SUMMARY
Visual information is mediated by two major thalamic pathways, which signal light decrements (OFF) and increments (ON) in visual scenes, the OFF pathway being faster than the ON. Here, we demonstrate that this OFF temporal advantage is transferred to visual cortex and has a correlate in human perception. OFF-dominated cortical neurons in cats responded ~3 ms faster to visual stimuli than ON-dominated cortical neurons, and dark-mediated suppression in ON-dominated neurons peaked ~14 ms faster than light-mediated suppression in OFF-dominated neurons. Consistent with the neuronal differences, human observers were 6–14 ms faster at detecting darks than lights, and better at discriminating dark than light flickers. Neuronal and perceptual differences both vanished if backgrounds were biased towards darks. Our results suggest that the cortical OFF pathway is faster than the ON pathway at increasing and suppressing visual responses; and these differences have parallels in the human visual perception of lights and darks.
Keywords: thalamus, LGN, area V1, receptive field, saliency, irradiation illusion, detection, reaction time
INTRODUCTION
Neurons in the visual pathway have different response time courses, which are likely to serve different functions. In cat, the fast, transient visual responses of Y thalamic cells are thought to be suitable for encoding motion, whereas the longer and more sustained responses of X cells are better suited to encode form (Derrington and Fuchs, 1979; Lehmkuhle et al., 1980; Sherman and Spear, 1982; Demb et al., 2001). In contrast to X and Y visual pathways, ON and OFF pathways were originally thought to have similar response time courses and differ only in their preferences for contrast polarity: ON neurons responding to light increments and OFF neurons to decrements (Hartline, 1938; Kuffler, 1953). This understanding of ON and OFF pathways in visual function has been changing over the past decades as new functional differences between the two emerge (Zemon et al., 1988; Chichilnisky and Kalmar, 2002; Zaghloul et al., 2003; Jin et al., 2008; Yeh et al., 2009; Liang and Freed, 2010; Pandarinath et al., 2010; Xing et al., 2010; Jin et al., 2011; Hesam Shariati and Freeman, 2012). There is evidence that OFF neurons respond faster to visual stimuli than ON neurons, in the retinae of salamanders, turtles and mice (Baylor and Fettiplace, 1977; Copenhagen et al., 1983; Burkhardt et al., 1998; Gollisch and Meister, 2008; Burkhardt, 2011; Nichols et al., 2013) and in the visual thalamus of cats (Jin et al., 2011).
The difference in response time-courses between ON and OFF pathways most likely originates in retinal bipolar cells that use slow metabotropic glutamate receptors (mGluR6) to generate ON responses and fast ionotropic receptors to generate OFF responses (Nakajima et al., 1993; Snellman et al., 2008; Koike et al., 2010). While these temporal differences seem to be preserved in the thalamocortical pathway (Jin et al., 2011), it remains unclear if they are transferred to visual cortex and influence perception. Because neurons in layer 4 of primary visual cortex receive convergent inputs from both ON and OFF thalamic cells (Tanaka, 1983; Reid and Alonso, 1995; Alonso et al., 2001), the thalamocortical convergence could remove the ON-OFF temporal differences imposed by the receptor kinetics in the retina. Alternatively, the thalamocortical convergence could preserve or even amplify the temporal differences, creating a temporal asymmetry in the perception of darks and lights. By taking advantage of multi-electrode recordings, we demonstrate that ON-OFF temporal differences are not only present in primary visual cortex but are likely amplified by thalamocortical convergence and intra-cortical suppression (Hirsch et al., 1998; Hirsch, 2003). Moreover, by using psychophysical measurements of temporal thresholds, we demonstrate that humans process darks 6–14 ms faster than lights, a temporal difference that is remarkably close to our physiological measurements of temporal differences in ON and OFF pathways. We also show that both temporal differences, in ON-OFF neuronal response latency and dark-light detection, vanish if the background is adjusted to compensate for the irradiation illusion, in which light stimuli on dark backgrounds appeared larger than physically equal dark stimuli on light backgrounds (Galilei, 1632). Finally, we show that dark-mediated cortical suppression is stronger and faster than light-mediated cortical suppression and, consequently, human observers take longer to perceive a stimulus after a light turns to dark than after a dark turns to light. These findings have important implications for our understanding of the functional organization of ON and OFF visual pathways and the perception of darks and lights in human observers.
RESULTS
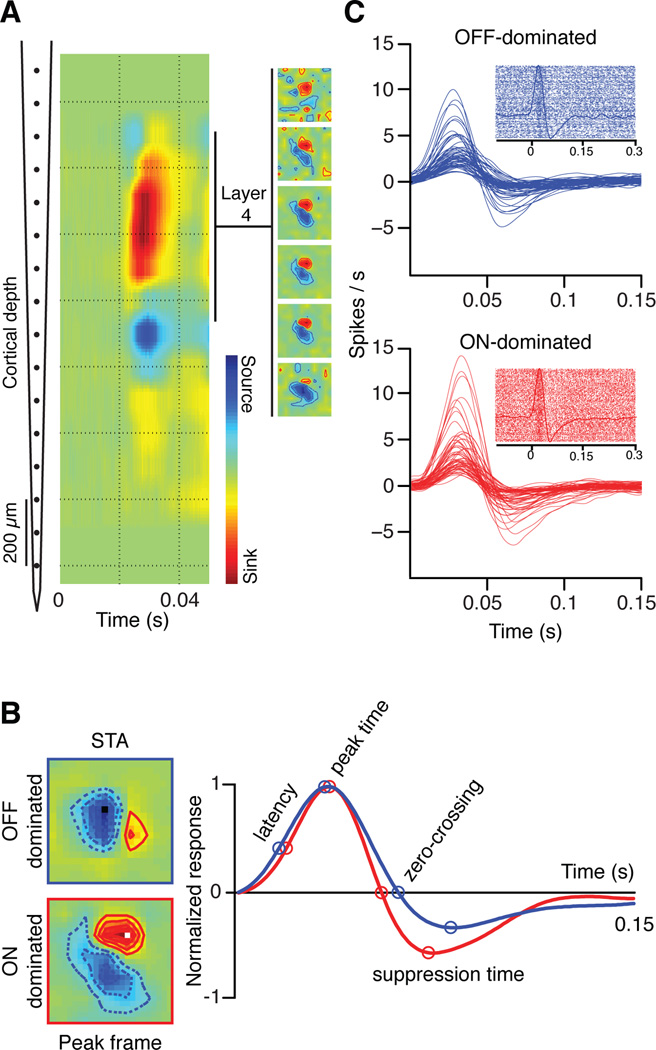
A 16 channel multi-electrode array was vertically introduced in cat visual cortex to record multiunit and single unit activity from cortical layer 4 (Figure 1a, left). Layer 4 was identified by current source density analysis (Jin et al., 2011) and the cortical receptive fields were mapped with binary white noise stimuli by spike-trigger averaging (STA) the stimulus (Figure 1a, right inset). Each multiunit receptive field in cortical layer 4 was classified as OFF-dominated (n=418) or ON-dominated (n=220) by measuring the contrast polarity of the stimulus pixel that generated the maximum response at the peak frame (Figure 1b, ON: red, OFF: blue). We recently demonstrated that visual response latencies are ~3 ms shorter in OFF than ON X cells of the lateral geniculate nucleus (LGN) (Jin et al., 2011), which are the main thalamic inputs to cat area 17 (Ferster, 1990). Because ON and OFF pathways converge in layer 4 cortical neurons, the ON-OFF latency differences could be reduced, preserved or amplified by intracortical processing. Our results support the notion that the ON-OFF temporal differences are amplified in cortex and influence visual perception.
Figure 1. Recordings from cortical layer 4 in anesthetized cats.
Multiple penetrations were made using a 16-channel probe (inter-electrode distance of 100 microns) in V1 of cat visual cortex. The receptive fields were mapped using binary white noise stimuli. (a) The depth of cortical layer 4 was identified as a strong current sink generated by a full-field flash presented at time 0 (left). Cortical receptive fields were measured in layer 4 with binary white noise by spike trigger averaging the stimulus (right). (b) The white noise pixel that generated the strongest response was used to determine the dominance polarity of the cortical receptive field (light for ON-dominated, dark for OFF-dominated). Four time points (latency, peak time, zero-crossing, and suppression time) were chosen to compare the temporal dynamics. (c) The time stamps of the white noise frames with the pixel that generated the strongest response were used as triggers to generate peri-stimulus time-histograms (PSTHs) and rasters for OFF-dominated (blue) and ON-dominated cells (red). The PSTHs were calculated with a 1 ms bin and smoothed using a moving average triangular filter of 21 ms width.
Time courses of ON and OFF responses in visual cortex
To measure the latency differences between ON-dominated and OFF-dominated cortical neurons, we first selected the receptive field pixel that generated the maximum response in the receptive field map: the preferred stimulus pixel. The preferred stimulus pixel was dark for OFF-dominated and light for ON-dominated neurons (position and polarity illustrated by small dark and light squares in Figure 1b, left). We then used the time stamps of the white-noise stimulus-frames with the preferred pixel as stimulus onset to generate peri-stimulus time histograms (PSTHs). The number of white noise frames with the preferred stimulus pixel was approximately half of the entire white noise sequence (32,767 white noise frames), therefore, we generated a PSTH from ~ 16,383 spike rasters (Figure 1c). As shown in this PSTH, the onset of the preferred white-noise pixel generates an increase in firing rate (peak) followed by a reduction in firing rate below baseline (suppression), as the preferred pixel reverses polarity (Figure 1b-c). The PSTHs revealed a great diversity of response magnitudes and time-courses in both ON- and OFF-dominated cortical neurons (Figure 1c).
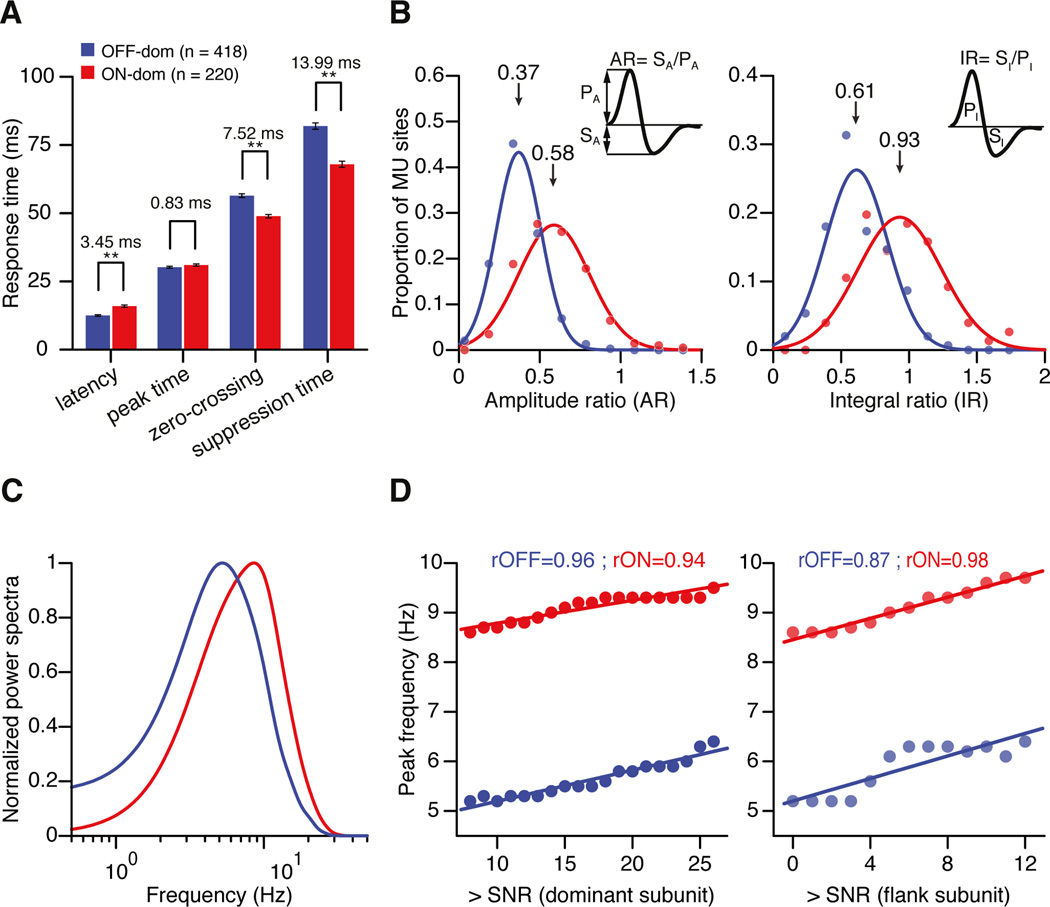
Similar to the properties of thalamic neurons, the response latency for cortical neurons was 3.45 ± 0.48 ms faster in OFF-dominated than ON-dominated cortical sites (p < 0.001) (Figure 2a). However, unlike the thalamus, the ON-OFF temporal difference was not significant at the response peak (0.83 ± 0.54 ms, p = 0.15), and was reversed at the zero-crossing with the baseline (7.52 ± 0.96 ms, p < 0.001), with the reversal reaching its maximum during the response suppression (13.99 ± 1.64 ms, p < 0.001; see distributions of temporal parameters in Supplemental Figure 1).
Figure 2. Response time courses of ON-dominated and OFF-dominated multiunit recording sites in cortical layer 4.
(a) Mean differences in response time course between ON-dominated (red) and OFF-dominated (blue) layer 4 recordings measured at the four time points described in Figure 1. Error bars show standard errors of mean (** indicates p < 0.001). (b) Distribution of ratios between response suppression and response increment in ON- and OFF-dominated cortical sites. The distributions were fitted with 1D Gaussian functions. Amplitude ratio (AR) was computed as the ratio of suppression amplitude to peak amplitude (SA / PA). Integral ratio (IR) (right) was computed as the ratio of the suppression integral to peak integral (SI / PI). (c) Fourier transform of the ON and OFF impulse responses represented as normalized power spectra. (d) Peak temporal frequency plotted as a function of signal-to-noise ratio of the dominant (left) and flank subregions (right).
When presented in an ON subregion, a light spot followed by a dark spot generates an increase in firing rate (peak) followed by a reduction in firing rate below baseline (suppression). To measure the relative amplitudes of peak and suppression, we used two different indices: the amplitude ratio (AR) and the integral ratio (IR) of response suppression to response peak (See Methods). Both indices were 1 when response peak and response suppression were equal and less than 1 when the suppression was smaller than the peak. Consistent with previous measurements in retinal ganglion cells and thalamic neurons (Zaghloul et al., 2003; Jin et al., 2011), the response suppression was significantly stronger in the ON than the OFF pathway (AR: ON = 0.58, OFF = 0.37, p < 0.001; IR: ON = 0.93, OFF = 0.61, p < 0.001) (Figure 2b), even if the background activity was not significantly different (36.62 spk/s vs 35.47 spk/s, p = 0.63). Moreover, the normalized power spectra revealed pronounced differences in ON and OFF temporal frequency tuning (Figure 2c). Importantly, the magnitude of the ON-OFF difference in peak frequency remained the same across different signal-to-noise ratios (SNRs), both in dominant and flank subregions (Figure 2d; see methods), indicating that the difference in suppression is robust and independent of the signal to noise. Notice that, because the response suppression is faster and stronger for the ON than the OFF pathways, the normalized power spectra predict a higher frequency peak for the ON pathway (Figure 2c). However, simple linear summation between PSTHs separated by short inter-stimulus intervals predicts exactly the opposite: higher temporal frequency peak for the OFF than the ON pathway (i.e. the stronger suppression prevents the ON pathway from responding to short inter-stimulus intervals). As we show below, our results are consistent with the predictions from linear summation while the predictions from the power spectra probably fail because they disregard phase information.
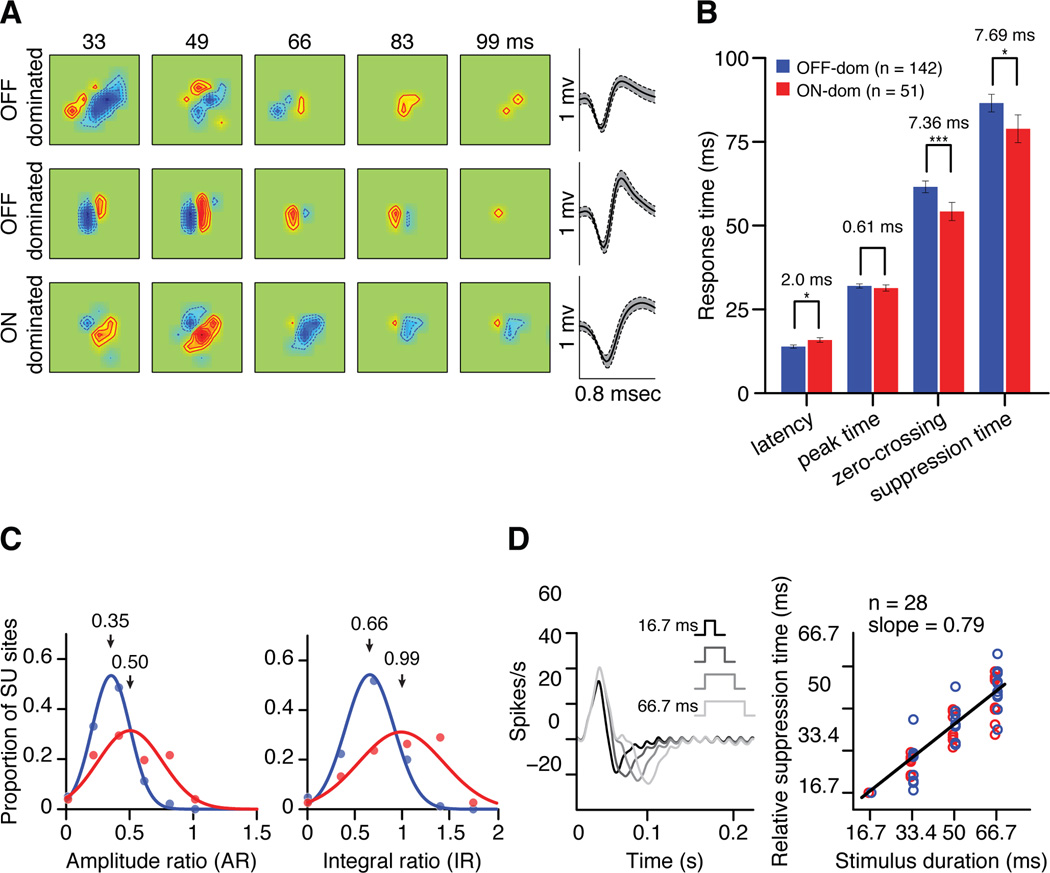
The ON-OFF temporal differences that we demonstrate with multiunit recordings could also be demonstrated in recordings from single layer 4 cortical neurons (see Supplemental Figure 2 for similar measurements using an LED monitor). As was the case for multiunit recordings, single neurons were classified as OFF-dominated (n=149) and ON-dominated (n=55) according to the contrast polarity of the strongest receptive field subregion (Figure 3a). Like for cortical multiunit measures, OFF-dominated cortical cells had faster response latencies than ON-dominated cortical cells (2.0 ± 0.84 ms, p < 0.05) but similar response peaks (0.61 ± 0.98 ms, p = 0.74). Moreover, ON-dominated cells decreased their responses more rapidly than OFF-dominated cells when their preferred pixel reversed polarity, reaching the zero-crossing and suppression peak ~ 7 ms faster (zero-crossing: 7.37 ± 3.21 ms, p < 0.005; suppression peak: 7.69 ± 3.65 ms, p < 0.05). The difference in zero-crossing time was similar between multiunit and single unit recordings (multiunit: 7.52 ms, single unit: 7.36 ms), however, the differences in suppression time were more pronounced in multiunit recordings (13.99 ms vs. 7.69 ms for measurements with CRT monitor and 10.35 ms vs. 3.15 ms for measurements with LED monitor). The more pronounced ON-OFF temporal differences in multiunit recordings were not caused by a sampling bias towards OFF-dominated cortical neurons, as the temporal differences remained the same when we randomly subsampled equal number of neurons (e.g. average latency difference: 3.45 ± 0.03; average suppression time difference: 14.01 ± 0.01; averaged across subsamples ranging from 100 to 220 pairs of ON-dominated and OFF-dominated neurons). Most likely, the ON-OFF temporal differences in multiunit recordings were more pronounced because multiunit activity provides a more homogenous sampling of different types of neurons than single neuron activity (less biased towards large neurons). Also, the reliability of the measurements is likely to increase with sample size and, consequently, we found that the larger the sample size, the larger the differences in suppression time (R2 = 0.9257, samples taken from CRT multiunit, LED multiunit, CRT multiunit and CRT single unit, Supplemental Figure 2). It should also be noted that, although the statistical errors in the ON-OFF comparisons are relatively small, the suppression response is much smaller in magnitude and noisier than the response peak (Figure 1C). Therefore, the measurements obtained from multiunit recordings are likely to provide the most accurate estimate of ON-OFF temporal differences in visual cortex.
Figure 3. Response time courses of ON-dominated and OFF-dominated single neurons in cortical layer 4.
(a) Example of receptive fields from OFF-dominated and ON dominated neurons in cortical layer 4 and spike waveforms (±1 SD envelope). (b) Mean differences in response time course between ON-dominated (red) and OFF-dominated (blue) layer 4 neurons. Error bars show standard errors of mean. * p < 0.05, ** p < 0.01, *** p < 0.005. (c) Distribution of ratios between response suppression and response peak in ON- and OFF-dominated cortical neurons. AR and IR were calculated as in Figure 2. (d) The cortical suppression time is locked to the time when the preferred pixel reverses polarity. (Left) PSTHs from an ON-dominated cortical cell triggered with the time stamps of white-noise stimulus-frames that had the preferred pixel as stimulus onset. In the sequence of white-noise frames, the preferred pixel could last from 2 monitor frames (16.7 ms, black) to 8 monitor frames (66.7 ms, light gray). In this PSTH example, the peak response was very transient and was similar across stimuli, however, the suppression time changed systematically with stimulus duration. The oscillation at the end of the PSTH is a response to the stimulus update (60 Hz), which is characteristic of transient neurons that follow high temporal frequencies. (Right) The stimulus duration was closely related with the relative suppression time with a slope close to 1. The slope was 0.79 for all neurons, 0.75 for ON-dominated (n=15, red circles) and 0.8 for OFF-dominated (n=13, blue circles). This relation is expected if the response suppression is triggered by a reversal in the polarity of the preferred pixel. The relative suppression time was measured as STi – ST1 + 16.7 msec where STi is the suppression time for stimulus durationi and ST1 is the suppression time for a stimulus lasting 16.7 msec.
As in multiunit recordings, the response suppression was significantly stronger in single ON- than OFF-dominated neurons (AR: OFF = 0.35, ON = 0.5, p < 0.001; IR: OFF = 0.66, ON = 0.99, p < 0.001) (Figure 3c), even if the background activity was not significantly different (4.96 spk/s vs 6.3 spk/s, p = 0.07). Intracellular recordings from layer 4 cortical neurons strongly suggest that the dark-mediated suppression of ON responses is driven by OFF inhibition and the light-mediated suppression of OFF responses is driven by ON inhibition (Hirsch, 2003). Consistent with this interpretation, the duration of a white noise pixel was correlated with the suppression time with a slope close to 1 (Figure 3d). If the push-pull mechanism is correct, our results indicate that the OFF pathway suppresses ON visual responses ~14 ms faster than the ON pathway suppresses OFF visual responses.
These results demonstrate that the temporal differences between ON and OFF pathways are preserved and amplified in primary visual cortex. The question is whether temporal differences in V1 affect visual perception. In the irradiation illusion, light spots on dark backgrounds are perceived as larger than dark spots of the same size on light backgrounds (Galilei, 1632). Moreover, white noise with the same number of dark and light pixels is perceived as having larger light than dark area (Komban et al., 2011). We have previously shown that the percentage of dark pixels has to be increased to 60% to eliminate the irradiation illusion in white noise (Komban et al., 2011). To investigate a possible physiological correlate for these psychophysical findings, we measured the responses of cat visual cortex to similar stimuli used in human psychophysical experiments: dark and light targets presented in binary white noise backgrounds.
V1 responses to dark and light targets on noisy backgrounds
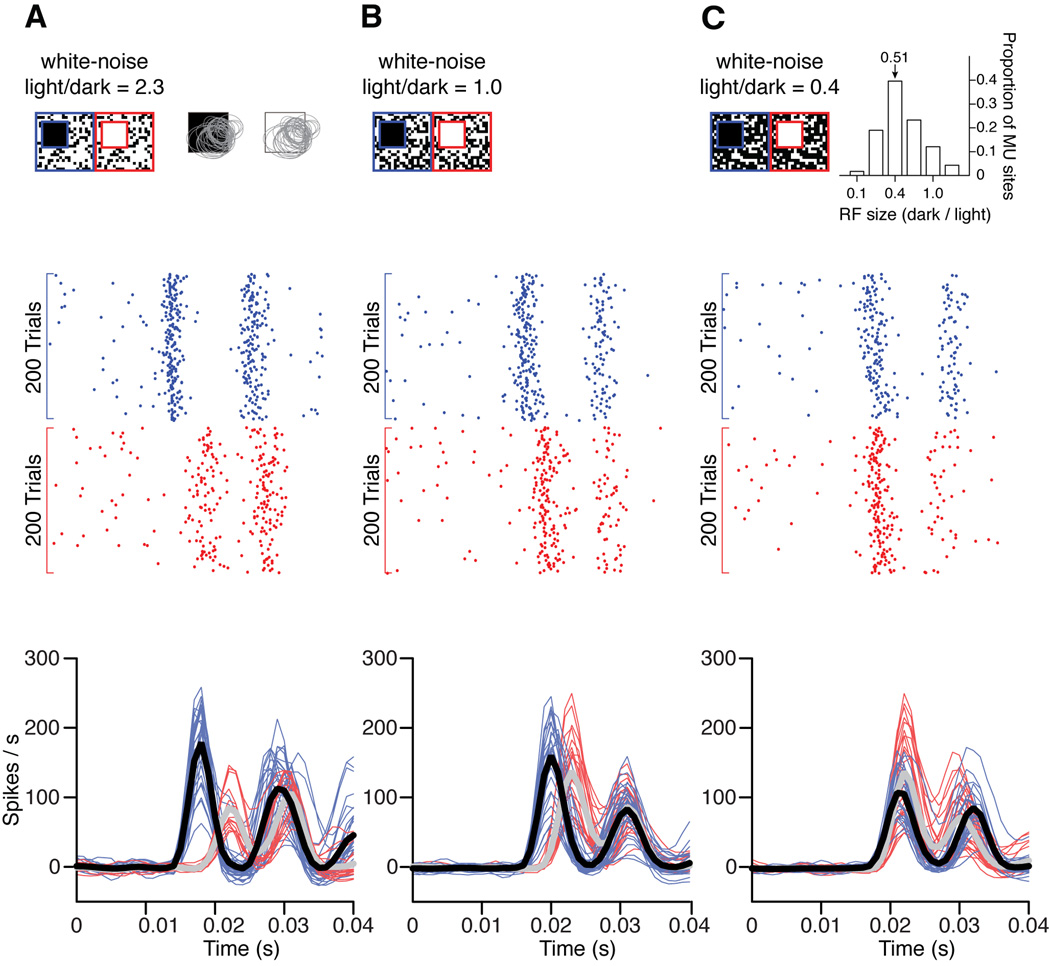
A 32-channel array was tangentially introduced in primary visual cortex to record multiunit activity across different cortical layers and different orientation columns. The receptive fields of all recording sites were mapped with sparse noise and the population receptive field was stimulated with a large square target superimposed on a white noise background (Figure 4a, top). We ensured that some surrounding regions of the population receptive field were stimulated by the white noise background and not only by the large square target. Target polarity and noise backgrounds were randomized for each trial. Consistent with our psychophysical measurements in humans (Komban et al., 2011), dark targets superimposed on binary white noise generated faster neuronal responses in visual cortex than did light targets. The temporal advantage for darks was very pronounced, both when the white noise had more light than dark pixels (Figure 4a, 9.11 ms; p < 0.001), and when dark and light pixels were equal in number (Figure 4b, 5.26 ms; p < 0.001). The temporal differences in the response to dark and light stimuli could be demonstrated in individual recording sites (Figure 4, rasters, middle panels) and in multiple simultaneously recorded sites (Figure 4, PSTHs, bottom panels, n=60). Consistent with the psychophysical findings of (Komban et al., 2011), the temporal advantage for darks disappeared when we reduced the light/dark ratio in white noise to 0.4 (Figure 4c, 0.56 ms; p = 0.4). Interestingly, this ratio is very close to the ratio of receptive field sizes mapped with dark and light stimuli at the same recording site in cat visual cortex (Figure 4c, inset at the top, n=116). Therefore, our results suggest that, the temporal asymmetry in the cortical responses to dark and lights can be eliminated by creating a spatial asymmetry in the ratio of light/dark pixels in the white noise background. Moreover, the light/dark asymmetry in the white noise background approaches the dark/light asymmetry in receptive field size. This result is very similar to our previous findings with human psychophysics (Komban et al., 2011): the temporal asymmetry in the detection of darks and lights can be eliminated by creating a spatial asymmetry in the ratio of light/dark pixels in the white noise background (light/dark ratio = 0.4). Moreover, the light/dark asymmetry in the white noise background eliminates the irradiation illusion.
Figure 4. Cortical responses to dark and light targets on noisy backgrounds.
(a) Top panel. The stimuli were large light and dark spots superimposed on a background of binary white noise. The white noise had 2.3 times more light than dark pixels. The dark and light spots were partially superimposed with the population of receptive fields from all cortical sites simultaneously recorded in a single penetration. Gray ellipses are 2D Gaussian fits to cortical receptive fields mapped with sparse noise stimuli. Middle panel. Rasters for 200 trials from a single cortical site in response to dark (blue) and light stimuli (red). Bottom panel. Cortical responses to the stimuli shown at the top, illustrated as PSTHs smoothed with a Gaussian kernel (width = 5 ms). Thin lines show responses of individual recording sites to dark (blue) and light (red) spots and thick lines show the average responses (black for dark spots and gray for light spots). (b) Same as in a, but using white noise with equal number of light and dark pixels. Notice that dark spots generated faster responses than light spots in both a and b. (c) Same as a and b, but using white noise with 0.4 times less light pixels than dark pixels. Notice that the white-noise light/dark ratio had to be reduced for dark and light stimuli to generate responses with similar latency. Interestingly, the value of the light/dark ratio for white noise was similar to the mean dark/light ratio for cortical receptive field size (inset, see methods). The double-peak PSTH is a slow oscillation that we observed when stimulating cortical layer 4 with large stimuli.
The ON-OFF temporal differences that we demonstrate in visual cortex are pronounced enough (~3 to 14 ms) to affect neuronal temporal integration; however, they are almost two orders of magnitude smaller than the temporal differences demonstrated in humans counting dark and light targets on noisy backgrounds (~200 ms, (Komban et al., 2011)). This large mismatch in temporal scale could be due to the visual search required in the psychophysical experiments from Komban et al (2011). Since our main interest was to find a perceptual correlate of ON-OFF temporal differences in visual detection, not visual search, we measured the temporal thresholds for darks and lights, which are more directly related to ON-OFF response latencies.
Psychophysical correlate of ON-OFF latency differences in V1
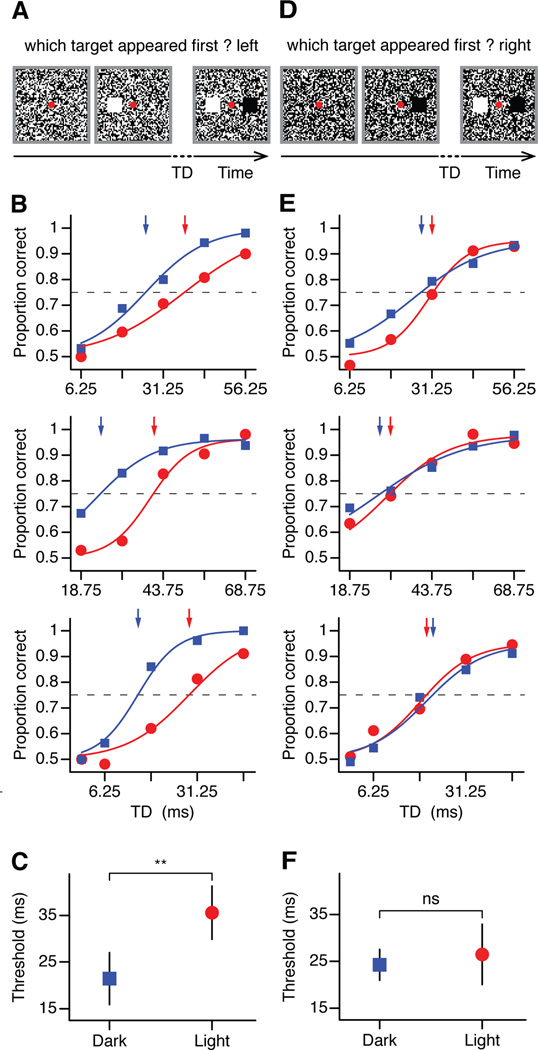
We presented two targets superimposed on white noise, one light and one dark, flanking the fixation point, vertically or horizontally. On each trial, one of the targets was presented with a random delay and the observer had to report the location of the target that appeared first (light target in the example from Figure 5a). All three observers (Figure 5b) detected 75% of the targets (dashed line) faster when they were dark (blue lines and arrows) than light (red lines and arrows). Moreover, the average temporal difference (Figure 5c) was close to the difference measured in visual cortical responses (Figure 5c, 14.05 ms± 6.36, p < 0.01, paired t-test). Similar results were obtained if we used a LED monitor instead of the CRT monitor (Supplemental Figure 3). The similarity between the temporal differences measured in physiological and psychophysical experiments is remarkable since the accuracy of the latency measures in humans is limited by the monitor frame rate (160 Hz, sampling every 6.25 ms). More importantly, the temporal difference disappeared when we increased the percentage of white noise dark pixels to 60% (Figure 5,d–f, 2.2 ± 5.93 ms, p= 0.37, paired t-test), a manipulation that also corrects for the irradiation illusion in humans. That is, while white noise with the same number of light and dark pixels is perceived as having larger light than dark area, the light and dark areas are perceived to be equal when 60% of the noise pixels are dark (Komban et al., 2011).
Figure 5. Human temporal thresholds in the detection of lights and darks.
(a) Observers were presented a pair of targets (one dark, one light) on a uniform noise background (light-to-dark ratio = 0.5), with variable amount of target delay (TD) between them. Size of targets and white noise pixels has been modified for illustration purposes. Observers were asked to report the location of the target that appeared first (light target in this example). (b) Correct responses of three observers to dark (blue) and light (red) targets as a function of TD. Threshold levels were defined as 75% of correct trials (dashed lines). Arrows show the target delay needed to reach the threshold level for dark (blue) and light (red) targets. (c) Thresholds for darks and lights averaged across observers. (d,e,f) Same as (a,b,c) but for uniform noise background adjusted for irradiation illusion (light/dark = 0.4). Error bars show standard errors of mean. Solid lines are psychometric fits to the data points.
Perceptual consequence of ON/OFF response suppression in visual cortex
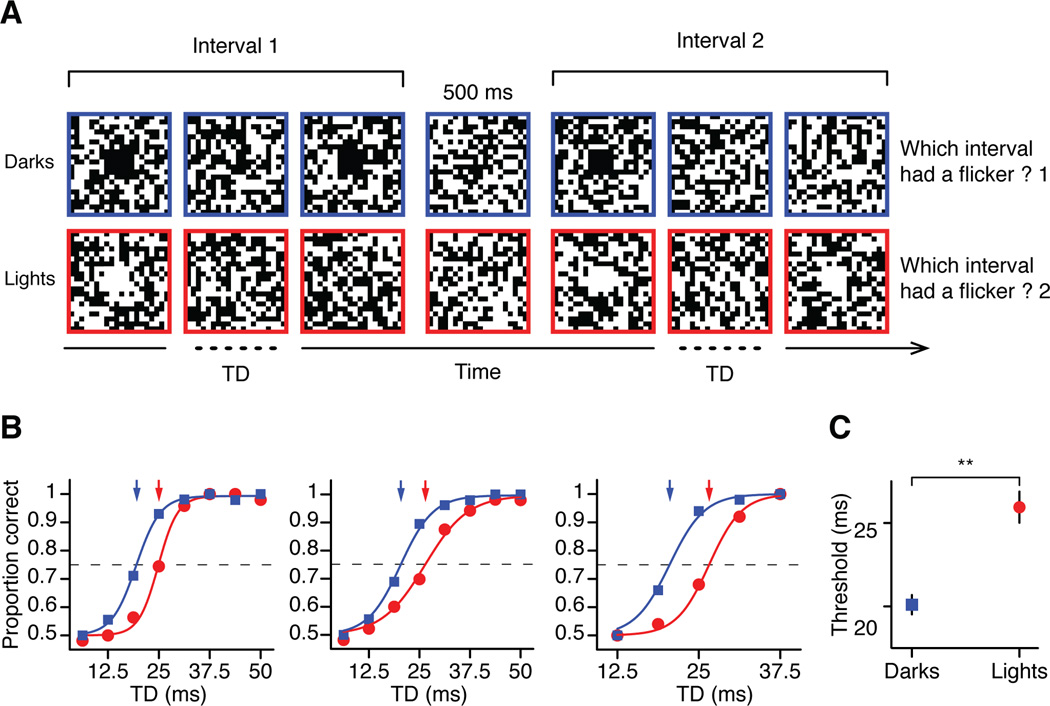
Our cortical measurements also demonstrate that dark targets suppress the response of the ON channel more than light targets suppress the response of the OFF channel. To investigate the possible psychophysical correlate of this ON-OFF difference in neuronal response suppression, we measured the temporal delay thresholds for light and dark flickers in human observers. Observers were presented with either dark or light targets in two consecutive temporal intervals. One interval had only a single target pulse and the other had two pulses separated by a variable temporal delay (flickering target). Observers were instructed to report the interval with the flickering target (Figure 6a). Notice that the minimum temporal delay in these experiments is one monitor frame (6.25 ms), which is the example represented in Figure 6a (interval 1 for darks and interval 2 for lights). Importantly, the durations of the two intervals were equal within each trial, but varied across trials, therefore, the observers could not use the interval duration to guess which interval had the flicker. Also, the observers could not use magnitude rather than inter-stimulus separation to detect the flicker. Otherwise, the proportion of correct trials for a temporal delay of 6.25 ms would be greater than 0.5, which was not the case for any of the observers. As illustrated in Figure 6b, observers saw a flickering dark target (blue squares and blue arrows) in 75% of the trials when the temporal delay (TD) was just 20 ms but needed a 26 ms interval for light flickering targets (red circles and red arrows). The average temporal difference between the temporal thresholds for lights and darks was 5.84 ± 2.34 ms (Figure 6c, p <0.01, paired t-test). This result is consistent with the physiological finding that OFF responses to dark spots are followed by less response suppression than ON responses to light spots. In a flicker, the response to the second pulse is also less attenuated in OFF (Supplemental Figure 4a) than ON thalamic neurons (Supplemental Figure 4b).
Figure 6. Human temporal delay thresholds in the detection of dark and light flickers.
(a) Stimulus paradigm. Observers were presented with either dark or light targets on uniform noise background in two consecutive temporal intervals. The figure uses the same ratio of target size to white noise pixel used in experiments. One interval had only one target (single target) and the other had two targets separated by a variable temporal delay (flickering target). Observers were instructed to report the interval in which they perceived a flicker. (b) Proportion of correct responses to dark (blue) and light (red) targets and (c) the average thresholds for three observers. Error bars show standard errors of mean. Solid lines are psychometric fits to the data points.
DISCUSSION
Our results demonstrate that OFF-dominated cortical neurons respond faster to visual stimuli than ON-dominated neurons, a difference that can be demonstrated both at the level of cell populations (multiunit activity) and single neurons. Like OFF- and ON-center X cells in visual thalamus, OFF-dominated cortical neurons responded ~3 ms faster than ON-dominated cortical neurons. In addition, dark stimuli suppressed ON cortical responses ~14 ms faster than light stimuli suppressed OFF cortical responses. Therefore, dark stimuli are faster than light stimuli at both increasing and suppressing visual responses in cortex. Importantly, we show that ON-OFF temporal differences measured in visual cortex have a psychophysical correlate in the detection of lights and darks in humans. Moreover, both the ON-OFF temporal differences in cortex and light-dark temporal differences in human vision disappear when the stimulus background is adjusted to compensate for the irradiation illusion.
The origin of the ON-OFF temporal difference that we demonstrate is likely to begin at the retina, where the metabotropic glutamate receptor (mGluR6) mediating ON responses has slower kinetics than the ionotropic receptor mediating OFF responses (Nakajima et al., 1993; Snellman et al., 2008; Koike et al., 2010). Previous retinal recordings in cold-blooded animals such as salamanders and turtles demonstrated that the OFF pathway is faster than the ON pathway (Baylor and Fettiplace, 1977; Copenhagen et al., 1983; Burkhardt et al., 1998; Gollisch and Meister, 2008; Burkhardt, 2011); however, evidence in mammals has remained more elusive. For example, primate recordings from retinal ganglion cells revealed either no ON-OFF temporal differences in vivo (Benardete and Kaplan, 1999) or a faster ON pathway in vitro (Chichilnisky and Kalmar, 2002), while cat recordings from thalamic neurons revealed a faster OFF pathway in vivo (Jin et al., 2011). Recent in vitro measurements in mice also found OFF retinal ganglion cells to be faster than ON retinal ganglion cells (Nichols et al., 2013). These contradictory findings may be explained by sampling differences across studies. While our recordings in both thalamus (Jin et al., 2011) and visual cortex were performed at eccentricities <10° of visual angle, the in vitro recordings in primate were more peripheral and included retinal regions of 20–35° (Chichilnisky and Kalmar, 2002). In the visual periphery, ON retinal ganglion cells have larger dendritic fields than OFF retinal ganglion cells (Dacey and Petersen, 1992) and may reach spike threshold faster by summing more inputs, as is also the case with parasol cells when compared with midget cells. The magnitude of ON-OFF temporal differences may also depend on the cell type studied. In the cat visual thalamus, ON-OFF temporal differences are smaller in X cells than Y cells (Jin et al., 2011) and it is possible that they are even smaller in primate midget cells recorded within the central 5° (Benardete and Kaplan, 1999). Future studies are needed to investigate how ON-OFF temporal differences change as a function of eccentricity and cell type in primate retina.
Although our previous work demonstrated that ON-OFF temporal differences are present in the thalamus (Jin et al., 2011), ON and OFF thalamic afferents converge on the same cortical neuron. Therefore, the ON-OFF thalamocortical convergence could provide an opportunity to eliminate temporal differences imposed by receptor kinetics in the retina, if they were not needed for visual processing. However, while ON and OFF thalamic afferents converge in single cortical neurons, their inputs are rarely balanced. Some neurons are OFF-dominated while others are ON-dominated, which enables the ON and OFF pathways to remain largely segregated and preserve their temporal differences in visual cortex. The average ON-OFF latency difference in cortex was similar to that measured in X cells of the cat visual thalamus (~3 ms), which are the main thalamic input to cat area 17 (Ferster, 1990). Unlike the thalamus (Figure 3b in ((Jin et al., 2011)) in visual cortex there was a large ON-OFF difference of ~14 ms in suppression time, which could originate from intracortical inhibition (Figure 3d) or temporal sorting of thalamic afferents (e.g. Figure 5b in (Jin et al., 2011)). The suppression caused by dark stimuli in ON cortical subregions and light stimuli in OFF cortical subregions is thought to be due to a push-pull mechanism that involves intracortical inhibition (Hirsch et al., 1998; Hirsch, 2003). If this push-pull mechanism is correct, our results suggest that the OFF pathway is faster than the ON pathway at both increasing and suppressing visual responses.
Although our results demonstrate that ON-OFF temporal differences are present in visual cortex, these differences would be irrelevant if they were not transmitted to further cortical stages to influence visual perception. In a previous study, we demonstrated that humans can discern the number of dark targets ~200 ms faster than the number of light targets in uniformly distributed noisy backgrounds, but this difference disappears when the background is corrected to compensate for the irradiation illusion described by (Galilei, 1632). Here, we provide a physiological correlate for these psychophysical experiments by showing that cortical neurons respond faster to darks than lights in noisy backgrounds. The ON-OFF temporal differences that we demonstrate in cat visual cortex (3–14 ms) are almost two orders of magnitude smaller than the temporal differences previously measured in humans by (Komban et al., 2011) with visual search tasks (200 ms). To better isolate the light-dark temporal differences in human perception, we used a new approach based on temporal thresholds. As a result, we found that the temporal differences in light-dark detection (6–14 ms) are remarkably close to the ON-OFF temporal differences measured in visual cortex (3–14 ms).
As with thalamic neurons (Jin et al., 2011), darks suppressed the responses of ON-dominated cortical neurons more than lights suppressed the responses of OFF-dominated cortical neurons. However, the response suppression in the OFF pathway was reduced in visual cortex when compared with the thalamus. While the ratio between the amplitude of response suppression and response peak (AR) was similar in ON-dominated cortical neurons and ON thalamic neurons (cortex: 0.58, thalamus: 0.53), OFF-dominated cortical neurons had smaller ratios than OFF thalamic neurons (cortex: 0.37, thalamus: 0.48) and, consequently, the ON-OFF difference in AR was ~4 times larger in cortex than thalamus (ON-OFF cortex: 0.21, ON-OFF thalamus: 0.05). Our psychophysical results suggest a possible perceptual consequence of this pronounced ON-OFF cortical difference in response suppression: humans can perceive dark flickers with significantly smaller inter-stimulus intervals than light flickers, and are better at detecting visual targets that follow darks than lights.
Taken together with previous studies, our results demonstrate that darks are processed faster and have access to more neuronal resources than lights in the early visual pathway (Ahmad et al., 2003; Jin et al., 2008; Balasubramanian and Sterling, 2009; Yeh et al., 2009; Ratliff et al., 2010; Xing et al., 2010; Jin et al., 2011), which could explain why darks appear more salient on noise backgrounds and are detected faster than lights (Chubb and Nam, 2000; Buchner and Baumgartner, 2007; Komban et al., 2011). We also show that the background correction needed to eliminate light-dark temporal differences is the same for a number of psychophysical tasks [temporal threshold (this manuscript) or suprathreshold detection (Komban et al., 2011) ] and matches the luminance profile of natural scenes (van Hateren et al., 2002; Balasubramanian and Sterling, 2009; Ratliff et al., 2010). Therefore, neural circuits in the early visual pathway may have evolved to match the distribution of darks and lights in natural scenes and, by doing so, treat darks and lights as equals in our visual environments.
EXPERIMENTAL PROCEDURES
Animal preparation
Adult male cats (n=15) were tranquilized with acepromazine (0.2 mg/Kg, IM) and ketamine (10 mg/Kg, IM) and anesthetized with propofol (2 mg/Kg, IV). An intravenous catheter was inserted into each hind limb to allow continuous infusions of propofol (5–6 mg/Kg/hr) and sufentanil (10–20 ng/Kg/hr) for anesthesia, vecuronium bromide (0.2 mg/Kg/hr) for muscle paralysis, and saline (1–3 ml/hr) for hydration. All vital signs were closely monitored and carefully maintained within normal physiological limits. The nictitating membranes were retracted with 2% neosynephrine and the pupils dilated with 1% atropine sulfate. Contact lenses were used to protect the corneas and focus visual stimuli on the retina. The positions of the optic disk and the area centralis were plotted on a screen in front of the animal by using a fiber-optic light source. All procedures were performed in accordance to the guidelines of the U.S. Department of Agriculture and approved by the Institutional Animal Care and Use Committee at the State University of New York, State College of Optometry.
Electrophysiological recording and receptive field mapping
Multi-electrode arrays with 16 or 32 channels (Neuronexus) were introduced vertically or tangentially through the cat primary visual cortex. The electrodes in each array were separated by 100 microns from each other. The voltage from the electrodes was amplified, filtered and collected via Plexon hardware and software. We performed multiple penetrations with the multi-electrode arrays, all restricted to the central 10° of the area centralis. As in previous studies (Swadlow et al., 2002; Jin et al., 2008), cortical layer 4 was identified in vertical penetrations as a strong current sink generated by a flash stimulus (Figure 1a, left).
The spatiotemporal receptive field (RF) of each cortical site (Figure 1a, right inset) was mapped using binary white noise and sparse noise by spike trigger averaging (STA) the stimulus (Jones et al., 1987; Reid et al., 1997). The white noise was used to map cortical receptive fields in layer 4 and the sparse noise to map receptive fields across different cortical layers. We used large stimulus targets to obtain robust responses and reliable measures of time course. The white noise was made of checkerboards with 16 × 16 light and dark pixels, with each pixel covering 1.83° × 1.83° of visual angle. The sparse noise consisted of light or dark squares presented in random positions on a dark or light background respectively, with each square covering 3.33° × 3.33° of visual angle and separated from each other by 1.66°. The stimuli were updated at 60 Hz for white noise and at 30 Hz for sparse noise on a cathode ray tube (CRT) monitor that refreshed at 120 Hz. The CRT monitor was located at a distance of 57 cm from the eye and had a mean luminance of 61 cd/m2. Cortical recording sites were classified as either ON-dominated or OFF-dominated based on the polarity of the most effective white noise pixel that generated an excitatory response (position and polarity illustrated by light and dark small squares in the receptive field maps shown in Figure 1b).
Time course of dark and light impulse responses
We measured the time course of the response generated by the most effective white noise pixel (light or dark) using a peri-stimulus time histogram (PSTH) averaged across trials (Figure 1b-c; see also (Jin et al., 2011)). We first calculated the spatial temporal receptive fields by spike trigger averaging (STA) white noise stimuli (Figure 1b, left). From the spatiotemporal receptive fields, we selected the pixel that generated the strongest response, which was dark for OFF-dominated and light for ON-dominated cortical cells (Figure 1b, position and polarity illustrated by small dark and light squares). Then, we used the time stamps of the white-noise stimulus-frames with the most effective pixel to generate PSTHs. Both ON- and OFF-dominated cortical cells responded to their most effective pixel with an increase in response (peak) followed by a decrease in response below baseline (suppression). The raw PSTHs were binned at 1 ms and smoothed using a moving average triangular filter 21 ms wide (Figure 1b-c).
The kinetics of ON and OFF responses were quantified at four different time points (Jin et al., 2011): latency, peak-time, zero-crossing, and suppression-time (Figure 1b). We defined the latency as the time to reach 40% of the maximum response, the peak-time as the time to reach the maximum response, the zero-crossing as the time to cross the baseline, and the suppression-time as the time to reach the minimum firing rate below baseline. The strength of the suppression was calculated using two indices, the amplitude ratio (AR) and the integral ratio (IR). The amplitude ratio was calculated as the ratio of suppression amplitude (SA) to peak amplitude (PA). The integral ratio was calculated as the ratio of suppression integral (SI) to peak integral (PI). To maximize the reliability of the temporal measurements, we selected the layer 4 recording sites with high signal-to-noise ratios (SNR > 8). Some analyses required calculating the SNR from both the dominant and flank subregions. The dominant subregion was defined as the subregion that generated the strongest response in the receptive field and the flank subregion as the strongest subregion with opposite sign to the dominant subregion. The SNR of each subregion was defined as the maximum pixel value at the peak frame of the subregion divided by the standard deviation of the pixel values at the time frame preceding the stimulus onset.
Measurements with LED monitor
CRTs are widely used in vision research to generate stimuli. However, CRT phosphors are known to have an asymmetric response profile with a rapid rise time and a slow decay time. Therefore, in a light-dark sequence, a response to dark could be triggered by the phosphor decay before the onset of the dark stimulus, particularly if the monitor refresh rate is low (Gawne and Woods, 2003) but see (Bair, 2004)). To rule out possible artifacts due to CRT pulses in the ON-OFF temporal differences that we describe, we repeated our measurements with stimuli presented on an LED monitor. The LED monitor was operated in tachistoscope mode using a fast shutter to control the backlight (ViewPixx /3D, VPixx Tech), which provided rapid turn-on and turn-off times and a steady luminance intensity profile. The measurements with the LED monitor were performed using the same stimuli (white noise and dark/light spots superimposed on white noise), the same stimulus update frequency (60 Hz for white noise) and the same viewing distance as for the CRT monitor (57 cm from the eye). The only differences between the two monitors were the mean luminance (LED: 112 cd/m2, CRT: 61 cd/m2) and the asymmetry between the rise and decay times of the light pulse (Supplemental Figure 2).
Responses to dark and light targets against noisy backgrounds
Tangential cortical recordings with a 32 channel multi-electrode array were used to study the responses to large dark and light targets (8.3° × 8.3°) against noisy backgrounds. The spatiotemporal receptive fields of cortical sites were mapped with sparse noise by spike-triggered averaging (STA) the stimulus. The STA, calculated around the peak response, was averaged across all cortical sites for each penetration to obtain the center of the population cortical receptive field. Dark and light targets were then presented roughly at the center of this population receptive field on a stationary background of binary white noise. Cortical receptive fields were larger when mapped with light sparse noise on dark background than dark sparse noise on light background. To measure the light/dark ratio in receptive field size, we fit each receptive field with a 2D Gaussian and calculated the ratio between the Gaussian standard deviation measured with lights and darks. For example, the dark/light ratio for receptive field size was 1 if the sizes mapped with dark and light stimuli were the same and 0.4 if the receptive field size was 0.4 times smaller when mapped with dark than light stimuli.
The responses to dark and light targets were measured on three different white-noise backgrounds with light/dark ratios of 2.3, 1.0 and 0.4. The light/dark ratio of white noise was defined as a ratio of number of light pixels divided by the number of dark pixels. For example, the light/dark ratio was 1 if the white noise had the same number of light and dark pixels and 0.4 if the number of light pixels was 0.4 times the number of dark pixels. The responses to dark and light targets were calculated as PSTHs binned at 1 ms and smoothed using a moving average triangular filter (width = 5 ms).
Psychophysical measurements
All stimuli were presented using MATLAB and Psychtoolbox 3 (Brainard, 1997) on a gamma corrected 21-inch CRT monitor. The monitor was placed at a distance of 1 m from the observer. The mean luminance and refresh rate of the monitor was held constant at 45 cd/m2 and 160 Hz, respectively. All experiments were carried out in a dark room. Observers used a numerical keypad to respond. Five observers (1 female and 4 males, including the author S.J.K.) with 20/20 or corrected vision participated in the experiments (one observer participated in both experiments). We used the likelihood ratio (LR) to test the null hypothesis that the true thresholds for darks and lights are identical. The LR test compares the log-likelihoods (LL) of two models, unrestricted and restricted, and tests whether their difference is statistically significant (equation 1). The probability density of this statistic under the null hypothesis is approximately a chi-square distribution (Hoel et al., 1971). Here, the unrestricted model represents the case in which data from the two conditions are fitted separately and the restricted model is the case where a single psychometric function is fitted to the joint data across the two conditions.
| (1) |
Psychophysical temporal threshold
We performed psychophysical measurements to investigate the perceptual correlate of the faster cortical response latency to darks than lights described in this paper. Subjects fixated on a red spot at the center of the screen while two targets (one dark and another light) appeared either above/below or left/right of the fixation spot. The dark and light targets were presented with a variable temporal delay (TD) with respect to each other (0–68.75 ms). The observers had to identify the location of the target that appeared first. Targets were presented on a stationary background of uniform binary white noise (each pixel subtended 0.05° of visual angle); each target covered 0.17° × 0.17° of visual angle and the inter-target separation was 2.3°. Before the experiments started, observers adapted to a binary white noise background for 120 s. Successive trials were initiated following the observer’s response. A sequence of 160 random binary white noise images was presented for 1 sec between trials to minimize the possibility of an afterimage bias. In the irradiation illusion, light spots on dark backgrounds are perceived as larger than dark spots of the same size in light backgrounds (Galilei, 1632). Moreover, white noise with the same number of dark and light pixels is perceived as having larger light than dark area (Komban et al., 2011). We previously showed that the ratio of light/dark pixels has to be reduced to roughly 40%/60% for subjects to report the same dark/light area in white noise (Komban et al., 2011). Therefore, in the experiments described here, we used two noise backgrounds: one with equal number of dark and light pixels and another with 40% light pixels and 60% dark pixels to correct for the irradiation illusion (Komban et al., 2011).
Psychophysical measurements of dark and light flickers
We performed psychophysical experiments to evaluate the perceptual correlates of the dark- and light-mediated suppression in neuronal responses. We used a two-interval forced choice paradigm. Both intervals had targets of 0.4° × 0.4° with the same contrast polarity (e.g., both dark) that were presented for 6.25 ms. One interval had only one target and the other had a pair of targets separated by a variable flicker interval (TD) of 6.25–50 ms. Observers were asked to indicate the interval in which they perceived a flicker. Targets were presented at the center of the screen on a stationary background of uniform white noise (each pixel subtended 0.05° of visual angle). At the start of the experiment, observers adapted to a binary white noise background for 120 s.
Supplementary Material
HIGHLIGHTS.
ON-OFF temporal differences are preserved and amplified in primary visual cortex.
ON-OFF differences in latency and suppression strength have perceptual consequences.
Neuronal and perceptual differences vanish on backgrounds biased towards darks.
Dark-mediated suppression in ON-dominated neurons is faster than light-mediated suppression in OFF-dominated neurons.
Acknowledgements
We are grateful to the observers who participated in the study and to NIH (J.M.A.: EY005253, EY020679; Q.Z.: EY013312, EY007556) and DFG Research Fellowship (J.K.) for funding support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests:
The authors declare no competing financial interests.
REFERENCES
- Ahmad KM, Klug K, Herr S, Sterling P, Schein S. Cell density ratios in a foveal patch in macaque retina. Vis Neurosci. 2003;20:189–209. doi: 10.1017/s0952523803202091. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J Neurosci. 2001;21:4002–4015. doi: 10.1523/JNEUROSCI.21-11-04002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W. No doubt about offset latency. Vis Neurosci. 2004;21:671–674. doi: 10.1017/S0952523804215012. [DOI] [PubMed] [Google Scholar]
- Balasubramanian V, Sterling P. Receptive fields and functional architecture in the retina. J Physiol. 2009;587:2753–2767. doi: 10.1113/jphysiol.2009.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fettiplace R. Kinetics of synaptic transfer from receptors to ganglion cells in turtle retina. J Physiol. 1977;271:425–448. doi: 10.1113/jphysiol.1977.sp012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E. Dynamics of primate P retinal ganglion cells: responses to chromatic and achromatic stimuli. J Physiol. 1999;519(Pt 3):775–790. doi: 10.1111/j.1469-7793.1999.0775n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [Google Scholar]
- Buchner A, Baumgartner N. Text - background polarity affects performance irrespective of ambient illumination and colour contrast. Ergonomics. 2007;50:1036–1063. doi: 10.1080/00140130701306413. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. Contrast processing by ON and OFF bipolar cells. Vis Neurosci. 2011;28:69–75. doi: 10.1017/S0952523810000313. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Fahey PK, Sikora M. Responses of ganglion cells to contrast steps in the light-adapted retina of the tiger salamander. Vis Neurosci. 1998;15:219–229. doi: 10.1017/s0952523898152021. [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002;22:2737–2747. doi: 10.1523/JNEUROSCI.22-07-02737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb C, Nam JH. Variance of high contrast textures is sensed using negative half-wave rectification. Vision Res. 2000;40:1677–1694. doi: 10.1016/s0042-6989(00)00007-9. [DOI] [PubMed] [Google Scholar]
- Copenhagen DR, Ashmore JF, Schnapf JK. Kinetics of synaptic transmission from photoreceptors to horizontal and bipolar cells in turtle retina. Vision Res. 1983;23:363–369. doi: 10.1016/0042-6989(83)90083-4. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Petersen MR. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proc Natl Acad Sci U S A. 1992;89:9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Sterling P. Cellular basis for the response to second-order motion cues in Y retinal ganglion cells. Neuron. 2001;32:711–721. doi: 10.1016/s0896-6273(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Fuchs AF. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1979;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. X- and Y-mediated current sources in areas 17 and 18 of cat visual cortex. Vis Neurosci. 1990;4:135–145. doi: 10.1017/s0952523800002297. [DOI] [PubMed] [Google Scholar]
- Galilei G. Dialogue concerning the Two Chief World Systems: Translated by Stillman Drake. 2nd rev ed. Berkeley: 1632. p. 1967. [Google Scholar]
- Gawne TJ, Woods JM. Video-rate and continuous visual stimuli do not produce equivalent response timings in visual cortical neurons. Vis Neurosci. 2003;20:495–500. doi: 10.1017/s0952523803205034. [DOI] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Rapid neural coding in the retina with relative spike latencies. Science. 2008;319:1108–1111. doi: 10.1126/science.1149639. [DOI] [PubMed] [Google Scholar]
- Hartline HK. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. American Journal of Physiology. 1938;121:400–415. [Google Scholar]
- Hesam Shariati N, Freeman AW. A multi-stage model for fundamental functional properties in primary visual cortex. PLoS One. 2012;7:e34466. doi: 10.1371/journal.pone.0034466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA. Synaptic physiology and receptive field structure in the early visual pathway of the cat. Cereb Cortex. 2003;13:63–69. doi: 10.1093/cercor/13.1.63. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Alonso JM, Reid RC, Martinez LM. Synaptic integration in striate cortical simple cells. J Neurosci. 1998;18:9517–9528. doi: 10.1523/JNEUROSCI.18-22-09517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoel PG, Port SC, Stone CJ. Introduction to Statistical Theory. 1971 [Google Scholar]
- Jin J, Wang Y, Lashgari R, Swadlow HA, Alonso JM. Faster thalamocortical processing for dark than light visual targets. J Neurosci. 2011;31:17471–17479. doi: 10.1523/JNEUROSCI.2456-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Weng C, Yeh CI, Gordon JA, Ruthazer ES, Stryker MP, Swadlow HA, Alonso JM. On and off domains of geniculate afferents in cat primary visual cortex. Nat Neurosci. 2008;11:88–94. doi: 10.1038/nn2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JP, Stepnoski A, Palmer LA. The two-dimensional spectral structure of simple receptive fields in cat striate cortex. J Neurophysiol. 1987;58:1212–1232. doi: 10.1152/jn.1987.58.6.1212. [DOI] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komban SJ, Alonso JM, Zaidi Q. Darks are processed faster than lights. J Neurosci. 2011;31:8654–8658. doi: 10.1523/JNEUROSCI.0504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S, Kratz KE, Mangel SC, Sherman SM. Spatial and temporal sensitivity of X- and Y-cells in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1980;43:520–541. doi: 10.1152/jn.1980.43.2.520. [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci. 2010;30:5533–5543. doi: 10.1523/JNEUROSCI.4733-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nichols Z, Nirenberg S, Victor J. Interacting linear and nonlinear characteristics produce population coding asymmetries between ON and OFF cells in the retina. J Neurosci. 2013;33:14958–14973. doi: 10.1523/JNEUROSCI.1004-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandarinath C, Victor JD, Nirenberg S. Symmetry breakdown in the ON and OFF pathways of the retina at night: functional implications. J Neurosci. 2010;30:10006–10014. doi: 10.1523/JNEUROSCI.5616-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff CP, Borghuis BG, Kao YH, Sterling P, Balasubramanian V. Retina is structured to process an excess of darkness in natural scenes. Proc Natl Acad Sci U S A. 2010;107:17368–17373. doi: 10.1073/pnas.1005846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Reid RC, Victor JD, Shapley RM. The use of m-sequences in the analysis of visual neurons: linear receptive field properties. Vis Neurosci. 1997;14:1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982;62:738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res. 2008;27:450–463. doi: 10.1016/j.preteyeres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG, Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. J Neurosci. 2002;22:7766–7773. doi: 10.1523/JNEUROSCI.22-17-07766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Cross-correlation analysis of geniculostriate neuronal relationships in cats. J Neurophysiol. 1983;49:1303–1318. doi: 10.1152/jn.1983.49.6.1303. [DOI] [PubMed] [Google Scholar]
- van Hateren JH, Ruttiger L, Sun H, Lee BB. Processing of natural temporal stimuli by macaque retinal ganglion cells. J Neurosci. 2002;22:9945–9960. doi: 10.1523/JNEUROSCI.22-22-09945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Shapley RM. Generation of black-dominant responses in V1 cortex. J Neurosci. 2010;30:13504–13512. doi: 10.1523/JNEUROSCI.2473-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CI, Xing D, Shapley RM. "Black" responses dominate macaque primary visual cortex v1. J Neurosci. 2009;29:11753–11760. doi: 10.1523/JNEUROSCI.1991-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemon V, Gordon J, Welch J. Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Vis Neurosci. 1988;1:145–150. doi: 10.1017/s0952523800001085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.