Abstract
Cognitive neuroscience has made considerable progress in understanding the neural architecture of human intelligence, identifying a broadly distributed network of frontal and parietal regions that support goal-directed, intelligent behavior. However, the contributions of this network to social and emotional aspects of intellectual function remain to be well characterized. Here we investigated the neural basis of emotional intelligence in 152 patients with focal brain injuries using voxel-based lesion-symptom mapping. Latent variable modeling was applied to obtain measures of emotional intelligence, general intelligence and personality from the Mayer, Salovey, Caruso Emotional Intelligence Test (MSCEIT), the Wechsler Adult Intelligence Scale and the Neuroticism-Extroversion-Openness Inventory, respectively. Regression analyses revealed that latent scores for measures of general intelligence and personality reliably predicted latent scores for emotional intelligence. Lesion mapping results further indicated that these convergent processes depend on a shared network of frontal, temporal and parietal brain regions. The results support an integrative framework for understanding the architecture of executive, social and emotional processes and make specific recommendations for the interpretation and application of the MSCEIT to the study of emotional intelligence in health and disease.
Keywords: emotional intelligence, general intelligence, voxel-based lesion-symptom mapping
INTRODUCTION
Accumulating evidence supports an integrative understanding of the human mind, uncovering the cognitive and emotional foundations of intelligent behavior, and their joint contributions to perception, memory, language and thought (for reviews, see Blakemore et al.., 2004; Ochsner, 2004; Barrett et al.., 2007; Lieberman, 2007; Adolphs, 2010). Parallel developments in cognitive neuroscience have motivated new perspectives about the information processing architecture of the mind, breaking away from the classic view that each identifiable function is localized to a single cortical area [reviewed in (Miller, 2000; Miller and Cohen, 2001; Miller and Phelps, 2010)]. One postulate of this approach is that each cortical region has more than one function, and that functions of distinct areas might overlap with one another to support a coordinated architecture for cognitive and emotional processes.
According to this emergent view, most neural computations should not be thought of as implemented by an individual area, but rather by the interaction and collaboration among multiple areas. Specific brain regions are thought to belong to several intersecting networks based on their structural topology and functional connectivity (Passingham et al.., 2002). Therefore, the impact of a brain region on behavior depends on its structural and functional connectivity as a member of a broader information processing network. Advances in network theory have shown that regions characterized by a high degree of functional connectivity are important in regulating the flow and integration of information among areas (Guimera and Amaral, 2005; Guimera et al.., 2007; Sporns et al.., 2007). A recent computational study indicates, for example, that the dorsolateral prefrontal cortex (BA 46) in the macaque links multiple functional clusters, supporting an integrative architecture for the coordination of multiple brain systems (Sporns et al.., 2007). These developments suggest that connectivity information will be particularly important in understanding a region’s role in the coordination of cognitive and emotional processes.
Together with cognitive intelligence, emotional and social intelligence form important components of general intelligence. One of the major differences between the two is that the former is thought to relate primarily to higher order mental processes like reasoning, while the latter focuses more on perceiving, immediate processing and applying emotional and social content, information and knowledge. It has also been suggested that another fundamental difference between the two may be that cognitive intelligence depends primarily on the prefrontal cortex, whereas emotional and social intelligence is more limbically tactical for immediate behavior suited more for survival and adaptation (Goleman, 1995; Bar-On et al.., 2000; Stein and Book, 2011). However, thus far these theories are supported more by supposition than by empirical findings.
One of the primary purposes of this study is to investigate whether the neural architecture of emotional and social intelligence is integrated with cognitive intelligence or instead depends on distinct brain systems. Of the neuropsychological patient studies that have examined the neural bases of general intelligence (Basso et al.., 1973; Black, 1976; Eslinger and Damasio 1985; Shallice and Burgess, 1991; Bechara et al.., 1994; Duncan et al.., 1995; Burgess and Shallice, 1996; Isingrini and Vazou, 1997; Parkin and Java, 1999; Blair and Cipolotti, 2000; Kane and Engle, 2002; Bugg et al.., 2006; Glascher et al.., 2009, 2010; Roca et al.., 2010; Barbey et al. 2012), social cognition (Kertesz et al.., 1979; Naeser et al.., 1982; Alexander et al.., 1989; Damasio, 1992; Kertesz et al.., 1993; Caplan et al.., 1996; Bates et al.., 2003; Dronkers et al.., 2004; Caplan et al.., 2007; Tyler and Marslen-Wilson, 2008) and emotion (Bechara et al. 1994; Devinsky et al.., 1995; Rowe et al.., 2001; Stuss et al.., 2001), all share one or more of the following features: diffuse (rather than focal) brain lesions, lack of comparison subjects carefully matched for pre- and post-injury performance measures and exclusive use of cognitive or emotional measures. As a consequence, there has been no comprehensive evaluation of these processes in a relatively large sample of patients with focal brain damage and across a broad range of tasks and stimulus material. The absence of such data represents a substantial gap in understanding the cognitive and neural architecture of human intelligence.
A central goal of the present study is to characterize the neural basis of emotional intelligence in a large sample of patients with focal brain injuries (n = 152), examining task performance on a comprehensive battery of tests designed to measure: (i) emotional intelligence (Mayer, Salovey, Caruso Emotional Intelligence Test; MSCEIT); (ii) general intelligence [(fluid ability, crystallized ability, working memory and processing speed; Wechsler Adult Intelligence Scale, third edition (WAIS-III)] and (iii) personality (extraversion, agreeableness, conscientiousness, neuroticism and openness to experience; NEO-PI-R). We investigated the neural substrates of each domain, examining the (i) selectivity of cortical networks for specific domains and (ii) the degree to which these systems engage common networks. We applied confirmatory factor analysis to obtain latent scores of each domain, followed by voxel-based lesion-symptom mapping.
MATERIALS AND METHODS
Participant data
Participants were drawn from the Phase 3 Vietnam Head Injury Study (VHIS) registry, which includes American male veterans who suffered brain damage from penetrating head injuries in the Vietnam War (n = 152). All subjects gave informed written consent. Phase 3 testing occurred between April 2003 and November 2006. Demographic and background data for the VHIS are reported in Supplementary Table 1 [see also (Koenigs et al.., 2009, 2001; Barbey et al.., 2011, 2012)]. No effects on test performance were observed in the VHIS sample on the basis of demographic variables (e.g., age, years of education, lesion size).
Lesion analysis
Computed tomography (CT) data were acquired during the Phase 3 testing period. The axial CT scans were acquired without contrast in helical mode on a GE Electric Medical Systems Light Speed Plus CT scanner at the Bethesda Naval Hospital. Structural neuroimaging data were reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm and a 1 mm slice interval. Lesion location and volume from CT images were determined using the interactive Analysis of Brain Lesions (ABLe) software implemented in MEDx, version 3.44 (Medical Numerics) (Makale et al.., 2002; Solomon et al.., 2007). Lesion volume was calculated by manually tracing the lesion in all relevant slices of the CT image in native space, and then summing the trace areas and multiplying by slice thickness. Manual tracing was performed by a trained psychiatrist with clinical experience of reading CT scans. The lesion tracing was then reviewed by an observer who was blind to the results of the clinical evaluation and neuropsychological testing enabling a consensus decision to be reached regarding the limits of each lesion. The CT image of each individual’s brain was normalized to a CT template brain image in Montreal Neurological Institute (MNI) space. The spatial normalization was performed with the automated image registration (AIR) algorithm (Woods et al.., 1993), using a 12-parameter affine fit. Note that both the patient’s brain and the CT template brain are first skull-stripped to maximize the efficacy of the AIR registration from native space to MNI space. In addition, voxels inside the traced lesion were not included in the spatial normalization procedure. For each subject, a lesion mask image in MNI space was saved for voxel-based lesion-symptom mapping (Bates et al.., 2003). The lesion overlap map for the entire VHIS patient sample is illustrated in Supplementary Figure 2.
Neuropsychological tests
We administered the MSCEIT (Mayer et al.., 2008), the WAIS-III (Wechsler, 1997) and the NEO-PI-R (Costa and McCrae, 1997) to investigate the common and specific neural substrates underlying emotional intelligence, psychometric intelligence and basic personality traits. Supplementary Table 2 summarizes the employed measures [for further detail concerning their standardization, reliability and validity, see (Wechsler, 1997; Mayer et al.., 2008)].
Confirmatory factor analysis
The measurement model for psychometric and emotional intelligence was tested (Supplementary Figure 1). Emotional intelligence was measured by the full MSCEIT inventory and psychometric intelligence was defined by the WAIS-III, which includes measures of fluid/perceptual ability, crystallized/verbal ability, working memory and processing speed.
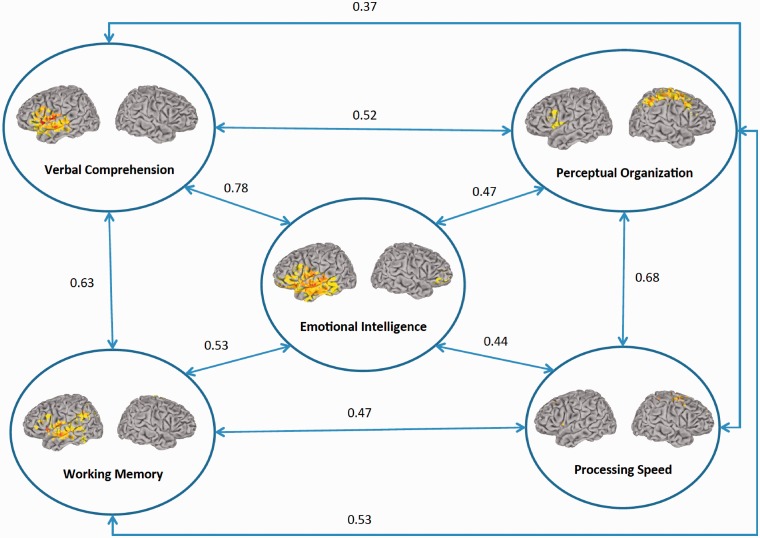
This five-factor model produced appropriate fit indices: χ2 = 344.9, degrees of freedom (DF) = 199, χ2/DF = 1.73, RMSEA = 0.07. All correlations among factors are statistically significant (P < 0.000). Nevertheless, the highest correlation was found for crystallized/verbal and emotional intelligence (r = 0.78).
Computation of scores of interest
Using the imputation function of the Analysis of moment structures (AMOS) program (Arbuckle, 2006), we obtained latent scores for the five factors depicted in Figure 1. The four latent scores derived from the WAIS-III subtests, along with the scores obtained from the NEO big five scales, were submitted to a stepwise regression analysis for predicting the latent score derived from the MSCEIT battery. Results showed that crystallized/verbal intelligence, processing speed and conscientiousness significantly contributed to the prediction of emotional intelligence (adjusted R2 = 0.81, P < 0.000). Emotional intelligence unpredicted defined a residual score reflecting its specific variance. This residual score supported an investigation of the neural basis of emotional intelligence while removing the variance shared with verbal intelligence, processing speed and conscientiousness.
Fig. 1.
Summary of lesion mapping and structural equation modeling results (n = 152). The statistical map is thresholded at 5% false discovery rate. In each map of the cortical surface, the left hemisphere is on the reader’s left.
Voxel-based lesion-symptom mapping
These scores were correlated to regional gray and white matter determined by voxel-based lesion-symptom mapping (Bates et al.., 2003). This method compares, for every voxel, scores from patients with a lesion at that voxel contrasted against those without a lesion at that voxel (applying a false discovery rate correction of q < 0.05). Unlike functional neuroimaging studies, which rely on the metabolic demands of gray matter and provide a correlational association between brain regions and cognitive processes, voxel-based lesion-symptom mapping can identify regions playing a causal role in human intelligence by mapping where damage can interfere with performance.
RESULTS
Emotional intelligence
Figure 1 depicts a summary of lesion mapping results keeping correlation values among fluid/perceptual intelligence, crystallized/verbal intelligence, working memory, processing speed and emotional intelligence, as obtained from the latent variable analysis shown in Supplementary Figure 1. Results for emotional intelligence removing its variance shared with the other factors are reported below.
Impairments in emotional intelligence were associated with selective damage to a social cognitive network [reviewed in (Saxe, 2006)]. This network comprised the extrastriate body area within left posterior temporal cortex, which is associated with perceiving the form of other human bodies; left posterior superior temporal sulcus, which is involved in interpreting the motions of a human body in terms of goals; left temporo-parietal junction, which supports the uniquely human ability to reason about the contents of mental states and left orbitofrontal cortex, which is known to support emotional empathy and triadic relations between two minds and an object, supporting shared attention and collaborative goals (Figure 1).
This network additionally engaged major white matter fiber tracts, including the superior longitudinal/arcuate fasciculus, which connects temporal, parietal and inferior frontal areas; the superior fronto-occipital fasciculus connecting dorsolateral prefrontal cortex and the frontal pole with the superior parietal cortex and the uncinate fasciculus, which connects anterior temporal cortex and amygdala with orbitofrontal and frontopolar regions (Figure 1). The necessity of the social cognitive network for emotional intelligence supports the integration of emotional and social processes at the neural level (Ochsner and Lieberman, 2001; Ochsner, 2004).
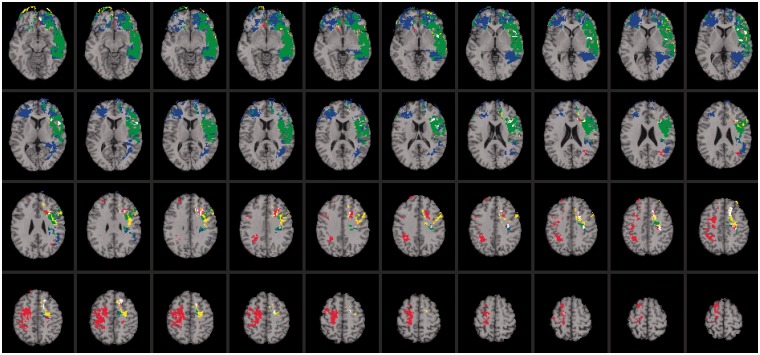
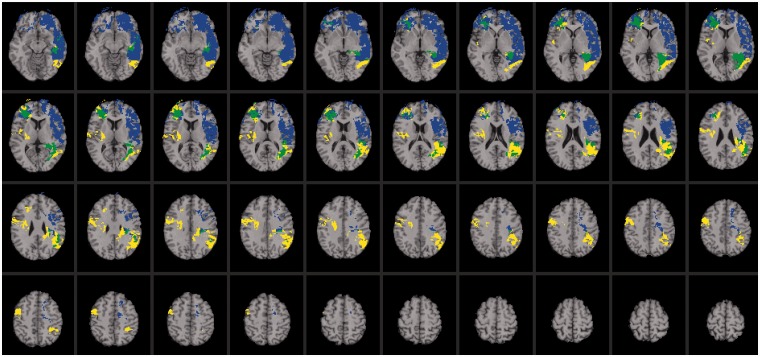
As expected, the neural system for emotional intelligence shared anatomical substrates with networks observed for psychometric intelligence. We focus on the independent predictors of emotional intelligence, as noted above, namely, verbal comprehension/crystallized intelligence (β = 0.796, P < 0.000) and processing speed (β = 0.175, P < 0.000). Impaired performance on measures of verbal comprehension was associated with selective damage to a left hemisphere perisylvian language network [reviewed in (Hickok and Poeppel 2007); Figure 2; regions highlighted in yellow]. This network is distributed throughout association areas in the left perisylvian cortex, recruiting a ventral pathway that maps sound to meaning (language comprehension) and a dorsal pathway that maps sound to action (language production). The ventral pathway comprised anterior middle temporal gyrus, posterior middle temporal gyrus and middle posterior superior temporal sulcus. These regions are known to support the process of mapping sensory or phonological representations onto lexical/conceptual representations in language comprehension (Hickok and Poeppel, 2007). The dorsal pathway engaged the anterior and posterior insula and an area at the parietal–temporal boundary, which are known to contribute to mapping sensory or phonological representations onto articulatory motor representations in language production (Hickok and Poeppel, 2007). Impaired performance on measures of verbal comprehension was also associated with damage to white matter fiber tracts that are widely implicated in language processing, including the arcuate fasciculus, which connects temporal, parietal and inferior frontal areas, and the uncinate fasciculus, which connects anterior temporal cortex and amygdala with orbitofrontal and frontopolar regions (Figure 2; regions highlighted in yellow). The anatomical extent of this network is consistent with the notion that crystallized intelligence is a complex process that derives from the coordinated activity of several brain regions. Critically, this network shared anatomical substrates with emotional intelligence (Figure 2; regions highlighted in green), engaging both the dorsal and ventral perisylvian language systems and indicating that emotional intelligence relies on neural systems for crystallized intelligence.
Fig. 2.
Voxel-based lesion-symptom mapping of emotional intelligence, verbal comprehension and processing speed. Lesion overlap map illustrating common and distinctive brain regions for emotional intelligence (blue), verbal comprehension (yellow) and processing speed (red) (n = 152). Overlap between emotional intelligence and perceptual organization is illustrated in green. Overlap between emotional intelligence and processing speed is illustrated in pink. Overlap between perceptual organization and processing speed is illustrated in orange. Overlap between all conditions is illustrated in white. The statistical map is thresholded at 5% false discovery rate. In each axial slice, the right hemisphere is on the reader’s left.
Deficits in processing speed were associated with damage to a bilateral network of frontal and parietal regions, including white matter fiber tracts that bind these areas into a unified system (Figure 2; regions highlighted in red). This network shared neural substrates with emotional intelligence (Figure 3; regions highlighted in pink), recruiting regions within the left dorsolateral prefrontal cortex (BA 9) that support the regulation and control of emotional and social behavior.
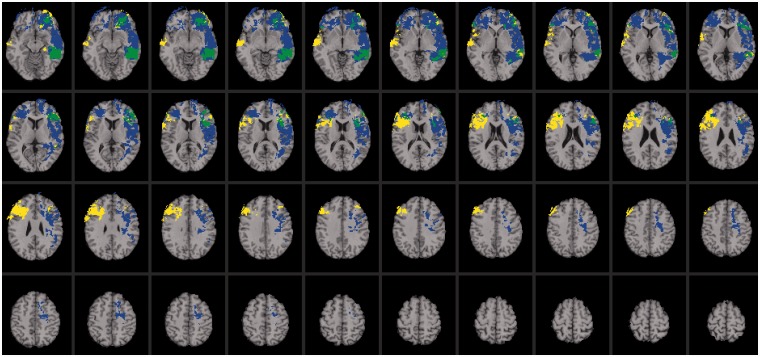
Fig. 3.
Voxel-based lesion-symptom mapping of emotional intelligence and conscientiousness. Lesion overlap map illustrating common and distinctive brain regions for emotional intelligence (blue) and conscientiousness (yellow) (n = 152). Overlap between emotional intelligence and conscientiousness is illustrated in green. The statistical map is thresholded at 5% false discovery rate. In each axial slice, the right hemisphere is on the reader’s left.
Personality traits and emotional intelligence
Computed regression analyses showed that only one of the big five contributed to the prediction of emotional intelligence, namely, conscientiousness (β = 0.15, P < 0.000). Therefore, we focus on this personality trait. Impairments in conscientiousness were associated with damage to regions of the social knowledge network that are important for the regulation and control of behavior, including the right dorsolateral prefrontal cortex (BA 9), left orbitofrontal cortex (BA 11) and left temporo-parietal junction (BA 22; Figure 3; regions highlighted in yellow). These regions have been widely implicated in social information processing networks [for a review, see (Saxe, 2006)] and share anatomical substrates with emotional intelligence, suggesting that these constructs may be closely related (Figure 3; regions highlighted in green).
Residual emotional intelligence scores
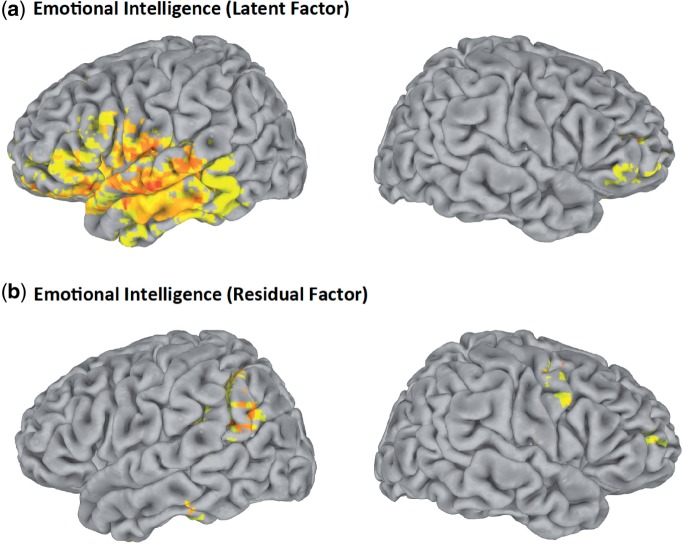
Finally, we analyzed the emotional intelligence residual scores removing variance shared with its significant predictors in both the psychometric intelligence and personality domains. Impairment in the emotional intelligence residual score was associated with selective damage to frontal and parietal brain structures that have been widely implicated in social information processing. These regions comprised the right orbitofrontal cortex (BA 10), left inferior (BA 40/39) and superior parietal cortex (BA 7), in addition to major white matter fiber tracts, including the superior longitudinal/arcuate fasciculus, the superior fronto-occipital fasciculus and the uncinate fasciculus (Figure 4). The necessity of the social cognitive network for emotional intelligence supports the integration of mechanisms for social and emotional information processing at the neural level (Ochsner and Lieberman, 2001; Ochsner, 2004).
Fig. 4.
Voxel-based lesion-symptom mapping of latent (a) and residual (b) emotional intelligence scores (n = 152). The statistical map is thresholded at 5% false discovery rate. In each map of the cortical surface, the left hemisphere is on the reader’s left.
DISCUSSION
In this study, we investigated the neural architecture of emotional intelligence and examined the degree to which this network engages systems for key competencies of psychometric intelligence (verbal comprehension/crystallized intelligence, perceptual organization/fluid intelligence, working memory and processing speed) and personality traits (extraversion, agreeableness, conscientiousness, neuroticism and openness to experience). We administered tests for measuring these psychological constructs to 152 patients with focal brain injuries and applied voxel-based lesion-symptom mapping to investigate their neural substrates.
We observed a significant effect on emotional intelligence with lesions in white matter sectors including the superior longitudinal/arcuate fasciculus that connect frontal and parietal cortices. Despite its distributed nature, the neural substrates of emotional intelligence were remarkably circumscribed, concentrated in the core of white matter and comprising a narrow subset of regions associated with social information processing. This finding suggests that emotional intelligence is supported by mechanisms for the regulation and control of social behavior and that the communication between areas associated with these abilities is of critical importance.
Orbitofrontal cortex is a central component of this network and has been increasingly implicated in emotional and social cognition (Kringelbach, 2005). This region is uniquely placed to integrate sensory and visceral motor information to modulate behavior through both visceral and motor systems. This has led to the proposal that orbitofrontal cortex is involved in the integration of bodily signals that help decision-making processes and have been termed, for example, ‘interoceptive’ (Nauta, 1971) or ‘somatic’ markers (Damasio, 1996). These proposals suggest that orbitofrontal cortex is an important part of the networks involved in emotional and social processing (Nauta, 1971; Rolls, 2000).
A growing body of evidence further supports the functional division of orbitofrontal cortex into orbital and medial sectors [for a review, see (Kringelbach, 2005)]. A large meta-analysis of the existing neuroimaging data was used to show that activity in the medial orbitofrontal cortex is related to the monitoring, learning and memory of the reward value of reinforcers, whereas lateral orbitofrontal cortex activity is related to the evaluation of punishers, which can lead to a change in ongoing behavior (Kringelbach and Rolls, 2004). Further studies have since confirmed the role of the medial orbitofrontal cortex in monitoring affective properties in olfaction (Anderson et al.., 2003; Rolls et al.., 2003), gustation (Small et al.., 2003) and for somatosensory (Rolls et al.., 2003) and multimodal stimuli (de Araujo et al.., 2003). These findings support the observed role of medial orbitofrontal cortex in emotional intelligence, indicating that this region is computationally necessary for monitoring affective properties of social and environmental stimuli. The involvement of lateral orbitofrontal cortex in the evaluation of punishers (Kringelbach and Rolls, 2004) elucidates the role of this region in executive function, suggesting that it is necessary for evaluative processes in goal-directed behavior and decision making (Barbey et al.., 2009). The findings reported here indicate that the orbitofrontal cortex plays a central role in the coordination of social and affective systems, providing a nexus for sensory integration and contributing to a fronto-parietal network for goal-directed behavioral control.
The neural system for emotional intelligence shared anatomical substrates with specific facets for psychometric intelligence, engaging perisylvian language areas (Figure 2; highlighted in green) and regions within the left anterior cingulate cortex (BA 32) and superior longitudinal fasciculus also implicated in processing speed (Figure 2; highlighted in pink). These findings are consistent with the observed pattern of correlations between these factors (Figure 1) and further suggest that emotional intelligence depends on key competencies for social information processing and psychometric intelligence. In addition, we found that emotional intelligence engaged brain regions implicated in conscientiousness, recruiting areas within the left orbitofrontal cortex (BA 10), anterior insula (BA 13) and inferior temporal cortex (BA 37) (Figure 3; highlighted in green). This result further indicates conscientiousness, or the degree of organization, persistence, control and motivation in goal-directed behavior, is a central feature of emotional intelligence.
Finally, we investigated the neural systems underlying emotional intelligence residual scores removing variance shared with its significant predictors in both the psychometric intelligence and personality domains. When compared to the neural system observed for emotional intelligence at the latent variable level, we see that the residual factor engages common and distinctive brain regions (Figures 4 and 5). Common regions include right dorsolateral PFC, left posterior superior temporal sulcus and left temporo-parietal junction (Figure 5; highlighted in green), while distinct areas reflect engagement of perisylvian language areas for emotional intelligence at the latent variable level (Figure 5; highlighted in blue) and recruitment of the right precentral gyrus and left superior parietal cortex for the residual emotional intelligence factor (Figure 5; highlighted in yellow).
Fig. 5.
Voxel-based lesion-symptom mapping of emotional intelligence (latent) and emotional intelligence (residual). Lesion overlap map illustrating common and distinctive brain regions for emotional intelligence latent (blue) and emotional intelligence residual (yellow) (n = 152). Overlap between these factors is illustrated in green. The statistical map is thresholded at 5% false discovery rate. In each axial slice, the right hemisphere is on the reader’s left.
CONCLUSION
Historically, cognitive and emotional processes have been viewed as separate constructs. Research in the past two decades, however, has increasingly shown that such a view may be limited and that, if we are to understand how complex behaviors are carried out in the brain, an understanding of their interactions is indispensable. The present study provides neuropsychological patient data to suggest that emotional and psychometric intelligence recruit shared neural systems for the integration of cognitive, social and affective processes. Although many behaviors might be reasonably well characterized in terms of cognitive–social interactions such that cognitive and social processes are partly separable, often true integration of cognitive and social processes takes place, blurring the distinction between these domains (Barbey et al.., 2009). We propose that one fruitful way to refine our understanding of their integration will involve a more quantitative analysis of structural and functional brain connectivity, with particular emphasis on the involvement of the observed social knowledge network.
The findings of the present study elucidate the neural foundations of emotional intelligence and provide evidence for the role of the social knowledge network in the coordination of cognitive, social, and affective processes. Damage to this network may produce impairments in emotional intelligence, which can have an ill effect on one's ability to effectively cope with daily demands. In particular, damage to this system may produce impairments in one's ability to: (i) be aware of and express oneself; (ii) function interpersonally; (iii) manage and control emotions; (iv) generate positive affect required in achieving personal goals and (v) cope with the immediate situation, make decisions and solve problems of a personal and interpersonal nature.
The data presented in this article are consistent with the view that behavior is a product of the orchestration of many brain regions and that the aggregate function of these areas supports emotional and cognitive processes. As the functional neuroanatomy of this network is further elucidated, we should try to understand the temporal dynamics that underlie its functions, and the structural and functional changes that occur during development. On a timescale of milliseconds, neuroimaging techniques such as magnetoencephalography (MEG) could be used to elucidate the precise role of this system in cognitive, social and emotional processes. The directionality and timing of neural activity between regions of the social knowledge network is not clear but could potentially be addressed with MEG by using sensitive measures such as Granger Causality (Granger and Hatanaka, 1969).
On a longer, developmental timescale, it would be interesting to investigate the role of this network in learning and, in particular, in the consolidation of learning. One hypothesis is that the rate of improvement in learning depends on emotional and motivational influences (Cattell and Cattell, 1987) and therefore on changes in functional activity in structures known to be involved in social and emotional processes, such as the orbitofrontal cortex. This approach will be particularly important in understanding goal-directed social behavior and cases of learning that depend on temporal and occipital brain structures that are initially set up through top–down interactions with frontal and parietal regions.
From a clinical perspective, understanding cognitive and emotional deficits in patients with brain damage may facilitate the design of appropriate assessment tools and rehabilitation strategies, with potential improvement in patients’ cognitive abilities and daily living. Our findings identify specific tests of the MSCEIT and WAIS that may be targeted in clinical investigations to assess the functioning of the social knowledge network, particularly, emotional intelligence tests of the MSCEIT and crystallized intelligence measures of the WAIS. These findings support predictions about the nature and significance of cognitive impairments that may result from damage to specific brain networks (Figure 1).
Many neurological disorders and mental illnesses are characterized by profound deficits in emotional and cognitive behaviors, including epilepsy, Alzheimer’s disease, autism and schizophrenia. Outstanding questions concerning these and many other debilitating conditions center on advancing our knowledge of how emotional and cognitive processes interact in both normal and abnormal circumstances. Understanding the neural mechanisms underlying these conditions will ultimately require a broader assessment that examines the functional organization of emotional and cognitive systems, and their interactive role in high-level processes. The reported finding contribute to this emerging research program by elucidating the role of the social knowledge network in the coordination of cognitive, social and affective processes, demonstrating that this system provides an integrative neural architecture for key competencies of human intelligence.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We are grateful to S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, V. Raymont, K. Reding and G. Tasick for their invaluable help with the testing of participants and organization of this study.
This work was supported by funding from the US National Institute of Neurological Disorders and Stroke intramural research program and a project grant from the United States Army Medical Research and Material Command administered by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: a 30year post-injury follow-up study, grant number DAMD17-01-1-0675). R. Colom was supported by grant PSI2010-20364 from Ministerio de Ciencia e Innovación [Ministry of Science and Innovation, Spain] and CEMU-2012-004 [Universidad Autonoma de Madrid].
REFERENCES
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–67. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP, Hiltbrunner B, Fischer RS. Distributed anatomy of transcortical sensory aphasia. Archives of Neurology. 1989;46:885–92. doi: 10.1001/archneur.1989.00520440075023. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Arbuckle J. Amos (Version 7.0) [Computer Program] Chicago: SPSS; 2006. [Google Scholar]
- Bar-On R, Brown JM, Kirkcaldy BD, Thome EP. Emotional expression and implications for occupational stress; an application of the emotional quotient inventory (EQ-i) Personality and Individual Differences. 2000;28:1107–18. [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012;135:1154–64. doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Orbitofrontal contributions to human working memory. Cerebral Cortex. 2011;21:789–95. doi: 10.1093/cercor/bhq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. An evolutionarily adaptive neural architecture for social reasoning. Trends in Neurosciences. 2009;32:603–10. doi: 10.1016/j.tins.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A, De Renzi E, Faglioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligence tasks. Brain. 1973;96:715–28. doi: 10.1093/brain/96.4.715. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Black FW. Cognitive deficits in patients with unilateral war-related frontal lobe lesions. Journal of Clinical Psychology. 1976;32:366–72. doi: 10.1002/1097-4679(197604)32:2<366::aid-jclp2270320234>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123(Pt 6):1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends in Cognitive Sciences. 2004;8:216–22. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Zook NA, DeLosh EL, Davalos DB, Davis HP. Age differences in fluid intelligence: contributions of general slowing and frontal decline. Brain and Cognition. 2006;62:9–16. doi: 10.1016/j.bandc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263–72. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Caplan D, Hildebrandt N, Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996;119(Pt 3):933–49. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters G, Kennedy D, et al. A study of syntactic processing in aphasia II: neurological aspects. Brain and Language. 2007;101:151–77. doi: 10.1016/j.bandl.2006.06.226. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell RB. Intelligence: Its Structure, Growth, and Action. Amsterdam: Elsevier Science Publications Co; 1987. [Google Scholar]
- Costa PT, Jr, McCrae RR. Stability and change in personality assessment: the revised NEO Personality Inventory in the year 2000. Journal of Personality Assessment. 1997;68:86–94. doi: 10.1207/s15327752jpa6801_7. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. The New England Journal of Medicine. 1992;326:531–9. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical transactions of the Royal Society of London Series B, Biological Sciences. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. The European Journal of Neuroscience. 2003;18:2059–68. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern. BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–8. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Glascher J, Rudrauf D, Colom R, et al. Distributed neural system for general intelligence revealed by lesion mapping; 2010. Proceedings of the National Academy of Sciences of the United States of America, 107, 4705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Tranel D, Paul LK, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–91. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleman D. Emotional Intelligence. New York: Bantam Books; 1995. [Google Scholar]
- Granger CWJ, Hatanaka H. Analyse Spectrale des Sâeries Temporelles en Âeconomie. Paris: Dunod; 1969. [Google Scholar]
- Guimera R, Amaral LAN. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Sales-Pardo M, Amaral LA. Classes of complex networks defined by role-to-role connectivity profiles. Nature Physics. 2007;3:63–9. doi: 10.1038/nphys489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Isingrini M, Vazou F. Relation between fluid intelligence and frontal lobe functioning in older adults. International Journal of Aging & Human Development. 1997;45:99–109. doi: 10.2190/WHWX-YNVB-079V-2L74. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–71. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain and Language. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Lau WK, Polk M. The structural determinants of recovery in Wernicke's aphasia. Brain and Language. 1993;44:153–64. doi: 10.1006/brln.1993.1010. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Acheson DJ, Barbey AK, Solomon J, Postle BR, Grafman J. Areas of left perisylvian cortex mediate auditory-verbal short-term memory. Neuropsychologia. 2011;49:3612–9. doi: 10.1016/j.neuropsychologia.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. Journal of Neuroscience. 2009;29:14980–6. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behavior Research Methods Instruments & Computers. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Emotional intelligence: new ability or eclectic traits? The American Psychologist. 2008;63:503–17. doi: 10.1037/0003-066X.63.6.503. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Phelps EA. Current opinion in neurobiology—cognitive neuroscience 2010. Current Opinion in Neurobiology. 2010;20:141–2. doi: 10.1016/j.conb.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Alexander MP, Helm-Estabrooks N, Levine HL, Laughlin SA, Geschwind N. Aphasia with predominantly subcortical lesion sites: description of three capsular/putaminal aphasia syndromes. Archives of Neurology. 1982;39:2–14. doi: 10.1001/archneur.1982.00510130004002. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. The problem of the frontal lobe: a reinterpretation. Journal of Psychiatric Research. 1971;8:167–87. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Current directions in social cognitive neuroscience. Current Opinion in Neurobiology. 2004;14:254–8. doi: 10.1016/j.conb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. The American Psychologist. 2001;56:717–34. [PubMed] [Google Scholar]
- Parkin AJ, Java RI. Deterioration of frontal lobe function in normal aging: influences of fluid intelligence versus perceptual speed. Neuropsychology. 1999;13:539–45. doi: 10.1037//0894-4105.13.4.539. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nature Reviews Neuroscience. 2002;3:606–16. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–47. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Precis of the brain and emotion. Behavioral and Brain Sciences. 2000;23:177–91. doi: 10.1017/s0140525x00002429. discussion 192–233. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cerebral Cortex. 2003;13:308–17. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Rowe AD, Bullock PR, Polkey CE, Morris RG. “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain. 2001;124:600–16. doi: 10.1093/brain/124.3.600. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social. Cognition, Current Opinion in Neurobiology. 2006;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Computer Methods and Programs in Biomedicine. 2007;86:245–54. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PloS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S, Book HE. The EQ Edge Emotional Intelligence and Your Success. 3rd edn. Mississauga, Ontario: Jossey-Bass; 2011. p 1 online resource (xiv, 354 p.) ill. [Google Scholar]
- Stuss DT, Gallup GG, Jr, Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124:279–86. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:1037–54. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. San Antonio, TX: The Psychology Corporation; 1997. Wechsler adult intelligence test administration and scoring manual. [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.