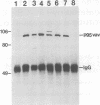
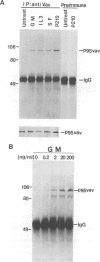
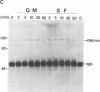
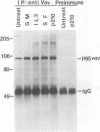
Abstract
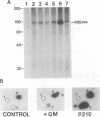
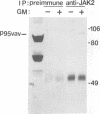
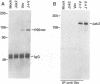
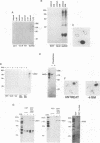
Vav is a recently described proto-oncogene expressed only in hematopoietic cells which contains an SH2 and two SH3 domains and shares homology with the Dbl GDP-GTP exchange factor and BCR. p95Vav is phosphorylated on tyrosine residues in response to stimulation of the T cell antigen receptor, cross-linking of IgE or IgM receptors and stimulation of immature hematopoietic cells by Steel factor. Monoclonal antibodies to human Vav were generated and used to examine the events which regulate tyrosine phosphorylation of p95Vav in myeloid cells. In the factor-dependent MO7e cell line, p95Vav was rapidly phosphorylated on tyrosine residues in a dose- and time-dependent manner by GM-CSF, IL-3 and Steel factor. Introduction of the BCR/ABL oncogene into this cell line resulted in factor-independent proliferation and constitutive phosphorylation of p95Vav. Tyrosine phosphorylation of p95Vav was also substantially increased by treatment of cytokine-deprived cells with the tyrosine phosphatase inhibitor sodium vanadate. Since many of the cytokines known to induce tyrosine phosphorylation of p95Vav are also known to activate JAK family tyrosine kinases, we looked for an interaction of p95Vav with JAK kinases. p95Vav co-precipitated with JAK2 in MO7e cells stimulated with GM-CSF, but not in unstimulated cells. Also, JAK2 was found to be constitutively associated with p95Vav in vivo when expressed at high levels in insect cells using baculovirus vectors. A fusion protein consisting of glutathione-S-transferase and the SH2 domain of p95Vav (GST-Vav-SH2) precipitated JAK2, suggesting that this interaction is mediated by the SH2 domain of p95Vav.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Houston H., Allen J., Lints T., Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992 Apr;7(4):611–618. [PubMed] [Google Scholar]
- Afar D. E., Goga A., McLaughlin J., Witte O. N., Sawyers C. L. Differential complementation of Bcr-Abl point mutants with c-Myc. Science. 1994 Apr 15;264(5157):424–426. doi: 10.1126/science.8153630. [DOI] [PubMed] [Google Scholar]
- Alai M., Mui A. L., Cutler R. L., Bustelo X. R., Barbacid M., Krystal G. Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J Biol Chem. 1992 Sep 5;267(25):18021–18025. [PubMed] [Google Scholar]
- Buday L., Egan S. E., Rodriguez Viciana P., Cantrell D. A., Downward J. A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. J Biol Chem. 1994 Mar 25;269(12):9019–9023. [PubMed] [Google Scholar]
- Bustelo X. R., Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992 May 22;256(5060):1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Ledbetter J. A., Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992 Mar 5;356(6364):68–71. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Suen K. L., Leftheris K., Meyers C. A., Barbacid M. Vav cooperates with Ras to transform rodent fibroblasts but is not a Ras GDP/GTP exchange factor. Oncogene. 1994 Aug;9(8):2405–2413. [PubMed] [Google Scholar]
- Carlesso N., Griffin J. D., Druker B. J. Use of a temperature-sensitive mutant to define the biological effects of the p210BCR-ABL tyrosine kinase on proliferation of a factor-dependent murine myeloid cell line. Oncogene. 1994 Jan;9(1):149–156. [PubMed] [Google Scholar]
- Cen H., Papageorge A. G., Zippel R., Lowy D. R., Zhang K. Isolation of multiple mouse cDNAs with coding homology to Saccharomyces cerevisiae CDC25: identification of a region related to Bcr, Vav, Dbl and CDC24. EMBO J. 1992 Nov;11(11):4007–4015. doi: 10.1002/j.1460-2075.1992.tb05494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola J., Bryant S., Koda T., Conway D., Barbacid M. Mechanism of activation of the vav protooncogene. Cell Growth Differ. 1991 Feb;2(2):95–105. [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Dosil M., Wang S., Lemischka I. R. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol Cell Biol. 1993 Oct;13(10):6572–6585. doi: 10.1128/mcb.13.10.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker B., Okuda K., Matulonis U., Salgia R., Roberts T., Griffin J. D. Tyrosine phosphorylation of rasGAP and associated proteins in chronic myelogenous leukemia cell lines. Blood. 1992 May 1;79(9):2215–2220. [PubMed] [Google Scholar]
- Ernst T. J., Slattery K. E., Griffin J. D. p210Bcr/Abl and p160v-Abl induce an increase in the tyrosine phosphorylation of p93c-Fes. J Biol Chem. 1994 Feb 25;269(8):5764–5769. [PubMed] [Google Scholar]
- Evans G. A., Howard O. M., Erwin R., Farrar W. L. Interleukin-2 induces tyrosine phosphorylation of the vav proto-oncogene product in human T cells: lack of requirement for the tyrosine kinase lck. Biochem J. 1993 Sep 1;294(Pt 2):339–342. doi: 10.1042/bj2940339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland F., Katzav S., Birnbaum D. The products of the mcf-2 and vav proto-oncogenes and of the yeast gene cdc-24 share sequence similarities. Oncogene. 1992 Mar;7(3):585–587. [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E., Coggeshall K. M., Baier G., Katzav S., Burn P., Altman A. Tyrosine kinase-stimulated guanine nucleotide exchange activity of Vav in T cell activation. Science. 1993 May 7;260(5109):822–825. doi: 10.1126/science.8484124. [DOI] [PubMed] [Google Scholar]
- Gulbins E., Coggeshall K. M., Langlet C., Baier G., Bonnefoy-Berard N., Burn P., Wittinghofer A., Katzav S., Altman A. Activation of Ras in vitro and in intact fibroblasts by the Vav guanine nucleotide exchange protein. Mol Cell Biol. 1994 Feb;14(2):906–913. doi: 10.1128/mcb.14.2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J. Molecular insights into the Philadelphia translocation. Hematol Pathol. 1991;5(1):1–10. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989 Aug;8(8):2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneuville P., Heisterkamp N., Groffen J. Expression of the chronic myelogenous leukemia-associated p210bcr/abl oncoprotein in a murine IL-3 dependent myeloid cell line. Oncogene. 1991 Feb;6(2):275–282. [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T., Salgia R., Hallek M., Eder M., Druker B., Ernst T. J., Griffin J. D. Shc phosphorylation in myeloid cells is regulated by granulocyte macrophage colony-stimulating factor, interleukin-3, and steel factor and is constitutively increased by p210BCR/ABL. J Biol Chem. 1994 Feb 18;269(7):5016–5021. [PubMed] [Google Scholar]
- Matulonis U., Salgia R., Okuda K., Druker B., Griffin J. D. Interleukin-3 and p210 BCR/ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line. Exp Hematol. 1993 Oct;21(11):1460–1466. [PubMed] [Google Scholar]
- Muller A. J., Pendergast A. M., Havlik M. H., Puil L., Pawson T., Witte O. N. A limited set of SH2 domains binds BCR through a high-affinity phosphotyrosine-independent interaction. Mol Cell Biol. 1992 Nov;12(11):5087–5093. doi: 10.1128/mcb.12.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Druker B., Kanakura Y., Koenigsmann M., Griffin J. D. Internalization of the granulocyte-macrophage colony-stimulating factor receptor is not required for induction of protein tyrosine phosphorylation in human myeloid cells. Blood. 1991 Oct 15;78(8):1928–1935. [PubMed] [Google Scholar]
- Pendergast A. M., Muller A. J., Havlik M. H., Maru Y., Witte O. N. BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL SH2 regulatory domain in a non-phosphotyrosine-dependent manner. Cell. 1991 Jul 12;66(1):161–171. doi: 10.1016/0092-8674(91)90148-r. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Quilliam L. A., Cripe L. D., Bassing C. H., Dai Z., Li N., Batzer A., Rabun K. M., Der C. J., Schlessinger J. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993 Oct 8;75(1):175–185. [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Callahan W., Witte O. N. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992 Sep 18;70(6):901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Rajan T. V., Yi T., Ihle J. N., Matthews R. J., Thomas M. L., Beier D. R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993 Jul 2;73(7):1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G. M., Adra C. N., Lim B. Inhibition of hematopoietic development from embryonic stem cells by antisense vav RNA. EMBO J. 1993 Dec 15;12(13):5065–5074. doi: 10.1002/j.1460-2075.1993.tb06200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]