Abstract
The mechanisms implicated in prion infection and tissue distribution are not completely understood. In this study we investigated the levels of 263K prions in brain and spleen of Syrian hamsters few days after intra-peritoneal challenge. For this purpose we utilized the PMCA technology which permits to detect as little as few PrPSc molecules. Our results show that peripherally administered prions directly reach the brain, although at levels below the minimum necessary to produce disease. PrPSc remains in the brain several days after administration suggesting inefficient clearance or early replication. Understanding the fate of the infectious agent after administration and its uptake in different organs and fluids may provide useful information to develop strategies to minimize further spreading of prion diseases.
Keywords: prions, Protein Misfolding Cyclic Amplification (PMCA), 263K, Syrian hamster
Introduction
Transmissible Spongiform Encephalopathies (TSEs) are a group of proteopathies affecting several mammalian species [1]. Creutzfeldt-Jakob disease (CJD) is the most prevalent disease in humans affecting one individual per million people every year [1;2]. Despite their low incidence, TSEs have been extensively studied due to their intriguing mechanisms of propagation and their zoonotic transmission potential [3–6]. As of today, TSEs are the only group of diseases transmitted by a protein-based infectious agent [7–9].
TSEs are characterized by long incubation periods followed by a short clinical phase that invariably leads to death [4]. In order to experimentally decrease these long incubation periods, intra-cerebral inoculations are used to assess infectivity in animal models. However, peripheral routes of administration are more relevant when assessing natural mechanisms of transmission. It is well documented that PrPSc accumulates in several peripheral tissues, much before the appearance of the first clinical signs [10;11]. Spleen and lymph nodes are important contributors to peripheral prion replication as well as to the transport of the agent to the brain, either by the vascular, sympathetic or parasympathetic systems [12;13]. Replication and accumulation of prions in peripheral organs do not produce any damage to the tissue and the clinical disease is only manifested when they extensively accumulate in the brain.
The long incubation periods and the fact that peripheral tissues, including blood, can harbor infectious quantities of prions [14–16] is a major concern for public health [3]. Reports of disease transmission by transfusion using blood from individuals silently incubating variant Creutzfeldt-Jakob disease (vCJD) urge the development of methods that can detect the infectious agent during its long and silent incubation period [3;15;17]. Animal bioassays and cell-based prion replication systems can be used to detect the infectious material. However, these assays take a long time to be completed and are often limited to only a subset of prion strains/animal species, reducing their applicability. In vitro assays such as Protein Misfolding Cyclic Amplification (PMCA, [8;18]) and Quaking Induced Conversion (QuIC, [19]) look promising as effective techniques for the identification of misfolded prions in different organs and fluids [20–22], even at pre-symptomatic stages of the disease [11;23].
To understand the mechanism of prion propagation within the body, it is important to study how PrPSc spreads and replicates in different tissues and organs after peripheral challenge. The purpose of this study was to analyze the tissue distribution and brain uptake of infectious prions in experimental hamsters few days after peripheral infection. Since the amount of prions in this organ is presumably very low at this stage, we estimated PrPSc levels using the PMCA technology. We compared the levels of infectious prions in this organ with the ones in spleen, where PrPSc replication appears to occur at earlier stages [24].
Materials and Methods
Samples
4 weeks old female Syrian Golden Hamsters (Harlan®) were intra-peritoneally (i.p.) injected with 75 µL of 10% brain homogenates (prepared as previously explained [8]) from a symptomatic 263K infected hamster. Hamsters were sacrificed at 0 (30 min), 2, 4 and 9 days after injection (n=5/group). Spleens and brains were collected and stored at −80°C until use. All animal manipulations were carried out in accordance to NIH regulations and approved by the Animal Welfare Committee of the University of Texas Medical School at Houston.
Preparation of tissue homogenates
Brain and spleen homogenates were prepared at 10% in phosphate saline buffer (PBS, MP Biomedicals, cat. no. 1860454) with a protease inhibitor cocktail (Roche Diagnostics, cat. no. 11 697 498 001). After a brief centrifugation to eliminate debris (805g for 45s in a Beckman-Coulter Allegra 25R centrifuge), supernatants were used for Western blot (WB) analysis. To concentrate PrPSc and remove tissue components that may interfere with PMCA, 500 µL of sample were mixed with a sarkosyl (Fisher Bioreagents, cat. no. BP234) solution prepared in PBS (final concentration, 10%) and centrifuged at 100,000g for 1 h at 4°C [25]. Supernatants were discarded and PBS was added to the pellets (without resuspension) in order to dilute out traces of detergent. Samples were centrifuged again at 100,000g for 30 min at 4°C. Final pellets were resuspended in 100 µL of normal hamster brain homogenate prepared at 10% in Conversion Buffer (PMCA substrate, 150 mM NaCl and 1% Triton X-100 in PBS) [8;26] and submitted to PMCA.
Protein Misfolding Cyclic Amplification and Western blotting
Spleen and brain pellets resuspended in PMCA substrate were submitted to 96 PMCA cycles. Serial rounds were performed by mixing 10µL of the resulting sample with 90µL of fresh PMCA substrate. Tissue homogenates and PMCA products were treated with 50 µg/mL of Proteinase K (PK, Sigma-Aldrich, cat. no. P2308) for 1 h at 37°C. PK digestion was stopped by adding LDS loading buffer (Invitrogen, cat. no. NP0007) and heating for 5 min at 100°C. Samples were fractionated by SDS-PAGE and transferred into nitrocellulose membranes. Membranes were probed with 3F4 antibody (Covance, cat. no. SIG-39600) and signal observed by using ECL Plus detection system (GE Healthcare, cat. no. RPN2132) as recommended by the manufacturer.
Results
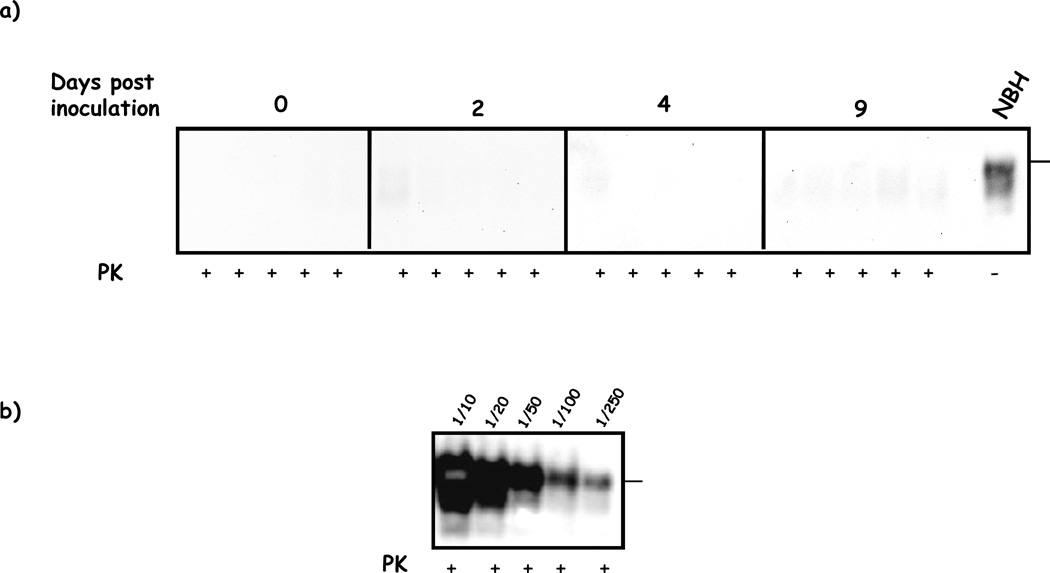
In order to analyze the distribution of PrPSc at early stages after infection we i.p. injected 263K prions in Syrian hamsters and brains and spleens were collected at several days (0, 2, 4 and 9) post-injection. Crude brain homogenates taken at these stages did not show any detectable amount of PrP27–30 after conventional PK digestion and Western blotting (Figure 1a). For comparison, figure 1b shows the PrP27–30 levels typically observed in the brain of a symptomatic 263K hamster inoculated with the same amount of prions by the same route.
Figure 1. Absence of detectable amounts of PrP27–30 in brain homogenates of 263K injected hamsters shortly after inoculation.
a) Brains from hamsters sacrificed at 0, 2, 4 and 9 days after i.p. infection with 263K prions were homogenized and analyzed by PK treatment and WB as explained in methods. All samples were negative for PrP27–30. b) Brain serial dilutions from a symptomatic 263K i.p. infected hamster analyzed in the same way as in a). NBH correspond to brain homogenates from healthy hamsters (no PK treated) used as a control of electrophoretic mobility. Horizontal lines at the right of each gel represent the position of a 36KDa (a) or 26KDa (b) molecular weight standard. Solid vertical lines depict separation of different gels.
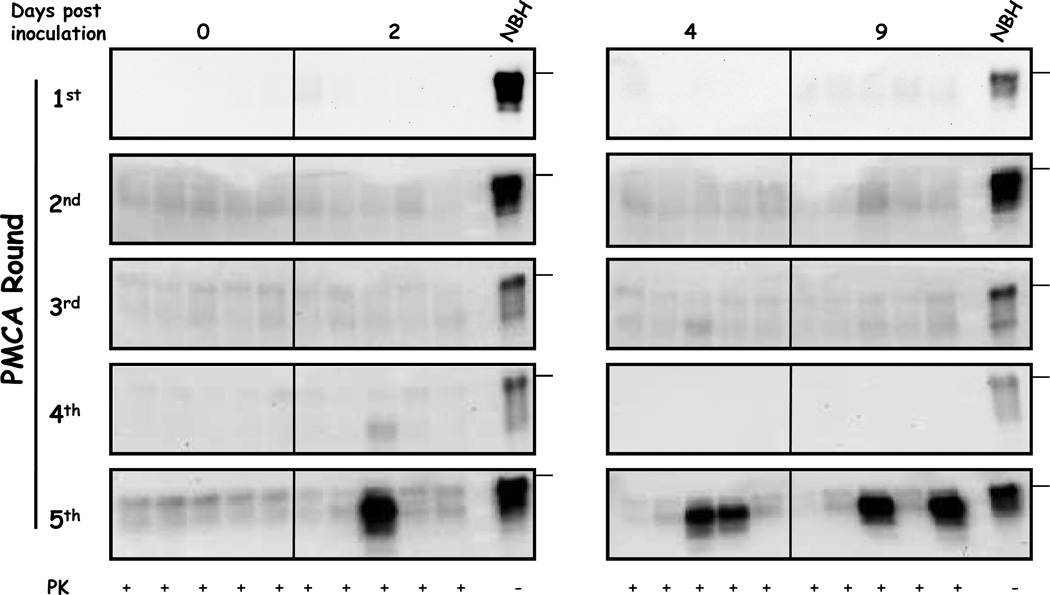
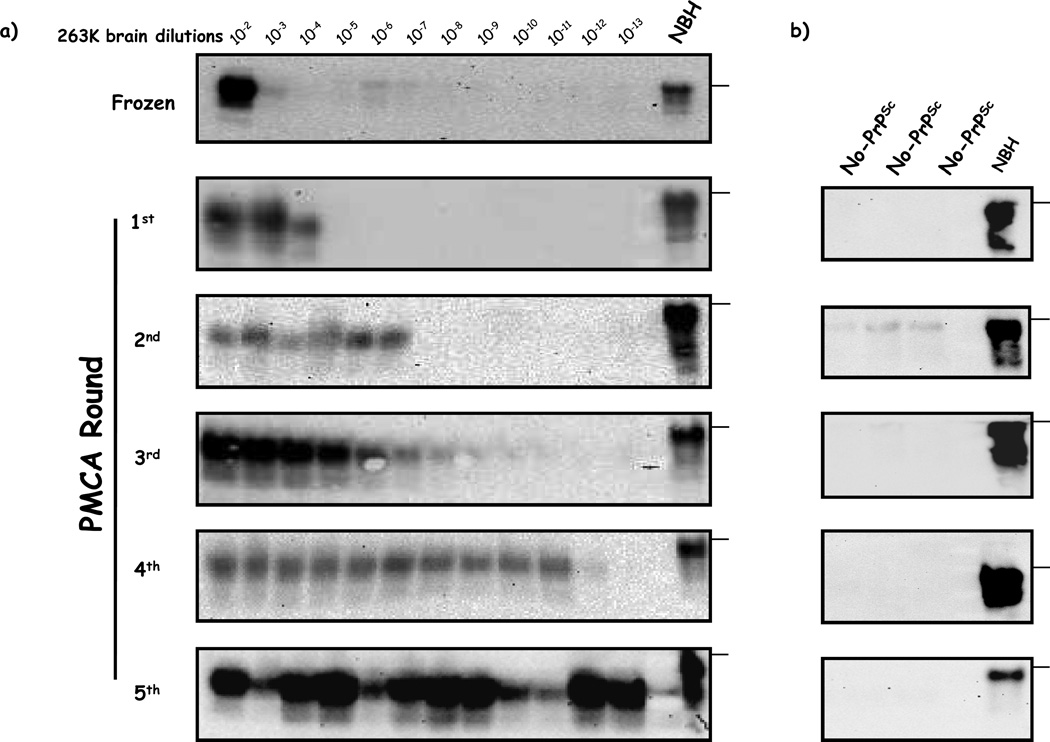
Previous reports showed that prions can rapidly distribute in several tissues, including the brain, after intra-venous (i.v.) injection [27;28]. In order to investigate the brain uptake of prions soon after exposure, we assessed the presence of PrPSc in this organ by PMCA [29]. This technique has been proven to detect as little as one particle of infectious prions [30]. Our results showed that we were able to detect PrPSc in brains after 5 rounds of PMCA in a fraction of animals sacrificed at 2, 4 and 9 days after treatment (figure 2). Only 1 out of 5 animals was positive 2 days after inoculation whereas no animals showed to be positive at day 0 (sacrificed 30 minutes after injection). The fraction of positive animals increased to 2/5 in 4 and 9 days post-challenge. This data suggest that after reaching the brain, PrPSc is not efficiently cleared. We analyzed the detection limit of our method by performing dilutions of 263K brain homogenates from a symptomatic hamster (figure 3a). According to this data we estimated that the PrPSc levels in the brain of PMCA-positive animals were equivalent to a brain dilution of 5×10−11 of a symptomatic hamster. It is important to highlight that control brain homogenates submitted to PMCA amplification in the absence of PrPSc seeds did not show any positive signal (figure 3b).
Figure 2. Detection of PrPSc by PMCA in brains of 263K infected hamsters sacrificed few days after inoculation.
Brain extracts were precipitated by centrifugation in the presence of sarkosyl, pellet resuspended in normal brain homogenates from healthy hamsters and submitted to serial PMCA. After PMCA, samples were PK treated and analyzed as described in methods. NBH correspond to brain homogenates from healthy hamsters (no PK treated) used as a control of electrophoretic mobility. Horizontal lines at the right of each gel represent the position of a 36KDa molecular weight marker. Solid vertical lines between different gels indicate blot splicing.
Figure 3. Serial PMCA of brain dilutions from a 263K symptomatic hamster.
a) 10-fold dilutions from a brain of a 263K symptomatic hamster were submitted to PMCA in order to assess the detection limit in each PMCA round. After the procedure, samples were PK digested and analyzed by WB. The WB shown here is representative of 4 independent assays. b) Serial PMCA of normal brain homogenates (without PrPSc seeds). NBH corresponds to brain homogenates from healthy hamsters (no PK treated) used as a control of electrophoretic mobility. Horizontal lines at the right of each gel represent the position of a 36KDa molecular weight marker.
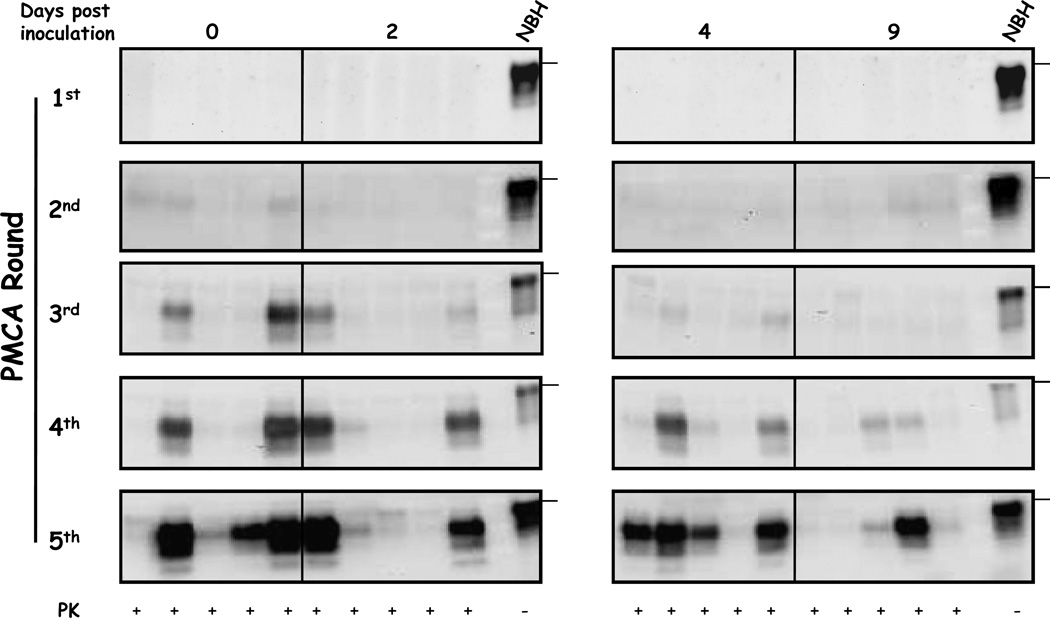
It is well documented that spleen is one of the most important peripheral organs in terms of prion replication. PrPSc generated in this tissue acts as a reservoir of infectious units that later on invade the central nervous system [31]. However, it is suggested that prions replicated in the periphery reach the brain only at later stages of their incubation periods. Using PMCA we detected the presence of PrPSc in spleen of some animals just after 3 PMCA rounds at the same day of injection (Figure 4). PrPSc detection was lower, but still positive, 2 days after infection (2/5 animals), but decreased at 4 days. At 9 days after challenge no PrPSc was detectable at the 3rd PMCA round. At the 5th PMCA round the tendency was even clearer: at 0 and 4 days post inoculation PrPSc was detected in the spleen of 4/5 animals, whereas a lower proportion of animals was detected at 9 days post inoculation (2/5). These results suggest that prions in spleen at these early time points correspond to the original inoculum injected, which is progressively cleared over time from this organ. Altogether, this data suggest that PrPSc detected in spleen and brain at these short time points correspond to the original inoculum administered.
Figure 4. Detection of PrPSc in spleens of 263K infected hamsters sacrificed at early time points after inoculation.
Spleen homogenates taken at the indicated time points were precipitated by centrifugation in the presence of sarkosyl, the pellet was resuspended in brain homogenate substrates and samples were submitted to serial PMCA assay. Samples were PK treated after amplification and analyzed by WB. NBH correspond to brain homogenates from healthy hamsters (no PK treated) used as a control of electrophoretic mobility. Horizontal lines at the right of each gel represent the position of a 36KDa molecular weight marker. Solid vertical lines between gels indicate splicing of different blots.
It is important to highlight that there appears to be a high animal-to-animal variability in PrPSc detection both in spleen and brain. This variability is not due to the PMCA reaction, since analysis of replicate samples in PMCA containing the same amount of diluted PrPSc showed little variability. This data indicate that distinct animals handle prions differently at the moment of infection.
Discussion
In this study we investigated the uptake of infectious prions in spleens and brains at early stages after peripheral challenge. The relatively rapid detection of prions in the brain suggests that PrPSc can directly reach this organ after inoculation. These results are in agreement with our previous observations using purified, radiolabeled PrPSc, which clearly demonstrated that prions can cross the blood-brain barrier to reach the brain parenchyma [27;28;32]. According to our amplification control shown in figure 3, the amount of prions we detected in brain few days after inoculation is equivalent to a 5×10−11 brain dilution of a symptomatic 263K infected hamster. Previous reports estimated that 1LD50 of 263K prions after i.c. infection is equivalent to a brain dilution of ~1×10−9 [8], although this estimation can vary between samples [33;34]. The higher infectious brain dilution for i.c inoculations has been determined in the same range for several other prion strains such as RML, ME7 and Hyper-TME as tested by in vivo and cell based assays [35–37]. The last infectious dilutions for peripheral routes are considerably lower than the ones described for direct i.c. inoculations [35]. Thus, our results indicate that the quantity of prions reaching the brain immediately after infection by peripheral routes is around 100 times lower than the ones necessary to produce disease.
Importantly, PrPSc was detectable in brain and spleen only in a fraction of the animals injected. This may explain the animal-to-animal variation in terms of incubation periods when infectivity bioassays are performed, especially when animals are inoculated with highly diluted material or are injected by peripheral routes (i.p., per os). This individual variation could result from dissimilar amounts of prions successfully reaching the brain, distinct rates of prion replication or different rates of clearance between animals, among others. Further research may clarify whether the inter-individual variability observed in our results has a long term repercussion in disease. Nevertheless, the amount of misfolded prions in animals having detectable levels of PrPSc was below infectious levels. This indicates that the direct uptake of prions to the brain may not be responsible for disease transmission, which would likely require peripheral replication of the agent followed by neuroinvasion, probably through peripheral nerves.
It is very well documented that several tissues other than brain can carry prion infectivity, albeit in lower titers. Among them, one of the most important and best characterized is the spleen [31]. According to our results, the amount of PrPSc detected in this organ at early time points after infection is approximately 100,000 times higher than in brains. It is important to note that 10% spleen homogenates from a 263K symptomatic hamster do not show any signal of PK resistant PrP by conventional WB (data not shown). In fact, it has been reported that spleens from 263K symptomatic hamsters have approximately 6log10 infectivity units less than brains [31]. In addition we observed that the presence of prion seeds in spleen progressively decreased in time (figure 4). This data suggest that the prions we are detecting in spleen at this early time points correspond to the original inoculum that was administrated. Nevertheless, previous studies by animal bioassays have shown that the amount of prions in spleen increases substantially at early points during the incubation period, reaching plateau levels much faster than those present later in the brain [31]. Our results are in agreement with a previous report showing that infectivity is found in the spleen of 263K infected hamsters few days after challenge [24].
It has been shown that PrPSc contains at least two population of molecules, differing in their resistance to protease degradation [38] and indeed it has been suggested that protease-sensitive PrPSc might be the predominant species for some prion strains [39;40]. PMCA is able to detect with similar efficiency both protease-resistant and protease-sensitive PrPSc forms [41]. Thus, we do not expect that determination of the amount of PrPSc present in a sample by PMCA will be underestimated. Nevertheless, coupling PMCA to detection methods that do not employ PK treatment (i.e. sedimentation, thermolysin treatment, conformational dependent immunoassay, etc) could provide an alternative evaluation of whether or not our results represent an underestimation of the total concentration of PrPSc present in the sample.
The findings presented in this study provide an initial description of the dynamic changes on PrPSc biodistribution soon after peripheral infection. Our results indicate that PMCA is a suitable technique to analyze the dynamics of prion replication during the whole time course of in vivo prion replication, from challenge to the symptomatic phase of the disease. Similar studies on various prion strains and animal species (including humans) may have important implications for understanding the mechanism of prion formation, metabolism, clearance and transport. This information would contribute to make informed decisions regarding risk assessment and may lead to the development of novel strategies for diagnosis and therapeutic intervention.
Highlights.
We identified sub-infectious presence of prions in brains and spleens after peripheral administration
We report the persistence of this material for up to 9 days after challenge in both, spleen and brain.
We describe a new methodology to detect infectious prions in tissues at early stages of in vivo replication.
Acknowledgments
We would like to thank Luis Concha-Marambio and María-José Liberona for critical review of this manuscript. This study was supported in part by the NIH grants NS078745 and NS049173 to CS and 948 projects (2013-S11; 2014-S9) to BC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflict of Interest
CS is inventor on several patents related to the PMCA technology and is currently Founder, Chief Scientific Officer and Vice-President of Amprion Inc., a biotech company focusing on the commercial exploitation of PMCA for prion diagnosis. BC and RM are listed as inventors on one patent application related to the PMCA technology.
References
- 1.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Harries-Jones R, Knight R, Will RG, Cousens S, Smith PG, Matthews WB. Creutzfeldt-Jakob disease in England and Wales, 1980-1984: a case-control study of potential risk factors. J. Neurol. Neurosurg. Psychiatry. 1988;51:1113–1119. doi: 10.1136/jnnp.51.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales R, González D, Soto C, Castilla J. Advances in prion detection. In: Wilson C, editor. Microbial food contamination. Boca Raton: CRC press; 2007. pp. 255–282. [Google Scholar]
- 4.Prusiner SB. Prions. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton DA. Pathogenesis and prevalence of variant Creutzfeldt-Jakob disease. J. Pathol. 2006;208:134–141. doi: 10.1002/path.1880. [DOI] [PubMed] [Google Scholar]
- 6.Barria MA, Telling GC, Gambetti P, Mastrianni JA, Soto C. Generation of a new form of human PrP(Sc) in vitro by interspecies transmission from cervid prions. J. Biol. Chem. 2011;286:7490–7495. doi: 10.1074/jbc.M110.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soto C, Castilla J. The controversial protein-only hypothesis of prion propagation. Nat. Med. 2004;10:S63–S67. doi: 10.1038/nm1069. [DOI] [PubMed] [Google Scholar]
- 8.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Wang X, Yuan C-G, Ma J. Generating a Prion with Bacterially Expressed Recombinant Prion Protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown P, Cervenakova L, Diringer H. Blood infectivity and the prospects for a diagnostic screening test in Creutzfeldt-Jakob disease. J. Lab Clin. Med. 2001;137:5–13. doi: 10.1067/mlc.2001.111951. [DOI] [PubMed] [Google Scholar]
- 11.Saa P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 12.Raeber AJ, Montrasio F, Hegyi I, Frigg R, Klein MA, Aguzzi A, Weissmann C. Studies on prion replication in spleen. Dev. Immunol. 2001;8:291–304. doi: 10.1155/2001/95404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nat. Rev. Microbiol. 2006;4:201–211. doi: 10.1038/nrmicro1346. [DOI] [PubMed] [Google Scholar]
- 14.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in sheep. Lancet. 2000;356:999–1000. doi: 10.1016/s0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 15.Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 16.Brown P, Rohwer RG, Dunstan BC, MacAuley C, Gajdusek DC, Drohan WN. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion. 1998;38:810–816. doi: 10.1046/j.1537-2995.1998.38998408999.x. [DOI] [PubMed] [Google Scholar]
- 17.Soto C. Diagnosing prion diseases: needs, challenges and hopes. Nat. Rev. Microbiol. 2004;2:809–819. doi: 10.1038/nrmicro1003. [DOI] [PubMed] [Google Scholar]
- 18.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 19.Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, Priola SA, Caughey B. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods. 2007;4:645–650. doi: 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008;582:3161–3166. doi: 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castilla J, Saa P, Soto C. Detection of prions in blood. Nat. Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 22.Shi S, Dong CF, Wang GR, Wang X, An R, Chen JM, Shan B, Zhang BY, Xu K, Shi Q, Tian C, Gao C, Han J, Dong XP. PrP(Sc) of scrapie 263K propagates efficiently in spleen and muscle tissues with protein misfolding cyclic amplification. Virus Res. 2009;141:26–33. doi: 10.1016/j.virusres.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Soto C, Anderes L, Suardi S, Cardone F, Castilla J, Frossard MJ, Peano S, Saá P, Limido L, Carbonatto M, Ironside J, Torres JM, Pocchiari M, Tagliavini F. Presymptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 2005;579:638–642. doi: 10.1016/j.febslet.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Kimberlin RH & Walker CA. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J. Gen. Virol. 1986;67(Pt 2):255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- 25.Morales R, Buytaert-Hoefen KA, Gonzalez-Romero D, Castilla J, Hansen ET, Hlavinka D, Goodrich RP, Soto C. Reduction of prion infectivity in packed red blood cells. Biochem. Biophys. Res. Commun. 2008;377:373–378. doi: 10.1016/j.bbrc.2008.09.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castilla J, Saa P, Morales R, Abid K, Maundrell K, Soto C. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 2006;412:3–21. doi: 10.1016/S0076-6879(06)12001-7. [DOI] [PubMed] [Google Scholar]
- 27.Banks WA, Niehoff ML, Adessi C, Soto C. Passage of murine scrapie prion protein across the mouse vascular blood-brain barrier. Biochem. Biophys. Res. Commun. 2004;318:125–130. doi: 10.1016/j.bbrc.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Urayama A, Morales R, Niehoff ML, Banks WA, Soto C. Initial fate of prions upon peripheral infection: half-life, distribution, clearance, and tissue uptake. FASEB J. 2011;25:2792–2803. doi: 10.1096/fj.11-180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B, Morales R, Barria MA, Soto C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat. Methods. 2010;7:519–520. doi: 10.1038/nmeth.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saa P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 31.Rubenstein R, Merz PA, Kascsak RJ, Scalici CL, Papini MC, Carp RI, Kimberlin RH. Scrapie-infected spleens: analysis of infectivity, scrapie-associated fibrils, and protease-resistant proteins. J. Infect. Dis. 1991;164:29–35. doi: 10.1093/infdis/164.1.29. [DOI] [PubMed] [Google Scholar]
- 32.Banks WA, Robinson SM, az-Espinoza R, Urayama A, Soto C. Transport of prion protein across the blood-brain barrier. Exp. Neurol. 2009;218:162–167. doi: 10.1016/j.expneurol.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meade-White KD, Barbian KD, Race B, Favara C, Gardner D, Taubner L, Porcella S, Race R. Characteristics of 263K scrapie agent in multiple hamster species. Emerg. Infect. Dis. 2009;15:207–215. doi: 10.3201/eid1502.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimberlin RH & Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J. Gen. Virol. 1977;34:295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- 35.Kincaid AE & Bartz JC. The nasal cavity is a route for prion infection in hamsters. J. Virol. 2007;81:4482–4491. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thackray AM, Klein MA, Aguzzi A, Bujdoso R. Chronic subclinical prion disease induced by low-dose inoculum. J. Virol. 2002;76:2510–2517. doi: 10.1128/jvi.76.5.2510-2517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klohn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc. Natl. Acad. SciUSA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 39.Cronier S, Gros N, Tattum MH, Jackson GS, Clarke AR, Collinge J, Wadsworth JD. Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem. J. 2008;416:297–305. doi: 10.1042/BJ20081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim C, Haldiman T, Surewicz K, Cohen Y, Chen W, Blevins J, Sy MS, Cohen M, Kong Q, Telling GC, Surewicz WK, Safar JG. Small protease sensitive oligomers of PrPSc in distinct human prions determine conversion rate of PrP(C) PLoS. Pathog. 2012;8:e1002835. doi: 10.1371/journal.ppat.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, Soto C, Requena JR. Isolation and Characterization of a Proteinase K-Sensitive PrP(Sc) Fraction. Biochemistry. 2006;45:15710–15717. doi: 10.1021/bi0615442. [DOI] [PubMed] [Google Scholar]