Abstract
Mental effort is a limited resource which must be invested to perform mental tasks. The amount of mental effort investment that an individual experiences during task performance can be measured afterwards with the help of self-rating scales. Earlier research suggests that integration of information about somatic state changes is crucial for the self-evaluation of mental effort investment. Damage to the pathways which convey information about somatic state changes can lead to an inability to self-evaluate mental effort investment, while conceptually similar evaluations of task difficulty can still be performed. We used functional magnetic resonance imaging to investigate brain activation, while subjects rated their mental effort investment and the difficulty of a previously performed task. Our results show stronger activation of the left anterior insular cortex (aIC) during evaluation of mental effort. Additionally, the activity in left aIC during task performance was modulated by changes in task demand in a similar way as the self-ratings of mental effort. We argue that aIC does not only play a role in the integration of self-related information during self-evaluation of mental effort investment, but that left aIC might also be involved in the experience of mental effort during task performance.
Keywords: anterior insular cortex, self-evaluation
INTRODUCTION
Scope of the study
Mental effort is generally experienced while performing any sort of mental task, from compiling a grocery list to planning a scientific study. It is described as spending energy and consequently experiencing a feeling of strain, thus ultimately investing a limited energetic resource to perform a mental task (Zijlstra, 1993). Damasio (1999) considered mental effort to be not an emotion as such, but rather a feeling which refers to a conscious appraisal of one’s own state. The amount of mental effort experienced while engaging in a task is determined by a variety of factors not limited to its mere computational demands (Mulder, 1986). Being aware of levels of mental effort investment provides information about the sustainability of a current activity. This awareness also makes it possible to evaluate the level of mental effort investment related to a task, and to communicate it.
The goal of this article is to identify neural structures that are involved in the process of mental effort evaluation. We will briefly describe the concept and measurement of mental effort. We will identify two crucial functions of a neural mechanism that enables mental effort evaluation. Evidence from research into brain areas with matching functional characteristics are then used to form hypotheses about the role of these areas in mental effort evaluation that will be tested using functional magnetic resonance imaging (fMRI).
The concept of mental effort and its implications for neural structures
Engaging in any mental task confronts an individual with a variety of demands. The integrated model of mental effort (Mulder, 1986) divides these in two classes of demands: the task load, which is related to the control of mental processes to produce the desired result and the state load, which refers to all compensatory control that needs to be invested to reach and maintain a suitable working state. The actively invested control that an individual deploys to meet the combination of these two loads is referred to as mental effort. Investment of mental effort is accompanied by a subjective feeling of strain and sustained high mental effort investment results in negative affect and fatigue (Hockey, 1997). The self-evaluation of mental effort investment has been described as a quasi-emotional appreciation of one’s own state, reflecting the changes that occur in the state due to meeting the mental workload (Damasio, 1999).
The ability to self-evaluate one’s own mental effort investment is necessary to manage one’s performance in a sustainable way regarding one’s own state. The existence of such an ability to intuitively self-assess the amount of mental effort related to the execution of a task has been demonstrated, and it is used routinely in occupational psychology. Modern tools measuring workload such as the rating scale mental effort (RSME) (Zijlstra, 1993) make use of this feature to provide an assessment of regulative effort in real-life work environments. Yet, to identify the impact of each of the many influences a subject faces in a work environment, it is necessary to understand the mechanism that leads to the coherent subjective experience of mental effort. Identifying the brain areas which are involved in the evaluation process provides an overview of the components of this mechanism.
By outlining necessary capacities of such an evaluative mechanism, it is possible to search for brain areas which have been shown to play a role in similar functions.
Two crucial capacities of a mechanism for evaluating mental effort can be derived from the theoretical model of mental effort and from research into its subjective measurement. On the one hand, the mechanism must have the capacity to monitor different information streams that carry information about the state of the individual and to integrate this information into a coherent impression. Stressors related to task execution can affect all domains in the experience of a subject, from bodily homeostasis to cognitive resources. Thus, the integration of information originating from different domains is necessary to give an indication of mental effort. A brain region involved in joining these different information streams will need input from the respective regions that are concerned with the lower processing stages of this information.
The second necessary capacity of the mechanism is to make the result of the integrative process available for self-evaluation. It enables the subject to become aware of recently experienced mental effort levels and to communicate it. Conceptionally, self-evaluation of mental effort investment is a recall of the effect of the act of performing a task on the state of the self. The state of the self is thus used as a reference point, and changes in this state due to task performance are attributed to the investment of mental effort. Scales such as the RSME rely on the fact that this self-evaluation of mental effort can be performed fast and intuitively.
A possible role for structures related to self-awareness
Evidence from earlier research [see Craig (2009) for a review] suggests the insular cortex (IC), especially the anterior part, as a likely candidate region to provide the described necessary functionality. The IC receives input from a vast number of cortical and subcortical regions that provide it with information about the somatic, emotional and cognitive aspects of the state of the individual. The IC integrates this information in a posterior to anterior order. The anterior IC (aIC) thus receives and integrates information about all major aspects of an individual’s state, which would enable it to determine the changes in this state due to mental effort investment. Thereby, it would fulfill the first criterion for a mental effort evaluation mechanism.
The second criterion, namely the capacity of a mechanism to perform a self-referential evaluation, initially makes the involvement of an additional number of regions seem plausible. In particular, a set of regions termed cortical midline structures (CMS) (Northoff and Bermpohl, 2004), consisting of the anterior and posterior cingulate cortices/precuneus and the medial prefrontal cortex have been found to be activated in paradigms contrasting self and other related information [see, van der Meer et al. (2010) or Lieberman (2010) for a review].
While these regions react to changes in the degree of general self-reference, studies specifically investigating emotional self-awareness and interoception rather point towards a prominent role of aIC in this specific sub-domain of self-awareness.
Craig (2009) compared the results of several studies which employed experimental paradigms centered around awareness of self-state or emotions. In his review, Craig proposed that due to the extended integrative nature of aIC, it possesses the ability to essentially create a comprehensive momentary meta-representation of the subjective state of the self. Of particular interest in this context is the notion that overlapping regions in aIC have been shown to be reactive to changes in emotion as well as to interoceptive awareness (Zaki et al., 2012). A strong relation of somatic information and the subjective self-appraisal of one’s state has been proposed earlier (Damasio, 1999). The results of Zaki et al. (2012) demonstrate that experience of emotion and attending to one’s heartbeat actually do activate overlapping areas in aIC in the same participants, while a number of earlier studies have shown a connection between interoceptive awareness and emotional reactivity [see Herbert and Pollatos (2012) for a review].
For one emotion, namely anxiety, a mechanism has been proposed in which aIC compares states of the bodily self at different times, signaling the occurrence of unexpected discrepancies between predicted and actual state (Paulus and Stein, 2006). Further evidence of such a functionality of aIC comes from its consistent, overlapping activation during both actual experience of pain and empathizing with other’s pain, the latter of which involves emulating the state changes caused by the pain stimulus (Singer et al., 2009). Such a state-comparing functionality of aIC would fit very closely with the conceptual description of self-evaluation of mental effort investment, especially as mental effort investment has long been known to cause changes in the somatic state of an individual (Fairclough and Mulder, 2011).
The involvement of several parts of the IC in evaluative processes has already been demonstrated in a number of paradigms. In a meta-analysis of studies investigating brain activation during explicit emotional evaluation (Lee and Siegle, 2009), identified the IC as being activated specifically during evaluation of one’s own emotion-related states by subjects. Although the locations of activated clusters in IC vary over different paradigms used in the analyzed studies, the computed peak activation is located towards aIC.
These two features, the integration of information from different domains and the proposed relation to emotional awareness and self-evaluation, provide the aIC with two prerequisites for mental effort evaluation according to the outlined theory above: first, necessary information about the state of the self, most important the bodily aspect, are projected to it. Second, the combined evidence suggests a functionality of aIC in which different states are compared, thereby enabling it of assessing the influence of task performance on the state of the self.
Findings from a patient case study (Naccache et al., 2005) provide further evidence supporting the crucial role of bodily state information in the self-evaluation of mental effort. In this case, it was demonstrated that mental effort evaluation is dissociable from other evaluations concerning a recently performed task. After suffering an extensive lesion in the left anterior cingulate cortex (ACC), a patient referred to as “RMB” was left with a specific impairment concerning her ability to evaluate her own invested mental effort. While she had no problems reasoning about the various difficulty levels of a task in terms of its computational demands, she did report to not feel any changes in her sense of mental effort investment. According to the interpretation of the authors, the function of the ACC is mainly the generation of emotional somatic markers in coordination with left ventromedial prefrontal regions; due to the lesion, these structures were disconnected which lead to the described inability to perform self-evaluation of mental effort investment. While in this case a lesion in ACC was responsible for the functional deficit, results from several studies reviewed by Craig (2009) showed no significant changes in ACC activation in relation to paradigms stimulating awareness of the self. Thus, in our view, ACC performs a necessary prerequisite role in self-related evaluation processes, but the main role of integrating and evaluating bodily information is performed by aIC.
Nevertheless, this case study presents a valuable observation that can be used to study the neural correlates of mental effort self-evaluation. The functional dissociation of evaluating task difficulty and invested effort offer a narrow contrast that can be used to assess the role of aIC in mental effort evaluation. While the evaluation of task difficulty seems similar to evaluating the mental effort, there is a crucial difference: difficulty concerns the objective demands of a task, as perceived and estimated by the subject in a purely rational manner. Mental effort evaluation concerns the same task-sided loads, but it adds the subject itself in the evaluation. The subjective appraisal of one’s own state is a necessary element in this process (Zijlstra, 1993). Therefore, the two key aspects of mental effort evaluation, namely integration of information across somatic/emotional/cognitive domains and the involvement of emotional self-awareness, do not play a prominent role in the evaluation of task difficulty. Thus, structures that support these two aspects of mental effort evaluation should be more active during the evaluation of mental effort than during task difficulty evaluation.
Still, both evaluations aim at the same recent task performance, and it has been demonstrated that nearly identical visual analog scale (VAS) interfaces can be used to assess the magnitudes of both effort and difficulty (Yeo and Neal, 2004). Among the available tools for measuring mental effort, a one-dimensional (1D) VAS has the advantage of minimal intrusion, while, as proven for the RSME, still providing high sensitivity (Verwey and Veltman, 1996).
Goal of the study and hypotheses
The goal of our study was to provide insight in the neural structures that are activated stronger during the evaluation of mental effort than during the evaluation of task difficulty. We devised a design that makes use of the contrast between difficulty and mental effort evaluation. The paradigm employed a common working memory task, the n-back task (Gevins and Cutillo, 1993). This task was used on three different levels of difficulty. Earlier findings demonstrated that ratings of both difficulty and amount of mental effort would be highly correlated (Yeo and Neal, 2004) and would correspond to the memory load of the n-back task in a parametric fashion.
Hypothesis 1
The difficulty and mental effort rating scores will show a significant correlation and will follow the parametric changes in task load.
The evaluation of a recent task using a VAS is in itself a complex process. The experience must be recalled, it must be evaluated, the outcome must be translated to a value on a scale, and finally a cursor must be moved to the chosen location on the scale. Lee and Siegle (2009) provided an overview of regions which, independent of the actual emotional content, would support the mere performance of a rating task as such. This includes expected task interface specific networks, which in our study would refer to networks engaged in visuo-motor coordination. The rating process itself relies on the recall of information over the task and on attention-related and higher cognitive functions to reflect and evaluate this information.
Hypothesis 2
During both rating tasks, areas associated with visual and motor activity, attention, working memory and higher cognitive functioning will be activated. Specifically, this should involve areas in the anterior and posterior cingulate cortex (ACC/PCC) and the dorsolateral prefrontal cortex (dlPFC).
The main focus of our study concerned the contrast between those two rating conditions. Both the theoretical accounts of mental effort (Mulder, 1986) and the findings in patient RMB of Naccache et al. (2005) make it plausible that integration of information is a crucial part of mental effort evaluation. We propose that the aIC is suited to fulfill this role. The role of the aIC in self-awareness (Craig, 2009) makes it furthermore plausible that it serves to make the outcome of this process accessible to the subject as a part of the awareness of one’s own state.
We, thus, expected this region to show stronger activation during mental effort evaluation than task difficulty evaluation.
Hypothesis 3
The aIC will be activated more during the evaluation of mental effort than during evaluation of task difficulty.
METHODS
Subjects
Fourteen Dutch-speaking subjects (three male, 11 female, mean age 21.8 years, 12 right-handed and 2 left-handed as measured by Edinburgh handedness inventory) were recruited using standard criteria for MRI safety and suitability for the experiment (no reports of mental health or extensive vision or motor problems). To minimize the chance for artifacts in the lower regions of the frontal cortex, we also excluded candidates with orthodontic retainers. Screened subjects were invited for the testing session. All subjects gave informed consent before the start of the experiment and were rewarded either monetary or with student participation points.
Two male, right-handed subjects were excluded from analysis because of movement artifacts in the functional scans that exceeded the limits described later in ‘Imaging data treatment’ section. This cut back our total sample size to 12, yet this reduction is far less severe than the effects of including low-quality datasets.
Task and procedure
At the beginning of the session, the task was explained and practiced in a short version (one block of each condition) under supervision of the experimenter to check proper understanding. Subsequently, subjects were placed in the MRI scanner and performed 2 × 15 blocks in a quasi-randomized fashion. Each run of 15 blocks took in total 28 min. Between the two blocks, a T1-weighted anatomical scan of the brain was carried out. The employed task was a version of the n-back task. Subjects saw letters appearing one after another on a computer screen. Subjects had to indicate if the letter currently presented on screen was identical to a letter that was presented either one, two or three letters back. These conditions were referred to as ‘1-back’, ‘2-back’ and ‘3-back’ conditions, respectively. N-back paradigms have been used to induce different levels of load in a number of previous studies (Owen et al., 2005). The conditions were quasi-randomized, with five instances of each condition in each of the two blocks of the experiment.
At the beginning of each block, an instruction screen told subjects which condition they would encounter. Subjects then had the task of responding to 20 letters with either left or right button press to indicate that the letters were identical or not. After each letter they received feedback by either being presented with a green ‘Right’ or a red ‘Wrong’ for 500 ms. The letters in question appeared on screen for 2000 ms, which was also the response window. After each block of 20 trials, the subjects were given two consecutive VAS to rate either experienced mental effort or task difficulty. The scales were presented in random order. Each scale was preceded by a rest period of 8000 ms and an instruction screen of 3000 ms, which primed subjects on which of the two VAS would be presented next. Following the instruction screen, the scales were presented for 10 000 ms. During this period, subjects could move a cursor on the scale and press a button to make a rating. Rating periods were kept at this standard duration to encourage actual rating instead of clicking on as fast as possible. For the subsequent analysis, only the actual time of rating up until the button press would be defined as the rating period in the fMRI protocol.
We employed the 150-point RSME (Zijlstra, 1993) to allow subjects to assess the amount of invested mental effort they experienced. To assess difficulty, we designed a simple 150-point scale with three anchor points, following the argumentation of Yeo and Neal (2004) that visual scales used in the same experiment should be similar in design and scaling. The RSME is a vertical 1D VAS, ranging from 0 to 150, with nine anchor points describing various levels of effort. These labels were carefully chosen and placed on the scale to ensure they have the same meaning for different subjects. The RSME assesses the combined regulative demands that are experienced by a subject as a result of both task load and state load. It is similar to other, routinely used instruments in measuring the construct of mental effort and is commonly used in the field of work psychology (Verwey and Veltman, 1996). The reliability of the RSME is given in the literature with r = 0.78 (Zijlstra, 1993). This apparently low value is explained by the author as resulting from the fact that, even under laboratory conditions, the subjectively experienced amount of effort will vary over time even in the same subject. Previous research has demonstrated that the RSME does not rely on obvious cues to indicate differences in levels of mental work load (Zijlstra, 1993). Therefore, it is unlikely that the obviousness of the differences between the conditions determines the resulting RSME scores. These obvious changes in task load were necessary for the evaluation of task difficulty.
The task and the two VAS were programmed in E-Prime. They were presented using E-Studio on a Windows XP PC connected to a MRI compatible optic system consisting of a projector and mirror goggles. Task and rating input was collected via an MRI compatible optical two-button Joystick (Current Designs Inc., Philadelphia, PA, USA). Subjects trained the handling of the Joystick for a brief period before the experiment by marking values on a VAS analog to the ones used in the actual experiment.
Magnetic resonance imaging was performed on a Siemens Allegra 3T head scanner (Siemens AG, Erlangen, Germany) at the facilities of the Maastricht Brain Imaging Center. Anatomical imaging was carried out with a standard ADNI T1 weighted sequence, voxel size = 1 mm3; flip angle = 9°; TR = 2250 ms; TE = 2.6 ms. Whole-brain echo-planar imaging (EPI) was performed using the following parameters: matrix size, 64 × 64; slice thickness, 3.5 mm; slice order descending and interleaved; no gap; FOV, 224 × 224 mm; TE = 30 ms; TR = 2000 ms. Slice orientation was tilted 30° backwards to minimize susceptibility artifacts in the orbitofrontal regions (Deichmann et al., 2003).
Analysis
Behavioral data were analyzed using SPSS 15.0. A mixed model analysis was carried out to reflect the nested structure of the data. To check for exhaustion effects, the number of the session was also included as a covariance factor.
Imaging data treatment
Analysis of fMRI data was performed in BrainVoyager QX 2.1 (Brain Innovation BV, Maastricht, The Netherlands). Anatomical images were individually preprocessed by inhomogeneity correction and extracranial noise filtering. The data were subsequently transformed into stereotactic space (Talairach and Tournoux, 1988). The transformed anatomical scans from all subjects were then averaged into a single anatomical dataset used as background for the visualization of group analyses.
The first three volumes of the functional scans were discarded because of magnetic saturation effects. The functional scans were preprocessed by slice scan time correction, motion correction and high pass filtering. Data of two subjects showed translation/rotation exceeding 3 mm/deg. Those datasets were excluded from further analysis. High pass filtering was performed using a General Linear Model (GLM) approach with a Fourier basis set which was adjusted to subtract the time course for predictors with up to two sine/cosine cycles per run and eventual linear trends from the time course of the data. Volume time course files were calculated for each separate run.
Statistical data analysis
The E-Primer script for BrainVoyager (Hester Breman, Brain Innovation B. V., 2009) was used to extract the timing information of the single conditions from the E-prime protocol files for each separate run. This timing information was used to build a design matrix. The single boxcar predictor time courses were adjusted for the shape and delay of the hemodynamic response by convolving them with a two-γ-function (Friston et al., 1998). Predictors for the translation/rotation of the subject’s head were derived during the motion correction of the functional data and added in the design matrix. All predictors were z-transformed.
A random effects GLM was computed for all runs of all subjects. For explorative analysis, the difficulty rating and the RSME rating condition were contrasted with the baseline. To explore the specific effect of the effort-related RSME evaluation, both ratings were contrasted against each other. The resulting activation map was adjusted to a single-voxel threshold of t = 3.13 (P < 0.0096). This map was subsequently corrected for multiple comparisons by using the Cluster Threshold estimation plugin of BrainVoyager. This plugin runs a Monte-Carlo-Simulation extension (Forman et al., 1995) to determine the minimal cluster size given a user-defined confidence level, which was set to α = 0.05. The minimal size for the current data was calculated to be 11 contiguous voxels.
Supra-threshold clusters of active voxels were labeled using a microatlas of the human brain (Mai et al., 2007) and the Talairach daemon applet (Research Imaging Center, TX, USA). Corresponding anatomical locations and approximate Broadmann areas (where applicable) were identified for each cluster.
RESULTS
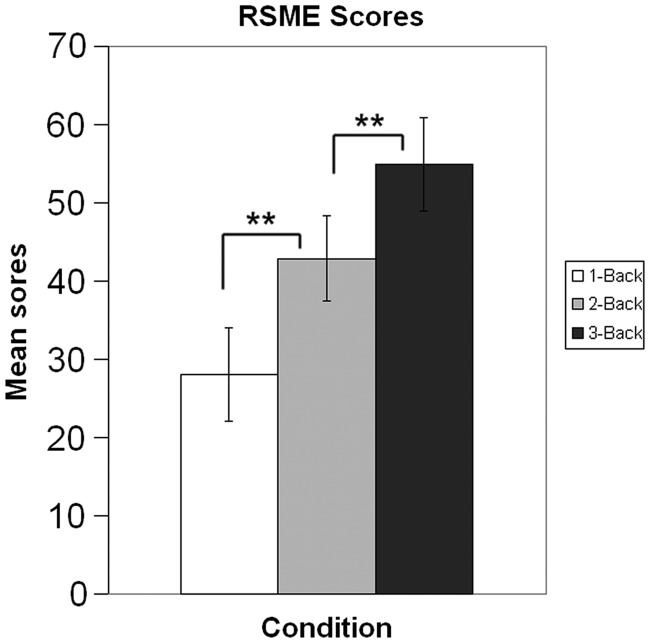
All subjects were able to perform the task at a sufficient level (mean score 18.29 correct out of 20 trials, s.d. = 0.91). Mean RSME scores for the single n-back conditions were 27.99 (s.d. = 20.68), 42.91 (s.d. = 18.98) and 54.94 (s.d. = 20.71), for 1-back, 2-back and 3-back, respectively. The according mean difficulty rating scores were 25.78 (s.d. = 23.81), 50.16 (s.d. = 24.92) and 66.65 (s.d. = 26.56) (Figure 1). There was a significant correlation (r = 0.88, P < 0.01) between the difficulty and RSME scores variables. There was no significant interaction effect between condition and the number of the session. Session did not have a significant main effect, either. The only significant main effect was elicited by condition: pairwise comparisons of subject’s evaluation scores on both the RSME and the difficulty scale showed significant differences (P < 0.01) between the conditions, confirming that the different n-back conditions indeed differed in perceived workload in a parametric fashion. This pattern could be expected from the original validation research of the RSME (Zijlstra, 1993).
Fig. 1.
Effort scores across different n-back conditions, illustrating the influence of task load on perceived effort. **P < 0.01.
As expected, the rating procedures as compared with baseline activated a large range of areas. Additionally to visual and motor areas, a number of working memory related areas in dlPFC and PPC became active during both rating tasks. Also, large clusters in the dorsal part of the mid-cingulate gyrus and in the anterior and posterior insula were activated. Furthermore, the inferior frontal gyrus (IFG) was activated. Various parts of the basal ganglia were activated, with strong bilateral thalamic activation present in both tasks. Bilateral activity was also found in the border region of external globus pallidus and putamen, albeit localized slightly more lateral in the difficulty rating condition.
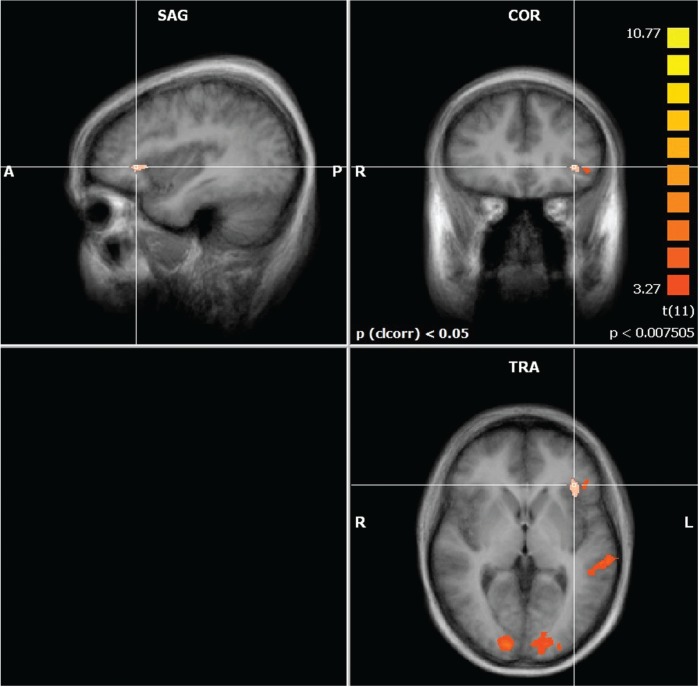
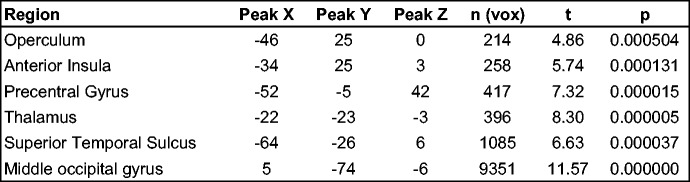
A contrast of the two rating conditions showed clusters of significantly stronger activation in the left aIC and IFG/operculum, as well as in the thalamus. Also, stronger activation was seen in the right inferior parietal sulcus (IPS), bilateral occipital gyrus and in the left superior temporal sulcus (STS). Separate fixed effects group analysis of the two left handed subjects did not reveal any results that would point towards differences in the lateralization of the reported clusters due to handedness.
DISCUSSION
Summary of the findings
We conducted this study to identify areas in the human brain that are relevant for the evaluation of mental effort. In particular, we investigated which areas would be significantly more active during mental effort evaluation when compared with a highly similar evaluation of task difficulty.
Our behavioral results confirmed our first hypothesis, regarding the correlation of difficulty and effort ratings and the induced differences in task load. As shown in earlier studies (Yeo and Neal, 2004), difficulty and effort ratings were correlated. Also, the scores followed the induced differences in a parametric fashion, illustrating the effectiveness of our task load manipulation (Figure 1).
The brain imaging results for both rating tasks were in line with our second hypothesis. Common activation in both rating tasks included generally expected areas recruited for the basic execution of the VAS rating task, namely various visual and motor areas (see appendix 1 and 2, respectively, for a list of clusters). Common activation also included anterior and posterior cingulate areas and dlPFC, i.e. results were very similar as those reported in several studies reviewed by Lee and Siegle (2009). During the rating task, we furthermore observed increased activation in parts of the basal ganglia, as can be expected for tasks involving motor output as response.
Activation in aIC
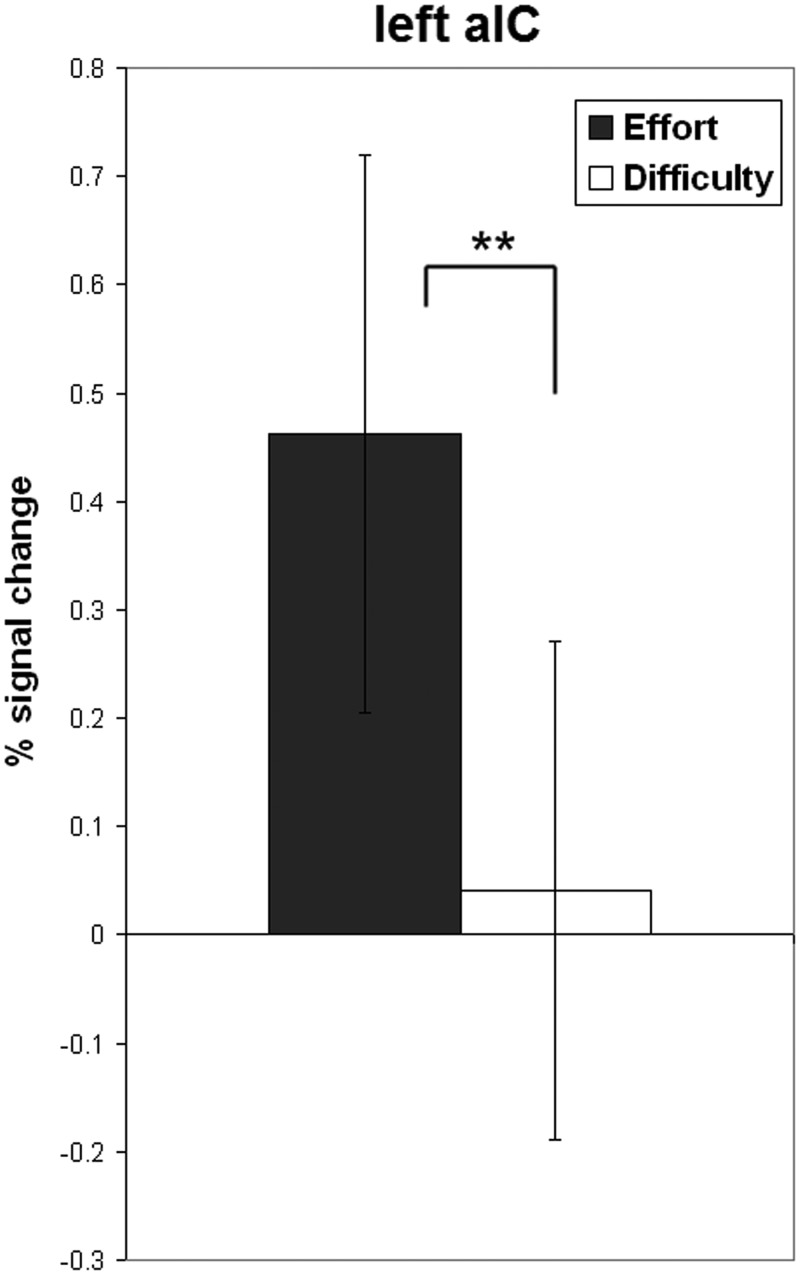
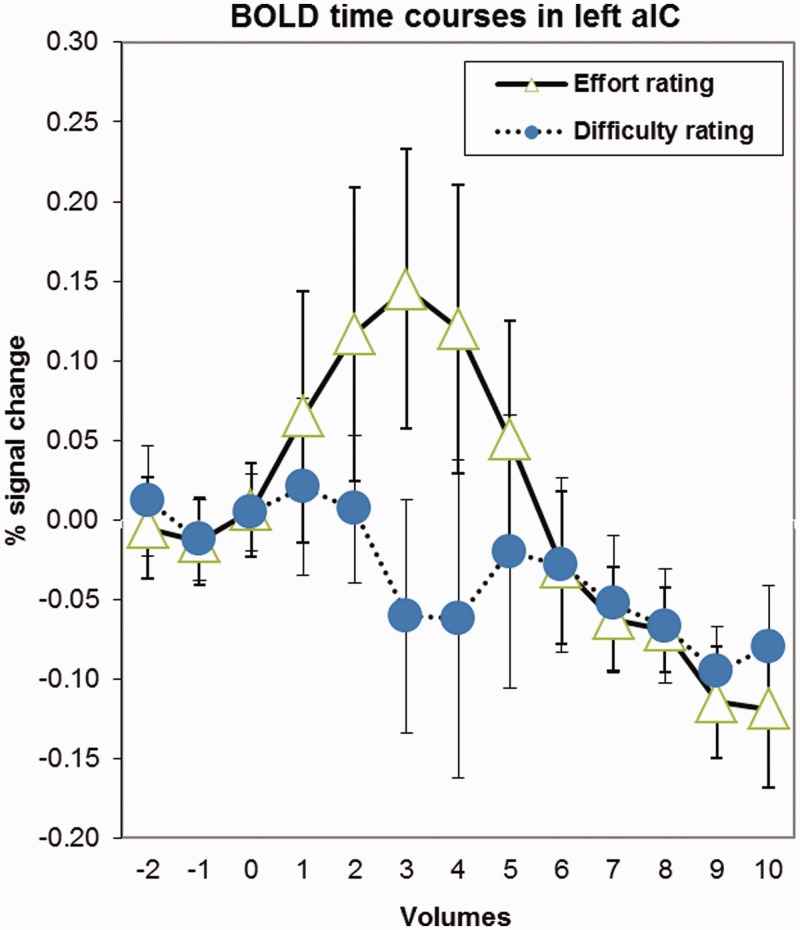
Our main hypothesis concerned the contrast of the two rating task conditions. This hypothesis was confirmed: we found a cluster of significantly stronger activated voxels in the left aIC during mental effort evaluation (Figures 2–5). The aIC has been proposed to combine two necessary capabilities for the evaluation of mental effort: integrating information from different domains and making the result accessible to the subject as a part of self-awareness (Craig, 2009). This is in line with our findings.
Fig. 2.
Significantly more active clusters and their approximate locations in a contrast of mental effort vs difficulty rating. P values represent the peak P-value of a cluster.
Fig. 3.
Activation map showing the contrast mental effort rating > difficulty rating.
Fig. 4.
Bar plot showing percent signal change during mental effort and difficulty rating in left aIC. **P < 0.01.
Fig. 5.
BOLD curves showing percent signal change in left aIC during mental effort and difficulty rating, relative to the start of the actual rating period.
We interpret the stronger activation in the left aIC in our subjects as evidence that mental effort evaluation indeed relies on this integrative process, compared with a more rational evaluation such as one of task difficulty.
The proposed function of aIC in self-awareness could also be related to the activation pattern we found. While both the assessments of difficulty and effort incorporate a cognitive appraisal of the task itself, mental effort evaluation additionally relies on emotional and somatic self-related factors. This difference in the self-referential aspect of our two evaluations resulted in a difference of aIC activation during the rating conditions. Similar results have been found in a study by Modinos et al. (2009), who investigated the neural correlates of self-evaluation. They employed a paradigm contrasting self-referential vs non-self-referential evaluation. Subjects had to evaluate the accuracy of various statements in relation to themselves or in relation to peers. The results of their study showed a cluster of activation in the left aIC, closely matching the location of the active cluster in our results. The left aIC showed a significantly stronger response when subjects were evaluating statements related to them contrasted with statements about peers. This was interpreted as evidence for the role of this region in self-related evaluations. In line with this evidence, we thus interpret the stronger activation in aIC found in our own results as reflecting the fact that mental effort evaluation relied more on the effect of the task on the state experienced by the subject during task performance. The contrast employed in our paradigm is, thus, more specific than the one used by Modinos et al. (2009), in the sense that it compared two self-centered evaluations. This explains why we did not find differences in activation in medial prefrontal regions as Modinos et al. reported for a contrast of self- vs other-related evaluations.
An alternative explanation given by Modinos et al. (2009) was that self-related evaluation merely elicits enhanced awareness of somatic and emotional information reflected in aIC activation. This alternative interpretation is also relevant for the interpretation of our results, as it would imply that aIC is not actually recruited in the rating of mental effort, but merely becomes more activated by the resulting increase in self-awareness of self-interrogated somatic and emotional state. However, the results of Naccache et al. (2005) lend evidence against this interpretation. The disturbance in the somatic feedback loop left patient RMB bare of the capability to evaluate mental effort. If increased self-awareness was a mere by-product of the evaluative process, the process itself should not be impaired by a disturbance of the information streams related to self-awareness. Also, no changes were noted in patient RMB’s general levels of self-awareness, demonstrating that other domains of self-referential processing which rely less on somatic information can still work normally in the light of this specific functional deficit. Our results support this interpretation, as our narrow experimental contrast of two self-centered evaluations did not elicit measurable differences in activation in the CMS, which is associated with differences in the level of general self-awareness. Our results illustrate the combination of processing capabilities of the aIC which are recruited significantly more during the evaluation of mental effort.
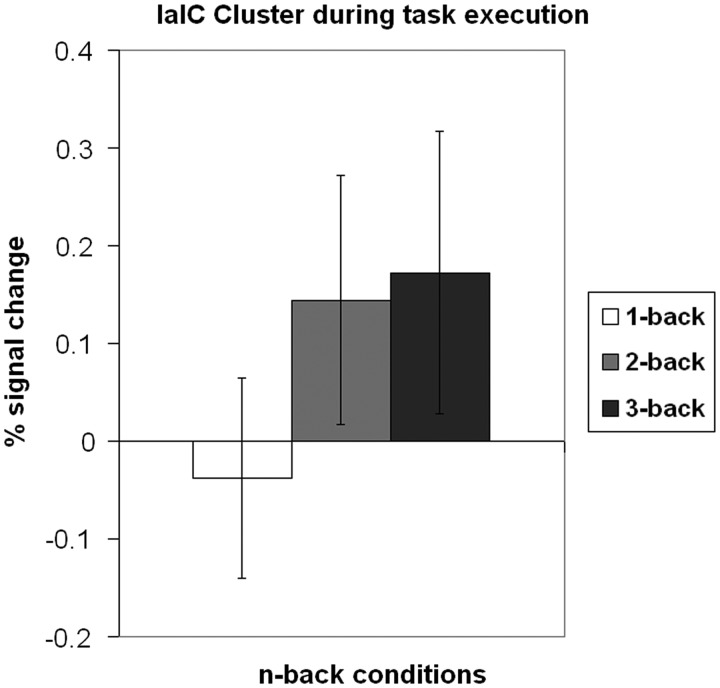
Additionally, our results suggest a more specific role of the left aIC connected to mental effort. When plotting the activation of the left aIC cluster during task execution, the activity varied in a linear fashion across the levels of task load, mirroring the pattern we observed in the corresponding RSME ratings (Figure 6). Similar observations have been reported earlier: Deary et al. (2004) found a change of activation in aIC as an effect of changing task difficulty, most prominent indeed in the left aIC. Although their design included no evaluations of task difficulty or mental effort, their behavioral results suggest effective changes in mental workload across the different levels of task difficulty. This indicates that in the case of mental effort, the left aIC has not only a role in offline evaluation, but also in experiencing mental effort during task execution. Shared insular regions that are recruited both during perceptual encoding of a self-related experience and during later evaluation with a VAS have been reported earlier (Kong et al., 2006).
Fig. 6.
Signal changes in left aIC during the execution of the n-back task. See also Figure 1.
Our findings are in line with evidence from these two studies, demonstrating that the experience of mental effort and later evaluations thereof share underlying neural structures.
Other involved brain areas
Additionally to the activation in left aIC, our maps showed a number of other areas that were stronger activated during mental effort evaluation than during task difficulty evaluation. We interpret the activity in the left IFG/operculum as a result of the proposed strong connectivity between aIC and IFG/operculum (Craig, 2009). The activity in the left thalamus is in our view a sign of the heightened activity of the somatic loop, which in this case conveys information about bodily states to higher cognitive areas. The thalamic activation in our data was most prominent in the posterior ventral part of the thalamus. This part has been shown to project towards the aIC and the operculum. These projections have been proposed to play an important role in the awareness and evaluation of bodily states (Craig, 2002).
We also found a substantial cluster of stronger activated voxels in the left STS. Modinos et al. also reported a cluster in the superior temporal region, albeit contralateral to our results, in the right superior temporal gyrus (STG).
Their interpretation is that self-evaluation includes the estimation of what others might think about one. The STG has been reported to play a role in such theory-of-mind-related processes (Frith and Frith, 2006).
Although we cannot rule out a similar explanation for our findings, our own interpretation is that the activation in STS is not related to a social feature of mental effort evaluation. The superior temporal area, especially the STS, is a functionally diverse region. It has been proposed that it might actually not be divided in specialized functional subregions, but that it provides a supportive role for different cognitive processes (Hein and Knight, 2008). Our interpretation of the activation in the superior temporal region in our results is thus that it provided a supportive role. Differences in the task difficulty VAS and the RSME might have accounted for part of the reported clusters in our results as well; the difficulty scale did not use the eleven anchor points of the RSME, but merely three indications of difficulty. This already resulted in a richer visual stimulation, which we attribute as the cause of the more active clusters in the occipital cortex. The effects of the more precise scaling of the RSME might have affected the rating as such as well. The subjects might have tried to place their rating more precisely on a designated point on the RSME, compared with placing it in a general area on the difficulty scale. This would explain the stronger recruitment of the primary motor cortex, but also of the IPS as an area which has been shown to be essential for numerical distance processing (Ansari et al., 2005).
CONCLUSION
The goal of this study was to investigate the neural correlates of mental effort evaluation. By employing the specific contrast between the objective evaluation of task difficulty and the subjective evaluation of mental effort, we were able to identify a number of brain areas that partake stronger in this process. The relation of mental effort evaluation with somatic awareness had been proposed earlier on the basis of the observations in patient RMB (Naccache et al., 2005). These findings, together with the theoretical implications of the integrated model of mental effort regarding integration and awareness, made an involvement of the aIC plausible. Our results support the view that the aIC unifies two important capabilities necessary for mental effort evaluation. This, together with the stronger activation in the left thalamus, demonstrates the importance of somatic information for the emotionally salient awareness and evaluation of the self.
The relation between the RSME scores and the activation in left aIC during task performance suggests further research in this direction. Future studies could investigate the effect of various manipulations of mental workload on this area, which would solve the question if the apparent similarity originates in changes of task load or total perceived mental workload. Manipulating the state load, a variable which remained constant in the present study, could help to attribute these effects.
Appendix 1.
Active clusters and their approximate locations during mental effort rating
| Region | Peak X | Peak Y | Peak Z | n (vox) | t | P |
|---|---|---|---|---|---|---|
| Inferior frontal gyrus, opercular part | −43 | 16 | 24 | 48 | 9.48 | 0.000001 |
| Anterior insula | 35 | 13 | 6 | 483 | 8.89 | 0.000002 |
| −37 | 13 | 6 | 47 | 8.11 | 0.000006 | |
| Precentral sulcus | 50 | 4 | 12 | 832 | 9.18 | 0.000002 |
| Precentral gyrus | −55 | 1 | 24 | 80 | 8.46 | 0.000004 |
| −52 | −2 | 33 | 56 | 6.70 | 0.000034 | |
| Precentral gyrus/superior frontal sulcus | 41 | −14 | 48 | 1835 | 10.28 | 0.000001 |
| −28 | −14 | 54 | 2551 | 9.78 | 0.000001 | |
| Postcentral sulcus | 32 | −41 | 39 | 2141 | 11.27 | 0.000000 |
| −34 | −38 | 42 | 3488 | 9.27 | 0.000002 | |
| Cingulate gyrus | −4 | −14 | 48 | 3890 | 11.99 | 0.000000 |
| Ventral lateral posterior thalamic nucleus | 14 | −23 | 9 | 692 | 8.57 | 0.000003 |
| −19 | −26 | 6 | 1160 | 9.29 | 0.000002 | |
| Parietooccipital trans. zone/Angular gyrus | 14 | −68 | 36 | 315 | 9.16 | 0.000002 |
| Intraparietal sulcus | −25 | −56 | 45 | 196 | 8.13 | 0.000006 |
| Lateral occipitotemporal sulcus | 38 | −62 | −9 | 906 | 10.36 | 0.000001 |
| −37 | −59 | −12 | 80 | 7.89 | 0.000007 | |
| Medial occipital gyrus | 26 | −83 | −6 | 4289 | 14.86 | 0.000000 |
| −4 | −77 | −12 | 815 | 10.03 | 0.000001 | |
| Lateral occipital gyrus | −28 | −71 | −15 | 496 | 8.00 | 0.000007 |
P values represent the peak P-value of a cluster.
Appendix 2.
Active clusters and their approximate locations during difficulty rating
| Region | Peak X | Peak Y | Peak Z | n (vox) | t | P |
|---|---|---|---|---|---|---|
| Inferior frontal gyrus, opercular part | 50 | 10 | 3 | 543 | 9.78 | 0.000001 |
| −55 | 10 | 0 | 177 | 6.94 | 0.000025 | |
| Anterior insula | 32 | 13 | 9 | 356 | 12.04 | 0.000000 |
| −37 | 13 | 6 | 77 | 7.78 | 0.000009 | |
| Mid-insula | 35 | −2 | 3 | 89 | 8.02 | 0.000006 |
| −34 | −2 | 3 | 85 | 7.72 | 0.000009 | |
| Precentral gyrus | 47 | 1 | 30 | 1480 | 10.82 | 0.000000 |
| Precentral gyrus/central sulcus | 41 | −14 | 48 | 3139 | 9.99 | 0.000001 |
| −25 | −14 | 57 | 3561 | 11.35 | 0.000000 | |
| Postcentral sulcus | 29 | −41 | 39 | 2686 | 11.18 | 0.000000 |
| −37 | −38 | 42 | 4783 | 13.82 | 0.000000 | |
| SFG (paracentr. lob.)/cing. gyrus | 5 | −5 | 45 | 5252 | 16.41 | 0.000000 |
| Ventral lateral ant. thalamic nucleus | 14 | −20 | 9 | 812 | 9.25 | 0.000002 |
| −19 | −26 | 6 | 1560 | 9.81 | 0.000001 | |
| Inferior frontal gyrus, opercular part | −3.14286 | −33.978 | 39.1319 | 3772.81 | 12.54 | −0.000002 |
| −4 | −37.4132 | 41.4923 | 4041.57 | 12.84 | −0.000003 | |
| Anterior insula | −4.85714 | −40.8484 | 43.8527 | 4310.33 | 13.13 | −0.000004 |
| −5.71429 | −44.2835 | 46.2132 | 4579.08 | 13.42 | −4.7E−06 | |
| Mid-insula | −6.57143 | −47.7187 | 48.5736 | 4847.84 | 13.71 | −5.5E−06 |
| −7.42857 | −51.1538 | 50.9341 | 5116.59 | 14.01 | −6.4E−06 | |
| Precentral gyrus | −8.28571 | −54.589 | 53.2945 | 5385.35 | 14.30 | −0.000007 |
| Precentral gyrus/central sulcus | −9.14286 | −58.0242 | 55.6549 | 5654.11 | 14.59 | −0.000008 |
| −10 | −61.4593 | 58.0154 | 5922.86 | 14.88 | −0.000009 | |
| Postcentral sulcus | −10.8571 | −64.8945 | 60.3758 | 6191.62 | 15.18 | −0.000010 |
P values represent the peak P-value of a cluster.
REFERENCES
- Ansari D, Garcia N, Lucas E, Hamon K, Dhital B. Neural correlates of symbolic number processing in children and adults. Neuroreport. 2005;16(16):1769. doi: 10.1097/01.wnr.0000183905.23396.f1. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio A. The Feeling of What Happens. New York: Harcourt Brace & Co.; 1999. [Google Scholar]
- Deary IJ, Simonotto E, Meyer M, et al. The functional anatomy of inspection time: an event-related fMRI study. Neuroimage. 2004;22(4):1466–79. doi: 10.1016/j.neuroimage.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19(2):430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Fairclough SH, Mulder LMJ. Psychophysiological processes of mental effort investment. In: Wright R, Gendolla G, editors. How Motivation Affects the Cardiovascular Response: Mechanisms and Applications. Washington DC: American Psychological Association; 2011. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–34. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalography and clinical Neurophysiology. 1993;87(3):128–43. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Hein G, Knight RT. Superior temporal sulcus—it’s my area: or is it? Journal of Cognitive Neuroscience. 2008;20(12):2125–36. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O. The Body in the Mind: On the Relationship Between Interoception and Embodiment. Topics in Cognitive Science. 2012;4(4):692–704. doi: 10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- Hockey GRJ. Compensatory control in the regulation of human performance under stress and high workload: a cognitive-energetical framework. Biological Psychology. 1997;45(1–3):73–93. doi: 10.1016/s0301-0511(96)05223-4. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Human Brain Mapping. 2006;27(9):715–21. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Social Cognitive and Affective Neuroscience. 2009;7(5):521–34. doi: 10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5th edn. New York: McGraw-Hill; 2010. pp. 143–93. http://onlinelibrary.wiley.com/mrw_content/socpsy/articles/socpsy001005/socpsy001005.pdf. [Google Scholar]
- Mai JK, Voss T, Paxinos G. Atlas of the Human Brain. 3rd edn. Amsterdam, Boston: Elsevier/Academic Press; 2007. [Google Scholar]
- Modinos G, Ormel J, Aleman A. Activation of anterior insula during self-reflection. PLoS One. 2009;4(2):e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder G. The concept and measurement of mental effort. In: Hockey GRJ, Gaillard AWK, Coles MGH, editors. Energetics and Human Information Processing. Dordrecht: Martinus Nijhoff; 1986. [Google Scholar]
- Naccache L, Dehaene S, Cohen L, et al. Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia. 2005;43(9):1318–28. doi: 10.1016/j.neuropsychologia.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience & Biobehavioral Reviews. 2010;34(6):935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Verwey WB, Veltman HA. Detecting short periods of elevated workload: a comparison of nine workload assessment techniques. Journal of Experimental Psychology: Applied. 1996;2(3):270–85. [Google Scholar]
- Yeo GB, Neal A. A multilevel analysis of effort, practice, and performance: effects; of ability, conscientiousness, and goal orientation. Journal of Applied Psychology. 2004;89(2):231–47. doi: 10.1037/0021-9010.89.2.231. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62(1):493–9. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra FRH. Efficiency in Work Behavior: A Design Approach for Modern Tools. Delft: Delft University Press; 1993. [Google Scholar]