Abstract
Event-related potential (ERP) approaches to social cognitive and affective neuroscience (SCAN) are not as widely used as other neuroimaging techniques, yet they offer several unique advantages. In particular, the high temporal resolution of ERP measures of neural activity make them ideally suited for studying the dynamic interplay of rapidly unfolding cognitive and affective processes. In this article, we highlight the utility of ERP methods for scientists investigating questions of SCAN. We begin with a brief description of the physiological basis of ERPs and discussion of methodological practices. We then discuss how ERPs may be used to address a range of questions concerning social perception, social cognition, attitudes, affect and self-regulation, with examples of research that has used the ERP approach to contribute important theoretical advances in these areas. Whether used alone or in combination with other techniques, the ERP is an indispensable part of the social and affective neuroscientist’s methodological toolkit.
Keywords: Event-related potentials, social cognition, social neuroscience, psychophysiology, brain imaging
INTRODUCTION
When most people think of neuroimaging data, they picture vivid brain slices with activations glowing in brilliant reds, yellows and blues, as if brain regions were actually alight with neural firing. Such visualizations represent data from functional magnetic resonance imaging (fMRI) or, less often, positron emission tomography (PET), both of which give the sensation of peering directly into a working brain. Far fewer people would imagine the relatively abstract waveform comprising the event-related potential (ERP), which represents changes in neural activity across time. Indeed, it is far easier to represent location than time in graphic form and for this reason, plots of ERP data are much less likely to induce the level of awe inspired by fMRI or PET results. But readers should not be fooled by their humbler appearance: ERP methods provide a powerful tool for probing the temporal dynamics of neural processes—a critical aspect of brain function that is most informative about psychological mechanisms. With exquisite temporal resolution (and spatial resolution that is often sufficient) and encumbered by fewer practical constraints than fMRI, ERP methods offer the best approach to testing many questions central to social cognitive and affective neuroscience (SCAN).
The goal of this article is to highlight the utility of ERP methods for scientists investigating SCAN-related questions. We begin with a brief description of the physiological basis of ERPs and discussion of methodological practices [for a more thorough treatment, see Luck (2005) or Fabiani et al. (2007)]. We then discuss how ERPs have been used to advance the understanding of issues important for social perception, social cognition, attitudes, affect and self-regulation. We conclude with some notes on practical considerations for using ERP methods in the SCAN laboratory.
WHAT ARE ERPS?
With apologies to our fMRI colleagues, we begin with a ‘bold’ statement: the images obtained from fMRI do not represent neural activity per se, nor do they represent accurately when neural activity has occurred. Rather, the blood oxygen level-dependent (BOLD) signal depicted in the striking renderings of fMRI data actually represents changes in blood flow, inferred to occur as the result of oxygen-dependent neural activity having taken place within the preceding 4–6 s [for in-depth comparison and analysis of neural firing vs the BOLD signal, see Logothetis (2002)]. Thus, the physiological signals captured by fMRI are best characterized as indirect measures of neural activation. In contrast, the ERP is a direct measure of neural activity—an electrical potential generated by the firing of cortical neurons in response to a specific event, such as the presentation of a stimulus or the delivery of a response—which precisely coincides with that neural activity.
Physiologically, ERPs represent the summation of post-synaptic potentials from populations of synchronously active neurons, located primarily in the cortex (see Allison et al., 1986; Coles and Rugg, 1995). The columnar structure of cortical neurons aligns the electrical field orientation of their potentials, creating a summated signal that is strong enough to be detected at the scalp. The measured ERP typically reflects one end of the electrical dipole produced by firing neurons (i.e. the positive or the negative end). The neural source of a given ERP component may be estimated by considering the orientation and strength of a dipole signal, as measured at multiple sites across the scalp (see Scherg and Picton, 1991; Huizenga and Molenaar, 1994). These techniques often permit reasonably accurate estimates of the underlying sources of scalp-recorded potentials and allow researchers to connect ERP results to findings from other methods (e.g. fMRI, PET or brain lesion studies).
ERP measurement
In this section, we provide a brief overview of ERP recording techniques and processing steps [see Luck (2005); Bartholow and Amodio (2009); Dickter and Kieffaber (2013) for more detailed reviews]. The goal of this section is to provide readers with a basis for understanding and interpreting ERP data in studies of SCAN-related topics. Most ERP experiments in social cognition and affective science are built upon a basic behavioral task design, which typically involves the carefully timed presentation of stimuli and the recording of behavioral responses (e.g. on a computer). But compared with behavioral experiments, ERP tasks often include more trials (e.g. 30–50 per trial type) and longer intertrial intervals (2–4 s), which may be jittered in timing to help clarify distinct patterns of brain activity (as in event-related fMRI designs).
During a recording session, participants are usually seated upright in a comfortable chair, approximately 1 m from a computer screen, often with a keyboard or button box placed on their lap or on a tray. Electroencephalography (EEG) signals are recorded non-invasively using electrodes, usually composed of silver/silver chloride or tin, placed on the surface of the scalp, typically according to standard placement guidelines (see American Encephalographic Society, 1994) and fixed within a stretch-nylon cap. The electrodes are connected to a set of pre-amplifiers located close to the participant’s scalp, which provide enough initial amplification to convey this very weak signal to the main amplifiers located more distally in the experimenter room. These main amplifiers magnify the electrical signals emitted by neurons by a factor of 10 000–50 000 so they can be measured accurately. The analog signals are then digitally sampled, usually at a frequency ranging from 250 to 1000 Hz (samples per second) and stored to a computer hard drive. With the high sampling rates afforded by modern computers, ERPs reflect the native temporal resolution of the post-synaptic potentials of interest.
Extracting the ERP signal: creating the waveform
As with fMRI, EEG recordings reflect ongoing activity from sources throughout the brain, much of which has little to do with the experimental task or psychological processes of interest. Thus, one must extract the ERP ‘signal’ from the background EEG ‘noise’. This is done by filtering the raw EEG signal, aligning the signals to events of interest and averaging.
Filtering is the process by which algorithms are applied to the analog signal to attenuate frequencies that are not of interest [see Marshall-Goodell et al., (1990) for a review of bioelectrical measurement]. The aligning and averaging process capitalizes on the principle that, once EEG signals are aligned to an event of interest (e.g. a stimulus onset), EEG activity unrelated to the processing of the event will vary randomly across trials and therefore will average to zero, whereas EEG activity elicited by the event will vary according to properties of that event and will stabilize when averaged across trials of the same type. After normalizing the amplitude of each individual epoch to zero, based on activity during an immediately preceding baseline period, epochs of EEG elicited during particular trial types are averaged together to form individual subject averages. These individual averages are then averaged together to create so-called grand averages, used to display sample-level responses. This process reveals the characteristic ERP waveform, with its sequence of positive and negative peaks unfolding across the timespan of the selected epoch.1
In general, two major classes of ERP waveforms are of interest for SCAN researchers. Aligning EEG epochs to the onset of stimuli renders a ‘stimulus-locked waveform’. Alternatively, one may align EEG epochs to the moment when a behavioral response is made, producing a ‘response-locked waveform’. Stimulus-locked ERPs arise in response to a specific stimulus, such as a visual image or auditory feedback. Most stimulus-locked ERP components reflect some aspect of perceptual or attentional processing and it is generally assumed that the earlier the deflection emerges following stimulus onset, the more likely it is to reflect an automatic or reflexive psychological process (see Fabiani et al., 2007). Naming conventions for stimulus-locked ERPs typically refer to the polarity (positive or negative) and either the ordinal position following the event (e.g. the first positive-going deflection following stimulus onset is P1, the first negative deflection is N1, etc.) or the approximate time at which the deflection typically peaks (P100, N100 and P200), as illustrated in Figure 1. Although the labels ascribed to ERP components (especially stimulus-aligned components) can sometimes be confusing to the uninitiated, ERP nomenclature is intended to be generically descriptive, as opposed to referring to a specific psychological function (e.g. like the ‘fusiform face area’). This convention acknowledges that any particular neural signal or structure is likely involved in multiple functions and that the function of a signal (or structure) elicited in one kind of task may be very different than a morphologically similar signal that is elicited by a different task.
Fig. 1.
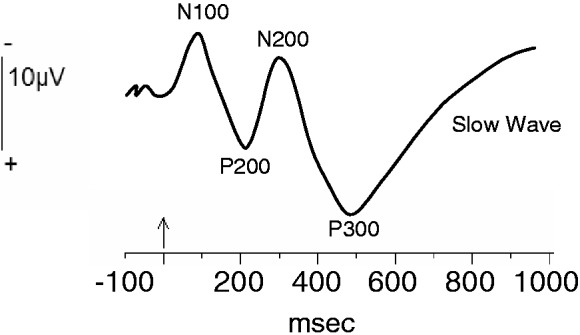
A schematic representation of an ERP waveform elicited by a novel visual stimulus. The vertical arrow on the timeline (horizontal axis) represents stimulus onset time. The positive and negative deflections in the waveform represent typical ERP components, named here according to their polarity (‘P’ for positive deflections and ‘N’ for negative deflections) and the approximate post-stimulus time (in milliseconds) of their peaks. Note, however, that this temporal naming convention is based on broad generalities such that the specific time varies considerably between different experimental tasks. Another method for component naming involves assigning numbers to the positive and negative deflections as a function of their ordinal position following stimulus onset (e.g. N1, P1, N2, etc.). Note, also, that negative voltages are plotted above zero on the Y-axis, following electrophysiological convention, although ERP waveforms are sometimes plotted with negative voltages displayed below zero.
Response-locked components are useful for examining mechanisms associated with the formation and regulation of a behavioral response. Response-locked waves tend to be named according to their polarity (positive vs negative) and the type of response that elicits them, such as the ‘error-related negativity’ (ERN) and ‘error-positivity’ (Pe). For a more in-depth discussion of ERP components and their interpretations, see Amodio and Bartholow (2011) or Luck and Kappenman (2012).
Quantifying ERPs
Once averaged waveforms are computed for each participant and condition, they can be scored for analysis. The most common method of scoring is to determine the time window in which a component of interest emerges (generally based on visual inspection of averaged waveforms or canonical time windows used in past research) and then to measure the average voltage within that window for each subject. Alternatively, researchers sometimes use an automated ‘peak-picking’ algorithm to determine the maximal voltage of a component of interest within a specified time range—a technique sometimes used on a subject-by-subject basis when there are notable individual differences in an ERP component’s timing. However, given that individual subject waveforms generally include more extreme values than are found in grand average waveforms (i.e. averaged across subjects) and that individual peak amplitudes are not linearly related to the average of the peaks, some theorists caution against the use of peak amplitude measures (see Fabiani et al., 1987; Luck, 2005). In addition to amplitude scoring, the peak latency of an ERP component may be quantified as the time point at which the component reaches its peak amplitude.
ERP STUDIES OF SOCIAL COGNITION AND AFFECT
How can ERPs be used to elucidate mechanisms of socio-cognitive and affective processes? It is often difficult to test hypotheses about the mechanisms underlying social and affective responses using only behavioral and self-report methods, given their relatively distal relation to mental events of interest [which, in some cases, may be unavailable to a participant’s introspection (Payne, 2001; Bartholow et al., 2009; Amodio and Mendoza, 2010)]. Finally, it is difficult to unobtrusively measure subtle online changes in mental processes using these traditional tools, as the measurement of these processes often interferes with their natural psychological function (i.e. when interrupting an ongoing response to have a participant complete an emotion questionnaire). Furthermore, fMRI is often unable to assess the neural activity underlying the dynamic mechanisms of a response, given its very coarse temporal resolution. ERPs offer a solution to these problems. In this section, we describe research that has used ERPs to overcome limitations of traditional methods to address some enduring questions about social cognitive and affective processes.
Attitudes and evaluative processes
Using ERPs to assess attitudes
In a seminal early report, Cacioppo et al. (1993) employed a novel use of the ERP to examine attitudes. The widely studied P3 (also sometimes referred to as the P300 or, more generically, as the late positive potential or LPP) is a relatively large, positive deflection that peaks between 300 and 800 ms post-stimulus. (For an in-depth review of P3, its putative neural sources and its hypothesized psychological function, see Nieuwenhuis et al., 2005.) Cacioppo et al. noted that P3 amplitude often is increased when a given stimulus represents a category different from that of preceding stimuli (e.g. Squires et al., 1976; Donchin and Coles, 1988). Their paradigm represented a modification of a classic ‘oddball’ task often used to elicit the P3, in which relatively infrequent target stimuli (i.e. oddballs) are presented among more frequent context stimuli. Cacioppo et al. (1993) applied the oddball paradigm to the categorization of evaluative (i.e. positive vs negative) words, and found that when a target word was evaluatively inconsistent with context words, such as when a negative word appeared within a series of positive words, a pronounced P3 was evoked. Thus, although the P3 does not represent evaluative processing directly, its sensitivity to contextual inconsistency combined with a well-designed experimental task provided a method for assessing attitudes that did not rely on participants’ self-reports (see also Cacioppo et al., 1994; Crites and Cacioppo, 1996; Ito et al., 1998).
Subsequent research has used this technique as a measure of implicit attitudes (for a review, see Ito and Cacioppo, 2007). For example, Crites et al., (1995) compared P3 amplitudes for conditions in which participants reported their attitudes toward target objects truthfully or falsely. Across conditions, the P3 was sensitive to the true evaluative nature of the stimuli and not to subjects’ explicit reports (see also Ito and Cacioppo, 2000).
Ito and colleagues have also used ERPs to better understand the relationship between spontaneous categorization processes and White participants’ explicit, self-reported evaluations of Black Americans as a group. Ito et al., (2004) presented participants with images of White people’s faces, Black people’s faces and positive objects, each of which appeared in the context of negative images. Using this task, the authors could test whether oddball ERPs elicited by either Black or White faces differed from ERPs to the negative word ‘context’ stimuli. Results revealed that pictures of White faces elicited larger amplitudes of two components, the P2 (also called the vertex positive potential) and the N2, than Black faces, suggesting the responses to White faces were more evaluatively discrepant from the negative context. A similar pattern was observed in the LPP component among participants who reported highly prejudiced attitudes on the Modern Racism Scale (McConahay et al., 1981). This research provides an example of how ERPs can be used to assess implicit racial attitudes in the context of categorizing faces.
Mechanisms of affective priming
Social psychologists have studied implicit attitudes for >30 years, yet the neural and psychological processes that drive them are still poorly understood. In an early demonstration, Fazio et al. (1986) showed that an affective target word is categorized in terms of its valence (positive or negative) more quickly when preceded by prime words of the same valence (i.e. congruent trials) than by prime words of the opposite valence (i.e. incongruent trials). Although this ‘affective congruency effect’ has since been replicated many times (e.g. see Klauer and Musch, 2003), researchers continue to debate its underlying mechanisms (e.g. Klauer et al., 2005).
Recently, some researchers have begun using ERPs to shed light on these underlying mechanisms. For example, Bartholow et al. (2009) used ERPs in an attempt to identify the locus of the affective congruency effect in the evaluative categorization process, the response output process or both (for discussion of this issue, see Klauer and Musch, 2003). The classic interpretation of the affective congruency effect is that evaluative categorization of congruent targets is facilitated, compared with incongruent targets, due to the relative proximity of congruent primes and targets in a semantic network (Fazio et al., 1986). This suggests a locus for the effect in the evaluative categorization process. As noted in the previous section, considerable research has linked P3 amplitude with the magnitude of change in evaluative categorization and peak P3 latency has been associated with stimulus evaluation time (see Kutas et al., 1977; McCarthy and Donchin, 1981). Thus, Bartholow et al. reasoned that if the affective congruency effect reflects differences in evaluative categorization across trial types, incongruent trials should elicit larger and/or slower P3 responses than congruent trials. However, this was not observed.
More recent accounts of the affective congruency effect posit a locus in the response output process such that incongruent trials elicit response conflict when the response associated with the prime conflicts with the response called for by the target (see Klinger et al., 2000; Wentura and Rothermund, 2003). If so, the amplitude of the lateralized readiness potential (LRP), a dynamic measure of motor cortex activation associated with preparation and initiation of behavioral responses (see Coles, 1989), should show evidence of response activation following the onset of the prime even before the target appears. This is indeed what Bartholow et al. (2009) found: under conditions in which congruent trials were as likely (50%) or more likely (80%) than incongruent trials, the amplitude of the LRP elicited by prime words showed that participants initially activated the response suggested by the prime (e.g. preparing to press the ‘positive’ key following positive primes).
Finally, Bartholow et al. (2009) measured the amplitude of the N2 following target onset. In previous studies, the N2 has been associated with the theorized conflict monitoring function of dorsal anterior cingulate cortex (ACC; see van Veen and Carter, 2002; Yeung et al., 2004), in that experimental conditions invoking conflicting response possibilities consistently lead to enhanced N2 amplitude (see Folstein and Van Petten, 2008). Bartholow et al. hypothesized that if primes elicit response activation (as indicated in the LRP), then incongruent prime target pairs should evoke response conflict as participants attempt to overcome their initial prime-driven tendency in order to correctly classify the valence of the target. Again, the data were consistent with this idea—the amplitude of the N2 component was larger on incongruent than on congruent trials (again, when the probability of congruent trials was either 50 or 80%).
Hence, overall these findings were consistent with the idea that affective congruency effects, at least within this classic paradigm, result from conflict during the response output stage rather than from ‘spreading of activation’ effects within a semantic network. Reaching this conclusion would be very difficult with fMRI, as the slow time course of the BOLD signal would not permit an accurate characterization of the rapidly unfolding neural responses associated with this effect.
Person perception
Face perception
Social interactions often begin with the perception of a face. Not surprisingly, much research on the initial stages of person perception has focused on face processing. In ERP research, the N170 component—an early negative deflection prominent over occipital-temporal scalp regions (particularly on the right side)—responds selectively to faces and is believed to reflect the early configural/holistic encoding of a face in visual perception (e.g. Bentin et al., 1996; Eimer, 2000). Research on the putative neural generator of the N170 suggests that it reflects multiple sources located in the occipito-temporal region of the brain, one of which is believed to be the fusiform gyrus (Deffke et al., 2007). The N170 component is of special interest for the study of social cognition because it presumably reflects the initial process of recognizing that an object is a conspecific—the earliest stage of social perception. In contrast to fMRI, which cannot discern the initial encoding of a face from more elaborative processing, the N170 can reveal differences in the extent to which an individual perceives another as a fellow human. Moreover, some recent evidence also suggests that the N170 is sensitive to higher level social/motivational factors (Ofan et al., 2011, in press; Ratner and Amodio, 2013). For these reasons, there has been a surge in research involving the N170 in social cognition.
Much recent research on the N170 component has examined responses to faces from different social groups, testing, e.g. whether ingroup/outgroup distinctions are made at this early processing stage. These investigations ask, in effect, whether social categories lead people to literally see ingroup and outgroup members’ faces differently. Initial findings from the N170 research on this topic were rather mixed, with some researchers observing larger N170 amplitudes to racial outgroups than ingroups (e.g. Stahl et al., 2008; Walker et al., 2008), others finding larger N170s to ingroup vs outgroup faces (Ito and Urland, 2005) and other studies finding no differences (e.g. Caldara et al., 2004; Wiese et al., 2009). It is notable, however, that the tasks and stimuli used to elicit the N170 varied considerably across these studies and therefore inconsistencies within this small literature were likely due to methodological variability (Ofan et al., 2011; Senholzi and Ito, 2013).
More recent work has clarified these issues by considering the effects of individual differences, social goals and situational factors associated with the processing of ingroup and outgroup faces. For example, Ratner and Amodio (2013) measured the N170 in response to faces classified according to an artificial, arbitrary group distinction (i.e. ‘minimal group’ categories; see Tajfel and Turner, 1986) and found larger N170 to ingroup compared with outgroup faces. This result is consistent with research showing that when a group distinction is arbitrary, and when intergroup competition is absent, people favor their own group members and are relatively indifferent to outgroup members (Brewer, 1999).
In the context of race, however, the outgroup may be threatening or otherwise noteworthy, leading to enhanced processing of outgroup faces. Indeed, greater processing of Black faces than White faces was observed among White subjects who scored higher on a behavioral measure of implicit prejudice (Ofan et al., 2011) and who viewed faces while anxious about appearing prejudiced (Ofan et al., in press). Other work shows that explicit instructions to attend to racial outgroup faces increases N170 responses to them, as compared with ingroup faces (Senholzi and Ito, 2013) (Figure 2). Together, these findings suggest that intergroup attitudes and goals can affect the way we see faces in the first place such that early perceptual biases may contribute to more elaborated forms of prejudice and stereotyping that have been traditionally found in social psychological research.
Fig. 2.
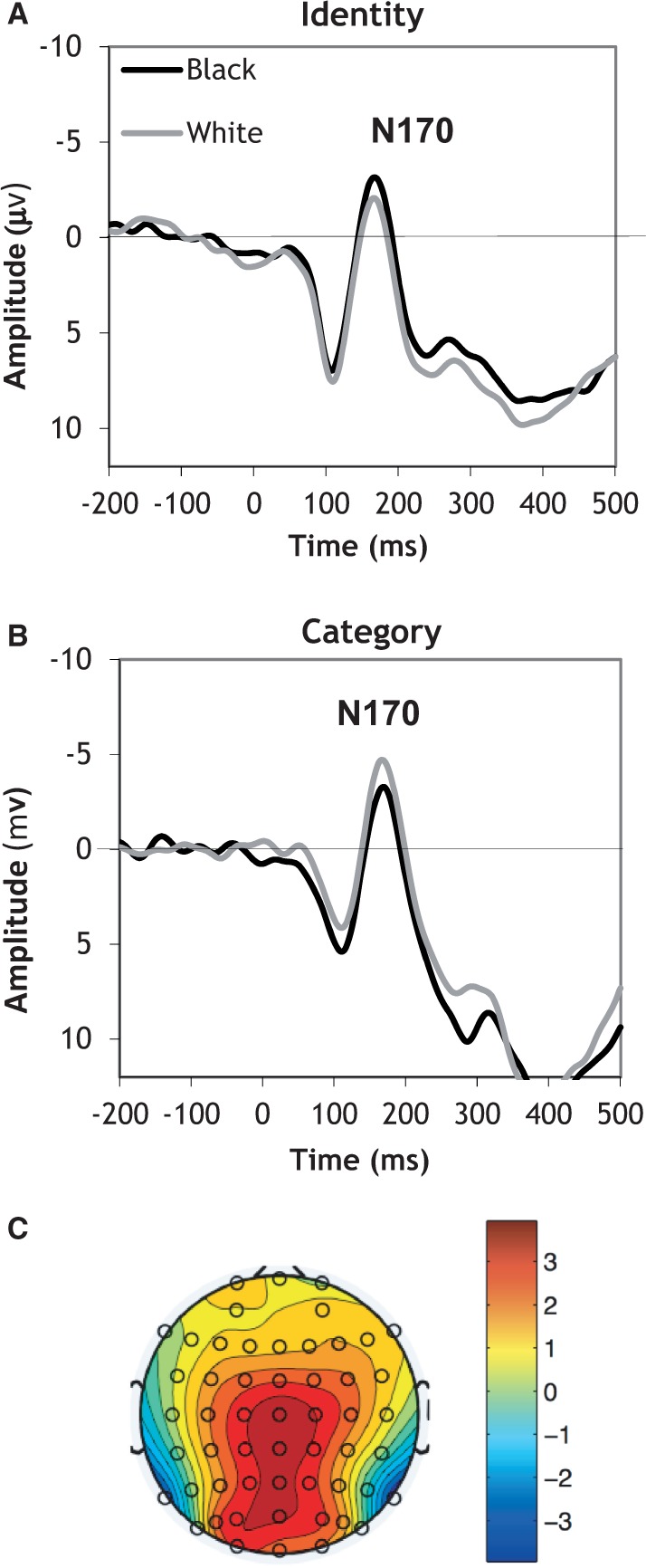
N170s elicited by Black and White faces from White subjects, showing the effect of social goals in the encoding of ingroup and outgroup faces. Panel A shows larger N170s to outgroup Black than ingroup White faces when subjects are motivated to attend to faces at the level of unique individuals. Panel B shows that the pattern reverses when subjects simply categorize the faces by race, resulting in larger N170s to ingroup White than outgroup Black faces. Panel C shows the topography of the N170 response (specifically over the 140–200 ms time window) (Senholzi and Ito, 2013). Structural face encoding: How task affects the N170’s sensitivity to race. Social Cognitive and Affective Neuroscience, by permission of Oxford University Press.
Social categorization
ERPs can also inform the process through which faces, once encoded, are categorized according to relevant social groups. Early research examined ERP responses as a function of a person’s gender, observing effects in negative polarity ERP components as early as ∼65 ms and then at ∼165 ms in parietal regions (Mouchetant-Rostaing et al., 2000). Given that these very early components precede the N170, they likely represent attention to coarse features of a face that are anticipated by top–down cognitive and attentional processes. Studies of racial categorization have observed similar patterns of early ERP responses. Ito and Urland (2003) used an oddball task in which target images were either consistent or inconsistent with the context images, as a function of both race (Black vs White) and gender. This study revealed that both gender and race were differentiated very rapidly, within 200 ms. Other studies have shown similar patterns of effects using variations of this task (e.g. Ito and Urland, 2005; Kubota and Ito, 2007) as well as several different types of implicit racial bias tasks (Amodio et al., 2004; Correll et al., 2006; Dickter and Bartholow, 2007, 2010; Hurtado et al., 2009). This literature of ERP findings has helped to delineate the timing and sequence of cognitive categorization in the rapidly unfolding process of person perception.
Stereotyping
To investigate the rapid activation of stereotypes, ERP components that respond to the semantic relationship between words in a sentence have been examined. In an early example of this research, Osterhout et al., (1997) recorded ERPs while participants read sentences that violated definitional (e.g. ‘the mailman took a shower after “she” got home’) or gender–stereotypical (e.g. ‘Our aerobics instructor gave “himself” a break’) noun–pronoun agreement (or violated neither). Their findings indicated that P3/LPP amplitude was enhanced to sentences containing definitional as well as stereotypical incongruities (compared with congruent control sentences), independent of participants’ overt judgments of the grammatical and syntactical correctness of the sentences. Bartholow et al., (2006, Experiment 1) subsequently demonstrated similar effects on P3 amplitude during processing of racial stereotype–incongruent trait words in a sequential priming task. This research demonstrated that social perceivers activated gender categories online as they evaluated new information about a person.
More recently, researchers have begun to investigate whether a different component—the N400 (or N4) known to be specifically sensitive to semantic processes (see Kutas and Federmeier, 2000)—is also sensitive to stereotyping. Kutas and Hillyard (1980) first famously reported that the N400 is larger to sentence-ending words that violate semantic context (e.g. ‘The pizza was too hot to “cry”’) compared with words congruent with semantic context (e.g. ‘The pizza was too hot to “eat”’). This feature has led some researchers to posit that the N400 might be sensitive to violations of semantic social knowledge, such as social stereotypes (see Bartholow et al., 2001). Consistent with this idea, White et al. (2009) recently observed larger N400 responses when subjects read sentences that, whereas semantically correct, violated rather than confirmed gender stereotypes. Together, these studies affirm findings from previous behavioral work (e.g. Gaertner and McLaughlin, 1983; Dovidio et al., 1986) indicating that stereotype-based categorizations occur very rapidly, but go beyond those previous reports by describing the time course over which such categorizations direct the online comprehension of subsequent information.
Self-regulation
Self-regulation refers broadly to the process of coordinating goal-directed responses. Research on self-regulation and cognitive control have focused on both corrective (i.e. bottom–up) and proactive (i.e. top–down) aspects of control and ERP research has been instrumental in delineating the specific mechanisms involved in these processes.
Amodio et al. (2004) used an ERP approach to identify the role of conflict monitoring in the regulation of social responses. Building on research in cognitive neuroscience (e.g. MacDonald et al., 2000; Botvinick et al., 2001), Amodio et al. (2004) suggested that the self-regulation of responses to stereotyped targets involves the coordination of two complementary processes: (i) an initial conflict monitoring mechanism, subserved by activity in the dorsal ACC, which monitors ongoing responses for conflict (e.g. between goal intentions and a race-biased tendency) and (ii) a regulative mechanism, associated with activity in lateral prefrontal cortex (PFC), which responds to the conflict by strengthening the influence of intentional responses to override an unwanted tendency (Kerns et al., 2004). In particular, Amodio et al. focused on the amplitude of the response-locked ERN component, a prominent fronto-central negativity that develops concurrently with the onset of a behavioral response (peaking around 50–80 ms post-response), which is always larger for incorrect than for correct responses (Figure 3). Considerable research has localized the ERN’s source to the dorsal ACC (Dehaene et al., 1994; van Veen and Carter, 2002), and its function to the operation of a conflict monitoring process (see Yeung et al., 2004).
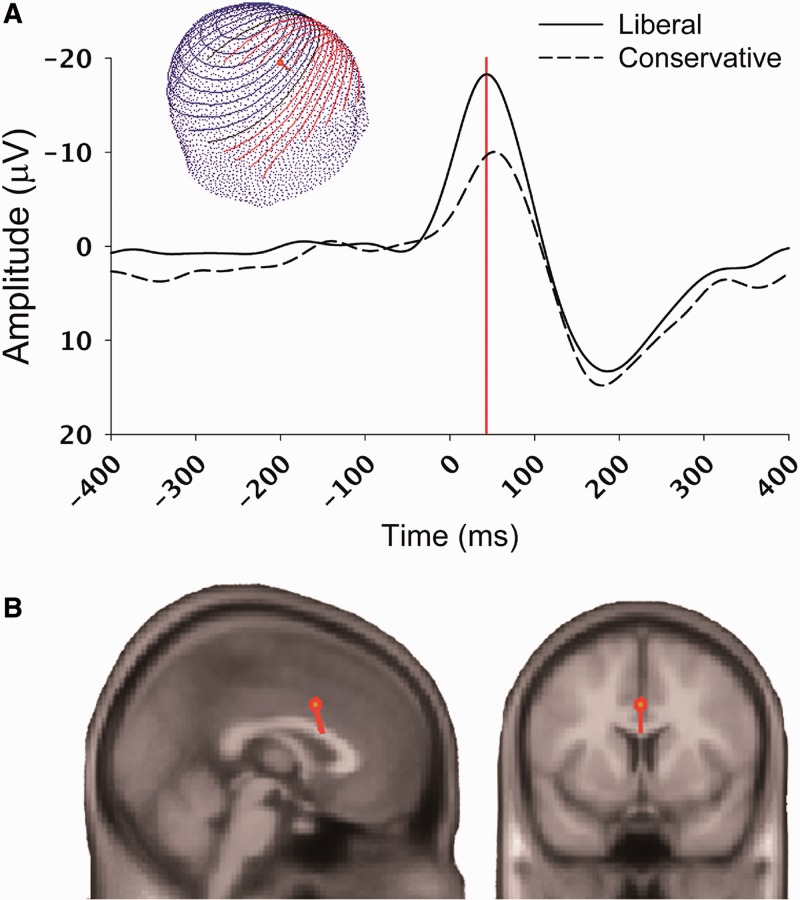
Fig. 3.
Illustration of the ERN component, as observed in a study on the relation between political orientation and individual differences in conflict monitoring processes (Amodio et al., 2007). Panel A depicts the ERN component in response to errors on No-Go trials of a Go/No-Go task for both liberal and conservative participants, showing greater responsiveness of the conflict monitoring process among liberals (response made at 0 ms; ERN peaked at 44 ms post-response). The inset shows the voltage map of the scalp distribution of the ERN. Panel B shows source localization maps, indicating a dorsal anterior cingulate generator for the ERN, computed at peak amplitude (red line in panel A).
By examining the ERN while participants completed a stereotype inhibition task, Amodio et al. found that the ACC was sensitive to unwanted stereotype-driven response tendencies, suggesting a role for conflict monitoring in the regulation of racial bias. Moreover, this research demonstrated that the monitoring of these responses occurred implicitly and operated independently of the regulative control process—a finding that relied on the high temporal resolution of ERP methods. This pattern has been replicated in other studies of racial stereotypes (Amodio et al., 2006, 2008a; Correll et al., 2006; Bartholow et al., 2012) and of gender stereotypes (Ma et al., 2008).
Whereas work by Amodio and colleagues (e.g. Amodio et al., 2004, 2008a) has focused on the conflict monitoring process, other ERP research has investigated the role of regulatory control in the expression of racial bias. For example, Bartholow et al. (2006) focused on the amplitude of a frontal negative slow wave (FSW or NSW), a late-developing, negative-going deflection believed to reflect PFC responses supporting regulative control processes (see West and Alain, 1999; West and Schwarb, 2006; Bailey et al., 2010; West and Bailey, 2012). In their study, Bartholow et al. used alcohol intoxication to manipulate control, and found that intoxicated subjects exhibited impaired regulative control (as indicated by reduced FSW amplitude on stereotypic inhibition trials) but not conflict monitoring (as indicated by the amplitude of the N2 component on those same trials). Importantly, the amplitude of the FSW on stereotypic inhibition trials correlated with participants’ ability to withhold stereotypic responses, suggesting a link between regulative control mechanisms in PFC and self-regulation of racial bias. Other research has used a combination of ERPs and online EEG measurement of frontal cortical activity to show that increases in PFC are associated with enhanced regulative control over stereotypes in behavior, and that this effect occurs by tuning participants’ perception of Black vs White faces (Amodio, 2010b).
Individual differences in self-regulatory processes
A number of studies have used ERP approaches to understand how individual differences, such as in one’s social beliefs or motivations, affect self-regulation. For example, building on their previous work (Devine et al., 2002; Amodio et al., 2003;), Amodio and colleagues (Amodio et al., 2008a) sought to understand why low-prejudice individuals who feel anxious about appearing prejudice in front of others have more trouble inhibiting stereotypes than those who do not feel anxious. These authors hypothesized that such anxious (i.e. externally motivated; Plant and Devine, 1998) individuals may be less sensitive to internal conflict monitoring signals associated with racial stereotypes. Indeed, this group evidenced smaller ERNs to response conflicts on a stereotype inhibition task than non-anxious individuals, and this ERN effect explained the difference observed in their behavioral control of stereotypes. Thus, the ERP approach provided a novel test of a social cognitive mechanism to explain why some people are more prone to expressing implicit stereotypes than others.
Other research has examined ERP responses on more basic conflict tasks, such as the Stroop or Go/No-Go tasks, as a means to test hypotheses about the roots of self-regulation in basic neurocognitive systems (e.g. Amodio et al., 2007; Forbes et al., 2008; Amodio et al., 2008b;). For example, Amodio et al. (2007) demonstrated that individual differences in liberal vs conservative political ideology was related to the sensitivity of the conflict monitoring system, as measured by the ERN, linking ideology to a more general underlying process of self-regulation (Figure 3). Other research by Inzlicht and Gutsell (2007) suggests that conflict monitoring capabilities, as measured by the ERN during the Stroop task, are diminished following the depletion of cognitive resources.
Another important feature of ERP research on self-regulation is the typical incorporation of behavioral measures, which may be used to validate interpretations of ERP effects. That is, self-regulation often pertains to action control and ERP methods can be used in combination with behavioral tasks and methods that have been developed to assess control in behavior. For example, Amodio et al. (2004) proposed that the ERN should be associated with controlled, but not automatic patterns of behavior during regulation of racial bias. Using a process dissociation method to model independent estimates of automatic and controlled responding (Jacoby, 1991; Payne, 2001), the authors demonstrated that ERN amplitudes were strongly associated with controlled processing but unrelated to automatic processing. Thus, by combining ERPs with the mathematical modeling of task behavior, researchers can achieve a high level of theoretical and methodological precision.
PRACTICAL CONSIDERATIONS FOR USING ERP METHODS AS A RESEARCH TOOL
Like virtually all methodologies, ERPs have advantages and disadvantages. A major advantage of ERPs as a dependent measure is their unrivaled capacity for tracking the precise timing of neural processes; ERPs currently represent one of the only direct measures of brain activity as it occurs in real time (see also Gratton and Fabiani, 2001). The relative sluggishness of the hemodynamic response measured with fMRI can limit the inferences that can be drawn from fMRI data, particularly for testing hypotheses about rapidly unfolding cognitive processes. Another major advantage of ERPs over traditional behavioral measures, as mentioned previously, is the ability to measure psychological processes independently from, or in the absence of, any behavioral response. This property allows researchers to separate, for example, the latency of overt responses from the timing of underlying cognitive processes on which those responses are thought to depend (see McCarthy and Donchin, 1981), as well as processes associated with ‘cognitive’ processing as opposed to response implementation (see Coles et al., 1995).
There are also several practical benefits, including substantially lower costs relative to neuroimaging methods frequently used in SCAN, and a data collection environment that may be less impactful on subtle social and affective processes of interest. An often overlooked advantage of ERP relative to fMRI is that participants in the ERP lab sit upright during data acquisition, whereas fMRI studies require participants to lie supine and motionless in a scanner bore. This distinction has a number of implications for SCAN research. Ecologically, an upright position more closely mimics how people typically interact in the social world than does a supine position. Perhaps more importantly, however, research has shown that certain psychological processes, especially those pertaining to approach motivation, do not operate in the same manner when people are lying down compared to when seated or standing (Harmon-Jones and Peterson, 2009). This finding suggests caution when interpreting the findings of all psychophysiological studies in which participants’ body posture and movements are restricted, but seem especially important for fMRI and other neuroimaging studies in which participants lie down or are otherwise restrained.
Of course, the major disadvantage of ERPs as a tool for SCAN research is their limited spatial resolution. This occurs partially because ERPs detect only neural activity of sufficient strength to be measurable at the scalp, rendering them insensitive to activity in subcortical structures like the amygdala. A second consideration is that the skull and scalp act as a spatial filter on neural activity, reducing the spatial resolution of the signal recorded with scalp electrodes. It is nevertheless possible to estimate the neural generators of ERPs using source localization procedures that consider the orientation and strength of a dipole signal, as measured at multiple sites across the scalp (see Scherg and Picton, 1991; Huizenga and Molenaar, 1994; Pascual-Marqui et al., 1994). Although the spatial resolution of such analyses is coarse relative to fMRI, these techniques often permit reasonably accurate estimates of the underlying sources of scalp-recorded potentials. In many instances, inferences about localization can be further strengthened by reference to converging fMRI and/or brain lesion data obtained using similar paradigms, as illustrated in our discussion of the self-regulation of racial bias in which converging ERP and fMRI research support a dorsal ACC generator for the ERN component.
It is also important to acknowledge the possibility of more directly leveraging the complementary strengths of ERP and fMRI in the temporal and spatial domains, respectively, through simultaneous (or sequential) measurement of the two (Mulert et al., 2004; Ullsperger and Debener, 2010). Consider an example in which ERP and fMRI were acquired simultaneously during a flanker task (Debener et al., 2005); errors on incompatible trials were associated with both increased ERNs and BOLD activity in the ACC, replicating findings obtained with each measure separately (e.g., Botvinick et al., 1999; Gehring et al., 1993). More importantly, trial-by-trial ERN amplitude co-varied with trial-by-trial hemodynamic activity in the ACC. This provided compelling evidence that the ERN is generated by the ACC and that this region contributes to very quickly occurring responses involved in behavior regulation. Nevertheless, questions regarding the precise location of a neural activation or regarding activity in regions that are not well represented by ERPs (e.g. subcortical structures), can be addressed more effectively using other methods such as fMRI.
Interpretational ssues
A final practical consideration concerns the mapping between psychological interpretations and ERP activity. As with most psychophysiological measures (including fMRI), a researcher must be very careful when making specific psychological interpretations of ERP components. As a general principle, the neural source of a particular ERP component is likely involved in multiple psychological functions and therefore a one-to-one mapping of a psychological construct onto a physiological indicator can never be assumed (Amodio, 2010a; Cacioppo and Tassinary, 1990). Therefore, as discussed by Folstein and van Petten (2008), readers are cautioned against assuming that, for example, the N2 associated with an ingroup attention bias in social categorization tasks (e.g. Ito and Urland, 2003, 2005) reflects the same neural source or represents similar information processing operations as the prominent N2 often seen in tasks involving response conflict or inhibition (Amodio et al., 2004; Dickter and Bartholow, 2010). Furthermore, although an ERP component may be described as reflecting a specific psychological process (see Fabiani et al., 2007) it is likely that any given component represents multiple simultaneously occurring neural activations, which together may comprise a circuit important for numerous information processing operations. Similar concerns pertain to fMRI, whereby a single observed activation may actually reflect activity in multiple subregions, due to threshold-based artifacts or the involvement of different populations of neurons underlying a similar pattern of BOLD activity. Furthermore, one can never be sure that an observed pattern of ERPs or BOLD signal (in fMRI) from two different tasks actually reflects the same collection of neurons (or the same psychological process) and so care is required in experimental design and data analysis to rule out potential alternative interpretations.
CONCLUSIONS
ERP methodology offers a powerful set of tools for probing the neural mechanisms underlying social cognition and affect. Here, we have provided an overview of the ways that ERPs may be used to address a range of critical questions concerning social and affective processes, highlighting issues of evaluation, social cognition and self-regulation as just a few examples. Given the advantages afforded by ERPs, such as exquisite temporal measurement of neural activation and their versatile use with a range of experimental tasks, ERP methods are likely to become increasingly prominent in SCAN laboratories, along with a host of other neural and physiological approaches that have emerged in the field of social neuroscience. As the questions of social and affective neuroscience continue to shift from neural location to neural process, ERP methods will become increasingly indispensable and more frequently integrated into the methodological toolkit of most neuroimaging labs.
Conflict of Interest
None declared.
Acknowledgments
National Institute on Alcohol Abuse and Alcoholism (R01 AA020970); National Science Foundation (BCS 0847350; BCS 0847872; RL 0910373); National Institute of Drug Abuse (DA 024002).
Footnotes
1There are several alternatives to the averaging approach to deriving ERP signals, such as principle components analysis (PCA) (e.g. see Dien and Frischkoff, 2005). However, in most situations averaging and PCA produce highly similar results.
REFERENCES
- Allison T, Wood CC, McCarthy GM. The central nervous system. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology: Systems, processes, and Applications. New York: Guilford Press; 1986. pp. 5–25. [Google Scholar]
- American Encephalographic Society. Guideline thirteen: Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–3. [PubMed] [Google Scholar]
- Amodio DM. Can neuroscience advance social psychological theory? Social neuroscience for the behavioral social psychologist. Social Cognition. 2010a;28:695–716. [Google Scholar]
- Amodio DM. Coordinated roles of motivation and perception in the regulation of intergroup responses: Frontal cortical asymmetry effects on the P2 event-related potential and behavior. Journal of Cognitive Neuroscience. 2010b;22:2609–17. doi: 10.1162/jocn.2009.21395. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Bartholow BD. Event-related potential methods in social cognition. In: Voss A, Stahl C, Klauer C, editors. Cognitive Methods in Social Psychology. New York: Guilford Press; 2011. pp. 303–39. [Google Scholar]
- Amodio DM, Devine PG, Harmon-Jones E. Individual differences in the regulation of intergroup bias: The role of conflict monitoring and neural signals for control. Journal of Personality and Social Psychology. 2008a;94:60–74. doi: 10.1037/0022-3514.94.1.60. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink responses and self-report. Journal of Personality and Social Psychology. 2003;84:738–53. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Hartley SL, Covert AE. Neural signals for the detection of unintentional race bias. Psychological Science. 2004;15:88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Jost JT, Master SL, Yee CM. Neurocognitive correlates of liberalism and conservatism. Nature Neuroscience. 2007;10:1246–7. doi: 10.1038/nn1979. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Kubota JT, Harmon-Jones E, Devine PG. Alternative mechanisms for regulating racial responses according to internal vs. external cues. Social Cognitive and Affective Neuroscience. 2006;1:26–36. doi: 10.1093/scan/nsl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology. 2008b;45:11–9. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Mendoza SA. Implicit intergroup bias: Cognitive, affective, and motivational underpinnings. In: Gawronski B, Payne BK, editors. Handbook of Implicit Social Cognition. New York: Guilford; 2010. pp. 353–74. [Google Scholar]
- Bailey K, West R, Anderson C. A negative association between video game experience and proactive cognitive control. Psychophysiology. 2010;47:34–42. doi: 10.1111/j.1469-8986.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Amodio DM. Using event-related brain potentials in social psychological research: A brief review and tutorial. In: Harmon-Jones E, Beer JS, editors. Methods in Social Neuroscience. New York: Guilford Press; 2009. pp. 198–232. [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: Cognitive control of inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90:272–87. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Fabiani M, Gratton G, Bettencourt BA. A psychophysiological analysis of cognitive processing of and affective responses to social expectancy violations. Psychological Science. 2001;12:197–204. doi: 10.1111/1467-9280.00336. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK. Alcohol effects on performance monitoring and adjustment: Affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology. 2012;121:173–86. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Riordan MA, Saults JS, Lust SA. Psychophysiological evidence of response conflict and strategic control of responses in affective priming. Journal of Experimental Social Psychology. 2009;45:655–66. doi: 10.1016/j.jesp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brewer MB. The psychology of prejudice: Ingroup love or outgroup hate? Journal of Social Issues. 1999;55:429–44. [Google Scholar]
- Cacioppo JT, Crites SL, Jr, Berntson GG, Coles MGH. If attitudes affect how stimuli are processed, should they not affect the event-related brain potential? Psychological Science. 1993;4:108–12. [Google Scholar]
- Cacioppo JT, Crites SL, Gardner WL, Berntson GG. Bioelectrical echoes from evaluative categorizations: I. A late positive brain potential that varies as a function of trait negativity and extremity. Journal of Personality and Social Psychology. 1994;67:115–25. doi: 10.1037//0022-3514.67.1.115. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG. Psychophysiology and psychophysiological inference. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. New York: Cambridge University Press; 1990. pp. 3–33. [Google Scholar]
- Caldara R, Rossion B, Bovet P, Hauert C. Event-related potentials and time course of the ‘other-race' face classification advantage. Neuroreport. 2004;15:905. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- Coles MGH. Modern mind-brain reading: Psychophysiology, physiology, and cognition. Psychophysiology. 1989;26:251–69. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Rugg MD. Event-related brain potentials: An introduction. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-related Brain Potentials and Cognition. New York: Oxford University Press; 1995. pp. 1–26. [Google Scholar]
- Coles MGH, Smid HGOM, Scheffers MK, Otten LJ. Mental chronometry and the study of human information processing. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-related Brain Potentials and Cognition. New York: Oxford University Press; 1995. pp. 86–131. [Google Scholar]
- Correll J, Urland GR, Ito TA. Event-related potentials and the decision to shoot: The role of threat perception and cognitive control. Journal of Experimental Social Psychology. 2006;42:120–8. [Google Scholar]
- Crites SL, Cacioppo JT. Electrocortical differentiation of evaluative and nonevaluative categorizations. Psychological Science. 1996;7:318–21. [Google Scholar]
- Crites SL, Cacioppo JT, Gardner WL, Berntson GG. Bioelectrical echoes from evaluative categorization: II. A late positive brain potential that varies as a function of attitude registration rather than attitude report. Journal of Personality and Social Psychology. 1995;68:997–1013. doi: 10.1037//0022-3514.68.6.997. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. The Journal of Neuroscience. 2005;25:11730–7. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffke I, Sander T, Heidenreich J, et al. MEG/EEG sources of the 170-ms response to faces are co-localized in the fusiform gyrus. Neuroimage. 2007;35:1495–501. doi: 10.1016/j.neuroimage.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–5. [Google Scholar]
- Devine PG, Plant EA, Amodio DM, Harmon-Jones E, Vance SL. The regulation of explicit and implicit race bias: The role of motivations to respond without prejudice. Journal of Personality and Social Psychology. 2002;82:835–48. [PubMed] [Google Scholar]
- Dickter CL, Bartholow BD. Event-related brain potential evidence of ingroup and outgroup attention biases. Social, Cognitive, and Affective Neuroscience. 2007;2:189–98. doi: 10.1093/scan/nsm012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickter CL, Bartholow BD. Ingroup categorization and response conflict: Interactive effects of target race, flanker compatibility and infrequency on N2 amplitude. Psychophysiology. 2010;47:596–601. doi: 10.1111/j.1469-8986.2010.00963.x. [DOI] [PubMed] [Google Scholar]
- Dickter CL, Kieffaber PK. EEG Methods for the Psychological Sciences. London: Sage Publications, Ltd; 2013. [Google Scholar]
- Dien J, Frischkoff GA. Prinicipal components analysis of ERP data. In: Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambridge, MA: MIT Press; 2005. pp. 189–207. [Google Scholar]
- Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- Dovidio JF, Evans N, Tyler RB. Racial stereotypes: The contents of their cognitive representations. Journal of Experimental Social Psychology. 1986;22:22–37. [Google Scholar]
- Eimer M. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clinical Neurophysiology. 2000;111(4):694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Federmeier K. Event related brain potentials. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd edn. New York: Cambridge University Press; 2007. pp. 85–119. [Google Scholar]
- Fabiani M, Gratton G, Karis D, Donchin E. The definition, identification, and reliability of measurement of the P300 component of the event-related brain potential. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Vol. 1. Greenwich, CT: JAI; 1987. pp. 1–78. [Google Scholar]
- Fazio RH, Sanbonmatsu DM, Powell MC, Kardes FR. On the automatic activation of attitudes. Journal of Personality and Social Psychology. 1986;50:229–38. doi: 10.1037//0022-3514.50.2.229. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–70. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes CE, Schmader T, Allen JJB. The role of devaluing and discounting in performance monitoring: A neurophysiological study of minorities under threat. Social Cognitive and Affective Neuroscience. 2008;3:253–61. doi: 10.1093/scan/nsn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner SL, McLaughlin JP. Racial stereotypes: Associations and ascriptions of positive and negative characteristics. Social Psychology Quarterly. 1983;46:23–30. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–90. [Google Scholar]
- Gratton G, Fabiani M. Shedding light on brain function: The event-related optical signal. Trends in Cognitive Sciences. 2001;5:357–63. doi: 10.1016/s1364-6613(00)01701-0. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Peterson CK. Supine body position reduces neural response to anger evocation. Psychological Science. 2009;20:1209–10. doi: 10.1111/j.1467-9280.2009.02416.x. [DOI] [PubMed] [Google Scholar]
- Huizenga HM, Molenaar PCM. Estimating and testing the sources of evoked potentials in the brain. Multivariate Behavioral Research. 1994;29:237–62. doi: 10.1207/s15327906mbr2903_3. [DOI] [PubMed] [Google Scholar]
- Hurtado E, Haye A, Gonzalez R, Manes F, Ibanez R. Contextual blending of ingroup/outgroup face stimuli and word valence: LPP modulation and convergence of measures. BMC Neuroscience, 10. 2009:69. doi: 10.1186/1471-2202-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Gutsell JN. Running on empty: Neural signals for self-control failure. Psychological Science. 2007;8:233–8. doi: 10.1111/j.1467-9280.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- Ito TA, Cacioppo JT. Electrophysiological evidence of implicit and explicit categorization processes. Journal of Experimental Social Psychology. 2000;35:660–76. [Google Scholar]
- Ito TA, Cacioppo JT. Attitudes as mental and neural states of readiness: Using physiological measures to study implicit attitudes. In: Wittenbrink B, Schwarz N, editors. Implicit Measures of Attitudes. New York: Guilford Press; 2007. pp. 125–58. [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Ito TA, Thompson E, Cacioppo JT. Tracking the timecourse of social perception: The effects of racial cues on event-related brain potentials. Personality and Social Psychology Bulletin. 2004;30:1267–80. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. Race and gender on the brain: Electrocortical measures of attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology. 2003;85(4):616–26. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–41. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring predicts adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Musch J. Affective priming: Findings and theories. In: Musch J, Klauer KC, editors. The Psychology of Evaluation: Affective Processes in Cognition and Emotion. Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 7–49. [Google Scholar]
- Klauer KC, Musch J, Eder A. Priming of semantic classifications: Late and response-related, or earlier and more central? Psychonomic Bulletin and Review. 2005;12:897–903. doi: 10.3758/bf03196783. [DOI] [PubMed] [Google Scholar]
- Klinger MR, Burton PC, Pitts G. Mechanisms of unconscious priming: Response competition, not spreading activation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:441–55. doi: 10.1037//0278-7393.26.2.441. [DOI] [PubMed] [Google Scholar]
- Kubota JT, Ito TA. Multiple cues in social perception: The time course of processing race and facial expression. Journal of Experimental Social Psychology. 2007;43:738–52. doi: 10.1016/j.jesp.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Science. 2000;4:463–70. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–5. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science. 1977;197:792–5. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philosophical Transactions of the Royal Society of London, B. 2002;357:1003–37. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ, Kappenman ES. Oxford Handbook of ERP Components. New York: Oxford University Press; 2012. [Google Scholar]
- Ma Q, Shu L, Wang X, Dai S, Che H. Error-related negativity varies with the activation of gender stereotypes. Neuroscience Letters. 2008;442:186–9. doi: 10.1016/j.neulet.2008.06.080. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of dorsolateral prefrontal cortex and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric of thought: A comparison of P300 latency and reaction time. Science. 1981;21:171–86. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McConahay JB, Hardee BB, Batts V. Has racism declined in America? It depends on who is asking and what is asked. Journal of Conflict Resolution. 1981;25:563–79. [Google Scholar]
- Marshall-Goodell BS, Tassinary LG, Cacioppo JT. Principles of bioelectrical measurement. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. New York: Cambridge University Press; 1990. pp. 113–48. [Google Scholar]
- Mouchetant-Rostaing Y, Girard MH, Bentin S, Aguera PE, Pernier J. Neurophysiological correlates of face gender processing in humans. European Journal of Neuroscience. 2000;12:303–10. doi: 10.1046/j.1460-9568.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, et al. Integration of fMRI and simultaneous EEG: Towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–32. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Ofan RH, Rubin N, Amodio DM. Seeing race: N170 responses to race and their relation to automatic racial attitudes and controlled processing. Journal of Cognitive Neuroscience. 2011;10:3153–61. doi: 10.1162/jocn_a_00014. [DOI] [PubMed] [Google Scholar]
- Ofan RH, Rubin N, Amodio DM. Situation-based social anxiety enhances the neural processing of faces: evidence from an intergroup context. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nst087. doi:10.1093/scan/nst087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout L, Bersick M, McLaughlin J. Brain potentials reflect violations of gender stereotypes. Memory & Cognition. 1997;25:273–85. doi: 10.3758/bf03211283. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Payne BK. Prejudice and perception: The role of automatic and controlled processes in misperceiving a weapon. Journal of Personality and Social Psychology. 2001;81:181–92. doi: 10.1037//0022-3514.81.2.181. [DOI] [PubMed] [Google Scholar]
- Plant EA, Devine PG. Internal and external motivation to respond without prejudice. Journal of Personality and Social Psychology. 1998;75:811–32. doi: 10.1177/0146167205275304. [DOI] [PubMed] [Google Scholar]
- Ratner KG, Amodio DM. Seeing “us vs. them”: Minimal group effects on the neural encoding of faces. Journal of Experimental Social Psychology. 2013;49:298–301. [Google Scholar]
- Scherg M, Picton TW. Separation and identification of event-related potential components by brain electrical source analysis. In: Brunia CHM, Mulder G, Verbaten MN, editors. Event-Related Brain Research. Amsterdam: Elsevier; 1991. pp. 24–37. [PubMed] [Google Scholar]
- Senholzi KB, Ito TA. Structural face encoding: how task affects the N170’s sensitivity to race. Social Cognitive and Affective Neuroscience. 2013;8:937–42. doi: 10.1093/scan/nss091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires KC, Wickens C, Squires NK, Donchin E. The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 1976;193:1142–6. doi: 10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- Stahl J, Wiese H, Schweinberger SR. Expertise and own-race bias in face processing: An event-related potential study. NeuroReport. 2008;19:583–58. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- Tajfel H, Turner JC. The social identity theory of intergroup behavior. In: Worchel S, Austin WG, editors. Psychology of Intergroup Relations. Chicago, IL: Nelson-Hall; 1986. pp. 7–24. [Google Scholar]
- Ullsperger M, Debener S. Simultaneous EEG and fMRI: Recording, Analysis, and Application. New York: Oxford University Press; 2010. [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Walker P, Silvert L, Hewstone M, Nobre A. Social contact and other-race face processing in the human brain. Social Cognitive and Affective Neuroscience. 2008;3:16. doi: 10.1093/scan/nsm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentura D, Rothermund K. The “meddling-in” of affective information: A general model of automatic evaluation effects. In: Musch J, Klauer KC, editors. The Psychology of Evaluation: Affective Processes in Cognition and Emotion. Mahwah: Lawrence Erlbaum Associates; 2003. pp. 51–86. [Google Scholar]
- West R, Alain C. Event-related neural activity associated with the Stroop task. Cognitive Brain Research. 1999;8:157–64. doi: 10.1016/s0926-6410(99)00017-8. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K. ERP correlates of dual mechanisms of control in the counting Stroop task. Psychophysiology. 2012;49:1309–18. doi: 10.1111/j.1469-8986.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–81. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- White KR, Crites SL, Jr, Taylor JH, Corral G. Wait, what? Assessing stereotype incongruities using the N400 ERP component. Social Cognitive and Affective Neuroscience. 2009;4:191–8. doi: 10.1093/scan/nsp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese H, Stahl J, Schweinberger S. Configural processing of other-race faces is delayed but not decreased. Biological Psychology. 2009;81:103–9. doi: 10.1016/j.biopsycho.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]