Abstract
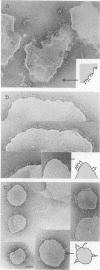
Activation of the membrane fusion potential of influenza haemagglutinin (HA) at endosomal pH requires changes in its structure. X-ray analysis of TBHA2, a proteolytic fragment of HA in the fusion pH conformation, indicates that at the pH of fusion the 'fusion peptide' is displaced by > 10 nm from its location in the native structure to the tip of an 11 nm triple-stranded coiled coil, and that the formation of this structure involves extensive re-folding or reorganization of HA. Here we examine the structure of TBHA2 with the electron microscope and compare it with the fusion pH structure of HA2 in virosomes, HA2 in aggregates formed at fusion pH by the soluble, bromelain-released ectodomain BHA and HA2 in liposomes with which BHA associates at fusion pH. We have oriented each HA2 preparation for comparison, using site-specific monoclonal antibodies. We conclude that the structural changes in membrane-anchored and soluble HA preparations at the pH of fusion appear to be the same; that in the absence of a target membrane, the 'fusion peptide' of HA in virosomes associates with the virosome membrane so that HA2 is membrane bound at both N- and C-termini, which implies that inversion of the re-folded HA can occur; and that the structural changes observed by X-ray analysis do not result from the proteolytic digestions used in the preparation of TBHA2.
Full text
PDF



Images in this article
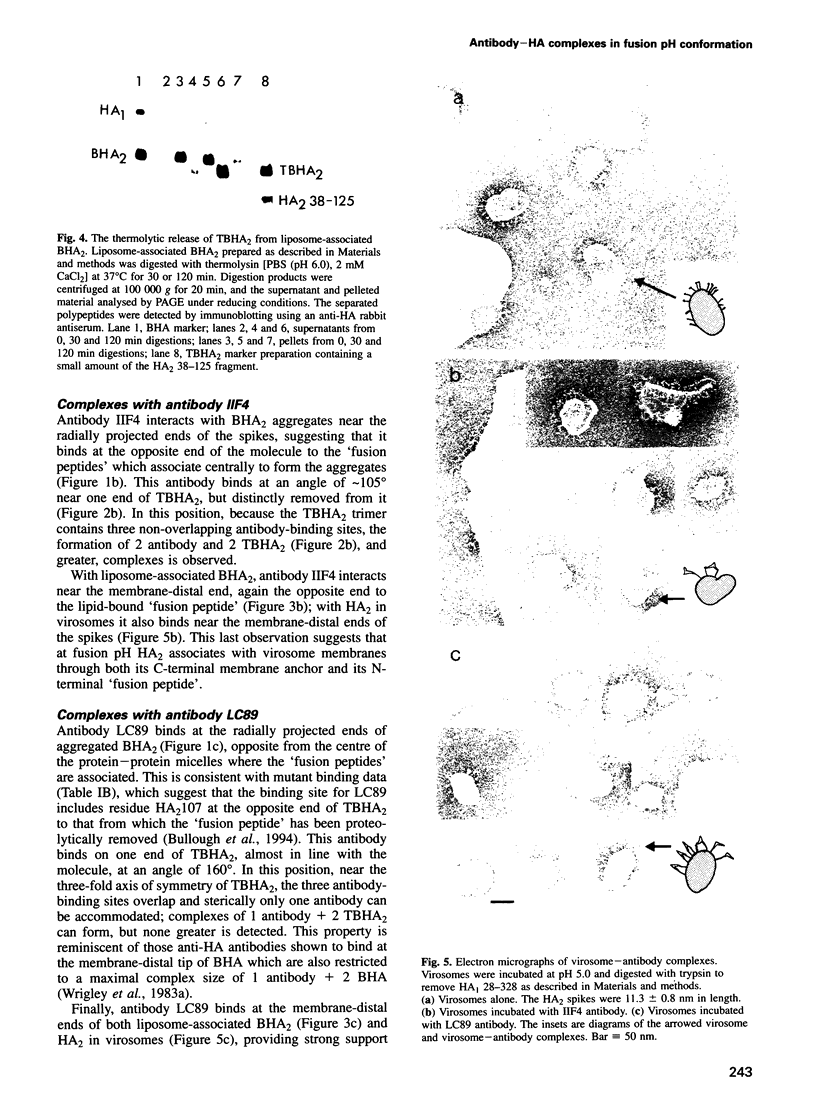
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos E. S., van der Doelen A. A., van Rooy N., Schuurs A. H. 3,3',5,5' - Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoassay. 1981;2(3-4):187–204. doi: 10.1080/15321818108056977. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994 Sep 1;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993 May 21;73(4):823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Daniels P. S., Jeffries S., Yates P., Schild G. C., Rogers G. N., Paulson J. C., Wharton S. A., Douglas A. R., Skehel J. J., Wiley D. C. The receptor-binding and membrane-fusion properties of influenza virus variants selected using anti-haemagglutinin monoclonal antibodies. EMBO J. 1987 May;6(5):1459–1465. doi: 10.1002/j.1460-2075.1987.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983 Aug;64(Pt 8):1657–1662. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Downie J. C., Hay A. J., Knossow M., Skehel J. J., Wang M. L., Wiley D. C. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985 Feb;40(2):431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Godley L., Pfeifer J., Steinhauer D., Ely B., Shaw G., Kaufmann R., Suchanek E., Pabo C., Skehel J. J., Wiley D. C. Introduction of intersubunit disulfide bonds in the membrane-distal region of the influenza hemagglutinin abolishes membrane fusion activity. Cell. 1992 Feb 21;68(4):635–645. doi: 10.1016/0092-8674(92)90140-8. [DOI] [PubMed] [Google Scholar]
- Knossow M., Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Three-dimensional structure of an antigenic mutant of the influenza virus haemagglutinin. Nature. 1984 Oct 18;311(5987):678–680. doi: 10.1038/311678a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Aitken A., Calder L. J., Martin S. R., Skehel J. J., Wharton S. A., Weis W., Wiley D. C. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J Gen Virol. 1988 Nov;69(Pt 11):2785–2795. doi: 10.1099/0022-1317-69-11-2785. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Wrigley N. G., Calder L. J., Cusack S., Wharton S. A., Brown E. B., Skehel J. J. Electron microscopy of the low pH structure of influenza virus haemagglutinin. EMBO J. 1986 Jan;5(1):41–49. doi: 10.1002/j.1460-2075.1986.tb04175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
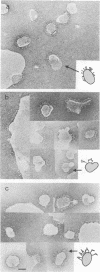
- Skehel J. J., Schild G. C. The polypeptide composition of influenza A viruses. Virology. 1971 May;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Varecková E., Mucha V., Ciampor F., Betáková T., Russ G. Monoclonal antibodies demonstrate accessible HA2 epitopes in minor subpopulation of native influenza virus haemagglutinin molecules. Arch Virol. 1993;130(1-2):45–56. doi: 10.1007/BF01318995. [DOI] [PubMed] [Google Scholar]
- Watowich S. J., Skehel J. J., Wiley D. C. Crystal structures of influenza virus hemagglutinin in complex with high-affinity receptor analogs. Structure. 1994 Aug 15;2(8):719–731. doi: 10.1016/s0969-2126(00)00073-3. [DOI] [PubMed] [Google Scholar]
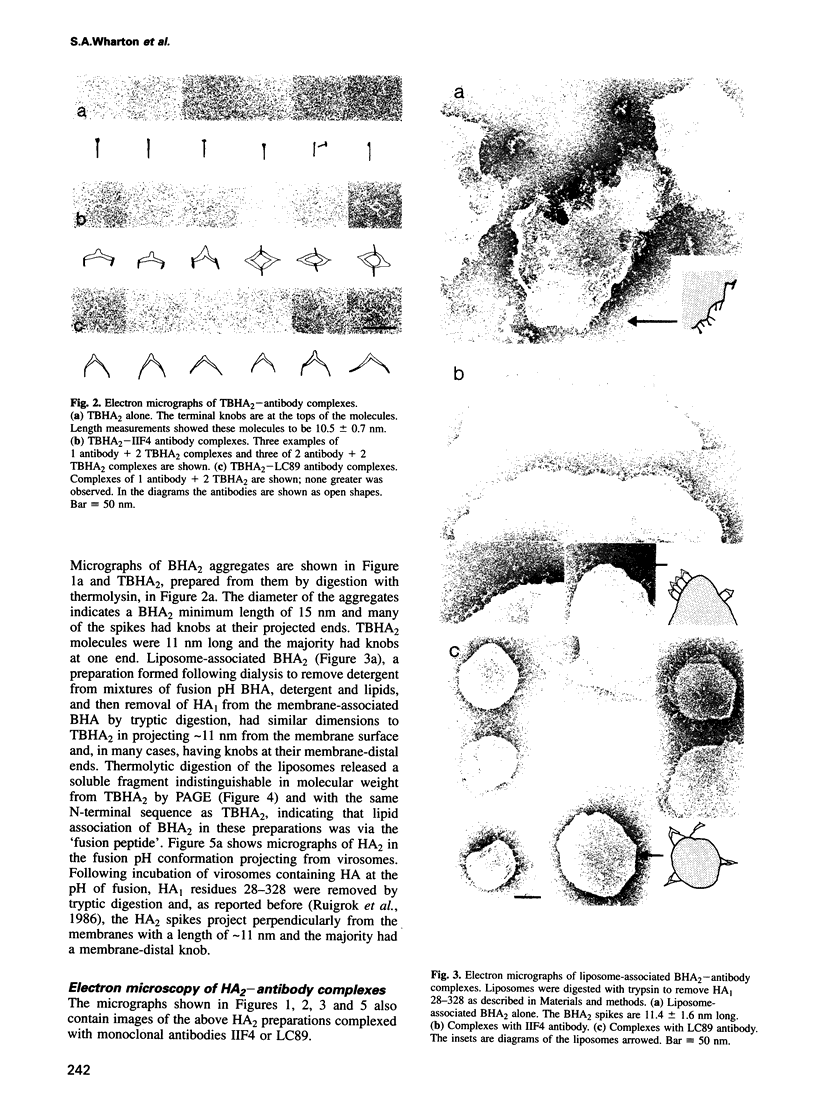
- Weber T., Paesold G., Galli C., Mischler R., Semenza G., Brunner J. Evidence for H(+)-induced insertion of influenza hemagglutinin HA2 N-terminal segment into viral membrane. J Biol Chem. 1994 Jul 15;269(28):18353–18358. [PubMed] [Google Scholar]
- Webster R. G., Brown L. E., Jackson D. C. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology. 1983 Apr 30;126(2):587–599. doi: 10.1016/s0042-6822(83)80015-4. [DOI] [PubMed] [Google Scholar]
- White J. M., Wilson I. A. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J Cell Biol. 1987 Dec;105(6 Pt 2):2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Brown E. B., Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Electron microscopy of influenza haemagglutinin-monoclonal antibody complexes. Virology. 1983 Dec;131(2):308–314. doi: 10.1016/0042-6822(83)90499-3. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Brown E., Chillingworth R. K. Combining accurate defocus with low-dose imaging in high resolution electron microscopy of biological material. J Microsc. 1983 May;130(Pt 2):225–232. doi: 10.1111/j.1365-2818.1983.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gerhard W., Bachi T. Monoclonal anti-hemagglutinin antibodies detect irreversible antigenic alterations that coincide with the acid activation of influenza virus A/PR/834-mediated hemolysis. J Virol. 1983 Oct;48(1):239–248. doi: 10.1128/jvi.48.1.239-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]