Abstract
The therapeutic potential of mixed micelles, made of PEG-PE and vitamin E co-loaded with curcumin and paclitaxel, was investigated against SK-OV-3 human ovarian adenocarcinoma along with its multi-drug resistant version SK-OV-3-paclitaxel-resistant (TR) cells in vitro and in vivo. The addition of curcumin at various concentrations did not significantly enhance the cytotoxicity of paclitaxel against SK-OV-3 in vitro. However, a clear synergistic effect was observed with the combination treatment against SK-OV-3TR in vitro. In vivo, this combination treatment produced a three-fold tumor inhibition with each of these cell lines. Our results indicate that such co-loaded mixed micelles could have significant clinical advantages for the treatment of resistant ovarian cancer.
Keywords: Curcumin, nuclear factor-kappa B (NF-κB), mixed micelles, multi-drug resistance, combination therapy, ovarian cancer
1. Introduction
Curcumin (CUR), a polyphenol known as diferuloylmethane extracted from the perennial herb Curcuma longa, has been extensively studied for its therapeutic efficacy for many disorders including several inflammatory diseases, Alzheimer’s, and cancer. The extensive research on CUR has revealed several of its important functions. It interacts with various proteins, inhibits the activity of various kinases, and controls the activation of transcription factors that are involved in cell proliferation and survival (Goel et al., 2008). Several groups have performed studies in which CUR was used as a chemotherapeutic agent, see (Anand et al., 2010; Anand et al., 2008; Nair et al., 2012) as examples. Notably, recent studies performed by Bava et al. demonstrated the use of CUR as a mediator of chemoresistance by sensitizing cancer cells to a conventional chemotherapeutic agent. Cervical cancer cells were sensitized by CUR to paclitaxel (PCL)-induced apoptosis through down-regulation of the nuclear factor (NF)-κB and Akt pathways, suggesting its use as a strong chemosensitizer to improve the therapeutic potential of PCL (Bava et al., 2011; Sreekanth et al., 2011). Despite the effectiveness of CUR, its use in the clinic has been limited due to its hydrophobicity (solubility in water is ~0.6 µg/mL) and low in vivo bioavailability (Sreekanth et al., 2011). However, because of the multiple therapeutic activities attributed to CUR, there has been a persistent search for a solution to these problems. Nano-sized drug delivery systems are being sought as a way to overcome these limitations, see for examples (Anand et al., 2010; Cui et al., 2009; Ganta and Amiji, 2009; Kunwar et al., 2006; Mohanty and Sahoo, 2010; Nair et al., 2012).
PCL, isolated from the Pacific yew tree Taxus Brevifolia, is one of the most prescribed conventional chemotherapeutic agents against a wide variety of cancers. It has been used in the clinic for more than two decades. Like CUR, PCL is also a poorly water soluble drug ~0.3 µg/ml, and in order to solubilize it for intravenous administration, cremophor EL produced from castor oil is utilized. Administering Cremophor EL often causes severe hypersensitivity reactions, abnormal lipoprotein patterns, hyperlipidaemia, and peripheral neuropathy (Gelderblom et al., 2001). Nano-sized carriers, such as polymeric micelles, have been widely explored for the delivery of poorly soluble drugs such as CUR and PCL (Lukyanov et al., 2003; Torchilin, 2004). Micelles prepared by us from conjugates of polyethylene glycol (PEG) and phosphatidylethanolamine (PE) together with vitamin E have a high solubilization efficiency towards various poorly soluble drugs, low immunogenicity, high stability, i.e. low critical micelle concentration (CMC) value (1.66×10−5 M), and small size (7–35 nm) that can utilize the enhanced permeability and retention (EPR) effect to preferentially accumulate at the tumor site (Sawant et al., 2008). The existence of the two fatty acid acyls in PEG-PE contributes considerably to the increase of the van der Waals interactions in the micelle’s core while the addition of vitamin E further increases the strength and size of the hydrophobic core thereby increasing drug solubilization and micelle stability (Sawant et al., 2008).
The development of multi-drug resistance (MDR) is one of the major factors leading to the failure of many conventional chemotherapies. There are three major mechanisms through which cancer cells acquire drug resistance: (1) decreased uptake of water-soluble drug; (2) intracellular changes that affect the activity of cytotoxic drugs in killing cells such as shifts in cell cycle and increased repair of damaged DNA; and (3) increased energy-dependent efflux of hydrophobic drugs from the cell (Szakacs etal., 2006). The third mechanism is the most commonly reported mechanism in MDR. The increased efflux of hydrophobic drugs from the cell by ATP-binding cassette (ABC) transporter trans-membrane proteins such as P-glycoprotein (P-gp, also known as MDR1) is an example of such transporters. PCL efflux from cancer cells generated by P-gp activity is the main factor limiting PCL’s clinical efficiency (Shapira et al., 2011; Sreekanth et al., 2011). Combination therapy has proven to be an effective way in dealing with resistant cancers. Several clinical trials confirmed the superiority of combination therapy versus monotherapy when the drugs chosen have non-overlapping resistance mechanisms and are effective as single agents (D. N. Waterhouse, 2006; Webb et al., 2007). MDR can also be reversed by priming the cells for a specific chemotherapeutic agent. For example, by downregulating the ABC transporters, reversal of MDR could be achieved and the selected drug can exert its cytotoxic effect on the once resistant cells (Patel et al., 2011).
We hypothesized that the combination therapy of CUR and PCL can be effective in treating MDR tumors since CUR enhances PCL-induced cytotoxicity via downregulation of nuclear factor (NF)-κB and the Akt pathways (Ganta and Amiji, 2009). CUR has al so shown great promise in vivo with minimal side-effects even at high doses (Goel et al., 2008). The goal of the present study was to co-load CUR and PCL into the mixed micelles made of PEG-PE/vitamin E and evaluate the anticancer efficacy of these micellar formulations in vitro as well as in vivo against SK-OV-3 and SK-OV-3TR human ovarian adenocarcinoma cells. Simultaneous delivery of both compounds in one micellar drug delivery system, while minimizing the number of drug administration events, may have the potential to be an effective treatment for resistant cancers.
2. Materials and methods
2.1 Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG2000–PE) was purchased from Corden Pharma International (Plankstadt, Germany). Curcumin (CUR) was purchased from Sigma (St. Louis, MO, USA catalog #C7727). Paclitaxel was purchased from LC laboratories (Woburn, MA catalog #P-9600). Vitamin E (>96% catalogue #T3251) was purchased from Sigma (St. Louis, MO, USA). Matrigel™ basement membrane matrix was purchased from BD Biosciences (Bedford, MA). CellTiter-blue® was purchased from Promega (Madison, WI). All buffer solution components were analytical grade preparations and deionized reverse osmosis treated water was used in all the experiments. All the organic solvents were HPLC grade.
2.2 Methods
2.2.1 Cell cultures
Cell culture media and supplements were purchased from Cell-Gro (Kansas City, MO, USA). The SK-OV-3 human ovarian adenocarcinoma cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). SK-OV-3TR, the taxol resistant variant of SK-OV-3, was a kind gift from Dr. Duan Zhenfeng (MGH, Boston, MA). SK-OV-3 cells were cultured in McCoy’s media while SKOV-3-TR cells were cultured in RPMI 1640 media (both supplemented with 10% fetal bovine serum and 1% penicillin, streptomycin, and amphotericin). Cells were maintained at 37°C in a humidified incubator with 5% CO2, and were passaged according to ATCC protocols.
2.2.2 Preparation of drug-loaded micelles
PCL and/or CUR drug-loaded mixed micelles were prepared by the thin film hydration method. Various weight % of PCL (1 mg/ml in 0.1% acetic acid methanol solution) and/or CUR (2 mg/ml in 0.1% acetic acid methanol solution) were added a to PEG2000-DSPE and vitamin E (89:11 molar ratio) solution in chloroform. A 5 mM concentration of micelle forming material was used in all experiments. The organic solvents were removed by the rotary evaporation to form a thin film of drug/micelle-forming material mixture. This film was further dried under high vacuum (2 × 10−3 mBar) for at least 12 hours to remove any remaining organic solvents (Freezone 4.5 Freeze Dry System Labconco, Kansas City, MO). Drug-loaded mixed micelles were formed by resuspending the film in phosphate buffered saline (PBS) pH 7.4. The mixture was incubated in water bath at 40°C for 10 min and then vortexed for at least 5 minutes to insure proper resuspension of the film. Excess non-incorporated drugs were separated by centrifugation (13,500 g) for 5 minutes followed by filtration through a 0.2 µm syringe filter (Nalgene, Rochester, NY) to remove any non-incorporated drug that is still present in the solution and to sterilize the solution before in vitro or in vivo use.
2.2.3 Characterization of micelles
2.2.3.1 Micelle size
The micelle size (hydrodynamic diameter) was measured by the dynamic light scattering (DLS) using a N4 Plus Submicron Particle System (Coulter Corporation, Miami, FL, USA). The micelles were diluted with deionized water until the concentration providing light scattering intensity was between 5×104 and 1×106 counts/second. Three samples from the same formulation were measured once to determine the particle size distribution. For the data in table 1, 6 different formulations were measured 3 times each.
Table 1.
Particle size in the different micellar formulations (mean diameter ± SD, n = 6)
| Formulation (89:11 Molar ratio) | Size (nm) |
|---|---|
| Empty PEG2000-PE / Vitamin E micelles | 15.6 ±1.9 |
| CUR-Loaded PEG2000-PE / Vitamin E micelles | 17.3 ± 3.0 |
| PCL-Loaded PEG2000-PE / Vitamin E micelles | 17.6 ± 1.7 |
| CUR+PCL co-loaded PEG2000-PE / Vitamin E micelles | 19.3 ± 1.9 |
2.2.3.2 Critical micelle concentration (CMC) determination
The CMC value of the mixed micelles was estimated by the standard pyrene method. Briefly, tubes containing 1 mg crystals of pyrene were prepared; a 10−4 to 10−6 M concentration of micelle forming material was added to these crystals. The mixtures were then incubated for 24 hrs with shaking at room temperature (~21 °C). Free pyrene was removed by filtration through a 0.2 µm polycarbonate syringe filter. The fluorescence of filtered samples was measured at the excitation wavelength of 339 nm and emission wavelength of 390 nm using an F-2000 fluorescence spectrometer (Hitachi, Japan). Once a sharp increase in fluorescence was observed, the concentration of the micelle components at this point correspond to the CMC value of the micellar formulation.
2.2.3.3 Drug solubilization efficiency
Drug incorporation efficiency was measured by the RP-HPLC using an Xbridge C18 (4.6 mm × 250 mm) column (Waters Corporation, Milford, MA) on a Hitachi Elite LaChrome HPLC equipped with an autosampler (Pleasanton, CA) and diode array detector. The mobile phase used was 60:40 acetonitrile:water with a flow rate of 1 ml/min. PCL was detected at a wavelength of 227 nm, while CUR was detected at 420 nm. Sample injection volume was kept constant at 50 µl and the sample run time was 8 min. The concentration of the drug was determined by measuring the area under curve of the corresponding peaks. Standard curves of stock drug solution, dissolved in the mobile phase, were used to determine the concentration of the incorporated drug in micelles. Drug-loaded micelles were diluted in the mobile phase to disrupt the micelles and release the incorporated drug for detection. All samples were analyzed in triplicate.
2.2.3.4 Micelle stability
The micelles were stored at 4°C for up to two months. The samples were monitored periodically (once a week) for any changes in appearance, particle size, and drug content. When the hydrophobic drugs are completely solubilized in the core of the micelle, the micellar solution is clear. However, when the drug is present outside of the micelle core, turbidity is observed and precipitation occurs. Therefore, any change in the appearance of the micellar solution indicates instability. In addition, the presence of free drug in the micellar solution alters the size measurements due to the presence of drug crystals so any change in the size distribution also indicates instability. Prior to the weekly determination of the drug content, the micellar solution were centrifugated (13,500 g) for 5 minutes followed by filtration through a 0.2 µm syringe filter before characterization (Nalgene, Rochester, NY) to remove any non-incorporated drug that is present in the solution followed by HPLC analysis as previously described.
2.2.3.5 Drug release from the micellar formulations
Three 1 ml aliquots of each formulation in a dialysis bag (molecular weight cutoff 3500 Da, Spectrum Labs) were placed in 1 liter of PBS (10 mM, pH 7.4) containing 0.2% Tween 80 to maintain sink conditions (Gill et al., 2012). Samples of 20 µL volume were withdrawn from the external medium after 0.5, 1, 2, 4, 8, 12, 24, and 48 hrs. The amount of the drug in each sample was then determined by the RP-HPLC analysis as previously described.
2.2.4 Cell viability assays
Viability of cells was measured using the CellTiter Blue® (Promega, Madison, WI) viability assay according to the manufacture’s protocol. Briefly, cells were seeded in 96-well plates at a density of 3,000 cells/well and grown for 24 hrs. Then cells were continuously incubated with the various formulations for 48 hrs in serum complete media. After 48 hrs of treatment, media was removed and the cells were washed with 200 µl serum complete media and then incubated with 100 µl of the media containing 20 µl of the Cell Titer-Blue® reagent. Cell viability was evaluated after 2 hrs of incubation by measuring the fluorescence (excitation 530 nm, emission 590 nm) using a Synergy HT multi-detection microplate reader (Biotek, Winooski, VT). PBS-treated cells were taken as controls to calculate % cell viability, and the treatment was carried out in triplicate and at least 3 different assays.
2.2.5 In vivo tumor inhibition study
The efficacy of CUR and PCL combination micelles was investigated on well-established tumors using SK-OV-3 and SK-OV-3TR cells. Female nude mice, about 6–8 weeks old, were used. Their weights were monitored throughout the study as weight loss is an indicator for toxicity. Cells were cultured to 70% confluency, harvested, and 3 million cells were resuspended in 200 µL of 1:1 matrigel:PBS solution and injected subcutaneously over the right flank of the animal. Tumor volume measured every two days and was estimated from the measurements in two perpendicular dimensions taken with vernier calipers by applying the formula (L × W2)/2, where L is the longest dimension and W is the dimension perpendicular to L. Once the tumors reached ~200 mm3, mice were randomly split into 4 different groups and micelles were administered intraperitoneally due to the high injection volume (~500µL) at a dose of 25 mg/kg CUR and 10 mg/kg PCL every 3 days and commenced once tumor volume reached to ~200 mm3 at day 10 post tumor inoculation. The dose ratio of CUR:PCL was 2.5:1 w/w as determined by in vitro assays. All animals were sacrificed when the tumor volume in the control group reached 1000 mm3.
2.2.6 Data analysis
Data were generated in multiples of triplicates for proper statistical analysis. In vitro experiments are reported as mean ± SD while in vivo experiments are reported as mean ± SEM. Comparisons between two groups were made using Student’s t-test and with more than two groups, one way ANOVA was used to compare results. Statistical significance was determined by a p-value < 0.05.
3. Results
3.1 Preparation and characterization of micelles
Our overall goals were to prepare and characterize of CUR-, PCL-, and CUR+PCL-loaded micelles and evaluate their in vitro cytotoxicity against SK-OV-3 and SK-OV-3TR cells to assess changes in anti-tumor activity and the possible reversal of MDR using these micelles loaded with the drug combination in vivo. PCL and/or CUR drug-loaded micelles were prepared by the thin film hydration method. At a 5 mM concentration of PEG2000-PE/vitamin E micelle-forming material (89:11 molar ratio), we successfully incorporated PCL at a concentration of ~600 µg/ml (~4.7% w/w). CUR was also effectively encapsulated at a concentration of ~1.2 mg/ml (8.7% w/w). The same concentrations of both drugs were achieved when co-loaded into the same micellar formulation. In addition, higher encapsulation can be achieved for both drugs individually. However, the stability of the micelles at higher concentrations is relatively compromised because turbidity is observed after a few hours of storage. The micelles sizes ranged from 15 – 20 nm (Table 1) with a zeta potential of −27.7 ± 1.7 mV.
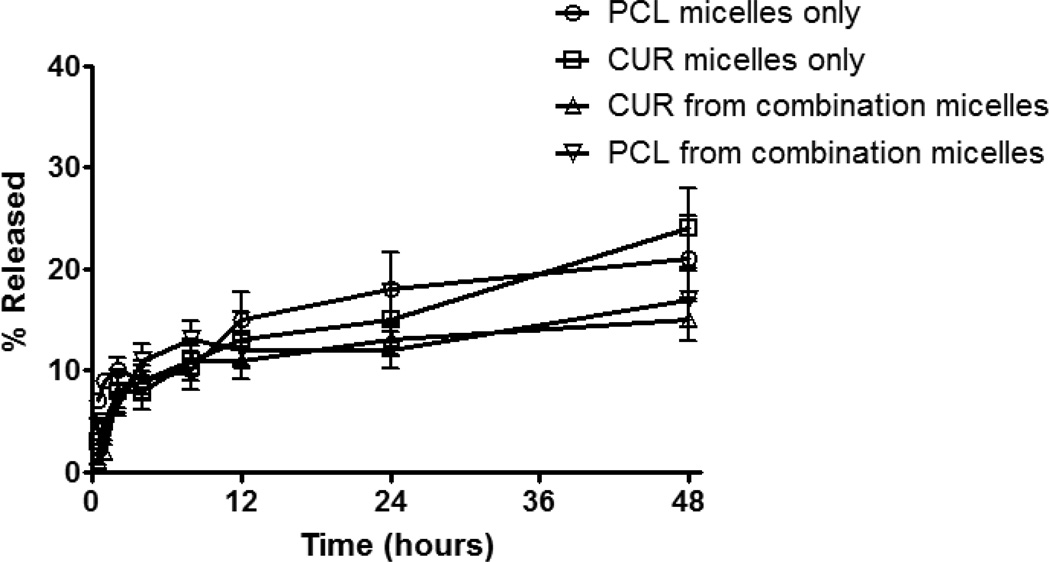
An HPLC method was developed to determine the drug concentrations in the micellar formulations simultaneously with a clear separation between the PCL and CUR peaks. Standard curves were prepared with an R2 value of 0.99 with linearity over the concentration range of 0.1–100 µg/ml and sensitivity of detection was 0.05 µg/ml. Using these standard curves, we calculated the loading efficiency, which was ~90% with the optimized amounts of drug. The drug loads were retained inside the micelles for at least 2 months at 4°C. This high encapsulation efficiency and stability is attributed to the hydrophobicity of the drugs and the effectiveness of these micelles in solubilizing such lipophilic drugs. Furthermore, size and zeta potential measurements monitored over a period a two months showed no difference in size or charge. The CMC value for PEG2000-PE/vitamin E micelles was 1.66×10−5 M. While following the in vitro release profiles, we observed a slow release pattern of the entrapped drug from the micelles. A rapid release of about 10% occurred in the first 4 hours and only 10% more released by 48 hours (Figure 1). This slow release confirms the stability of the drug-loaded micelles. In addition, the release profile from the co-loaded micelles versus single drug-containing micelles was not different.
Figure 1.
Release profiles of the drug-loaded micelles. Drug-loaded micelles (1 ml) placed in 1 L of PBS pH 7.4 supplied with 0.2% tween-80 to maintain sink conditions at 37°C using a 3500 Da MWCO membrane.
3.2 Cell viability assays
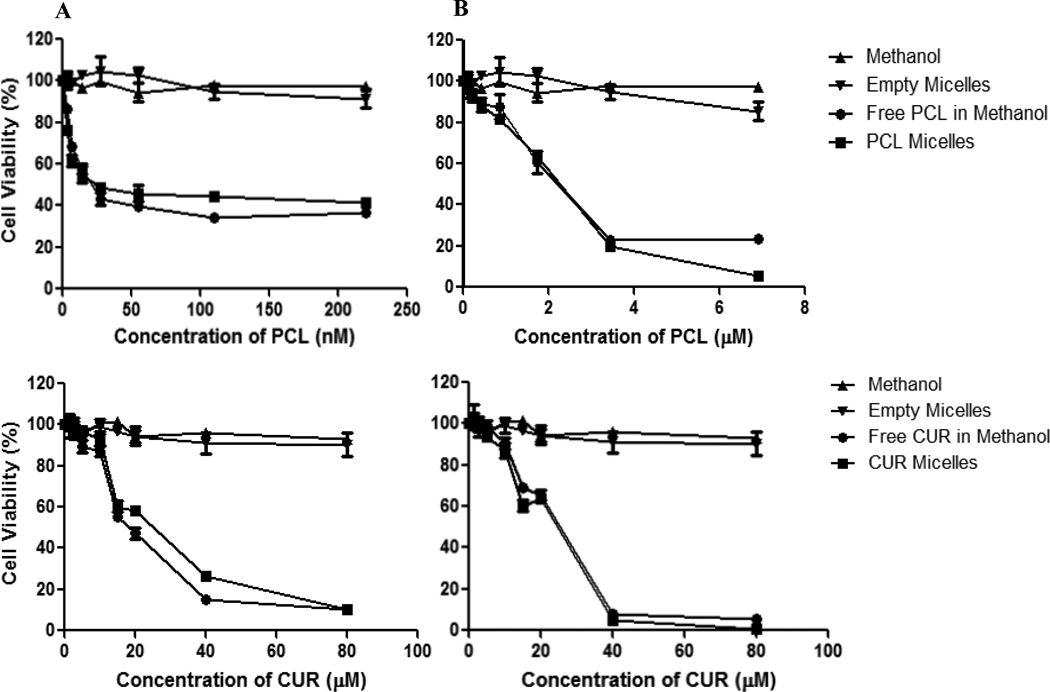
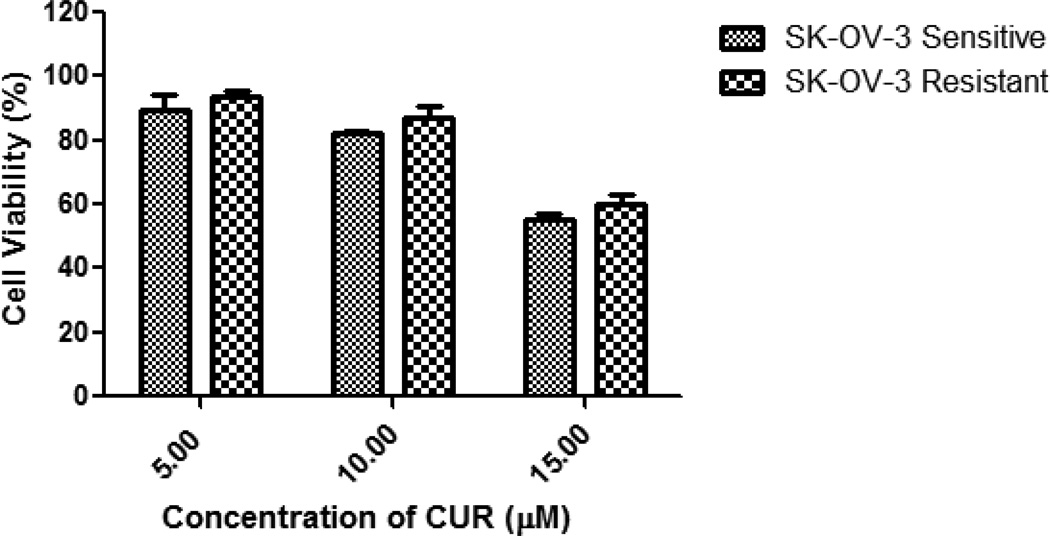
The in vitro cytotoxicity of the different micellar formulations was investigated against SK-OV-3 and SK-OV-3TR cell lines. Empty PEG–PE/vitamin E micelles had minimal cytotoxic effects on the cells at the corresponding concentrations used. The results of the dose-response studies with PCL and CUR as a single agent are shown in Figure 2. The toxicity of the PCL- or CUR-loaded micelles was equivalent to the toxicity observed with the free drugs (drugs dissolved in methanol). In other words, the micelles delivered their cargo into the cell and the drugs retained their activity post encapsulation. The PCL IC50 on SK-OV-3 and SK-OV-3TR cells was determined to be ~10 nM and 2.1 µM respectively. The resistant cells required ~210 fold higher dose of PCL to achieve the same level of cell death as the sensitive variant. The CUR IC50 was determined to be ~21.7 and 23.6 µM respectively. At doses below 20 µM of CUR, no difference in toxicity was observed between SK-OV-3 and SK-OV-3TR cells with minimal toxicity observed below 10 µM in both cell lines (Fig. 3). Thus, the toxicity of CUR did not depend on the cellular resistance towards PCL.
Figure 2.
Cell viability of SK-OV-3 (panel A) and SK-OV-3TR (panel B) cells after 48 hrs of continuous incubation with free PCL/CUR or micellar PCL/CUR at various concentrations. Cell viability was determined using CellTiter Blue cell viability assay. Data shown are representative of 3 independent experiments performed in triplicate.
Figure 3.
Cell viability of SK-OV-3 and SK-OV-3TR after 48 hrs of continuous incubation with micellar CUR at various concentrations. Cell viability was determined using CellTiter Blue cell viability assay. Data shown are representative of 3 independent experiments performed in triplicate.
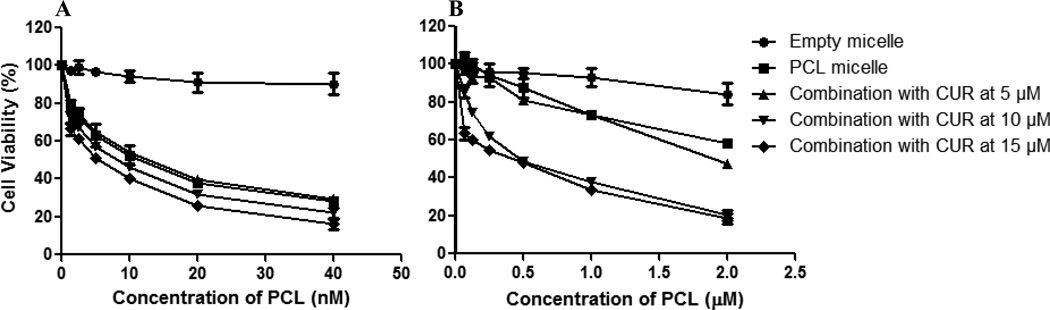
The addition of CUR at different concentrations did not significantly enhance the cytotoxic effect of PCL on the sensitive cell line (Figure 4). To test for synergism, PCL and CUR combination index (CI) was determined with the classic isobologram equation of Chou and Talalay (Chou TC, 1984). CI = a/A + b/B, where “a” is the PCL IC50 in combination with CUR at concentration “b”; A is the PCL IC50 and B is the CUR IC50. When the CI is less than 1, a synergistic effect is observed; CI=1 corresponds to an additive effect, and when the CI is greater than 1 an antagonistic effect is observed. With respect to the sensitive cell line, at a CUR concentration of 5 µM, the CI was 1.26 at variable concentrations of PCL suggesting that the combination treatment at this concentration was antagonistic. However, an additive effect was observed at 10 µM of CUR at variable concentrations of PCL since the CI was 1.
Figure 4.
Cell viability of SK-OV-3 (A) and SK-OV-3TR (B) cells after 48 hrs of continuous incubation with combination micelles at various concentrations of PCL and CUR. Cell viability was determined using CellTiter Blue cell viability assay. Data shown are representative of 3 independent experiments performed in triplicate.
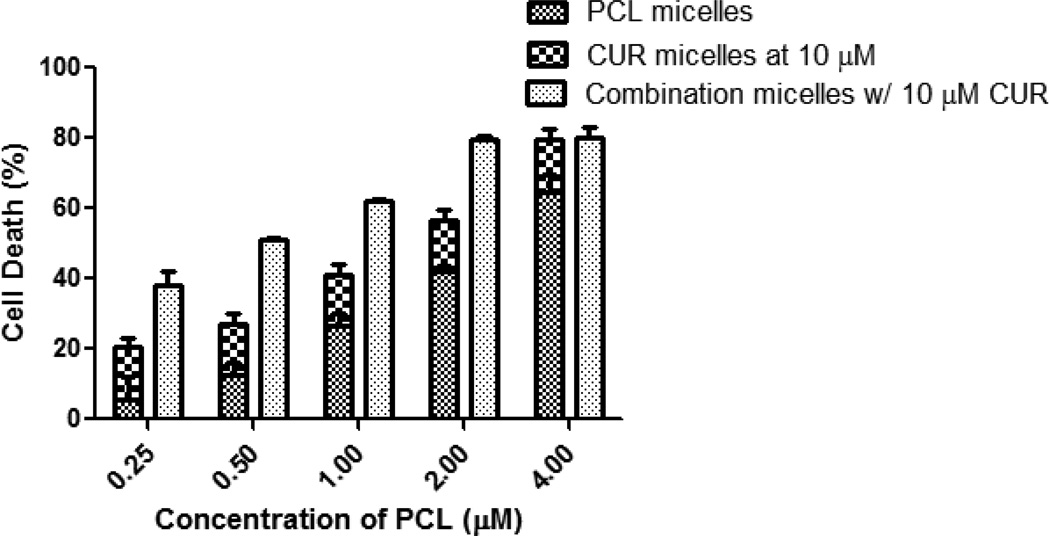
On the other hand, the micellar co-delivery of CUR and PCL to the resistant cell line resulted in three distinct outcomes: (1) an antagonistic effect was observed at the concentration of 5 µM CUR with CI = 1.1; (2) at concentrations of 10 µM CUR, a synergistic effect was observed with CI = 0.78; (3) additive toxicity was noted with a CUR concentration of 15 µM CI = 1.0 (Figure 4). Accordingly, a dramatic improvement in the efficacy of PCL was observed at the 10 µM concentration. The IC50 significantly decreased to 0.68 µM from 2.1 µM, a 3-fold decrease in the amount drug required to achieve a similar cytotoxic outcome.
The MDR reversal capability of CUR was best demonstrated at concentration of 10 µM. While varying the concentration of PCL in the combination micelles and keeping CUR constant, the combination treatment resulted in significantly higher toxicity compared to the additive toxicity of individual drugs except at high concentrations of PCL (Figure 5). SK-OV-3TR cells overexpress the P-gp transporter (MDR-1), which is a known efflux protein responsible for pumping PCL out of the cell. Here, CUR effectively inhibited the function of the ABC drug transporters and was able to sensitize the cells to PCL even at a dose where CUR has minimal cytotoxicity on its own as a single agent. Furthermore, since CUR itself showed an anticancer effect as a single agent at higher doses, we decided to evaluate this combination treatment in vivo.
Figure 5.
Comparison of cell death of SK-OV-3TR cells after the treatment with various concentrations of PCL, 10 µM CUR, or combination treatment. MDR reversal capability of CUR was best demonstrated at a concentration of 10 µM. Cell viability was determined using CellTiter Blue cell viability assay. Data shown are representative of 3 independent experiments and each performed in triplicate.
3.3 Tumor inhibition study
Nude mice bearing ~200 mm3 SK-OV-3 sensitive and resistant tumors were treated with 25 mg/kg CUR and 10 mg/kg PCL as a single treatment or in combination every 3 days till the end of the study. From the in vitro cytotoxicity data, we concluded that a 2.5:1 w/w ratio of CUR:PCL was optimal at demonstrating the synergistic effect between the two compounds. Even though we were administering the formulations intraperitoneally, we wanted to keep the injection volume below 500 µL to minimize stress on the animals. At a PCL concentration of 600 µg/ml in the micelles, a ~416 µL injection volume is required to achieve a 10 mg/kg dose in a 25 gram mouse. At a CUR concentration of 1200 µg/ml, a 520 µL injection volume is required to achieve a 25 mg/kg target dose. For these reasons, we selected 25 mg/kg CUR and 10 mg/kg PCL as a single treatment or in combination which is the maximum dose that can be achieved with ~500 µL injection volume. At the doses chosen, the formulations produced no toxicity in vivo as indicated by no significant decrease in body weight throughout the study. Empty micelles were chosen as control for these studies.
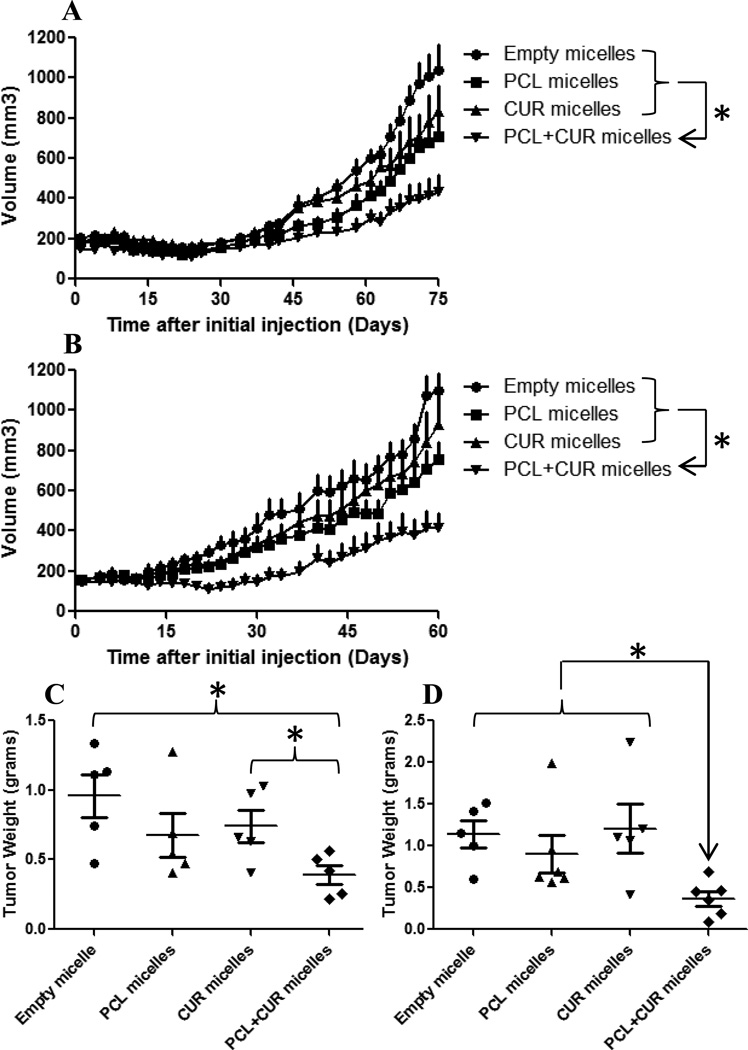
CUR at a dose of 25 mg/kg produced no significant tumor growth inhibition versus the control group in the SK-OV-3 sensitive study. Tumor volume of the PCL group at a 10 mg/kg dose was significantly lower than the empty micelle control group but demonstrated no significant difference versus the CUR group. However, the combination treatment exhibited superior tumor inhibition properties. By the end of the study, the combination treatment of PCL and CUR was successful at inhibiting tumor growth by almost 3-fold (Figure 6A).
Figure 6.
Tumor inhibition studies with various micellar formulations. Nude mice bearing ~200 mm3 SK-OV-3 (A) and SK-OV-3TR (B) tumors were treated every 3 days at a dose of 25 mg/kg CUR and 10 mg/kg PCL IP starting at day zero. Empty micelle dose was equivalent to the amount of micelle-forming material from the drug-loaded micelle groups. (One way ANOVA was performed from day 45–75 on A and from day 30–60 on B, * p<0.05 with n ≥5 /group and all values are expressed as mean ± SEM. SK-OV-3 (C) and SK-OV-3TR (D) tumors were harvested when the average tumor volume in the control group reached 1000 mm3. (Student’s two tailed unpaired T-test, * p<0.05) n ≥5/group ± SEM.
In the tumor resistant study, CUR at a dose of 25 mg/kg showed no significant tumor growth inhibition versus control group as well. Similar to the sensitive study, tumor volume of the PCL group at a 10 mg/kg dose was significantly lower than the empty micelle control group but demonstrated no significant difference versus the CUR group. Again, the combination treatment exhibited superior tumor inhibition properties suggesting that CUR reversed MDR and this combination treatment was also successful at inhibiting tumor growth by 3-fold as well (Figure 6B).
As seen in figure 6A, tumor growth in the SK-OV-3 sensitive study group was minimal for the first 45 days in all treatment groups. The accelerated tumor growth phase did not start until ~ day 50. We hypothesize that the continuous treatment with PCL every 3 days led the tumor to develop resistance as evident from other clinical studies and its use in the clinic. We initially expected the PCL treatment in the sensitive group to have a much higher tumor inhibition effect compared to the resistant study. For that reason, the resemblance in the results between the two studies led us to believe that the resistance could be the factor contributing to this similarity.
At the end of the in vivo studies, the tumors were excised and weighed. As expected, the combination treatment tumor weights were significantly lower from the control and CUR groups in the sensitive study. Additionally, the combination treatment tumor weights in the resistant study were significantly lower compared to all other treatment groups (Fig. 6C/D).
From the in vitro data, the 10 µM CUR concentration exhibited a minimal cytotoxic effect as a single agent, but when combined with PCL, a 3-fold reduction in the PCL IC50 was observed on SK-OV-3TR cells. The in vivo data showed a remarkable similarity to the in vitro data. Even though the 25 mg/kg dose of CUR did not cause a significant increase in tumor inhibition as a single agent; however, when combined with PCL, the combination treatment was successful at inhibiting tumor growth by 3-fold and was statistically significant versus the PCL-treated group.
4. Discussion
The development of multi-drug resistance (MDR) is one of the major factors leading to the failure of many conventional chemotherapies. The two intrinsic properties of CUR, its toxicity towards cancer cells and MDR reversal capability, may have great potential in the clinic when used in combination with chemotherapeutic agents. Here, we demonstrated the effectiveness of PEG-PE/vitamin E mixed micelles in solubilizing CUR and the ability to co-load it with PCL in the same formulation. These combination micelles have significant advantages in vitro and in vivo compared to individual drug therapy and especially when dealing with resistant tumors. Combining CUR, a safe and effective NF-κB inhibitor, with PCL was successful in reversing MDR in a resistant human ovarian adenocarcinoma model as shown by the dramatic improvement of PCL efficacy. We believe this combination therapy modality could have significant clinical advantages for the treatment of resistant ovarian cancer and deserves further investigation.
Acknowledgements
This work was supported by the NIH/NCI CCNE grant 5U54CA151881 to Vladimir P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest is declared.
Contributor Information
Abraham H. Abouzeid, Northeastern University, Department of Pharmaceutical Sciences, Center for Pharmaceutical Biotechnology and Nanomedicine, 140 The Fenway, Room 236, 360 Huntington Ave, Boston, MA 02115
Niravkumar R. Patel, Northeastern University, Department of Pharmaceutical Sciences, Center for Pharmaceutical Biotechnology and Nanomedicine, 140 The Fenway, Room 236, 360 Huntington Ave, Boston, MA 02115
Vladimir P. Torchilin, Distinguished Professor, Northeastern University, Department of Pharmaceutical Sciences, Center for Pharmaceutical Biotechnology and Nanomedicine, 140 The Fenway, Room 211/214, 360 Huntington Ave, Boston, MA 02115, V.Torchilin@neu.edu, Fax number: 617-373-7509
References
- Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochemical pharmacology. 2010;79:330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer letters. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Bava SV, Sreekanth CN, Thulasidasan AK, Anto NP, Cheriyan VT, Puliyappadamba VT, Menon SG, Ravichandran SD, Anto RJ. Akt is upstream and MAPKs are downstream of NF-kappaB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. The international journal of biochemistry & cell biology. 2011;43:331–341. doi: 10.1016/j.biocel.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Chou TC TP. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, Zhai G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. International journal of pharmaceutics. 2009;371:148–155. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Waterhouse DN, G KA, Klasa R, Chi K, Huntsman D, Ramsay E, Wasan E, Edwards L, Tucker C, Zastre J, Zhang YZ, Yapp D, Dragowska W, Dedha S, Bally MB. Development and Assessment of Conventional and Targeted Drug Combinations for Use in the Treatment of Aggressive Breast Cancers. Current Cancer Drug Targets. 2006;6:455–489. doi: 10.2174/156800906778194586. [DOI] [PubMed] [Google Scholar]
- Ganta S, Amiji M. Coadministration of Paclitaxel and Curcumin in Nanoemulsion Formulations To Overcome Multidrug Resistance in Tumor Cells. Molecular pharmaceutics. 2009;6:928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. European Journal of Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Gill KK, Kaddoumi A, Nazzal S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2012;46:64–71. doi: 10.1016/j.ejps.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Kunwar A, Barik A, Pandey R, Priyadarsini KI. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochimica et biophysica acta. 2006;1760:1513–1520. doi: 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Gao Z, Torchilin VP. Micelles from polyethylene glycol/phosphatidylethanolamine conjugates for tumor drug delivery. Journal of Controlled Release. 2003;91:97–102. doi: 10.1016/s0168-3659(03)00217-7. [DOI] [PubMed] [Google Scholar]
- Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. International journal of pharmaceutics. 2012;425:44–52. doi: 10.1016/j.ijpharm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Patel NR, Rathi A, Mongayt D, Torchilin VP. Reversal of multidrug resistance by codelivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. International journal of pharmaceutics. 2011;416:296–299. doi: 10.1016/j.ijpharm.2011.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant RR, Sawant RM, Torchilin VP. Mixed PEG-PE/vitamin E tumor-targeted immunomicelles as carriers for poorly soluble anti-cancer drugs: improved drug solubilization and enhanced in vitro cytotoxicity. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2008;70:51–57. doi: 10.1016/j.ejpb.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2011;14:150–163. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Sreekanth CN, Bava SV, Sreekumar E, Anto RJ. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene. 2011;30:3139–3152. doi: 10.1038/onc.2011.23. [DOI] [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature reviews. Drug discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. CMLS, Cell. Mol. Life Sci. 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PubMed] [Google Scholar]
- Webb MS, Johnstone S, Morris TJ, Kennedy A, Gallagher R, Harasym N, Harasym T, Shew CR, Tardi P, Dragowska WH, Mayer LD, Bally MB. In vitro and in vivo characterization of a combination chemotherapy formulation consisting of vinorelbine and phosphatidylserine. European Journal of Pharmaceutics and Biopharmaceutics. 2007;65:289–299. doi: 10.1016/j.ejpb.2006.10.007. [DOI] [PubMed] [Google Scholar]