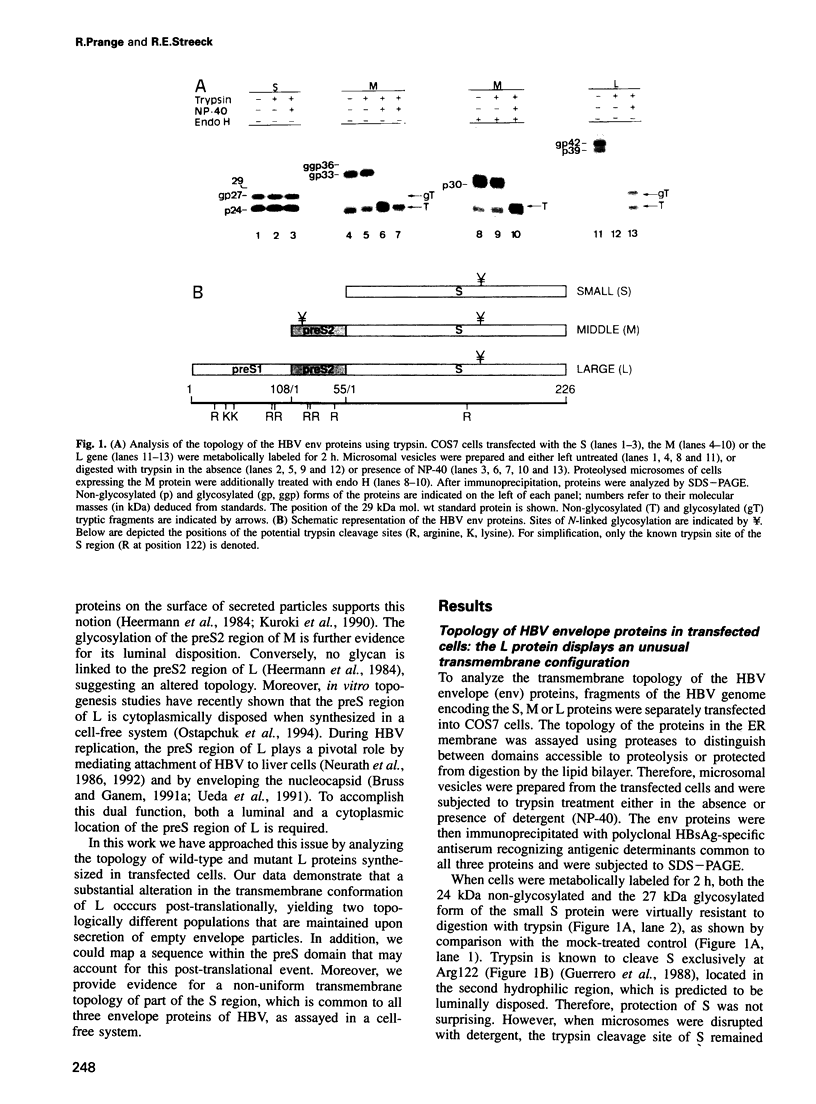
Abstract
The small (S), middle (M) and large (L) envelope proteins of the hepatitis B virus (HBV) are initially synthesized as multispanning membrane proteins of the endoplasmic reticulum membrane. We now demonstrate that all envelope proteins synthesized in transfected cells or in a cell-free system adopt more than one transmembrane orientation. The L protein disposes its N-terminal preS domain both to the cytoplasmic and the luminal side of the membrane. This unusual topology does not depend on interaction with the viral nucleocapsid, but is preserved in secreted empty envelope particles. Pulse-chase analysis suggests a novel process of post-translational translocation leading to the non-uniform topology. Analysis of L deletion mutants indicates that the block to co-translational translocation can be attributed to a specific sequence within preS, suggesting that translocation of L may be regulated. Additional topological heterogeneity is displayed in the S region of the envelope proteins and in the S protein itself, as assayed in a cell-free system. S proteins integrated into microsomal membranes exhibit both a luminal and a cytoplasmic orientation of the internal hydrophilic region carrying the major antigenic determinants. This may explain the unusual partial glycosylation of the HBV envelope proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bruss V., Ganem D. Mutational analysis of hepatitis B surface antigen particle assembly and secretion. J Virol. 1991 Jul;65(7):3813–3820. doi: 10.1128/jvi.65.7.3813-3820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Lu X., Thomssen R., Gerlich W. H. Post-translational alterations in transmembrane topology of the hepatitis B virus large envelope protein. EMBO J. 1994 May 15;13(10):2273–2279. doi: 10.1002/j.1460-2075.1994.tb06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Thomssen R. Mapping a region of the large envelope protein required for hepatitis B virion maturation. J Virol. 1994 Mar;68(3):1643–1650. doi: 10.1128/jvi.68.3.1643-1650.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Thrift R. N., Wu C. C., Howell K. E. Apolipoprotein B is both integrated into and translocated across the endoplasmic reticulum membrane. Evidence for two functionally distinct pools. J Biol Chem. 1990 Jun 15;265(17):10005–10011. [PubMed] [Google Scholar]
- Delpeyroux F., Chenciner N., Lim A., Malpièce Y., Blondel B., Crainic R., van der Werf S., Streeck R. E. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science. 1986 Jul 25;233(4762):472–475. doi: 10.1126/science.2425433. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986 May;6(5):1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. The N-terminal (pre-S2) domain of a hepatitis B virus surface glycoprotein is translocated across membranes by downstream signal sequences. J Virol. 1990 Mar;64(3):1414–1419. doi: 10.1128/jvi.64.3.1414-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., MacRae D. R., Lingappa V. R., Ganem D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol Cell Biol. 1987 Oct;7(10):3591–3601. doi: 10.1128/mcb.7.10.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilanes F., Gonzalez-Ros J. M., Peterson D. L. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J Biol Chem. 1982 Jul 10;257(13):7770–7777. [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila A. P., Eder A. M., Fuller S. D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992 Sep;118(6):1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki K., Floreani M., Mimms L. T., Ganem D. Epitope mapping of the PreS1 domain of the hepatitis B virus large surface protein. Virology. 1990 Jun;176(2):620–624. doi: 10.1016/0042-6822(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Kuroki K., Russnak R., Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989 Oct;9(10):4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt O., Heermann K. H., Seifer M., Gerlich W. H. Cell-type dependent expression and secretion of hepatitis B virus pre-S1 surface antigen. Postgrad Med J. 1987;63 (Suppl 2):41–50. [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986 Aug 1;46(3):429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Strick N., Sproul P. Search for hepatitis B virus cell receptors reveals binding sites for interleukin 6 on the virus envelope protein. J Exp Med. 1992 Feb 1;175(2):461–469. doi: 10.1084/jem.175.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Hearing P., Ganem D. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 1994 Mar 1;13(5):1048–1057. doi: 10.1002/j.1460-2075.1994.tb06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Rutter W. J. Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J Virol. 1987 Mar;61(3):782–786. doi: 10.1128/jvi.61.3.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Hull J. D., Lamb R. A. Transposition of domains between the M2 and HN viral membrane proteins results in polypeptides which can adopt more than one membrane orientation. J Cell Biol. 1989 Nov;109(5):2023–2032. doi: 10.1083/jcb.109.5.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange R., Clemen A., Streeck R. E. Myristylation is involved in intracellular retention of hepatitis B virus envelope proteins. J Virol. 1991 Jul;65(7):3919–3923. doi: 10.1128/jvi.65.7.3919-3923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange R., Nagel R., Streeck R. E. Deletions in the hepatitis B virus small envelope protein: effect on assembly and secretion of surface antigen particles. J Virol. 1992 Oct;66(10):5832–5841. doi: 10.1128/jvi.66.10.5832-5841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh O., Umeda M., Imai H., Tunoo H., Inoue K. Lipid composition of hepatitis B virus surface antigen particles and the particle-producing human hepatoma cell lines. J Lipid Res. 1990 Jul;31(7):1293–1300. [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Stirk H. J., Thornton J. M., Howard C. R. A topological model for hepatitis B surface antigen. Intervirology. 1992;33(3):148–158. doi: 10.1159/000150244. [DOI] [PubMed] [Google Scholar]
- Ueda K., Tsurimoto T., Matsubara K. Three envelope proteins of hepatitis B virus: large S, middle S, and major S proteins needed for the formation of Dane particles. J Virol. 1991 Jul;65(7):3521–3529. doi: 10.1128/jvi.65.7.3521-3529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]