with palladium (II) has been demonstrated through the use of 2,6-bis-(2-ethylhexyl)pyridine as a ligand.[78] The oxidation of 61 occurs with 5:1 selectivity, relative to the para position. There are numerous instances of the use of directing groups to oxidize the ortho position of substituted aromatics and only a few of these cases are highlighted below. Remarkably, palladation can be directed by secondary hydroxyls (62) even though this functionality is prone to oxidation.[79] In the oxidative annulation reaction between acetanilide 63 and 1-phenyl-1-propyne to form an indole, excellent regioselectivity is observed for C–H activation of the less hindered C–H bond with [Cp*Rh(MeCN)3][SbF6]2.[80]
The ability to select between electronically similar sites with only subtle differences in steric environment has been achieved through palladium-catalyzed C–H activation with protected amino acids as ligands (64).[81] In this instance, almost no selectivity was observed in the absence of ligands and a preliminary mechanistic investigation and improvement to these original conditions has recently been disclosed.[82]
Furthermore, there are also many beautiful examples of activation of the sp2 and sp3 C–H bonds in heterocyclic substrates and this subject has recently been reviewed.[83]
7. Case Studies
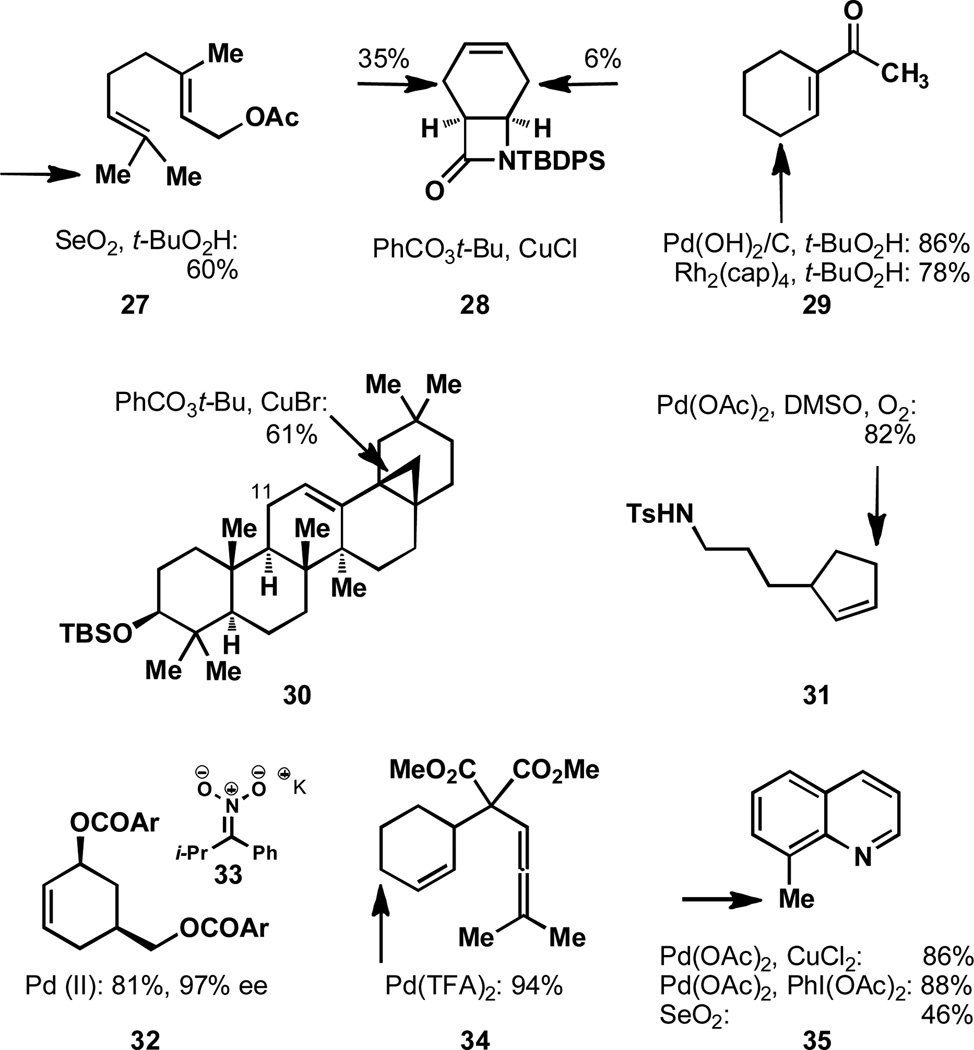
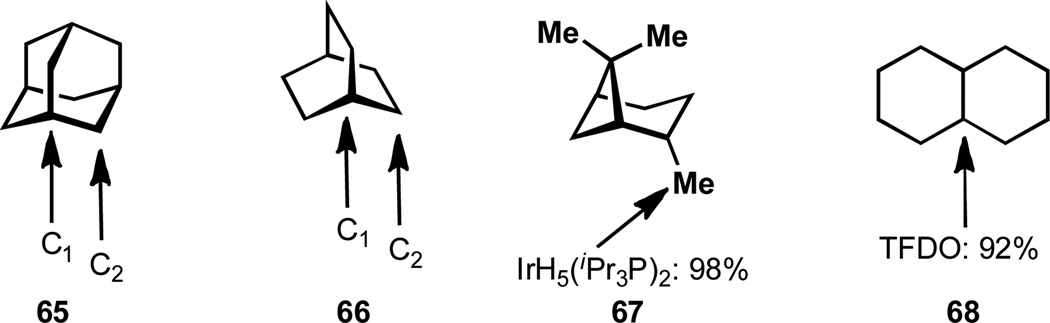
The oxidation of bicyclic systems represents an interesting case of site-selectivity in C–H activation. The well-known propensity of adamantane, 65, to undergo oxidation is an interesting peculiarity that has made it a yard-stick for C–H activation and numerous examples of its oxidation have been documented.[84] The ease of oxidation of the tertiary bridgehead position of this substrate has resulted in a common misperception that the oxidation of bridgehead positions (C1) in general is a relatively simple task. The reduced selectivity observed for bicyclo[2.2.2]octanes (66)[18] and the reversal of selectivity observed for bicyclo[3.1.1]heptanes[85] (67) and bicyclo[2.2.1]heptanes (not shown)[84a] illustrates the challenges associated with oxidizing bridgehead positions. The reactivity of a number of other unique polycyclic systems has been studied and it is worth noting that these selectivities are also reagent dependent.[86] The oxidation of ring junctions that are not bridgehead positions does not pose the same degree of difficulty. For example, the oxidation of cis- or trans-decalin (68) generally proceeds at the most electron rich tertiary position.[18]
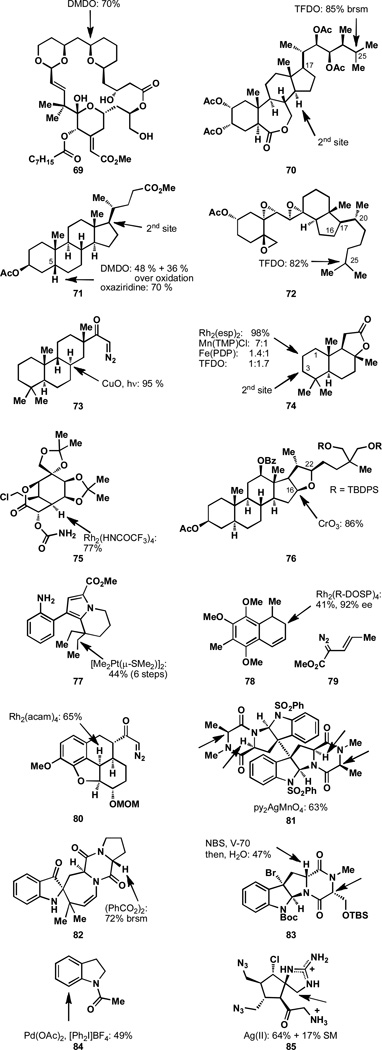
The selectivity observed in extremely complicated systems cannot always be rationalized since detailed structural information about solution-state conformation is frequently unavailable. Even though some solution structural information is known for bryostatin analogue 69,[87–88] rationalizing the observed selectivity is difficult (Figure 11). While it could be said that the most electron rich pyran is oxidized at the hyperconjugatively activated axial C–H bond, other factors certainly play a role. The unusual tolerance of olefins and a primary alcohol in this transformation and the pronounced selectivity for the C–H bond that is oxidized likely results from steric effects imparted by the structural complexity and its global conformation.
Figure 11.
Additional examples of C–H oxidation.
In the case of the B-ring seco steroidal substrate 70, the superb selectivity observed is the result of a number of different factors. While there are twelve methines present in this substrate, one position is selectively oxidized.[89] The most dominant effect in this case is the result of the inductive effect of the acetylated alcohols and the lactone. The electron-withdrawing nature of these functionalities prevents oxidation of the nearby positions, leaving only a few positions that could potentially be oxidized. Steric effects then eliminate the possibility of oxidation at C17. The difficulty associated with oxidation of this position is well-documented and is seen in subsequent examples in Figure 11. Since methylenes and methyl groups are not preferred sites in dioxirane-mediated oxidations, the methine at C25 is oxidized selectively.
The oxidation of 71 illustrates the propensity of equatorial C–H bonds to be activated, since the two equatorial positions are the ones oxidized, even though there are a number of axial C–H bonds that are more distal to the EWGs.[90–91] Even though C17 is extremely hindered, some oxidation of the initial product is observed at this position rather than one of the axial C–H bonds. In the case of 72, a combination of the electron-withdrawing influence of the epoxides and the sterically hindered C17 and C20 positions, results in selective oxidation at C25.[92] In this example, there is a tertiary position which is both distal from EWGs and relatively unhindered and consequently this position is selectively oxidized with TFDO.
The tethered oxidation of substrate 73 represents a complicated case wherein a directing group allows for the desired oxidation event.[93] It might be predicted that the A ring would otherwise be oxidized on this substrate due to the electron-withdrawing nature of the acyl group on the C ring. Yet, a highly selective transformation occurs due to the geometric requirements of the intramolecular oxidation reaction.
Sclareolide, 74, has recently become a popular substrate to probe C–H activation methodologies. It was observed that amination in the presence of Rh2(esp)2 occurs with exceptional selectivity for the C2 position.[57] This is primarily the result of the electron-withdrawing lactone functionality directing oxidation away from the B ring. Likewise the C1 position is disfavored due to its proximity to the lactone, leaving only two more positions. A combination of the fact that C2 is less hindered than C3 and that oxidation of C2 results in the relief of two 1,3-syndiaxial interactions (albeit between a hydrogen and methyl groups), results in its selective oxidation. Although a 7:1 (C2:C3) selectivity has been observed in the chlorination of 74,[94] a 1.4:1 selectivity was found for the hydroxylation of 74 by Fe(PDP) and hydrogen peroxide,[23c] and TFDO oxidation afforded a ratio of 1:1.7,[95] highlighting the present difficulties of selective C–H hydroxylation.
En route to the total synthesis of tetrodotoxin, a selective oxidation of 75 was demonstrated.[96] In this case, a directing group allowed for the selective amination of the bridgehead position, despite the presence of a number of other tertiary and hyperconjugatively activated ethereal positions. Even though this position is deactivated by the nearby lactone functionality, an oxidation event is affected with a high degree of selectivity.
Another example of oxidation of a steroidal substrate by CrO3/Bu4NIO4, 76, illustrates that a number of factors are at play in the oxidation of complex systems.[97] In this case, EWGs on the A and C rings direct oxidation away from these sites, while oxidation is promoted at the α positions of the tetrahydrofuran ring. Oxidation selectively occurs at the C16 position even though the other α position (C22) is more electron rich when considering inductive effects. The steric influence of the two TBDPS ethers effectively shields the C22 α positions and this hypothesis was confirmed by the unselective oxidation of the corresponding TMS ethers (consistent with the steric effect noted in Figure 5).
In a rare example of a dehydrogenation reaction applied to total synthesis, the stoichiometric oxidation of substrate 77 was achieved in 44% yield over a six-step process, including the installation and removal of a directing group to allow for complexation of a platinum reagent.[98] An excellent example of allylic oxidation comes from the oxidative kinetic resolution of racemic 78 by the rhodium carbenoid formed from 79. After allylic oxidation occurs a Cope rearrangement ensues providing the rearranged product in 41% yield and 92% ee.[99] An example of a rhodium-mediated diazoketone decompostion and C–H insertion is shown with substrate 80. Selective insertion occurs in this case, despite the presence of a number of other reactive sites.[100]
A silver-mediated method of hydroxylation was recently demonstrated to be an effective means to oxidize 81 at the four α positions of the diketopiperazines.[101] Similarly, the oxidation of diketopiperzine 82 has been accomplished using benzoyl peroxide. Notably this oxidation occurs selectively in the presence of an olefin.[102] Yet another example of diketopiperazine oxidation is shown with the hydroxylation of 83,[103] which proceeds with NBS and the radical initiator V-70.[104] Arylation of 84 is possible by a directed palladation and it was noted that the remainder of the mass balance was starting material.[105] Another silver-mediated method was also used to hydroxylate 85 in the context of the total synthesis of the axinellamines.[106] This oxidation reaction is highly selective for hemiaminal formation, rather than spiro-2-aminoimidazolinone formation. This method has also been applied to the total syntheses of the massadines and palau’amine.[107]
8. Conclusion
While numerous other excellent examples of selective C–H activations have been reported, it is hoped that the few examples discussed here will serve as a useful guide and a springboard for interpreting the chemical expression of C–H bonds. C–H activation is a century old field that has seen an incredible resurgence in the past two decades as a result of the enormous benefit that such reactions can provide in synthesis. The well-known trends summarized in this review serve to highlight some of the exciting challenges that remain. For example, what yet undiscovered processes will exploit the inherent reactivity of C–H bonds to reach exquisite levels of selectivity? How can this reactivity be overridden through the use of reagents and directing groups? How can organic chemists harness the selectivity factors discussed herein to complex problems in total synthesis? Through countless studies over the past century, the “thoughts” and “stories” of C–H bonds have been illuminated – giving chemists the ability to understand factors that affect positional selectivity and inherent preferences in C–H activation.
Figure 10.
Unique geometries of bicyclic systems results in varied selectivity.
Acknowledgments
Funding for this work was provided by Bristol-Myers Squibb and the NIH/NCI (CA134785).
Biographies
Phil S. Baran was born in New Jersey in 1977 and received his undergraduate education from New York University with Professor David I. Schuster in 1997. After earning his Ph.D. with Professor K. C. Nicolaou at The Scripps Research Institute in 2001 he pursued postdoctoral studies with Professor E. J. Corey at Harvard University until 2003 at which point he began his independent career at Scripps, rising to the rank of Professor in 2008. His laboratory is dedicated to the study of fundamental organic chemistry through the auspices of natural product total synthesis.
Timothy Newhouse received his B.A. in chemistry from Colby College (2005), conducting research under the supervision of Professor Dasan M. Thamattoor. Under the guidance of Professor Phil. S. Baran, he earned his Ph.D. in chemistry from The Scripps Research Institute (2010). He is now conducting postdoctoral studies in the laboratories of Professor E. J. Corey at Harvard University.
References
- 1.For representative examples and the importance of C–H activation in chemical synthesis, see: Gaich T, Baran PS. J. Org. Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812.
- 2.Newhouse T, Baran PS, Hoffmann RW. Chem. Soc. Rev. 2009;39:3010–3021. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For an overview with respect to taxol, see: Ishihara Y, Baran PS. Synlett. 2010;12:1733–1745.
- 4.For reviews on C–H oxidation, see: Barton DHR, Doller D. Acc. Chem. Res. 1992;25:504–512. Barton DHR. Tetrahedron. 1998;54:5805–5817. Doyle MP, Forbes DC. Chem. Rev. 1998;98:911–935. doi: 10.1021/cr940066a. Müller P, Fruit C. Chem. Rev. 2003;103:2905–2919. doi: 10.1021/cr020043t. Davies HML, Beckwith REJ. Chem. Rev. 2003;103:2861–2904. doi: 10.1021/cr0200217. Punniyamurthy T, Velusamy S, Iqbal J. Chem. Rev. 2005;105:2329–2363. doi: 10.1021/cr050523v. Godula K, Sames D. Science. 2006;312:67–72. doi: 10.1126/science.1114731. Dick AR, Sanford MS. Tetrahedron. 2006;62:2439–2463. Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3271. doi: 10.1039/b816707a. Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem. Rev. 2010;110:704–724. doi: 10.1021/cr900239n.
- 5.For reviews on C–H oxidation from an organometallic perspective, see: Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc. Chem. Res. 1995;28:154–162. Shilov AE, Shul’pin GB. Chem. Rev. 1997;97:2879–2932. doi: 10.1021/cr9411886. Stahl SS, Labinger JA, Bercaw JE. Angew. Chem. Int. Ed. 1998;37:2180. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2180::AID-ANIE2180>3.0.CO;2-A. Jones WD. Top. Organomet. Chem. 1999;3:9–46. Crabtree RH. J. Chem. Soc., Dalton Trans. 2001:2437. Pamplin CB, Legzdins P. Acc. Chem. Res. 2003;36:223–233. doi: 10.1021/ar0202215. Lersch M, Tilset M. Chem. Rev. 2005;105:2471–2526. doi: 10.1021/cr030710y. Campeau L-C, Stuart DR, Fagnou K. Aldrichimica Acta. 2007;40:35–41. Kulkarni AA, Daugulis O. Synthesis. 2009:4087–4109. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890–931. doi: 10.1021/cr900206p. Shul’pin GB. Org. Biomol. Chem. 2010;8:4217–4228. doi: 10.1039/c004223d. Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654–2672. doi: 10.1002/chem.200902374. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. Yu J-Q, Shi Z, editors. C–H Activation, Top. Curr. Chem. 2010:292.
- 6.For reviews on C–H oxidation from a computational perspective, see: Lin Z. Coord. Chem. Rev. 2007;251:2280–2291. Boutadla Y, Davies DL, Macgregor SA, Poblador-Bahamonde AI. Dalton. Trans. 2009:5820–5831. doi: 10.1039/b904967c. Balcells D, Clot E, Eisenstein O. Chem. Rev. 2010;110:749–823. doi: 10.1021/cr900315k.
- 7.For reviews on enzymatic and biomimetic C–H oxidation, see: Breslow R. Chem. Soc. Rev. 1972;1:553–580. Breslow R. Acc. Chem. Res. 1980;13:170–177. Breslow R. Pure Appl. Chem. 1990;62:1859–1866. Breslow R. Acc. Chem. Res. 1995;28:146–153. Barton DHR, Taylor DK. Pure Appl. Chem. 1996;68:497–504. Que L, Jr., Ho RYN. Chem. Rev. 1996;96:2607–2624. doi: 10.1021/cr960039f. Mahato SB, Garai S. Steroids. 1997;62:332–345. doi: 10.1016/s0039-128x(96)00251-6. Costas M, Chen K, Que L., Jr Coord. Chem. Rev. 2000;200–202:517–544. Breslow R. Chemtracts. 2002;15:59–68. Meunier B, de Visser SP, Shaik S. Chem. Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. Costas M, Mehn MP, Jensen MP, Que L., Jr Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. Lippard SJ. Phil. Trans. R. Soc. A. 2005;363:861–877. doi: 10.1098/rsta.2004.1532. Que L., Jr Acc. Chem. Res. 2007;40:493–500. doi: 10.1021/ar700024g.
- 8.For reviews on the factors that affect C–H activation by radical reactions, see: Tedder JM, Walton JC. Tetrahedron. 1980;36:701–707. Tedder JM. Tetrahedron. 1982;38:313–329. Tedder JM. Angew. Chem. Int. Ed. 1982;21:401–410. Fokin AA, Schreiner PR. Chem. Rev. 2002;102:1551–1593. doi: 10.1021/cr000453m.
- 9.For example, see: Hass HB, McBee ET, Weber P. Ind. Eng. Chem. 1936;28:333–339.
- 10. Singh H, Tedder JM. J. Chem. Soc. 1964:4737–4741. For examples of oxidations at particularily remote sites, see: Eck CR, Hunter DJ, Money T. J. Chem. Soc., Chem. Commun. 1974:865–866. Eigendorf GK, Ma CL, Money T. J. Chem. Soc., Chem. Commun. 1976:561–562. Eigendorf GK, Ma CL, Money T. J. Chem. Soc., Perkin. Trans. 1. 1979:896–904.
- 11.Minisci F, Galli R, Galli A, Bernardi R. Tetrahedron Lett. 1967;8:2207–2209. [Google Scholar]
- 12.Asensio G, Castellano G, Mello R, González Núñez ME. J. Org. Chem. 1996;61:5564–5566. [Google Scholar]
- 13.Brodsky BH, Du Bois J. J. Am. Chem. Soc. 2005;127:15391–15393. doi: 10.1021/ja055549i. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen NA, Chen MS, White MC. Tetrahedron. 2009;65:3078–3084. [Google Scholar]
- 15.McNeill E, Du Bois J. J. Am. Chem. Soc. 2010;132:10202–10204. doi: 10.1021/ja1046999. [DOI] [PubMed] [Google Scholar]
- 16.Bovicelli P, Lupattelli P, Mincione E, Prencipe T, Curci R. J. Org. Chem. 1992;57:5052–5054. [Google Scholar]
- 17.Sicinski RR, Deluca HF. Bioorg. Med. Chem. Lett. 1995;5:159–162. [Google Scholar]
- 18.Mello R, Fiorentino M, Fusco C, Curci, R R. J. Am. Chem. Soc. 1989;111:6749–6757. [Google Scholar]
- 19. Kamata K, Yonehara K, Nakagawa Y, Uehara K, Mizuno N. Nat. Chem. 2010;2:478–483. doi: 10.1038/nchem.648. and references therein.
- 20.For a review on agostic interactions, see: Brookhart M, Green MLH. J. Organomet. Chem. 1983;250:395–408. Also see: Kubas GJ, Ryan RR, Swanson BI, Vergamini PJ, Wasserman HJ. J. Am. Chem. Soc. 1984;106:451–452. Crabtree RH, Holt EM, Lavin M, Morehouse SM. Inorg. Chem. 1985;24:1986–1992. and references therein Periana RA, Bergman RG. J. Am. Chem. Soc. 1986;108:7332–7346. Davies DL, Donald SMA, Macgregor SA. J. Am. Chem. Soc. 2005;127:13754–13755. doi: 10.1021/ja052047w. Häller LJL, Page MJ, Macgregor SA, Mahon MF, Whittlesey MK. J. Am. Chem. Soc. 2009;131:4604–4605. doi: 10.1021/ja900953d. Rousseaux S, Davi M, Sofack-Kreutzer J, Pierre C, Kefalidis CE, Clot E, Fagnou K, Baudoin O. J. Am. Chem. Soc. 2010;132:10706–10716. doi: 10.1021/ja1048847.
- 21. Chen H, Schlecht S, Semple TC, Hartwig JF. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. Lawrence JD, Takahashi M, Bae C, Hartwig JF. J. Am. Chem. Soc. 2004;126:15334–15335. doi: 10.1021/ja044933x. Murphy JM, Lawrence JD, Kawamura K, Incarvito C, Hartwig JF. J. Am. Chem. Soc. 2006;128:13684–13685. doi: 10.1021/ja064092p. For mechanistic studies on the origins of related transition metal mediated insertions, see: Bromberg SE, Yang H, Asplund MC, Lian T, McNamara BK, Kotz KT, Yeston JS, Wilkens M, Frei H, Bergman RG, Harris CB. Science. 1997;278:260–263. Sawyer KR, Cahoon JF, Shanoski JE, Glascoe EA, Kling MF, Schlegel JP, Zoerb MC, Hapke M, Hartwig JF, Webster CE, Harris CB. J. Am. Chem. Soc. 2010;132:1848–1859. doi: 10.1021/ja906438a. Wei CS, Jiménez-Hoyos CA, Videa MF, Hartwig JF, Hall MB. J. Am. Chem. Soc. 2010;132:3078–3091. doi: 10.1021/ja909453g.
- 22.a) Doyle MP, Westrum LJ, Wolthuis WNE, See MM, Boone WP, Bagheri V, Pearson MM. J. Am. Chem. Soc. 1993;115:958–964. [Google Scholar]; b) Wang P, Adams J. J. Am. Chem. Soc. 1994;116:3296–3305. [Google Scholar]; c) Doyle MP, Kalinin AV, Ene DG. J. Am. Chem. Soc. 1996;118:8837–8846. [Google Scholar]; d) Fiori KW, Du Bois J. J. Am. Chem. Soc. 2007;129:562–568. doi: 10.1021/ja0650450. [DOI] [PubMed] [Google Scholar]; e) Yun SY, Zheng J-C, Lee D. J. Am. Chem. Soc. 2009;131:8413–8415. doi: 10.1021/ja903526g. [DOI] [PubMed] [Google Scholar]
- 23.a) Proksch E, de Meijere A. Angew. Chem. Int. Ed. 1976;15:761–762. [Google Scholar]; b) D’Accolti L, Dinoi A, Fusco C, Russo A, Curci R. J. Org. Chem. 2003;68:7806–7810. doi: 10.1021/jo034768o. [DOI] [PubMed] [Google Scholar]; c) Chen MS, White MC. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 24.a) Hehre WJ. Acc. Chem. Res. 1975;8:369–376. [Google Scholar]; b) de Meijere A. Angew. Chem. Int. Ed. 1979;18:809–826. [Google Scholar]; c) Olah GA, Reddy VP, Prakash GKS. Chem. Rev. 1992;92:69–95. [Google Scholar]
- 25.The following references suggest that cyclopropanes do not exert significant long range hyperconjugative effects in such a setting and that the selectivity in the case of 15 is primarily the result of a steric effect: Vinkovic V, Mlinaric-Majerski K, Marinic Z. Tetrahedron Lett. 1992;33:7441–7444. Zefirova ON, Nurieva EV, Zyk NV. Russ. J. Org. Chem. 2005;41:1286–1288.
- 26.Barton DHR, Eaton W-G, Liu PE. Tetrahedron Lett. 1991;32:6263–6264. [Google Scholar]
- 27.For representative examples of Gif chemistry, see: a) Refs 4a and b Stavropoulos P, Celenligil-Cetin R, Tapper AE. Acc. Chem. Res. 2001;34:745–752. doi: 10.1021/ar000100+. About-Jaudet E, Barton DHR, Csuhai E, Ozbalik N. Tetrahedron Lett. 1990;31:1657–1660. Barton DHR, Csuhai E, Doller D. Tetrahedron. 1992;48:9195–9206. Barton DHR, Chavasiri W. Tetrahedron. 1994;50:19–30. Barton DHR, Chavasiri W. Tetrahedron. 1994;50:47–60.
- 28.For oxidation of the tertiary positions, see: Pramod K, Eaton PE, Gilardi R, Flippen-Anderson JL. J. Org. Chem. 1990;55:6105–6107. D’Accolti L, Fusco C, Lucchini V, Carpenter GB, Curci R. J. Org Chem. 2001;66:9063–9066. doi: 10.1021/jo0109671.
- 29.Banwell MG, Haddad N, Huglin JA, MacKay MF, Reum ME, Ryan JH, Turner KA. J. Chem. Soc., Chem. Commun. 1993:954–957. [Google Scholar]
- 30.a) Shiue G-H, Misslitz U, Ding X-t, Jones M, Jr, de Meijere A. Tetrahedron Lett. 1985;26:5399–5402. [Google Scholar]; b) Brinker UH, Lin G, Xu L, Smith J-L, Mieusset WB. J. Org. Chem. 2007;72:8434–8451. doi: 10.1021/jo7013356. [DOI] [PubMed] [Google Scholar]
- 31.Coudret JL, Zöllner S, Ravoo BJ, Malara L, Hanisch C, Dörre K, de Meijere A, Waegell B. Tetrahedron Lett. 1996;37:2425–2428. [Google Scholar]
- 32.González-Núñez ME, Royo J, Mello R, Baguena M, Martínez Ferrer J, Ramírez de Arellano C, Asensio G, Prakash GKS. J. Org. Chem. 2005;70:7919–7924. doi: 10.1021/jo0509511. [DOI] [PubMed] [Google Scholar]
- 33.a) Díaz-Requejo MM, Belderrain TR, Nicasio MC, Trofimenko S, Pérez PJ. J. Am. Chem. Soc. 2002;124:896–897. doi: 10.1021/ja016798j. [DOI] [PubMed] [Google Scholar]; b) Curci R, D’Accolti L, Fiorentino M, Fusco C. Tetrahedron Lett. 1992;33:4225–4228. [Google Scholar]
- 34.For further examples of heteroatom-directed C–H oxidation, see: Yoshifuji S, Arakawa Y, Nitta Y. Chem. Pharm. Bull. 1987;35:357–363. Doyle MP, Kalinin AV. Synlett. 1995:1075–1076. Davies HML, Hansen T. J. Am. Chem. Soc. 1997;119:9075–9076. Davies HML, Hansen T, Hopper DW, Panaro SA. J. Am. Chem. Soc. 1999;121:6509–6510. Davies HML, Hansen T, Churchill MR. J. Am. Chem. Soc. 2000;122:3063–3070. Davies HML, Ni A. Chem. Commun. 2006:3110–3112. doi: 10.1039/b605047f. Davies HML, Jin Q. Org. Lett. 2004;6:1769–1772. doi: 10.1021/ol0495467.
- 35.Davies HML, Beckwith REJ, Antoulinakis EG, Jin Q. J. Org. Chem. 2003;68:6126–6132. doi: 10.1021/jo034533c. [DOI] [PubMed] [Google Scholar]
- 36.Liang R-P, Fabien C, Fruit C, Müller P, Dodd RH, Dauban P. Angew. Chem. Int. Ed. 2006;45:4641–4644. doi: 10.1002/anie.200601248. [DOI] [PubMed] [Google Scholar]
- 37. Ferreira EM, Stoltz BM. J. Am. Chem. Soc. 2001;123:7725–7726. doi: 10.1021/ja015791z. Bagdanoff JT, Ferreira EM, Stoltz BM. Org. Lett. 2003;5:835–837. doi: 10.1021/ol027463p. Cheng Q, Deng F, Xia C, Sun W. Tetrahedron: Asymmetry. 2008;19:2359–2362. Brown MK, Blewett MM, Colombe JR, Corey EJ. J. Am. Chem. Soc. 2010;133:11165–11170. doi: 10.1021/ja103103d. For reviews, see: Sigman MS, Jensen DR. Acc. Chem. Res. 2006;39:221–229. doi: 10.1021/ar040243m. Ebner DC, Bagdanoff JT, Ferreira EM, McFadden RM, Caspi DD, Trend RM, Stoltz BM. Chem. Eur. J. 2009;15:12978–12992. doi: 10.1002/chem.200902172.
- 38.Barton DHR, Boivin J, Gaudin D, Jankowski K. Tetrahedron Lett. 1989;30:1381–1382. [Google Scholar]
- 39.Moriarty RM, Vaid RK, Duncan MP, Ochiai M, Inenaga M, Nagao Y. Tetrahedron Lett. 1988;30:6913–6916. [Google Scholar]
- 40.For reviews, see: Rawlinson DJ, Sosnovsky G. Synthesis. 1972:1–28. Clennan EL. Tetrahedron. 2000;56:9151–9179. Eames J, Watkinson M. Angew. Chem. Int. Ed. 2001;40:3567–3571. doi: 10.1002/1521-3773(20011001)40:19<3567::AID-ANIE3567>3.0.CO;2-C. Andrus MB, Lashley JC. Tetrahedron. 2002;58:845–866. Moiseev II, Vargaftik MN. Coord. Chem. Rev. 2004;248:2381–2391.
- 41.Umbreit MA, Sharpless KB. J. Am. Chem. Soc. 1977;99:5526–5528. [Google Scholar]
- 42.a) Kharasch MS, Fono A. J. Org. Chem. 1958;23:324. [Google Scholar]; b) Kharasch MS, Fono A. J. Org. Chem. 1958;23:324–325. [Google Scholar]; c) Kharasch MS, Sosnovsky G. J. Am. Chem. Soc. 1958;80:756. [Google Scholar]; d) Bateson JH, Robins AM, Southgate R. J. Chem. Soc., Perkin Trans. 1. 1991:2399–2405. [Google Scholar]
- 43.a) Yu J-Q, Corey EJ. Org. Lett. 2002;4:2727–2730. doi: 10.1021/ol0262340. [DOI] [PubMed] [Google Scholar]; b) Yu J-Q, Corey EJ. J. Am. Chem. Soc. 2003;125:3232–3233. doi: 10.1021/ja0340735. [DOI] [PubMed] [Google Scholar]; c) Catino AJ, Forslund RE, Doyle MP. J. Am. Chem. Soc. 2004;126:13622–13623. doi: 10.1021/ja045330o. [DOI] [PubMed] [Google Scholar]; d) Yu J-Q, Wu H-C, Corey EJ. Org. Lett. 2005;7:1415–1417. doi: 10.1021/ol050284y. [DOI] [PubMed] [Google Scholar]
- 44.Corey EJ, Lee J. J. Am. Chem. Soc. 1993;115:8873–8874. [Google Scholar]
- 45.a) Åkermark B, Zetterberg K. Tetrahedron Lett. 1975;16:3733–3736. [Google Scholar]; b) Rönn M, Bäckvall J-E, Andersson PG. Tetrahedron Lett. 1995;36:7749–7752. [Google Scholar]; c) Larock RC, Hightower TR, Hasvold LA, Peterson KP. J. Org. Chem. 1996;61:3584–3585. doi: 10.1021/jo952088i. [DOI] [PubMed] [Google Scholar]; d) Brice JL, Harang JE, Timokhin VI, Anastasi NR, Stahl SS. J. Am. Chem. Soc. 2005;127:2868–2869. doi: 10.1021/ja0433020. [DOI] [PubMed] [Google Scholar]; e) Reed SA, Mazzotti AR, White MC. J. Am. Chem. Soc. 2009;131:11701–11706. doi: 10.1021/ja903939k. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Yin G, Wu Y, Liu G. J. Am. Chem. Soc. 2010;132:11978–11987. doi: 10.1021/ja1030936. [DOI] [PubMed] [Google Scholar]
- 46.Trost BM, Richardson J, Yong K. J. Am. Chem. Soc. 2006;128:2540–2541. doi: 10.1021/ja057163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franzén J-E, Bäckvall J. J. Am. Chem. Soc. 2003;125:6056–6057. doi: 10.1021/ja029505a. [DOI] [PubMed] [Google Scholar]
- 48.a) Seyhan M, Fernelius WC. J. Org. Chem. 1957;22:217–219. [Google Scholar]; b) Ceder RM, Gómez M, Sales J. J. Organomet. Chem. 1989;361:391–398. [Google Scholar]; c) Holcomb HL, Nakanishi S, Flood TC. Organometallics. 1996;15:4228–4234. [Google Scholar]
- 49.a) Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300–2301. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]; b) Kalyani D, Dick AR, Anani WQ, Sanford MS. Tetrahedron. 2006;62:11483–11498. [Google Scholar]; c) Zhang J, Khaskin E, Anderson NP, Zavalij PY, Vedernikov AN. Chem. Commun. 2008:3625–3627. doi: 10.1039/b803156h. [DOI] [PubMed] [Google Scholar]
- 50.For more examples of “pyridine-like” nitrogen directed C–H oxidation reactions and related mechanistic studies, see: Dick AR, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2005;127:12790–12791. doi: 10.1021/ja0541940. Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391–3394. doi: 10.1021/ol061389j. Hull KL, Ananni WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134–7135. doi: 10.1021/ja061943k. Hull KL, Lanni EL, Sanford MS. J. Am. Chem. Soc. 2006;128:14047–14049. doi: 10.1021/ja065718e. Hull KL, Sanford MS. J. Am. Chem. Soc. 2007;129:11904–11905. doi: 10.1021/ja074395z. Whitfield SR, Sanford MS. J. Am. Chem. Soc. 2007;129:15142–15143. doi: 10.1021/ja077866q. Desai LV, Stowers KJ, Sanford MS. J. Am. Chem. Soc. 2008;130:13285–13293. doi: 10.1021/ja8045519. Racowski JM, Dick AR, Sanford MS. J. Am. Chem. Soc. 2009;131:10974–10983. doi: 10.1021/ja9014474. Hull KL, Sanford MS. J. Am. Chem. Soc. 2009;131:9651–9653. doi: 10.1021/ja901952h. Deprez NR, Sanford MS. J. Am. Chem. Soc. 2009;131:11234–11241. doi: 10.1021/ja904116k. Arnold PL, Sanford MS, Pearson SM. J. Am. Chem. Soc. 2009;131:13912–13913. doi: 10.1021/ja905713t. Powers DC, Ritter T. Nat. Chem. 2009;1:302–309. doi: 10.1038/nchem.246. Powers DC, Geibel MAL, Klein JEMN, Ritter T. J. Am. Chem. Soc. 2009;131:17050–17051. doi: 10.1021/ja906935c. Powers DC, Benitez D, Tkatchouk E, Goddard WA, III, Ritter T. J. Am. Chem. Soc. 2010;132:14092–14103. doi: 10.1021/ja1036644. Powers DC, Xiao DY, Geibel MAL, Ritter T. J. Am. Chem. Soc. 2010;132:14530–14536. doi: 10.1021/ja1054274. Ye Y, Ball ND, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2010;132:14682–14687. doi: 10.1021/ja107780w. Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p.
- 51.a) Tenaglia A, Terranova E, Waegell B. Tetrahedron Lett. 1989;30:5271–5274. [Google Scholar]; b) Tenaglia A, Terranova E, Waegell B. J. Org. Chem. 1992;57:5523–5524. [Google Scholar]
- 52.Taber DF, Ruckle RE. J. Am. Chem. Soc. 1986;108:7686–7693. doi: 10.1021/ja00284a037. [DOI] [PubMed] [Google Scholar]
- 53.For further examples, see: Müller P, Fernandez D. Helv. Chim. Acta. 1995;78:947–958. Taber DF, Song Y. J. Org. Chem. 1996;61:6706–6712. doi: 10.1021/jo960758u. Taber DF, Malcom SC. J. Org. Chem. 1998;63:3717–3721. Kurosawa W, Kan T, Fukuyama T. J. Am. Chem. Soc. 2003;125:8112–8113. doi: 10.1021/ja036011k.
- 54.Davies HML, Venkataramani C. Angew. Chem. Int. Ed. 2002;41:2197–2199. [PubMed] [Google Scholar]
- 55.Gómez L, Garcia-Bosch I, Company A, Benet-Buchholz J, Polo A, Sala X, Ribas X, Costas M. Angew. Chem. Int. Ed. 2009;48:5720–5723. doi: 10.1002/anie.200901865. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber J, Eschenmoser A. Helv. Chim. Acta. 1955;38:1529–1536. [Google Scholar]
- 57.Chen K, Eschenmoser A, Baran PS. Angew. Chem. Int. Ed. 2009;48:9705–9708. doi: 10.1002/anie.200904474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasuya S, Kamijo S, Inoue M. Org. Lett. 2009;11:3630–3632. doi: 10.1021/ol901367m. [DOI] [PubMed] [Google Scholar]
- 59.a) Chen K, Richter JM, Baran PS. J. Am. Chem. Soc. 2008;130:7247–7249. doi: 10.1021/ja802491q. [DOI] [PubMed] [Google Scholar]; b) Hofmann AW. Ber. 1883;16:558–560. [Google Scholar]; c) Hofmann AW. Ber. 1885;18:5–23. [Google Scholar]; d) Hofmann AW. Ber. 1885;18:109–131. [Google Scholar]; e) Wolff ME. Chem. Rev. 1963;63:55–64. [Google Scholar]; f) Mackiewicz P, Furstoss R. Tetrahedron. 1978;34:3241–3260. [Google Scholar]
- 60.a) Breslow R, Corcoran RJ, Snider BB, Doll RJ, Khanna PL, Kaleya R. J. Am. Chem. Soc. 1977;99:905–915. doi: 10.1021/ja00445a038. [DOI] [PubMed] [Google Scholar]; b) Breslow R, Heyer D. J. Am. Chem. Soc. 1982;104:2045–2046. [Google Scholar]
- 61.a) Breslow R, Dale JA, Kalicky P, Liu SY, Washburn WN. J. Am. Chem. Soc. 1972;94:3276–3278. doi: 10.1021/ja00764a085. [DOI] [PubMed] [Google Scholar]; b) Breslow R, Baldwin S, Flechtner T, Kalicky P, Liu S, Washburn W. J. Am. Chem. Soc. 1973;95:3251–3262. doi: 10.1021/ja00791a031. [DOI] [PubMed] [Google Scholar]
- 62. Wong M-K, Chung N-W, He L, Yang D. J. Am. Chem. Soc. 2003;125:158–162. doi: 10.1021/ja028357l. Wong M-K, Chung N-W, He L, Wang X-C, Yan Z, Tang Y-C, Yang D. J. Org. Chem. 2003;68:6321–6328. doi: 10.1021/jo0347011. For a unique example of an intramolecular oxidative cyclization using oxaziridines, see: Allen CP, Benkovics T, Turek AK, Yoon TP. J. Am. Chem. Soc. 2009;131:12560–12561. doi: 10.1021/ja906183g.
- 63.Asensio G, González-Núñez ML, Bernardini CB, Mello R, Adam W. J. Am. Chem. Soc. 1993;115:7250–7253. [Google Scholar]
- 64.Chen K, Baran PS. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 65. Dangel BD, Godula K, Youn SW, Sezen B, Sames D. J. Am. Chem. Soc. 2002;124:11856–11857. doi: 10.1021/ja027311p. For example, see: Taber DF, Petty EH. J. Org. Chem. 1982;47:4808–4809.
- 66.a) Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; b) Chiong HA, Pham Q-N, Daugulis O. J. Am. Chem. Soc. 2007;129:9879–9884. doi: 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]
- 67.a) Espino CG, When PM, Chow J, Du Bois J. J. Am. Chem. Soc. 2001;123:6935–6936. [Google Scholar]; b) Fleming JJ, Du Bois J. J. Am. Chem. Soc. 2006;128:3926–3927. doi: 10.1021/ja0608545. [DOI] [PubMed] [Google Scholar]
- 68.Giri R, Chen X, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 69. Giri R, Liang J, Lei J-G, Li J-J, Wang D-H, Chen X, Naggar IC, Guo C, Foxman BM, Yu J-Q. Angew. Chem. Int. Ed. 2005;47:7420–7424. doi: 10.1002/anie.200502767. Giri R, Chen X, Hao X-S, Li J-J, Liang J, Fan Z-P, Yu J-Q. Tetrahedron: Asymmetry. 2005;16:3502–3505. Chen X, Li J-J, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:78–79. doi: 10.1021/ja0570943. Giri R, Wasa M, Breazzano SP, Yu J-Q. Org. Lett. 2006;8:5685–5688. doi: 10.1021/ol0618858. Yu J-Q, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041–4047. doi: 10.1039/b611094k. Li J-J, Giri R, Yu J-Q. Tetrahedron. 2008;64:6979–6987. Giri R, Maugel NL, Foxman BM, Yu J-Q. Organometallics. 2008;27:1667–1670. For related palladium-mediated C–H activations with oxime directing groups, see Carr K, Sutherland JK. J. Chem. Soc., Chem. Commun. 1984:1227. Baldwin JE, Jones RH, Najera C, Yus M. Tetrahedron. 1985;41:699–711. Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542–9543. doi: 10.1021/ja046831c. Neufeldt SR, Sanford MS. Org. Lett. 2010;12:532–535. doi: 10.1021/ol902720d.
- 70. Wasa M, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2010;132:3680–3681. doi: 10.1021/ja1010866. For similar selectivity seen in a carbonylation reaction, see: Yoo EJ, Wasa M, Yu J-Q. J. Am. Chem. Soc. 2010;132:17378–17380. doi: 10.1021/ja108754f.
- 71.For two other notable means of effecting directed oxidations, see: Concepción JI, Francisco CG, Hernández R, Salazar JA, Suárez E. Tetrahedron Lett. 1984;25:1953–1956. Schönecker B, Lange C, Zheldakova T, Günther W, Görls H, Vaughan G. Tetrahedron. 2005;61:103–114.
- 72.For an early review on Friedel-Crafts reactions, see: Calloway NO. Chem. Rev. 1935;17:327–392. For reviews on directed metalation, see: Snieckus V. Chem. Rev. 1990;90:879–933. Schlosser M. Angew. Chem. Int. Ed. 2005;44:376–393. doi: 10.1002/anie.200300645. Epsztanjn J, Józwiak A, Szczesniak AK. Curr. Org. Chem. 2006;10:1817–1848. For seminal contributions to the Heck reaction, see: Heck RF. J. Am. Chem. Soc. 1968;90:5518–5526. Heck RF. J. Am. Chem. Soc. 1969;91:6707–6714. Mizoroki T, Mori K, Ozaki A. Bull. Chem. Soc. Jpn. 1971;44:581. Heck RF, Nolley, Jr. JP. J. Org. Chem. 1972;37:2320–2322.
- 73. Padwa A, Austin DJ, Hornbuckle SF, Semones MA, Doyle MP, Protopopova MN. J. Am. Chem. Soc. 1992;114:1874–1876. Padwa A, Austin DJ, Price AT, Semones MA, Doyle MP, Protopopova MN, Winchester WR, Tran A. J. Am. Chem. Soc. 1993;115:8669–8680. See also References 22a-d.
- 74. Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J. Am. Chem. Soc. 2002;124:390–391. doi: 10.1021/ja0173019. Ishiyama T, Takagi J, Hartwig JF, Miyaura N. Angew. Chem. Int. Ed. 2002;41:3056–3058. doi: 10.1002/1521-3773(20020816)41:16<3056::AID-ANIE3056>3.0.CO;2-#. For an example of meta arylation, see: Phipps RJ, Gaunt MJ. Science. 2009;323:1593–1597. doi: 10.1126/science.1169975.
- 75.For an excellent discussion on steric effects, see: Chotana GA, Rak MA, Smith MR., III J. Am. Chem. Soc. 2005;127:10539–10544. doi: 10.1021/ja0428309.
- 76.For an ortho iridium-mediated C–H insertion of haloarenes, see: Ben-Ari E, Gandelman M, Rozenberg H, Shimon LJW, Milstein D. J. Am. Chem. Soc. 2003;125:4714–4715. doi: 10.1021/ja028362p.
- 77.a) Iverson CN, Smith MR., III J. Am. Chem. Soc. 1999;121:7696–7697. [Google Scholar]; b) Cho J-Y, Iverson CN, Smith MR., III J. Am. Chem. Soc. 2000;122:12868–12869. [Google Scholar]; c) Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr, Smith MR., III Science. 2002;295:305–308. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y-H, Shi B-F, Yu J-Q. J. Am. Chem. Soc. 2009;131:5072–5074. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu Y, Wang D-H, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2010;132:5916–5921. doi: 10.1021/ja101909t. Wang X, Lu Y, Dai H-X, Yu J-Q. J. Am. Chem. Soc. 2010;132:12203–12205. doi: 10.1021/ja105366u. For triflamide directed oxidations, see: Wang X, Mei T-S, Yu J-Q. J. Am. Chem. Soc. 2009;131:7520–7521. doi: 10.1021/ja901352k. Shi BF, Maugel N, Zhang Y-H, Yu J-Q. Angew. Chem. Int. Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030.
- 80.a) Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J. Am. Chem. Soc. 2008;130:16474–16475. doi: 10.1021/ja806955s. [DOI] [PubMed] [Google Scholar]; b) Stuart DR, Alsabeh P, Kuhn M, Fagnou K. J. Am. Chem. Soc. 2010;132 doi: 10.1021/ja1082624. In Press, [DOI] [PubMed] [Google Scholar]
- 81.Wang D-H, Engle KM, Shi B-F, Yu J-Q. Science. 2010;327:315–319. doi: 10.1126/science.1182512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engle KM, Wang D-H, Yu J-Q. J. Am. Chem. Soc. 2010;132:14137–14151. doi: 10.1021/ja105044s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.For representative reviews, see: Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174–238. doi: 10.1021/cr0509760. Campos KR. Chem. Soc. Rev. 2007;36:1069–1084. doi: 10.1039/b607547a. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n.
- 84.For an excellent study and discussion, see: Likhotvorik IR, Yuan K, Brown DW, Krasutsky PA, Smyth N, Jones M., Jr Tetrahedron Lett. 1992;33:911–914. For other selected adamantane oxidations, see: Cohen Z, Keinan E, Mazur Y, Varkony TH. J. Org. Chem. 1975;40:2141–2142. Groves JT, Nemo TE, Myers RS. J. Am. Chem. Soc. 1979;101:1032–1033. Barton DHR, Gastiger MJ, Motherwell WB. J. Chem. Soc., Chem. Commun. 1983:41–43. Muzart J, Aijou AN. J. Mol. Catal. 1993;84:L15–L19. Minisci F, Fontana F, Zhao L. Tetrahedron Lett. 1994;35:8033–8036. Murray RW, Iyanar K, Chen J, Wearing JT. Tetrahedron Lett. 1995;36:6415–6418. Groves JT, Bonchio M, Carofiglio T, Shalyaev K. J. Am. Chem. Soc. 1996;118:8961–8962. Schreiner PR, Lauenstein O, Butova ED, Gunchenko PA, Kolomitsin IV, Wittkopp A, Feder G, Fokin AA. Chem. Eur. J. 2001;7:4996–5003. doi: 10.1002/1521-3765(20011203)7:23<4996::aid-chem4996>3.0.co;2-p. Wang C, Shalyaev KV, Bonchio M, Carofiglio T, Groves JT. Inorg. Chem. 2006;45:4769–4782. doi: 10.1021/ic0520566. Bianchini G, Crucianelli M, Crestini C, Saladino R. Top. Catal. 2006;40:221–227. For an overview of reagent systems for adamantane oxidation, see: Ishii Y, Sakaguchi S. In: Modern Oxidation Methods. Bäckvall J-E, editor. WILEY-VCH: Weinheim; 2004. pp. 119–163.
- 85.Lin Y, Dawei M, Lu X. J. Organomet. Chem. 1987;323:407–419. [Google Scholar]
- 86.For some interesting examples, see: Fokin AA, Tkachenko BA, Korshunov OI, Gunchenko PA, Schreiner PR. J. Am. Chem. Soc. 2001;123:11248–11252. doi: 10.1021/ja0158096.
- 87.Wender PA, Hilinski MK, Mayweg AVW. Org. Lett. 2005;7:79–82. doi: 10.1021/ol047859w. [DOI] [PubMed] [Google Scholar]
- 88.For solution structures, see: Kamano Y, Zhang H-p, Morita H, Itokawa H, Shirota O, Pettit GR, Herald DL, Herald CL. Tetrahedron. 1996;52:2369–2376. Wender PA, DeBrabander J, Harran PG, Jimenez J-M, Koehler MFT, Lippa B, Park C-M, Siedenbiedel C, Pettit GR. Proc. Natl. Acad. Sci. USA. 1998;95:6624–6629. doi: 10.1073/pnas.95.12.6624.
- 89.a) Voigt B, Porzel A, Golsch D, Adam W, Adam G. Tetrahedron. 1996;52:10653–10658. [Google Scholar]; b) Seto H, Fujioka S, Koshino H, Yoshida S, Tsubuki M, Honda T. Tetrahedron. 1999;55:8341–8352. [Google Scholar]
- 90.a) Bovicelli P, Gambacorta A, Lupattelli P, Mincione E. Tetrahedron Lett. 1992;33:7411–7412. [Google Scholar]; b) Arnone A, Cavicchioli M, Montanari V, Resnati G. J. Org. Chem. 1994;59:5511–5513. [Google Scholar]; c) Iida T, Yamaguchi T, Nakamori R, Hikosaka M, Mano N, Goto J, Nambara T. J. Chem. Soc., Perkin Trans. 1. 2001;18:2229–2236. [Google Scholar]
- 91.For an example of an enzymatic oxidation with similar preference, see: Carr RT, Balasubamanian S, Hawkins PCD, Benkovic SJ. Biochemistry. 1995;34:7525–7532. doi: 10.1021/bi00022a028.
- 92.Curci R, Detomaso A, Prencipe T, Carpenter GB. J. Am. Chem. Soc. 1994;116:8112–8115. [Google Scholar]
- 93.Kitadani M, Ito K, Yoshikoshi A. Bull. Chem. Soc. Jap. 1971;44:3431–3434. [Google Scholar]
- 94.Liu W, Groves JT. J. Am. Chem. Soc. 2010;132:12847–12849. doi: 10.1021/ja105548x. [DOI] [PubMed] [Google Scholar]
- 95.Newhouse T, Lockner JW, Chen K, Eschenmoser A, Baran PS. unpublished results. [Google Scholar]
- 96.Hinman A, Du Bois J. J. Am. Chem. Soc. 2003;125:11510–11511. doi: 10.1021/ja0368305. [DOI] [PubMed] [Google Scholar]
- 97.Lee S, Fuchs PL. Org. Lett. 2004;6:1437–1440. doi: 10.1021/ol049712a. [DOI] [PubMed] [Google Scholar]
- 98.a) Johnson JA, Sames D. J. Am. Chem. Soc. 2000;122:6321–6322. [Google Scholar]; b) Johnson JA, Li N, Sames D. J. Am. Chem. Soc. 2002;124:6900–6903. doi: 10.1021/ja026130k. [DOI] [PubMed] [Google Scholar]
- 99.Davies HML, Dai X, Long MS. J. Am. Chem. Soc. 2006;128:2485–2490. doi: 10.1021/ja056877l. [DOI] [PubMed] [Google Scholar]
- 100.White JD, Hrnciar P, Stappenbeck F. J. Org. Chem. 1999;64:7871–7884. [Google Scholar]
- 101.Kim J, Ashenhurst JA, Movassaghi M. Science. 2009;324:238–241. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.a) Baran PS, Corey EJ. J. Am. Chem. Soc. 2002;124:7904–7905. doi: 10.1021/ja026663t. [DOI] [PubMed] [Google Scholar]; b) Hutchison AJ, Kishi Y. J. Am. Chem. Soc. 1979;101:6786–6788. [Google Scholar]
- 103.Iwasa E, Hamashima Y, Fujishiro S, Higuchi E, Ito A, Yoshida M, Sodeoka M. J. Am. Chem. Soc. 2010;132:4078–4079. doi: 10.1021/ja101280p. [DOI] [PubMed] [Google Scholar]
- 104.Kita Y, Sano A, Yamaguchi T, Oka M, Gotanda K, Matsugi M. Tetrahedron Lett. 1997;38:3549–3552. [Google Scholar]
- 105.Kalyani D, Deprez NR, Desai LV, Sanford MS. J. Am. Chem. Soc. 2005;127:7330–7331. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]
- 106.Yamaguchi J, Seiple IB, Young IS, O’Malley DP, Maue M. Angew. Chem. Int. Ed. 2008;47:3578–3581. doi: 10.1002/anie.200705913. [DOI] [PubMed] [Google Scholar]; b) O’Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2008;47:3581–3583. doi: 10.1002/anie.200801138. [DOI] [PubMed] [Google Scholar]
- 107.a) Su S, Seiple IB, Young IS, Baran. PS. J. Am. Chem. Soc. 2008;130:16490–16491. doi: 10.1021/ja8074852. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seiple IB, Su S, Young IS, Lewis CA, Yamaguchi J, Baran PS. Angew. Chem. Int. Ed. 2010;49:1095–1098. doi: 10.1002/anie.200907112. [DOI] [PMC free article] [PubMed] [Google Scholar]