Abstract
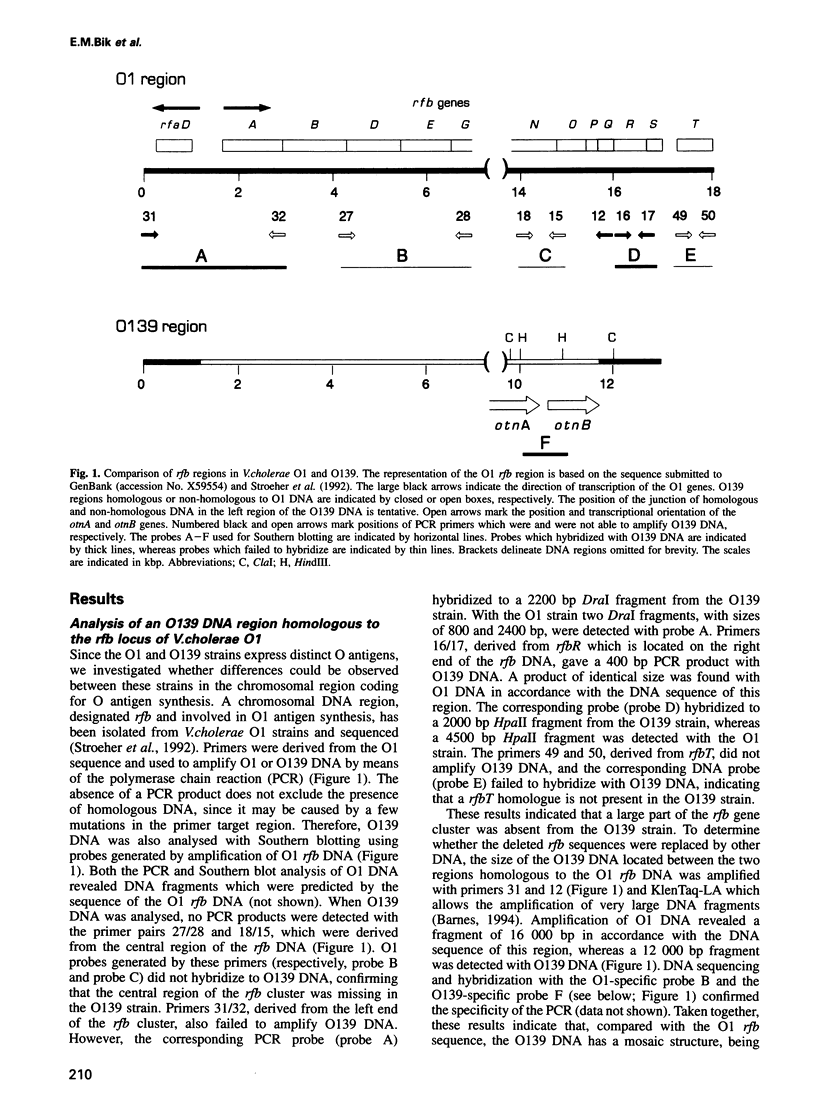
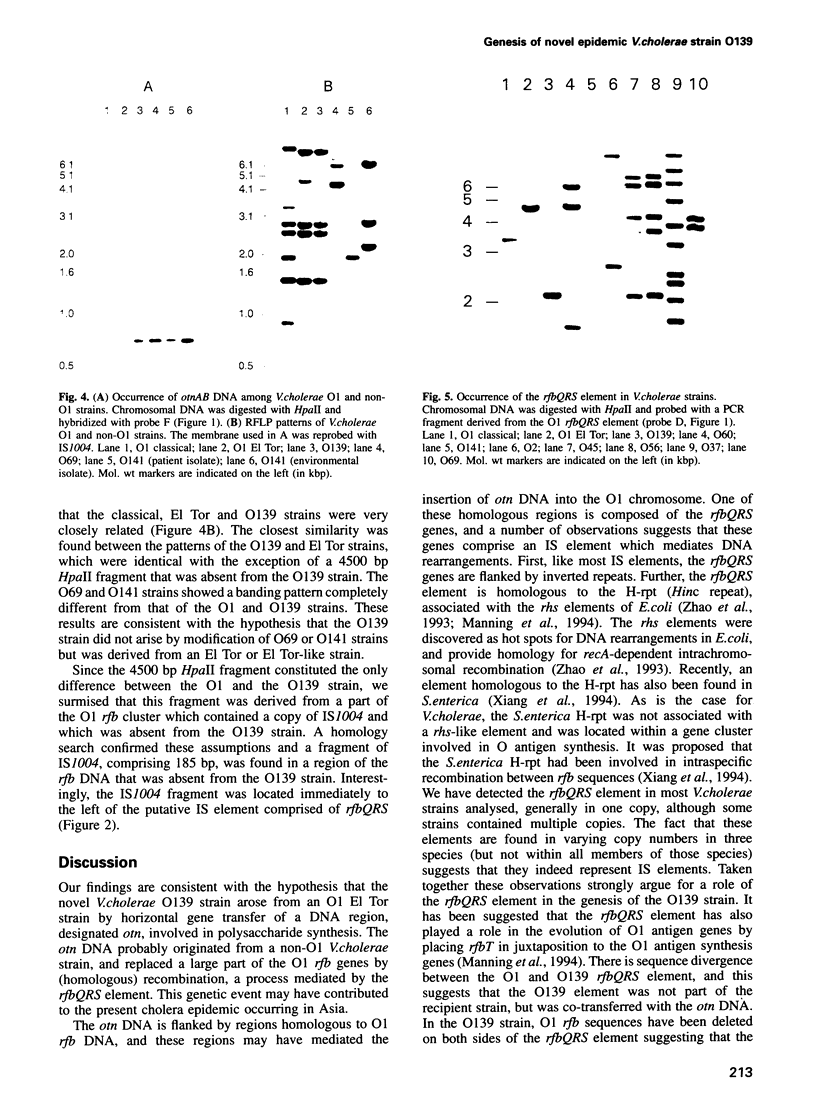
Only Vibrio cholerae strains of serotype O1 are known to cause epidemics, while non-O1 strains are associated with sporadic cases of cholera. It was therefore unexpected that the recent cholera epidemic in Asia was caused by a non-O1 strain with the serotype O139. We provide evidence that O139 arose from a strain closely related to the causative agent of the present cholera pandemic, V. cholerae O1 El Tor, by acquisition of novel DNA which was inserted into, and replaced part of, the O antigen gene cluster of the recipient strain. Part of the novel DNA was sequenced and two open reading frames (otnA and otnB) were observed, the products of which showed homology to proteins involved in capsule and O antigen synthesis, respectively. This suggests that the otnAB DNA determines the distinct antigenic properties of the O139 cell surface. The otnAB DNA was not detected in O1 strains, but was present in two non-O1 V. cholerae strains with serotypes O69 and O141. In the O69 and O139 strains the otnAB genes were located proximate to the putative insertion sequence (IS) element rfbQRS, which is associated with O antigen synthesis genes in O1 strains, and may have played a role in the insertion of the otnAB DNA in the recipient chromosome. Our results suggest that the O139 strain arose by horizontal gene transfer between a non-O1 and an O1 strain. The acquired DNA has altered the antigenic properties of the recipient O1 strain, providing a selective advantage in a region where a large part of the population is immune to O1 strains.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Alam K., Ansaruzzaman M., Qadri F., Sack R. B. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 (Bengal strain) after oral immunization of rabbits with V. cholerae O1 vaccine strain CVD103-HgR. J Infect Dis. 1994 Jan;169(1):230–231. doi: 10.1093/infdis/169.1.230. [DOI] [PubMed] [Google Scholar]
- Albert M. J., Siddique A. K., Islam M. S., Faruque A. S., Ansaruzzaman M., Faruque S. M., Sack R. B. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993 Mar 13;341(8846):704–704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin D. A., Stevenson G., Brown P. K., Haase A., Reeves P. R. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol. 1993 Mar;7(5):725–734. doi: 10.1111/j.1365-2958.1993.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Batchelor R. A., Alifano P., Biffali E., Hull S. I., Hull R. A. Nucleotide sequences of the genes regulating O-polysaccharide antigen chain length (rol) from Escherichia coli and Salmonella typhimurium: protein homology and functional complementation. J Bacteriol. 1992 Aug;174(16):5228–5236. doi: 10.1128/jb.174.16.5228-5236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M. K., Bhattacharya S. K., Garg S., Saha P. K., Dutta D., Nair G. B., Deb B. C., Das K. P. Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet. 1993 May 22;341(8856):1346–1347. doi: 10.1016/0140-6736(93)90855-b. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M. K., Bhattacharya S. K., Garg S., Saha P. K., Dutta D., Nair G. B., Deb B. C., Das K. P. Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet. 1993 May 22;341(8856):1346–1347. doi: 10.1016/0140-6736(93)90855-b. [DOI] [PubMed] [Google Scholar]
- Bronner D., Sieberth V., Pazzani C., Roberts I. S., Boulnois G. J., Jann B., Jann K. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J Bacteriol. 1993 Sep;175(18):5984–5992. doi: 10.1128/jb.175.18.5984-5992.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calia K. E., Murtagh M., Ferraro M. J., Calderwood S. B. Comparison of Vibrio cholerae O139 with V. cholerae O1 classical and El Tor biotypes. Infect Immun. 1994 Apr;62(4):1504–1506. doi: 10.1128/iai.62.4.1504-1506.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Snyder M. J., Wenzel R. P., Hornick R. B. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974 Jan;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Faruque S. M., Abdul Alim A. R., Roy S. K., Khan F., Nair G. B., Sack R. B., Albert M. J. Molecular analysis of rRNA and cholera toxin genes carried by the new epidemic strain of toxigenic Vibrio cholerae O139 synonym Bengal. J Clin Microbiol. 1994 Apr;32(4):1050–1053. doi: 10.1128/jcm.32.4.1050-1053.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Saha P. K., Ramamurthy T., Deb B. C., Nair G. B., Shimada T., Takeda Y. Nationwide prevalence of the new epidemic strain of Vibrio cholerae O139 Bengal in India. J Infect. 1993 Jul;27(1):108–109. doi: 10.1016/0163-4453(93)94398-u. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Rijpkema S. G. Infection and immunity to Vibrio cholerae, Salmonella typhimurium and Escherichia coli in a rabbit model. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;270(1-2):260–269. doi: 10.1016/s0176-6724(88)80162-7. [DOI] [PubMed] [Google Scholar]
- Hall R. H., Khambaty F. M., Kothary M., Keasler S. P. Non-O1 Vibrio cholerae. Lancet. 1993 Aug 14;342(8868):430–430. doi: 10.1016/0140-6736(93)92839-l. [DOI] [PubMed] [Google Scholar]
- Hisatsune K., Kondo S., Isshiki Y., Iguchi T., Kawamata Y., Shimada T. O-antigenic lipopolysaccharide of Vibrio cholerae O139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1309–1315. doi: 10.1006/bbrc.1993.2395. [DOI] [PubMed] [Google Scholar]
- Jesudason M. V., Cherian A. M., John T. J. Blood stream invasion by Vibrio cholerae O139. Lancet. 1993 Aug 14;342(8868):431–431. doi: 10.1016/0140-6736(93)92842-h. [DOI] [PubMed] [Google Scholar]
- Jesudason M. V., John T. J. Major shift in prevalence of non-O1 and El Tor Vibrio cholerae. Lancet. 1993 Apr 24;341(8852):1090–1091. doi: 10.1016/0140-6736(93)92447-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Panigrahi P., Morris J. G., Jr Non-O1 Vibrio cholerae NRT36S produces a polysaccharide capsule that determines colony morphology, serum resistance, and virulence in mice. Infect Immun. 1992 Mar;60(3):864–869. doi: 10.1128/iai.60.3.864-869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Salles C. A., Panigrahi P., Albert M. J., Wright A. C., Johnson R. J., Morris J. G., Jr Vibrio cholerae O139 synonym bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect Immun. 1994 May;62(5):2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Cholera Working Group, International Centre for Diarrhoeal Diseases Research, Bangladesh. Lancet. 1993 Aug 14;342(8868):387–390. [PubMed] [Google Scholar]
- Lawrence J. G., Dykhuizen D. E., DuBose R. F., Hartl D. L. Phylogenetic analysis using insertion sequence fingerprinting in Escherichia coli. Mol Biol Evol. 1989 Jan;6(1):1–14. doi: 10.1093/oxfordjournals.molbev.a040531. [DOI] [PubMed] [Google Scholar]
- Meier-Dieter U., Barr K., Starman R., Hatch L., Rick P. D. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. Molecular cloning of the rfe-rff gene cluster. J Biol Chem. 1992 Jan 15;267(2):746–753. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy T., Garg S., Sharma R., Bhattacharya S. K., Nair G. B., Shimada T., Takeda T., Karasawa T., Kurazano H., Pal A. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993 Mar 13;341(8846):703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- Rich J. J., Willis D. K. A single oligonucleotide can be used to rapidly isolate DNA sequences flanking a transposon Tn5 insertion by the polymerase chain reaction. Nucleic Acids Res. 1990 Nov 25;18(22):6673–6676. doi: 10.1093/nar/18.22.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrin S., Morris J. G., Jr, Adams M., Pons V., Jacobs R., Conte J. E., Jr Non-O:1 Vibrio cholerae bacteremia: case report and review. Rev Infect Dis. 1988 Sep-Oct;10(5):1012–1017. doi: 10.1093/clinids/10.5.1012. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Vann W. F. Translocation of capsular polysaccharides in pathogenic strains of Escherichia coli requires a 60-kilodalton periplasmic protein. J Bacteriol. 1987 Dec;169(12):5489–5495. doi: 10.1128/jb.169.12.5489-5495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeher U. H., Karageorgos L. E., Morona R., Manning P. A. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Mekalanos J. J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994 Jan;62(1):72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Mekalanos J. J. Vibrio cholerae O139 specific gene sequences. Lancet. 1994 May 28;343(8909):1366–1366. doi: 10.1016/s0140-6736(94)92504-6. [DOI] [PubMed] [Google Scholar]
- Xiang S. H., Hobbs M., Reeves P. R. Molecular analysis of the rfb gene cluster of a group D2 Salmonella enterica strain: evidence for its origin from an insertion sequence-mediated recombination event between group E and D1 strains. J Bacteriol. 1994 Jul;176(14):4357–4365. doi: 10.1128/jb.176.14.4357-4365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Sandt C. H., Feulner G., Vlazny D. A., Gray J. A., Hill C. W. Rhs elements of Escherichia coli K-12: complex composites of shared and unique components that have different evolutionary histories. J Bacteriol. 1993 May;175(10):2799–2808. doi: 10.1128/jb.175.10.2799-2808.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]