Abstract
Bariatric surgery improves glucose homeostasis and alters gut hormones partly independent of weight loss. Leptin plays a role in these processes; levels are decreased following bariatric surgery, creating a relative leptin insufficiency. We previously showed that leptin administration in a weight-reduced state after Roux-en-Y gastric bypass (RYGB) caused no further weight loss. Here, we discuss the impact of leptin administration on gut hormones, glucostasis, and appetite. Weight stable women after RYGB were randomized to receive placebo or recombinant human metreleptin (0.05 mg/kg twice daily). At weeks 0 and 16, a liquid meal challenge was performed. Glucose, insulin, C-peptide, GLP-1, PYY, glucagon, and ghrelin (total, acyl, and desacyl) were measured fasting and postprandially. Appetite was assessed using a visual analog scale. Mean post-op period was 53 ± 2.3 months; mean BMI was 34.6 ± 0.2 kg/m2. At 16 weeks, there was no significant change in weight within or between groups. Fasting PYY was significantly different between groups and the leptin group had lower sweets craving at week 16 than the placebo group (P < 0.05). No other differences were observed. Leptin replacement does not alter gut hormones or glucostasis but may diminish sweet cravings compared to placebo in this population of post-RYGB women.
1. Introduction
Roux-en-Y gastric bypass (RYGB) surgery results in a reduction of approximately 38% of total body weight at one year that, unlike diet therapy alone, is mostly maintained over the long term [1]. In addition to weight reduction, improvement in glucose homeostasis has also been observed, which may be partly independent of reduced body weight. Unique alterations in circulating levels of gut hormones, such as ghrelin, peptide YY (PYY), and glucagon-like peptide 1 (GLP-1), also occur after RYGB that create an environment favoring decreased appetite, weight reduction, maintenance of a reduced body weight, and improved glucose tolerance. Ghrelin, an orexigenic hormone produced in cells of the oxyntic glands of the stomach, was found to decrease or remain the same following RYGB [2, 3], in contrast to the usual increase in ghrelin levels that occurs after diet or gastric banding. PYY is secreted by intestinal L cells in response to food intake, leading to a decrease in gastrointestinal motility and increased satiety. Postprandial PYY levels are markedly increased after RYGB [2, 4]. Circulating concentrations of GLP-1, also produced by L cells, are increased following RYGB, contributing directly to reduced appetite, increased satiety, and weight loss as well as increases in glucose-stimulated insulin release following food ingestion [4, 5].
Many individuals who have undergone RYGB experience a plateau in weight loss with a body mass index (BMI) still within the obese range [6]. Counterregulatory hormones may impede further loss despite the presence of excess of body fat [7]. Leptin is a critical afferent component of a regulatory loop linking fat mass to food intake and energy expenditure and has also been shown to play an important role in glucose homeostasis through its effects on insulin as well as other mediators of glucose metabolism [8, 9]. Following weight loss, leptin levels decrease out of proportion to the amount of fat mass [10]. Leptin levels in those having lost weight following RYGB are less than levels in BMI-matched individuals who have not undergone weight loss [11], putting the former in a state of relative leptin insufficiency, which may be an important factor contributing to their inability to lose more weight.
Leptin is thought to modulate a number of hormones involved in appetite regulation and food metabolism, which are themselves altered by weight loss [12, 13]. While its relationship with some appetitive hormones is as yet unclear, animal studies have suggested that GLP-1 as well as PYY are increased following leptin administration [14–16], favoring appetite reduction and weight loss. Leptin and ghrelin have opposing actions and leptin administration in animal models has resulted in a reduction in ghrelin levels [17, 18]. Leptin administration has been shown to increase satiety and satiation in mouse models of obesity as well as in humans with leptin insufficiency [19–21], suggesting that it may affect the secretion and/or function of such hormones to promote weight reduction. Insulin sensitivity is improved following leptin administration [22], and leptin has been found to decrease glucagon levels in rat and mouse models of both type 1 and type 2 diabetes, contributing to the improvement in glycemic status [23, 24].
Leptin replacement therapy has been used in humans with congenital leptin deficiency and has resulted in weight loss when prescribed in physiologic doses. However, high pharmacologic levels of leptin are required to induce weight loss in otherwise healthy obese individuals; physiologic replacement of leptin has led to minimal to no weight loss [25–31]. In contrast, administration of physiologic replacement doses of leptin that restore circulating concentrations to preweight loss levels reverses many of the manifestations characteristic of the weight-reduced state, in some cases, irrespective of further weight loss [25–27]. Animal models of weight loss have suggested that leptin interacts with appetitive hormones in a manner that promotes further weight reduction [14, 15, 18]. Such interactions have yet to be studied in humans after RYGB.
We previously reported that, contrary to our expectation, leptin administration did not lead to further weight loss in women who had undergone weight loss after RYGB and whose leptin levels were lower than that predicted for a nonreduced individual with the same BMI [32]. This paper examines our secondary objective, which was to establish whether leptin administration in this weight-reduced state would be associated with changes in hormones involved in nutrient metabolism as well as in satiation. We hypothesized that leptin administration would lead to alteration in gut hormones that would promote further weight reduction and improve glucose homeostasis.
2. Materials and Methods
2.1. Study Subjects
Women between the ages of 25 and 65 years who were at least 18 months after RYGB, had a percent total weight loss from the highest presurgical weight to current weight between 18% and 45%, and had a current BMI of 28–50 kg/m2 were invited to participate. Subjects were considered for enrollment if their plasma leptin level was less than the level predicted from the regression equation generated using leptin levels and BMI from a non-weight-reduced cohort of 55 women who had participated in previous studies from our group: (0.991 × BMI) − 3.37. [JK, unpublished data]. Exclusion criteria have been described elsewhere [32]. This study is in accordance with the guidelines of the Declaration of Helsinki and was approved by the Columbia University Institutional Review Board. All subjects provided written informed consent.
Thirty-five of the 69 subjects screened met enrollment criteria. Eight subjects failed to have at least one follow-up visit after randomization and were excluded from the analysis. Of the remaining 27 subjects, 22 completed the test meal at baseline and 16 weeks after treatment [32].
2.2. Protocol
The study protocol has been described previously [32]. Briefly, subjects who met criteria entered a 2 week single-blind placebo run-in period, after which they were randomized to receive either placebo or recombinant human metreleptin. Metreleptin, referred to as “leptin,” and placebo were generously donated by Amylin Pharmaceuticals (San Diego, CA). The dose of leptin (0.05 mg/kg body weight self-administered via subcutaneous injection twice daily) was expected to raise maximum plasma leptin levels to high physiologic/low pharmacologic levels yet would not be expected to cause clinically significant weight loss in a person who had not undergone weight reduction [25].
At weeks 0 and 16, a meal challenge (Optifast; Novartis, Minneapolis MN; 474 mL, 320 Kcal, 50% carbohydrate, 35% protein, and 15% fat) was performed. PYY, insulin, glucose, insulin, C-peptide, and ghrelin (total, acyl, and desacyl) were measured in the fasted state as well as 15, 30, 60, 90, and 120 minutes after consumption of the liquid beverage, with the exception of total GLP-1 that was measured in the fasted state as well as 15 and 30 minutes postprandially. Appetite was assessed using a validated visual analog scale [33] with questions about a subjective feeling written below a 100 mm line anchored on either end with opposite descriptors (not at all, extremely). Subjects were asked to make a vertical mark across the line corresponding to their feelings. The answer was quantified by measuring the distance from the left end of the line to the mark. The VAS was administered in the fasted state as well as 60, 90, and 120 minutes after consumption of the liquid meal.
Venous blood samples were collected in EDTA tubes that were centrifuged for 15 minutes at 4°C and stored at −80°C until assayed in duplicate. Glucose, insulin, leptin, and total ghrelin were measured as described elsewhere [11]. Assays for total PYY and total GLP-1 were previously described as well [34]. C-peptide was measured with the Immulite Analyzer (Diagnostic Products Corp., Los Angeles, CA). Glucagon was measured by RIA as per manufacturer's instructions (Millipore Corporation, Billerica, MA). Blood samples for the measurement of acyl and desacyl-ghrelin were collected in EDTA tubes containing AEBSF, were centrifuged, and then acidified with HCl prior to measurement by sandwich assays [35]. Plasma was diluted as necessary to obtain readings within the assay range.
2.3. Statistical Analysis
Data were evaluated for normality with the Kolmogorov-Smirnov test and none were found to require transformation. Raw score differences and percent change from baseline were calculated. For repeatedly measured postprandial samples, area-under-the-curve was calculated using the trapezoidal rule. Group differences at baseline were evaluated with independent t-tests for continuous measures. Group, time, and group by time interactions were estimated with linear mixed models for repeated measures as fixed effects, the value of the outcome at baseline entered as a continuous covariate, and a compound symmetry covariance structure for the autocorrelation of measures within subject. The covariance structure was selected prior to inferential testing from empirical evaluation of alternative structures. Model estimated mean and standard errors for differences between times within group and between groups at specific times were used for the specific comparisons. P values for differences are based on the method of simultaneous confidence intervals. Least squares regression was used to assess the association of weight loss to initial leptin levels, number of months between surgery and study entry, and percent weight loss from presurgical maximum weight. No adjustment for multiplicity was employed for the multiple endpoints assessed.
3. Results
Prior to RYGB, BMI was similar between groups (Table 1). Baseline characteristics, with the exception of age, were also similar at the time of this study. Duration of postoperative period was a mean of 53 ± 2.3 months for the study cohort. Mean percent weight loss from the highest preoperative weight to weight at time of screening visit was 30.7 ± 0.33%, with a range of 18.2–44.7%. The mean BMI for the study cohort at the time of screening was 34.6 ± 0.2 kg/m2, with a range of 28.4–41.7 kg/m2.
Table 1.
Baseline characteristics of study participants.
| Parameter | Placebo | Leptin | P value* |
|---|---|---|---|
| Age (y) | 42.2 ± 2.8 | 51.4 ± 2.0 | 0.02 |
| Pre-RYGB BMI (kg/m2) | 48.6 ± 1.9 | 47.1 ± 1.8 | 0.58 |
| BMI at screen (kg/m2) | 35.0 ± 1.1 | 33.3 ± 1.4 | 0.35 |
| Wt Loss (%) | 30.2 ± 2.3 | 30.7 ± 2.1 | 0.88 |
| Post-op period (mo) | 44.2 ± 7.4 | 64.6 ± 8.6 | 0.09 |
| Leptin (ng/mL) | 27.1 ± 3.2 | 21.8 ± 2.5 | 0.20 |
| Leptin/kg FM (ng/mL/kg) | 0.70 ± 0.06a | 0.66 ± 0.06b | 0.61 |
Results are expressed as mean ± SEM. n = 11 subjects per group except as follows: a n = 9; b n = 10. *P value obtained by two-tailed independent t-test.
At 16 weeks there was no significant change in weight within or between groups (Table 2). As expected, leptin concentrations increased in the treated group. Group by time interaction testing revealed significant differences between leptin and placebo treated groups for fasting PYY and insulin AUC (Table 2); however, the latter difference was driven by one subject who had an unusually elevated postprandial insulin and C-peptide response in the placebo group at week 16 (Figure 1). Otherwise, there were no changes in glucostatic parameters that were different between the groups. Fasting ghrelin and the ghrelin response to the test meal were similar between groups (Figure 2). Similarly, acyl-ghrelin, desacyl-ghrelin, and the ratio of desacyl-to acyl-ghrelin in the fasted and postprandial state did not change with leptin treatment.
Table 2.
Changes in weight, glucostatic, and appetitive hormones.
| Placebo | Leptin | P* | |||
|---|---|---|---|---|---|
| Week 0 | Week 16 | Week 0 | Week 16 | ||
| Wt (kg) | 91.5 ± 4.9 | 91.1 ± 5.1 | 86.2 ± 3.8 | 86.4 ± 3.9 | 0.74 |
| Leptin (ng/mL) | 27.1 ± 3.2 | 29.8 ± 4.5 | 21.8 ± 2.5 | 223.4 ± 66ab | 0.01 |
| Glucose (mg/dL) | 86.2 ± 1.8 | 84.4 ± 2.5 | 88.0 ± 1.7 | 88.0 ± 2.1 | 0.49 |
| Insulin (μIU/mL) | 3.0 ± 0.4 | 3.6 ± 0.6 | 4.2 ± 0.7 | 4.1 ± 0.8 | 0.34 |
| Glucose AUC | 10354 ± 599 | 11506 ± 294 | 11718 ± 338b | 11814 ± 438 | 0.20 |
| Insulin AUC | 2990 ± 327 | 4693 ± 888a | 4553 ± 455 | 4181 ± 561 | 0.04 |
| HOMA-IR | 0.63 ± 0.08 | 0.76 ± 0.13 | 0.90 ± 0.14 | 0.89 ± 0.19 | 0.40 |
| CPEP (ng/mL) | 1.31 ± 0.11 | 1.30 ± 0.15 | 1.42 ± 0.14 | 1.40 ± 0.17 | 0.94 |
| CPEP AUC | 8.5 ± 0.7 | 10.3 ± 1.4 | 10.9 ± 1.3 | 9.8 ± 1.2 | 0.07 |
| GLP-1 (pg/mL) | 11.5 ± 2.1 | 9.7 ± 2.2 | 6.8 ± 1.6 | 7.1 ± 1.4 | 0.54 |
| GLP-1 AUC | 1332 ± 166 | 1136 ± 84 | 1957 ± 395 | 1171 ± 198a | 0.21 |
| PYY (pg/mL) | 69.1 ± 12.6 | 43.0 ± 10.6a | 47.9 ± 10.4 | 58.5 ± 15.6 | 0.04 |
| PYY AUC | 19536 ± 2483 | 16968 ± 2793 | 19557 ± 3405 | 21619 ± 3380 | 0.15 |
| Glucagon (pg/mL) | 79 ± 7 | 75 ± 7 | 71 ± 7 | 82 ± 5 | 0.10 |
| Glucagon AUC | 197 ± 20 | 231 ± 20a | 216 ± 15 | 251 ± 10a | 0.96 |
| Ghrelin (pg/mL) | 379 ± 54 | 379 ± 53 | 343 ± 48 | 326 ± 43 | 0.42 |
| Acyl-ghrelin (pg/mL) | 34.1 ± 13.5 | 31.7 ± 9.9 | 18.8 ± 5.4 | 21.9 ± 4.4 | 0.59 |
| Desacyl-ghrelin (pg/mL) | 64.7 ± 16.3 | 68.4 ± 19.0 | 41.4 ± 0.4 | 37.2 ± 3.2 | 0.63 |
Results are expressed as mean ± SEM. n = 11 subjects per group except for ghrelin, acyl-ghrelin, and desacyl-ghrelin, where n = 9 for placebo group. Hormone measurements are from the fasted state unless otherwise indicated. a P < 0.05; week 16 is statistically different from week 0 within group. b P < 0.05; difference in values is statistically significant between groups at the same week. *P < 0.05; statistically significant values for group by time interaction.
Figure 1.
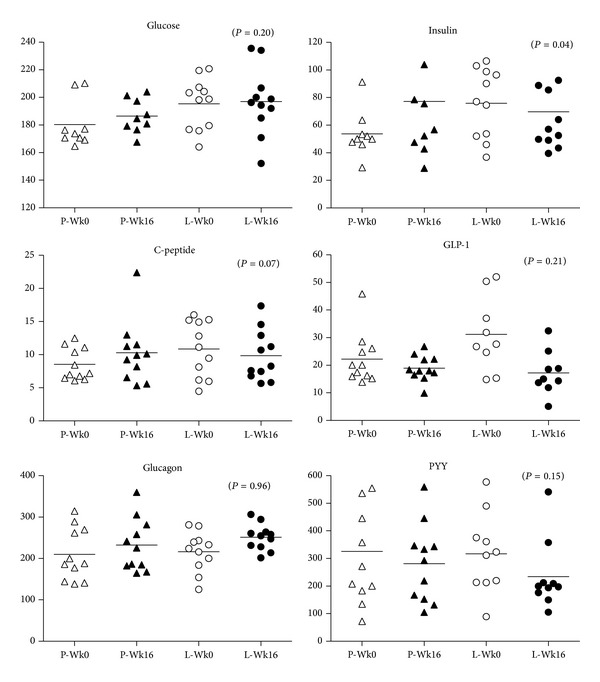
Levels of glucose, insulin, C-peptide, GLP-1, glucagon, and PYY. GLP-1 in placebo and leptin treated groups at weeks 0 and 16 (triangles, placebo group; circles, leptin group). Individual values and group mean (solid line) are represented.
Figure 2.

Fasting and postprandial plasma levels of total ghrelin, acyl-ghrelin, and desacyl-ghrelin (open circles and dashed line, week 0; closed circles and solid line, week 16).
One question on the VAS scale showed a statistically significant difference between the leptin and placebo treated groups at week 16 (P = 0.05; Table 3). “How much do you crave something sweet right now?” The leptin treated group had a significantly lower rating for this question than did the placebo treated group.
Table 3.
Visual analog scale.
| Placebo | Leptin | |||
|---|---|---|---|---|
| Week 0 | Week 16 | Week 0 | Week 16 | |
| How hungry are you? | 2460 ± 479 | 3761 ± 569 | 2747 ± 524 | 3200 ± 592 |
| How satisfied are you? | 7606 ± 819 | 6537 ± 924 | 5283 ± 885 | 4719 ± 969 |
| How full do you feel? | 7428 ± 785 | 6241 ± 890 | 5286 ± 848 | 5197 ± 933 |
| How much do you crave something sweet? | 2120 ± 517 | 2898 ± 563* | 2391 ± 543 | 1461 ± 585* |
| How much can you eat now? | 3221 ± 454 | 4264 ± 529 | 3453 ± 492 | 3246 ± 557 |
| How much nausea or discomfort do you feel? | 2958 ± 508 | 2330 ± 577 | 3056 ± 549 | 2888 ± 604 |
Values represent mean ± SEM AUC from fasting to 120 min after consumption of the test meal. *P < 0.05 for within-group change.
4. Discussion
Our previously published report of this investigation showed that physiological leptin replacement in weight stable women who are in a state of relative leptin insufficiency after RYGB does not result in further weight loss [32]. Absence of an effect on body weight allowed for the unique opportunity to examine the role of leptin on gut hormone and glucose regulation independent of weight loss. Our findings indicate that leptin replacement does not lead to alterations in gut hormone physiology that would favor a further reduction in weight or improvement in glucose homeostasis compared to placebo in this population of obese women after RYGB.
Ghrelin is an orexigenic hormone that has an opposing effect to the anorexigenic properties of leptin. Leptin has been found to decrease ghrelin release from the stomach in vitro and suppress ghrelin secretion from wild type and ob/ob mouse models as well as diabetic rat models [17, 18, 36]. However, this effect was observed only transiently in adult rats [37]. Ghrelin O-acyl transferase (GOAT), the enzyme that octanoylates ghrelin from the desacylated to the acylated form, is increased by leptin in vitro in stomach cells, thus increasing expression of the more potent acylated form and promoting food intake [38]. Desacyl-ghrelin has been shown in some, but not all, studies to favor insulin sensitivity and lack of weight gain [18, 36, 38]. Glucose-stimulated insulin secretion was observed in rats following central desacyl-ghrelin administration but not following peripheral administration [39]. Our findings showed no changes in ghrelin levels after leptin administration, suggesting that modulation of ghrelin levels or acylation may not be dependent upon leptin in the weight-reduced state or that our subjects were resistant to the effects of leptin.
Leptin and PYY both play roles in appetite reduction yet it is unclear whether they work synergistically or independently toward this common goal. Ad libitum fed rats administered PYY followed by leptin had prolonged satiation compared to when administered PYY alone [40], suggesting that the two hormones work synergistically. Leptin administration to humans following diet-induced weight loss did not increase PYY levels following a short term fast [40], which is similar to our findings in surgically induced weight loss, suggesting that PYY is not dependent on leptin to exert satiating effects.
Postprandial GLP-1 levels have been found to increase following RYGB [41] and may contribute to a reduction in appetite, weight loss, and improved glucose homeostasis. GLP-1 and leptin interact to cause a reduction in food intake in ob/ob mice and rat models, with GLP-1 secretion being enhanced following leptin administration [15, 16]. We did not observe an increase in GLP-1 levels after leptin treatment. Although AUC measurement of GLP-1 decreased after leptin treatment, which is contrary to animal models and counter to what we expected based on the role of GLP-1, the group by time interaction was not significant. A possible limitation of this study is that total, and not active, GLP-1 was measured.
Leptin, glucagon, and insulin work collaboratively to maintain normoglycemia through inhibitory and stimulatory effects on each other. The relationship between insulin and leptin has been heavily studied, with findings most recently suggesting that leptin has an inhibitory effect on insulin secretion and reduces glucagon secretion in rodents with type 1 diabetes, resulting in improved glycemic control in the absence of insulin [24]. Restoration of leptin receptors on proopiomelanocortin neurons in obese mice normalized blood glucose and ameliorated hepatic insulin resistance and hyperglucagonemia independent of changes in body weight [24]. In humans, leptin administration had a minimal impact on glycemic control (HbA1c decreased from 8.01% to 7.96%) in patients with type 2 diabetes who maintained a stable weight over the 4 month study period [42]. Similarly, leptin treatment did not have a clinically important effect on insulin action in obese people with newly diagnosed type 2 diabetes [43]. Our findings are in disagreement with mouse studies showing a reduction in glucagon secretion in the presence of leptin. A limitation of our study is that although subjects were obese, they were not hyperglycemic or insulin resistant as evaluated by HOMA-IR; thus, results may not be indicative of results that might be obtained using leptin therapy in a population with glucose intolerance or before diabetes. Also, the small study size would not be able to detect very small changes.
Feelings of hunger and satiety data were not different between groups with the exception of a decreased craving for something sweet in the leptin treated group at week 16 compared to placebo treated subjects at that time point. Leptin has been shown to modulate sweet sensitivity at the level of the taste receptors of the tongue. Ob/ob mice were found to have increased cravings for sweet items that were decreased following administration of leptin [44]. This did not occur in the leptin receptor deficient db/db mice. Similarly, obese humans were found to have obliterated diurnal variation in sweet cravings that are present in nonobese humans and to have a higher threshold for sensitivity to the effects of leptin on sweet cravings, thought to be due to a higher basal leptin level [45]. Our findings suggest that this threshold can be overcome with leptin administration; however, such decreases in cravings were unable to elicit significant alterations in weight or glucose homeostasis but might conceivably play a role in the maintenance of weight loss over time.
Our study subjects were still overweight or obese at the time of study entry, and while they had lost a considerable amount of weight and had leptin levels below those predicted for their current BMI, there is a possibility that they continued to be leptin resistant, manifesting no significant changes to gut related hormones or feelings of satiety and satiation. Our results are specific to a stable weight-reduced state and, thus, may not indicate whether leptin administration can modulate hormone levels during active weight loss. The question then becomes, at what level of leptin or at what weight or at what amount of weight loss would the resistance begin to subside, thus allowing those undergoing weight loss to experience the weight regulating benefits of leptin and possibly of appetitive hormones regulated by leptin. There may also be subpopulations, such as individuals with sweet cravings, who may benefit from longer exposure to exogenous leptin administration in order to help maintain a reduced body weight.
5. Conclusion
Leptin administration to women after RYGB who are in a state of relative leptin insufficiency does not lead to alterations in gut hormones or hormones that control glucose homeostasis in the absence of weight reduction, suggesting that the effects of these hormones to promote weight loss are not necessarily leptin-dependent. Sweet cravings were found to be decreased in those subjects receiving leptin, suggesting a possible therapeutic benefit of leptin administration in a subset of individuals.
Acknowledgments
The authors would like to gratefully acknowledge the participants of this study. This work was supported by NIH/NIDDK Grant R21DK081050 (Judith Korner), Grant no. UL1 RR024156 from NCATS-NCRR/NIH, National Center for Advancing Translations Sciences Grant UL1 TR000040UL1 (Columbia University), and Institutional Research Training Grant (T32) in Pediatric Endocrinology T32 DK065522-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Metreleptin was generously provided by Amylin Pharmaceuticals, Inc. (San Diego, CA).
Conflict of Interests
Dr. Korner receives research support from Covidien, has served as consultant for the Federal Trade Commission, Office of Professional Misconduct, Expert Network Group, and Unigene Laboratories, and was on the scientific advisory board for Nutrisystem. Dr. L. Aronne does contracted research with Amylin Pharmaceuticals Inc., Aspire Bariatrics Inc., GI Dynamics, High Point Pharmaceuticals LLC, Medical University of South Carolina (MUSC), and Novo Nordisk. He is on the advisory board of Amylin Pharmaceuticals Inc., Ethicon Endo-Surgery Inc., GlaxoSmithKline Consumer Healthcare LP, Novo Nordisk, Orexigen Therapeutics Inc., VIVUS Inc., Takeda Pharmaceuticals, and Zafgen Inc. Dr. L. Aronne is on the speakers bureau for VIVUS Inc. and has ownership interest in Cardiometabolic Support Network, LLC, Myos Corporation. The rest of the authors have nothing to disclose. Amylin Pharmaceuticals Inc. generously supplied placebo and leptin for this trial.
References
- 1.Myers MG, Jr., Heymsfield SB, Haft C, et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metabolism. 2012;15(2):150–156. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. International Journal of Obesity. 2009;33(7):786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obesity Surgery. 2012;22(7):1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 4.le Roux CW, Aylwin SJB, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Annals of Surgery. 2006;243(1):108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coll AP, Farooqi IS, O’Rahilly SO. The hormonal control of food intake. Cell. 2007;129(2):251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Annals of Surgery. 2006;244(5):734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah M, Simha V, Garg A. REVIEW: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. The Journal of Clinical Endocrinology & Metabolism. 2006;91(11):4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 8.Haddad N, Howland R, Baroody G, Daher C. The modulatory effect of leptin on the overall insulin production in ex-vivo normal rat pancreas. Canadian Journal of Physiology and Pharmacology. 2006;84(2):157–162. doi: 10.1139/y06-006. [DOI] [PubMed] [Google Scholar]
- 9.Nakano M, Asakawa A, Inui A. Long-term correction of type 1 and 2 diabetes by central leptin gene therapy independent of effects on appetite and energy expenditure. Indian Journal of Endocrinology and Metabolism. 2012;16(9):556–561. doi: 10.4103/2230-8210.105572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klempel MC, Varady KA. Reliability of leptin, but not adiponectin, as a biomarker for diet-induced weight loss in humans. Nutrition Reviews. 2011;69(3):145–154. doi: 10.1111/j.1753-4887.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 11.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 12.Dardeno TA, Chou SH, Moon H-S, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Frontiers in Neuroendocrinology. 2010;31(3):377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janeckova R. The role of leptin in human physiology and pathophysiology. Physiological Research. 2001;50(5):443–459. [PubMed] [Google Scholar]
- 14.Unniappan S, Kieffer TJ. Leptin extends the anorectic effects of chronic PYY(3-36) administration in ad libitum-fed rats. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2008;295(1):R51–R58. doi: 10.1152/ajpregu.00234.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. International Journal of Obesity. 2012;36:1522–1528. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52(2):252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 17.Kohno D, Nakata M, Maekawa F, et al. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148(5):2251–2263. doi: 10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- 18.Kalra SP, Ueno N, Kalra PS. Stimulation of appetite by ghrelin is regulated by leptin restraint: peripheral and central sites of action. The Journal of Nutrition. 2005;135(5):1331–1335. doi: 10.1093/jn/135.5.1331. [DOI] [PubMed] [Google Scholar]
- 19.Dhillon H, Kalra SP, Kalra PS. Dose-dependent effects of central leptin gene therapy on genes that regulate body weight and appetite in the hypothalamus. Molecular Therapy. 2001;4(2):139–145. doi: 10.1006/mthe.2001.0427. [DOI] [PubMed] [Google Scholar]
- 20.Kissileff HR, Thornton JC, Torres MI, et al. Leptin reverses declines in satiation in weight-reduced obese humans. The American Journal of Clinical Nutrition. 2012;95(2):309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDuffie JR, Riggs PA, Calis KA, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. The Journal of Clinical Endocrinology & Metabolism. 2004;89(9):4258–4263. doi: 10.1210/jc.2003-031868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berglund ED, Vianna CR, Donato J, Jr., et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. The Journal of Clinical Investigation. 2012;122(3):1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings BP, Bettaieb A, Graham JL, et al. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14670–14675. doi: 10.1073/pnas.1107163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Park B-H, Wang M-Y, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. The Journal of the American Medical Association. 1999;282(16):1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. The Journal of Clinical Endocrinology & Metabolism. 2002;87(5):2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. The Journal of Clinical Investigation. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paz-Filho G, Mastronardi C, Delibasi T, Wong M-L, Licinio J. Congenital leptin deficiency: diagnosis and effects of leptin replacement therapy. Arquivos Brasileiros de Endocrinologia & Metabologia. 2010;54(8):690–697. doi: 10.1590/s0004-27302010000800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. The New England Journal of Medicine. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 30.Javor ED, Cochran EK, Musso C, Young JR, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 31.Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korner J, Conroy R, Febres G, et al. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity. 2013;21(5):951–956. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 34.Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity. 2011;19(11):2149–2157. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prudom C, Liu J, Patrie J, et al. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. The Journal of Clinical Endocrinology & Metabolism. 2010;95(5):2351–2358. doi: 10.1210/jc.2009-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsubone T, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Leptin downregulates ghrelin levels in streptozotocin-induced diabetic mice. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2005;289(6):R1703–R1706. doi: 10.1152/ajpregu.00773.2004. [DOI] [PubMed] [Google Scholar]
- 37.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. The Journal of Clinical Investigation. 2012;122(1):4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirchner H, Heppner KM, Holland J, Kabra D, Tschop MH, Pfluger PT. Ablation of ghrelin O-acyltransferase does not improve glucose intolerance or body adiposity in mice on a leptin-deficient ob/ob background. PLoS ONE. 2013;8(4, article e61822) doi: 10.1371/journal.pone.0061822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heppner KM, Piechowski CL, Müller A, et al. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2013 doi: 10.2337/db13-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan JL, Stoyneva V, Kelesidis T, Raciti P, Mantzoros CS. Peptide YY levels are decreased by fasting and elevated following caloric intake but are not regulated by leptin. Diabetologia. 2006;49(1):169–173. doi: 10.1007/s00125-005-0041-2. [DOI] [PubMed] [Google Scholar]
- 41.Rhee NA, Vilsboll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes, Obesity and Metabolism. 2012;14(4):291–298. doi: 10.1111/j.1463-1326.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 42.Moon H-S, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60(6):1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60(5):1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida R, Niki M, Jyotaki M, Sanematsu K, Shigemura N, Ninomiya Y. Modulation of sweet responses of taste receptor cells. Seminars in Cell & Developmental Biology. 2013;24(3):226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Horio N, Jyotaki M, Yoshida R, Sanematsu K, Shigemura N, Ninomiya Y. New frontiers in gut nutrient sensor research: nutrient sensors in the gastrointestinal tract: modulation of sweet taste sensitivity by leptin. Journal of Pharmacological Sciences. 2010;112(1):8–12. doi: 10.1254/jphs.09r07fm. [DOI] [PubMed] [Google Scholar]


