Abstract
The incidence of breast cancer brain metastases has increased in recent years, largely due to improved control of systemic disease with human epidermal growth factor receptor 2 (HER2)-targeted agents and the inability of most of these agents to efficiently cross the blood–blood barrier (BBB) and control central nervous system disease. There is, therefore, an urgent unmet need for treatments to prevent and treat HER2+ breast cancer brain metastases (BCBMs). Aberrant activation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway is frequently observed in many cancers, including primary breast tumors and BCBMs. Agents targeting key components of this pathway have demonstrated antitumor activity in diverse cancers, and may represent a new treatment strategy for BCBMs. In preclinical studies, several inhibitors of PI3K and mTOR have demonstrated an ability to penetrate the BBB and down-regulate PI3K signaling, indicating that these agents may be potential therapies for brain metastatic disease. The PI3K inhibitor buparlisib (BKM120) and the mTOR inhibitor everolimus (RAD001) are currently under evaluation in combination with trastuzumab in patients with HER2+ BCBMs.
Keywords: Phosphatidylinositol 3-kinase (PI3K), Mammalian target of rapamycin (mTOR), Breast cancer, Brain metastases, Human epidermal growth factor receptor 2 (HER2)
Introduction
Roughly one-quarter of all breast cancers are driven by amplification of the human epidermal growth factor receptor 2 (HER2) gene (HER2-positive [HER2+] breast cancers) [1]. Since the introduction of the first anti-HER2 therapy, trastuzumab, in the 1990s, multiple new targeted therapeutics have been developed. The regulatory approval of lapatinib, an oral dual tyrosine kinase inhibitor of HER2 and epidermal growth factor receptor (EGFR), was followed by pertuzumab, a monoclonal antibody directed against HER2 that blocks heterodimerization, and most recently trastuzumab emtansine (T-DM1), the first targeted chemotherapy in any solid malignancy [2–4]. As systemic therapy of HER2+ breast cancer has progressed, however, the treatment of brain metastases is, for the most part, unaddressed.
HER2+ breast cancer is associated with a higher risk of brain metastases than HER2-negative disease, with incidence ranging 30–53% [5–7] compared with approximately 10% in other breast cancers [6]. Median time for patients with metastatic HER2+ breast cancer to develop brain metastasis is 18 months [8] while the 1-year survival rate is only approximately 20% after brain metastases are known to be present [9]. In one study, up to 50% of patients with metastatic HER2+ breast cancer eventually died of central nervous system (CNS) progression [10]. The high rate of HER2+ breast cancer brain metastases (BCBMs) is likely multifactorial in etiology, owing to improvements in the control of systemic disease with patients living longer and therefore being more likely to develop brain metastases, as well as a limited ability of systemic therapies to cross the blood – brain barrier (BBB), making the brain a ‘sanctuary site’ for metastatic cells [9]. Furthermore, data from animal models indicate that HER2+ breast cancer cells carry an innate predilection to metastasize to visceral sites, including the brain [11]. There is therefore an urgent unmet need to develop systemic treatments that can cross the BBB and prevent or treat HER2+ BCBMs. This review will summarize the current treatment landscape of HER2+ BCBMs, the role of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway in BCBMs, and the potential for inhibitors of this pathway in this setting.
Treatment of CNS metastatic HER2+ breast cancer
The current treatment paradigm for symptomatic brain metastases in patients with HER2+ breast cancer includes stereotactic radiosurgery, surgical resection, and/or whole-brain radiation (WBRT) [7]. Although the BBB is probably impaired with development of metastatic disease, many drugs that are effective systemically, do not appear to reach therapeutic CNS levels, therefore, arguing that the BBB is still a major factor. Trastuzumab, a humanized monoclonal antibody, for example, forms the backbone of systemic anti-HER2 therapy but has limited ability to cross the BBB [12]. Some benefit with the intrathecal use of trastuzumab, first reported in 2001, has been demonstrated in case reports, but no clinical trials have been performed and no commercial intrathecal formulation exists [13–16]. Pertuzumab and T-DM1 are similarly large molecules and are not expected to cross the BBB significantly, though data is scarce; patients with CNS metastatic disease were excluded from the phase III trial of pertuzumab [3], while patients with symptomatic or recently treated CNS metastases were excluded from the EMILIA trial of T-DM1 [4].
Lapatinib, a small-molecule EGFR/HER2 inhibitor and the second anti-HER2 therapy approved for use by the US Food and Drug Administration (FDA) after trastuzumab, is the only directed HER2 therapy that has shown some ability to penetrate the BBB in preclinical models [17, 18]. In a phase III study comparing lapatinib plus capecitabine with capecitabine in 342 patients with metastatic HER2+ breast cancer refractory to trastuzumab, four patients (2%) in the combination-therapy group were reported to have symptomatic CNS progression as part of their first progression event versus 13 patients (6%) in the monotherapy group (p = 0.045), suggesting that lapatinib may be able to delay or prevent metastatic spread to the CNS [19]. In a phase II study of 242 patients with HER2+ CNS metastases whose disease had progressed on trastuzumab and had been treated with cranial radiation (reported by Lin et al.), a modest CNS response (defined as either a complete response or ≥50% reduction in the volumetric sum of all measurable CNS lesions) of 6% for single-agent lapatinib was reported [20]. Furthermore, in an extension to this study, in which 50 patients were treated with lapatinib in combination with capecitabine, a 20% objective response rate and a ≥20% reduction in disease volume in 40% of patients was reported [20]. The clinical benefit of lapatinib plus capecitabine in patients with treatment-naïve, HER2+ BCBMs was examined in the LANDSCAPE study [21]. In this single-arm, phase II study of 45 patients with previously untreated HER2+ brain metastases, 66% achieved a partial response (PR; defined as more than 50% volumetric reduction in brain metastatic lesions) following treatment with lapatinib plus capecitabine. Median time to progression was 5.5 months, and median time to WBRT was 8.3 months. Treatment in both this trial and the trial reported by Lin et al. was associated with significant toxicity, with 49% of patients in the LANDSCAPE trial having grade 3 or grade 4 treatment-related adverse events, of which the most common were diarrhea and hand–foot syndrome [21].
Overall, despite the clinical benefits observed with lapatinib in patients with HER2+ BCBMs, progression-free survival is still poor, and combination with capecitabine is required for maximal effect, increasing the risk of diarrhea as well as other chemotherapy-related side effects.
The PI3K pathway in HER2+ breast cancer
The PI3K/AKT/mTOR pathway, which plays a key role in regulating cell survival, growth, and proliferation, has also been shown to be involved in the development, progression, and treatment resistance of multiple cancers, including HER2+ breast cancer, and has been the subject of several recent thorough reviews [22–24]. PI3Ks are a family of lipid kinases comprising three classes, with class I being the most studied. Class IA PI3Ks are activated by receptor tyrosine kinases (such as EGFR and HER2), G protein-coupled receptors, and some oncogenes (such as Ras), and comprise of a regulatory subunit (p85) and a catalytic subunit (p110α, p110β, or p110δ). Upon activation, PI3K converts phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-bisphosphate (PIP3), which in turn activates a downstream signaling cascade involving the serine– threonine protein kinases AKT and mTOR complex 1 (mTORC1; Fig. 1) [22].
Fig. 1. The PI3K pathway in HER2+ breast cancer.
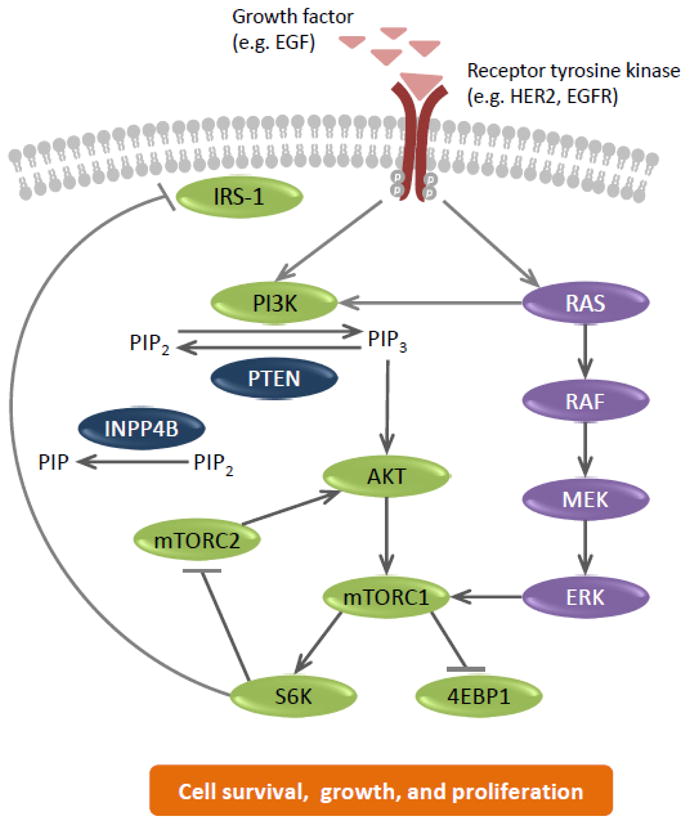
4EBP1, eukaryotic initiation factor 4E-binding protein 1; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular signal-related kinase; HER2, human epidermal growth factor receptor; INPP4B, inositol polyphosphate-4-phosphatase; IRS-1, insulin receptor substrate 1; MEK, mitogen-activated protein/ERK kinase; mTORC, mammalian target of rapamycin complex; PI3K, phosphatidylinositol 3-kinase; PIP, phosphatidylinositol 3-bisphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-bisphosphate; PTEN, phosphatase and tensin homolog; S6K, ribosomal protein S6 kinase.
The major negative regulator of the pathway is the tumor suppressor phosphatase and tensin homolog (PTEN), which converts PIP3 to PIP2 [22]. In addition, inositol polyphosphate-4-phosphatase type II (INPP4B) also negatively regulates the pathway by converting PIP2 to phosphatidylinositol 3-bisphosphate [25]. Another level of negative feedback is mediated through the activation of S6 kinase (S6K) by mTORC1; S6K inhibits another mTOR complex, mTORC2, whose activity is required for phosphorylation of AKT at S473 and full activation of the kinase [26]. In addition, S6K negatively regulates the PI3K/AKT/mTOR pathway through inhibition of insulin receptor substrate-1 (Fig. 1) [27].
Alterations that activate the PI3K/AKT/mTOR pathway have been linked to various neoplasms, including HER2+ breast cancer [22, 28, 29]. PIK3CA, the gene encoding p110α (a catalytic subunit of class IA PI3K), is one of the most commonly mutated genes found in breast cancers [23, 29]. In a recent comprehensive genomic analysis of HER2-enriched primary breast cancers, activating mutations of PIK3CA and PIK3R1 (a gene encoding the regulatory subunit p85) were identified in 39% and 7% of tumors, respectively, while PIK3CA was also amplified in 29% of tumors. In addition, homozygous or hemizygous deletions of the tumor suppressors PTEN and INPP4B were observed in 16% and 29% of tumors, respectively [29]. In another report, activation of the PI3K/AKT/mTOR pathway (defined as PIK3CA alteration, PTEN loss, or AKT activation) was reported to be as high as 75% [28]. Activation of the pathway has been associated with poor prognosis in patients with HER2+ breast cancer following trastuzumab treatment, and has been implicated in resistance to HER2-targeted therapies, including trastuzumab and lapatinib [30, 31]. Furthermore, in one study of 52 BCBMs, the PI3K/AKT/mTOR pathway was found to be active in approximately 70% of BCBMs [32]. In another study sequencing 110 primary breast tumors and BCBMs, alterations in PTEN were found in a significantly larger fraction of BCBM tumor tissues compared with samples from primary tumors with good prognosis, bone relapse, or other distant metastases [33]. Activation of the pathway in BCBMs validates it as a potential therapeutic target.
PI3K/AKT/mTOR pathway inhibitors in HER2+ BCBMs
Various drugs targeting key components of the PI3K/AKT/mTOR pathway are currently in development and include PI3K, mTORC1, dual mTORC1/2, AKT, and dual PI3K and mTORC1/2 inhibitors. Here we will review the data for those drugs that have shown preliminary efficacy in the treatment of cancer involving the CNS in clinical or preclinical models (Table 1).
Table 1.
Inhibitors of the PI3K/AKT/mTOR pathway with preclinical or clinical evidence of activity in the central nervous system
| Inhibitor | References | Ongoing clinical trials in HER2+ BCBMs | |
|---|---|---|---|
| Preclinical | Clinical | ||
| mTOR inhibitors | |||
| Everolimus | [34] | [35] | Ph II study of everolimus in combination with trastuzumab and vinorelbine in HER2+ BCBMs (NCT01305941) |
| Temsirolimus | [40] | [42] | |
| Dual mTORC1/2 inhibitors | |||
| Palomid 529 | [43] | ||
| PI3K inhibitors | |||
| Buparlisib (BKM120) | [46–48] | [49–51] | Ph I/II study in combination with trastuzumab in patients with trastuzumab-resistant HER2+ BCBMs (expansion cohort; NCT01132664) |
| PX-886 | [54] | ||
| SAR245408 | [57] | ||
| Dual PI3K/mTOR inhibitors | |||
| BEZ235 | [58] | [59] | |
| SAR245409 | [57] | ||
| GNE-317 | [61] | ||
BCBM, breast cancer brain metastasis; HER2, human epidermal growth factor receptor 2; mTORC, mammalian target of rapamycin complex; PI3K, phosphatidylinositol 3-kinase.
mTOR inhibitors
Everolimus (RAD001), a rapamycin analog, is an oral allosteric mTORC1 inhibitor. There is evidence in animal studies that this lipophilic compound can cross the BBB [34]. In mouse studies, everolimus uptake in the brain was modest but dose dependent and with a longer half-life compared with that in the systemic circulation [34].
The clearest clinical evidence for activity of everolimus in the CNS in humans comes from its use in the treatment of subependymal giant-cell astrocytomas associated with tuberous sclerosis. In tuberous sclerosis, mTOR is constitutively expressed leading to various tumors. A phase III trial, in which 117 patients with tuberous sclerosis complex and at least one subependymal giant-cell astrocytoma lesion with a diameter of 1 cm or greater were randomized to receive either everolimus or placebo, found that 35% of patients treated with everolimus achieved at least a 50% reduction in the size of their subependymal giant-cell astrocytomas compared with none in the placebo arm. Furthermore, the majority (78%) of patients treated with everolimus had at least a 30% reduction in tumor volume [35]. Everolimus in now approved for this indication.
Everolimus has also been shown to have activity in estrogen receptor-positive breast cancer and in 2012 was approved for use in combination with an aromatase inhibitor in post-menopausal patients with hormone receptor-positive (HR+) advanced disease that has progressed on or after a non-steroidal aromatase inhibitor as a result of the phase III BOLERO-2 trial [36]. Early-phase clinical data also suggested activity in HER2+ advanced breast cancer [37, 38], and more recently data from the BOLERO-3 trial assessing the triple combination of vinorelbine, trastuzumab, and everolimus versus vinorelbine, trastuzumab, and placebo in trastuzumab-resistant advanced HER2+ breast cancer demonstrated that the addition of everolimus significantly prolonged progression-free survival, with a 22% decreased risk of disease progression or death [39]. However, in most of these studies, including the pivotal BOLERO-2 trial, patients with CNS metastases were either excluded or CNS-specific responses were not reported. Despite this, a phase II study of everolimus, trastuzumab, and vinorelbine is currently recruiting patients with HER2+ BCBMs (NCT01305941).
Temsirolimus, another mTORC1 inhibitor, has also demonstrated ability to cross the BBB in preclinical models, and in combination with the MEK inhibitor SL327 significantly reduced brain metastases in a triple-negative breast cancer mouse-xenograft model [40]. In the clinic, although it has not yet shown definitive efficacy in patients with breast cancer [41], temsirolimus may have efficacy in patients with glioblastoma [42].
A potential limitation to the use of mTORC1 inhibitors is the loss of negative feedback regulation. Dual inhibitors of mTORC1/2, therefore, have potential for improved pathway inhibition, as they prevent mTORC2-dependent AKT activation, which may be upregulated following treatment with mTORC1 inhibitors due to the loss of the inhibitory effect of S6K on mTORC2 [27]. The dual mTORC1/2 inhibitor palomid 529, which is in the early stages of development, has been shown to bypass the ATP-binding cassette (ABC) drug efflux transporters ABCB1 (P-glycoprotein) and ABCG2 (breast cancer-resistant protein), and to effectively inhibit orthotopic glioblastoma cells in mice [43]. This drug has not yet been tested in humans but represents the first mTOR inhibitor, which bypasses the major transporter systems that constitute the BBB.
In addition to the potential for increased mTORC2-mediated AKT activation following treatment with mTORC1 inhibitors, the loss of negative feedback regulation on receptor tyrosine kinases via S6K inhibition of insulin receptor substrate 1 is another potential limitation of mTOR inhibitors [27, 44, 45]. Co-targeting the pathway upstream (e.g. with anti-HER2 therapy) or targeting the pathway higher up (e.g. with PI3K inhibitors) or at several nodes (e.g. with dual PI3K/mTOR inhibitors) may therefore result in improved pathway inhibition.
PI3K inhibitors
Buparlisib (BKM120) is a recently developed oral pan-PI3K inhibitor with enhanced activity against tumors harboring PIK3CA mutations when tested in immunodeficient mice bearing HER2+ breast cancer xenografts [46]. Preclinical studies in rodents have demonstrated that buparlisib can penetrate the BBB, and inhibit the PI3K/AKT/mTOR pathway, as evidenced by reduced phosphorylated AKT (pAKT) in the brains of mice treated with buparlisib [47, 48]. Furthermore, in a mouse model recapitulating widely metastatic HER2+ breast cancer, buparlisib effectively controlled metastatic growth in multiple organs, including the brain [47, 48]. In fact, in the brains of mice treated with buparlisib, >90% inhibition in the number of metastatic human cells was found [47]. In the clinic, buparlisib has also been shown to penetrate the BBB in patients with glioblastoma, as evidenced by reduced pAKT levels in biopsies taken before and after treatment [49]. It is noteworthy that the ability to cross the BBB is not common to all PI3K inhibitors [47, 48].
In a phase I dose-escalation study of buparlisib in advanced solid tumors, the maximum tolerated dose was determined to be 100 mg/day [50]. Of 31 evaluable patients, there was one PR (triple-negative breast cancer) and 16 patients (52%) had stable disease, including five patients with breast cancer. Of note, a 28% reduction in a CNS lesion was observed in one patient with metastatic breast cancer [50]. In another phase Ib/II study of buparlisib plus trastuzumab in patients with locally advanced or metastatic HER2+ breast cancer resistant to trastuzumab, two out of four patients with measurable CNS disease had stable disease at the time of study withdrawal [51]. An expansion arm of this study is investigating buparlisib plus trastuzumab, specifically in patients with HER2+ BCBMs (NCT01132664).
Common adverse events reported with buparlisib include rash, hyperglycemia (due to inhibition of PI3K-dependent insulin signaling), diarrhea, anorexia, and nausea. Also of note, altered mood and anxiety were reported in 20% and 17% of patients, respectively [50]. Mood alterations may be due to PI3K inhibition in the CNS, as decreased PI3K activity has been shown to be associated with decreased serotonin in the amygdala and psychiatric disturbances such as anxiety and depression in mice [52, 53]. Importantly, changes in mood were reversible upon buparlisib discontinuation and were responsive to treatment with selective serotonin reuptake inhibitors [50].
Other PI3K inhibitors in development with evidence of brain penetration include PX-866 and SAR245408 (XL147). PX-866 is an oral irreversible PI3K inhibitor that was found to have antitumor activity in xenograft models of glioblastoma, where it inhibited subcutaneous tumor growth and increased the median survival time of animals with intracranial tumors [54]. A phase I study has found it to be safe in 84 patients with advanced tumors, including three patients with breast cancer [55]. A phase II study in patients with recurrent glioblastoma is ongoing (NCT01259869). SAR245408 has also been tested in a phase I trial in patients with advanced solid tumors, where it demonstrated robust pharmacodynamic activity across diverse tumors, and preliminary efficacy [56]. In patients with glioblastoma, SAR245408 was taken up into CNS tumors (mean tumor:plasma ratio = 0.27), and resulted in a reduction in S6K, indicative of an inhibitory effect on PI3K signaling [57].
Dual PI3K/mTOR inhibitors
The catalytic domains of mTORC1/2 and the p110 subunit of PI3K are similar and therefore present a unique opportunity for development of drugs that target both molecules [24], and several dual PI3K and mTORC1/2 inhibitors are currently in development. BEZ235 is one such inhibitor and it has shown promise in preclinical models of gliomas [58]. In a recently reported phase I/Ib study in patients with metastatic HER2+ breast cancer with PIK3CA or PTEN alterations, oral BEZ235 was given in combination with trastuzumab [59]. Of the 15 patients evaluated at various doses, one patient with lung and brain metastases had a PR in brain lesions, indicating some BBB penetration, and four patients had disease stabilization for at least four cycles (16 weeks). Several studies examining the potential of BEZ235 in patients with breast cancer are ongoing, though none are focusing on brain metastases at this time (NCT01495247, NCT01300962).
Another dual PI3K and mTOR inhibitor SAR245409 (XL765), which had previously demonstrated antitumor activity in patients with metastatic or unresectable solid tumors, is currently being evaluated in patients with glioblastoma [60]. In a phase I study, oral SAR245409 was shown to effectively cross the BBB, with mean tumor:plasma ratios of 0.38 and 0.40 with once-daily and twice-daily dosing regimens, respectively [57]. Furthermore, inhibition of the PI3K/AKT/mTOR pathway, as evidenced through reductions in pS6K, were also observed [57].
GNE-317 is a dual PI3K/mTORC1/2 inhibitor that was specifically designed to cross the BBB by bypassing the two main transporters that constitute the BBB, ABCB1, and ABCG2 [61]. In preclinical studies, GNE-317 achieved potent suppression of the PI3K/AKT/mTOR pathway in the brains of mice with an intact BBB. pAKT, p4EBP1, and pS6, downstream targets of PI3K, were suppressed by 80%, 84%, and 92% respectively, following treatment with a single oral dose of GNE-317 (50 mg/kg). In the same study, GNE-317 also demonstrated efficacy in three distinct orthotopic mouse models of glioblastoma, where it reduced tumor volumes by 50–90%. A similar dual mTOR/PI3K inhibitor with reported ability to cross the BBB, GDC-0084, is being evaluated in a phase I trial of patients with glioblastoma (NCT01547546). It remains to be seen whether either drug has benefit in breast cancer cell lines or patients.
Summary
Activation of the PI3K/AKT/mTOR pathway occurs in approximately 75% of HER2+ breast cancers and a similar rate of HER2+ BCBMs [28, 32]. In patients with refractory HR+ breast cancer, inhibition of this pathway has already proved beneficial, resulting in FDA approval of everolimus in combination with an aromatase inhibitor. In HER2+ breast cancer, especially with brain metastatic disease, the targeting of this pathway represents an exciting new potential route of therapy. Several PI3K/AKT/mTOR pathway inhibitors have demonstrated an ability to cross the BBB and to inhibit PI3K/AKT/mTOR signaling, and combinations of PI3K/AKT/mTOR inhibitors with agents that target HER2 are currently in various stages of clinical development in patients with HER2+ BCBMs.
Acknowledgments
We thank Amanda Quinn for her medical editorial assistance with this manuscript. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Funding
Dr Hurvitz receives support from the National Cancer Institute of the National Institutes of Health under Award Number P30CA016042.
Footnotes
Conflict of interest
Dr. Hurvitz has received Novartis reimbursement for travel to international conference. Dr. Peddi has no conflict of interest to report.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Peddi receives support from the Conquer Cancer Foundation of the American Society of Oncology through a Young Investigator Award for year 2013-2014.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 6.Souglakos J, Vamvakas L, Apostolaki S, et al. Central nervous system relapse in patients with breast cancer is associated with advanced stages, with the presence of circulating occult tumor cells and with the HER2/neu status. Breast Cancer Res. 2006;8:R36. doi: 10.1186/bcr1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–5286. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 8.Berghoff A, Bago-Horvath Z, De Vries C, et al. Brain metastases free survival differs between breast cancer subtypes. Br J Cancer. 2012;106:440–446. doi: 10.1038/bjc.2011.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 10.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 11.Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 12.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 13.Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clin Breast Cancer. 2001;2:235. doi: 10.1016/S1526-8209(11)70419-0. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira M, Braga S, Passos-Coelho JL, Fonseca R, Oliveira J. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Res Treat. 2011;127:841–844. doi: 10.1007/s10549-011-1417-2. [DOI] [PubMed] [Google Scholar]
- 15.Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006;7:778–780. doi: 10.1016/S1470-2045(06)70864-6. [DOI] [PubMed] [Google Scholar]
- 16.Mir O, Ropert S, Alexandre J, Lemare F, Goldwasser F. High-dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2 overexpressing breast cancer. Ann Oncol. 2008;19:1978–1980. doi: 10.1093/annonc/mdn654. [DOI] [PubMed] [Google Scholar]
- 17.Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29:770–781. doi: 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 20.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 21.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16 (suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 24.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates AKT. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Knowles E, O'Toole SA, McNeil CM, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer. 2010;126:1121–1131. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Jegg AM, Ward TM, Iorns E, et al. PI3K independent activation of mTORC1 as a target in lapatinib- resistant ERBB2+ breast cancer cells. Breast Cancer Res Treat. 2012;136:693–692. doi: 10.1007/s10549-012-2252-9. [DOI] [PubMed] [Google Scholar]
- 32.Adamo B, Deal AM, Burrows E, et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125. doi: 10.1186/bcr3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohensee I, Lamszus K, Riethdorf S, et al. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183:83–95. doi: 10.1016/j.ajpath.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 34.O'Reilly T, McSheehy PM, Kawai R, et al. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother Pharmacol. 2010;65:625–639. doi: 10.1007/s00280-009-1068-8. [DOI] [PubMed] [Google Scholar]
- 35.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 36.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andre F, Campone M, O'Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 38.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Regan R, Ozguroglu M, Andre F, et al. Phase III, randomized, double-blind, placebo-controlled multicenter trial of daily everolimus plus weekly trastuzumab and vinorelbine in trastuzumab-resistant, advanced breast cancer (BOLERO-3) J Clin Oncol. 2013;31(suppl):abstr 505. [Google Scholar]
- 40.Zhao H, Cui K, Nie F, et al. The effect of mTOR inhibition alone or combined with MEK inhibitors on brain metastasis: an in vivo analysis in triple-negative breast cancer models. Breast Cancer Res Treat. 2012;131:425–436. doi: 10.1007/s10549-011-1420-7. [DOI] [PubMed] [Google Scholar]
- 41.Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 43.Lin F, Buil L, Sherris D, Beijnen JH, van Tellingen O. Dual mTORC1 and mTORC2 inhibitor Palomid 529 penetrates the Blood-Brain Barrier without restriction by ABCB1 and ABCG2. Int J Cancer. 2013;133(5):1222–1233. doi: 10.1002/ijc.28126. [DOI] [PubMed] [Google Scholar]
- 44.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-Kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 47.Nanni P, Nicoletti G, Palladini A, et al. Multiorgan Metastasis of Human HER-2(+) Breast Cancer in Rag2(−/−);Il2rg(−/−) Mice and Treatment with PI3K Inhibitor. PLoS One. 2012;7:e39626. doi: 10.1371/journal.pone.0039626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maira M, Schnell C, Lollini P, et al. Preclinical and preliminary clinical activity of NVP-BKM120, an oral pan-class I PI3K inhibitor, in the brain. Ann Oncol. 2012;23(suppl 9):abstr 1675. [Google Scholar]
- 49.Wen P, Yung W, Mellinghoff I, et al. Phase II trial of the phosphatidyinositol-3 kinase (PI3K) inhibitor BKM120 in recurrent glioblastoma (GBM) J Clin Oncol. 2013;31(suppl):abstr 2015. [Google Scholar]
- 50.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 51.Pistilli B, Urruticoechea A, Chan S, et al. Ph Ib/II study of BKM120 plus trastuzumab (T) in patients with T-resistant HER2+ advanced breast cancer (BC) Ann Oncol. 2012;23(suppl 9):abstr 3180. [Google Scholar]
- 52.Tohda C, Nakanishi R, Kadowaki M. Hyperactivity, memory deficit and anxiety-related behaviors in mice lacking the p85alpha subunit of phosphoinositide-3 kinase. Brain Dev. 2009;31:69–74. doi: 10.1016/j.braindev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Ackermann TF, Hortnagl H, Wolfer DP, et al. Phosphatidylinositide dependent kinase deficiency increases anxiety and decreases GABA and serotonin abundance in the amygdala. Cell Physiol Biochem. 2008;22:735–744. doi: 10.1159/000185557. [DOI] [PubMed] [Google Scholar]
- 54.Koul D, Shen R, Kim YW, et al. Cellular and in vivo activity of a novel PI3K inhibitor, PX-866, against human glioblastoma. Neuro Oncol. 2010;12:559–569. doi: 10.1093/neuonc/nop058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong DS, Bowles DW, Falchook GS, et al. A multicenter Phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–4182. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 56.Edelman G, Bedell C, Shapiro G, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(suppl):abstr 3004. [Google Scholar]
- 57.Cloughesy TF, Mischel P, Omuro A, et al. Tumor pharmacokinetics (PK) and pharmacodynamics (PD) of SAR245409 (XL765) and SAR245408 (XL147) administered as single agents to patients with recurrent glioblastoma (GBM): An Ivy Foundation early-phase clinical trials consortium study. J Clin Oncol. 2013;31(suppl):abstr 2012. [Google Scholar]
- 58.Liu TJ, Koul D, LaFortune T, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krop I, Saura C, Rodon J, et al. A Phase I/Ib dose-escalation study of BEZ235 in combination with trastuzumab in patients with PI3-kinase or PTEN altered HER2+ metastatic breast cancer. J Clin Oncol. 2012;30(suppl):abstr 508. [Google Scholar]
- 60.Brana I, LoRusso P, Baselga J, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(suppl):abstr 3030. [Google Scholar]
- 61.Salphati L, Heffron TP, Alicke B, et al. Targeting the PI3K Pathway in the Brain--Efficacy of a PI3K inhibitor optimized to cross the blood–brain barrier. Clin Cancer Res. 2012;18:6239–6248. doi: 10.1158/1078-0432.CCR-12-0720. [DOI] [PubMed] [Google Scholar]


