Abstract
The choroid plexus plays a wide range of roles in brain development, maturation, aging process, endocrine regulation, and pathogenesis of certain neurodegenerative diseases. To facilitate in vitro study, we have used a gene transfection technique to immortalize murine choroidal epithelial cells. A viral plasmid (pSV3neo) was inserted into the host genome of primary choroidal epithelia by calcium phosphate precipitation. The transfected epithelial cells, i.e., Z310 cells, that survived from cytotoxic selection expressed SV40 large-T antigen throughout the life span, suggesting a successful gene transfection. The cells displayed the same polygonal epithelial morphology as the starting cells by light microscopy. Immunocytochemical studies demonstrate the presence of transthyretin (TTR), a thyroxine transport protein known to be exclusively produced by the choroidal epithelia in the CNS, in both transfected and starting cells. Western blot analyses further confirm the production and secretion of TTR by these cells. The mRNAs encoding transferrin receptor (TfR) were identified by Northern blot analyses. The cells grow at a steady rate, currently in the 110th passage with a population doubling time of 20–22 h in the established culture. When Z310 cells were cultured onto a Trans-well apparatus, the cells formed an epithelial monolayer similar to primary choroidal cells, possessing features such as an uneven fluid level between inner and outer chambers and an electrical resistance approximately 150–200 Ω-cm2. These results indicate that immortalized Z310 cells possess the characteristics of choroidal epithelia and may have the potential for application in blood-CSF barrier (BCB) research.
Keywords: Choroid plexus, Choroidal epithelial cell, Blood–CSF barrier, Transthyretin, Immortalization, Z310 cells, Transferrin receptor, Sucrose, Z310 cells
1. Introduction
The epithelial layer of the choroid plexus constitutes the barrier between the blood and cerebrospinal fluid (CSF). Under the microscope, the choroid plexus consists of three cellular layers: the apical epithelial cells, the underlying supporting connective tissue, and the inner layer of endothelial cells. The apical layer consists of closely packed, cuboidal or columnar epithelial cells. These cells possess the tight junctions near their apical surface, which seal one epithelial cell to another and provide a structural basis for the blood–CSF barrier [5,14]. The functions of the choroid plexus include restriction of the entrance of substances from the blood to the CSF, active production and secretion of CSF to the cerebral compartment, transport and supply of useful substances to the brain, as well as a cleansing role via removal of unwanted materials from the CSF. In addition, the choroidal epithelial cells play a neuroen-docrine regulatory function in the CNS such as production and secretion of transthyretin, vasopressin, and transferrin, into the brain [2,26].
Cumulative evidence suggests that the choroid plexus serves as a target for toxicities associated with environmental exposure to heavy metals [8,26]. The importance of BCB in the etiology of certain neurodegenerative disorders has also received increasing attention [27]. Moreover, the BCB has been suggested to be a notable determinant in certain brain tumors, AIDS dementia, and ischemic stroke [28,29].
Progress in understanding biochemical and molecular functions of the choroid plexus demands an in vitro model system for mechanistic approaches. Several methods for establishment of primary culture of choroidal epithelial cells have been reported in literature [19,24,31]. However, the choroidal epithelial cells in the primary culture have a limited ability to differentiate and a rather short life span, lending themselves inapt to studies that demand multiple treatments and prolonged time courses. In addition, the small mass of the plexus tissue requires the experiments to pool a large quantity of tissues at the cost of considerable numbers of animals. These disadvantages have limited the application of primary cultures to wider investigations.
The purpose of this study was to use gene transfection techniques to establish an immortal cell line of murine choroidal epithelial cells. We used simian virus 40 large T antigen (SV40) to integrate the immortalizing gene into the host genome. The large T antigen encoded by the early region of the SV40 genome is thought to interact with the growth suppressors pRB and p53 during cell immortalization [13]. Upon selection of immortalized cells, we characterized the cell line based on its morphology, its genotypic properties of expression of SV40 large T and resistance to cytotoxic G418, and its phenotypic characteristics via expression of transthyretin and transferrin receptor. In addition, the culture was adapted onto a two-chamber system in order to evaluate the tightness and transport properties of the epithelial barrier.
2. Materials and methods
2. 1. Materials
Chemicals were obtained from the following sources: Dulbecco’s modified essential medium (DMEM), Hanks’ balanced salt solution (HBSS), fetal bovine serum (FBS), antibiotic-antimycin, penicillin, and G418-neomycin from Gibco (Grand Island, NY); pronase from CalBiochem–Novabiochem (La Jolla, CA); laminin, mouse epidermal growth factor (EGF), gentamycin, diethylpyrocarbonate (DEPC), phenylmethylsulfonylfluoride (PMSF), bovine serum albumin (BSA), fibroblast growth factor (FGF), and insulin from Sigma (St. Louis, MO); Escherichia coli containing the pSV3-neoplasmid (ATCC #37150) from American Type Culture Collection (Rockville, MD); monoclonal anti-SV-40 large T antibody from Oncogene Science (Uniondale, NY); biotinylated anti-secondary anti-bodies from Vector Laboratories (Burlingame, CA); RNA Zol™ B test kit from Tel-Test, (Friendswood, TX); Transwell-COL culture wells from Costar (Cambridge, MA); and [14C]sucrose (specific activity: 401 mCi/mmol) from Perkin-Elmer Life Sciences (Boston, MA). All reagents were of analytical grade, HPLC grade or the best available pharmaceutical grade.
The recombinant plasmid used for transfection, pSV3-neo, contains both the large and small T-antigens of the early region of SV40 and the bacterial neomycin phosphotransferase gene which confers resistance to the antibiotic geneticin (G418) [21]. Purified rat plasma TTR and rabbit anti-rat TTR polyclonal antibody were the gifts of Dr. W. Blaner at the Institute of Human Nutrition, Columbia University.
2. 2. Primary cultures of choroidal epithelial cells
Choroidal epithelial cells were cultured using the method established in this laboratory [31]. In short, plexus tissues were collected from Sprague–Dawley rats (4–6 weeks old, both sexes), purchased from Hilltop (Cottdale, PA). The protocol that involves use of animals for primary culture has been approved by the Institutional Animal Care & Use Committee at Columbia University. The plexuses were dissected, washed in Hank’s buffer, chopped with scissors, and digested in HBSS containing 0.2% pronase at 37 °C for 5 min. After centrifugation, the pellet was collected, washed with HBSS, and resuspended in DMEM with 10% FBS. The cells were further mechanically dissociated by seven to eight forced passages through a 20-gauge needle. The dissociated cells were washed in DMEM with 100 units/ml each of penicillin and streptomycin, and re-suspended in normal growth medium of DMEM supplemented, in addition to antibiotics, with 10% FBS and 10 ng/ml EGF. The cells were plated in 100-mm Petri dishes at the density of 0.5–1×106 cells/ml and cultured in a humidified incubator with 95% air–5% CO2 at 37°C. Unattached cells were discarded after 48 h. The growth medium was replaced 2 days after the initial seeding and every 2–3 days thereafter. The culture from 5-week-old rats showed a dominant polygonal type of epithelial cells for at least 7–10 days. A 5-day culture was used in the following study.
2. 3. Transfection of SV40 large T into choroidal epithelial cells
Choroidal epithelial cells were transfected with the pSV3-neoplasmid by calcium phosphate coprecipitation method using a calcium phosphate transfection system (Gibco-BRL) [9]. Choroidal epithelial cells from the primary culture were plated on a 100-mm Petri dish at a density of 0.5×106 cells/ml. Transfection was performed according to the procedure suggested by the manufacturer. Briefly, a total of 20 µg of DNA (15 µg of pSV3-neoplasmid DNA and 5 µg of carrier salmon sperm DNA) was mixed with a calcium solution to prepare Ca–DNA precipitates. An aliquot (1 ml) of this calcium phosphate–DNA suspension was added dropwise into the cells cultured in 10 ml of primary culture medium. The transfection continued for 24 h at 37 °C. At the end of the transfection, the medium containing the precipitate was removed and the cells were cultured in the primary culture medium for another 24 h for the selection of stable colonies as described below.
2. 4. Selection of neomycin-resistant cells
The transfected cells were cultured in a selection medium (primary culture medium plus 200 µg/ml G418) for 4 days to select neomycin-resistant cells, which expressed the bacterial neomycin phosphotransferase. The medium was changed every 2 days thereafter. After 2–3 weeks under G418 selection, single colonies were isolated using cloning rings and separately subcultured in the maintenance medium consisting of DMEM supplemented with 10% FBS, 200 µg/ml G418, and 10 ng/ml EGF. After characterization, the candidate clones were expanded to yield independent choroidal epithelial cell line in the maintenance medium through over 110 passages up to the present.
2. 5. Determination of cloning efficiency and cell growth rate
To estimate the immortalizing efficiency, the transfected cells were seeded in the maintenance medium containing G418. Two weeks after initial seeding, the number of large, round viable colonies were counted. The immortalizing efficiency was determined as the percentage of seeded cells which formed the colonies.
To determine the cumulative cell growth pattern, transfected cells were seeded at 3×106 cells/ml in the maintenance medium and passaged at 1:4–6 ratio every 3–4 days (Tuesday and Friday of each week). Total cell numbers from each passage were then calculated and added to the cumulated cell numbers from the previous passages to produce the cumulative cell numbers. For determination of cell growth rate, the established immortal cells were seeded at 0.5×105 and 1×105 cells/dish in 1 ml of medium. After initial seeding, the cells were treated with Trypan blue and counted for both viable and dead cells every day for 5 days. Population doubling time was calculated from the log-phase of the growth curve.
2. 6. Immunocytochemistry of SV40 large T antigen
Cells were grown on coverslips, rinsed with phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. The fixed cells were pretreated with 0.3% H2O2 for 15 min, washed, and preabsorbed with 0.3% horse serum in PBS for 1 h at room temperature. The cells were then incubated with monoclonal anti-SV40 large T antigen antibody for 1 h at 37 °C, followed by incubation with biotinylated anti-mouse IgG secondary antibody for 30 min at room temperature. The specimens were washed another four times with PBS, stained with ABC reagent (VECTASTAIN®, Vector Lab-oratories), reacted with 3,3-diaminobenzidine, and examined under a microscope.
2. 7. Immunocytochemistry of TTR
The cells to be stained were cultured on a glass coverslip for 2–3 days. The cells were fixed in 4% paraformaldehyde in PBS solution followed by three washes with PBS. The cell monolayers that attached to the glass coverslips were pretreated with 0.3% H2O2 in PBS for 15 min at room temperature and incubated with 0.3% normal rabbit serum for 30 min. The cells were incubated with rabbit anti-rat TTR antiserum (1:250 dilution) in PBS at room temperature for 30 min and rinsed with PBS for three times. The cultures were then incubated for 30 min with biotinylated goat anti-rabbit secondary antibody diluted 1:200 in PBS. The sections were further stained with ABC reagent to form avidin–biotin–horseradish peroxidase complex and examined under a microscope.
2. 8. Western blot analysis of TTR
Total proteins were extracted from the cultured cells according to the procedure previously described [30,31]. The cells were harvested and homogenized (1:10, w/v) in a buffer composed of 20 mM Tris (pH 8), 2 mM EDTA, 1 mM DTT, 0.25 mM PMSF, 1% Triton X-100, 150 mM NaCl, and 0.05% SDS on ice. Following centrifugation at 13 600×g at 4 °C for 1 h, aliquots (35 µg) of cellular proteins, purified rat TTR standard, and protein molecular weight markers were loaded onto an 8.5% SDS–polyacrylamide gel, and subjected to electrophoresis under constant current of 30 mA per gel. The serum-free culture media from the dishes (100-mm) were collected at 48 h after the seeding (2×105 cells/ml in 10 ml of medium) and centrifuged at 13 600×g at 4 °C for 30 min. An aliquot (60 µl) of media was loaded onto the gel. At the end of the electrophoresis, the proteins were transferred onto a PVDF membrane. The membrane was first incubated with 5% milk in PBS and then immunoblotted with rabbit anti-rat TTR antibody (1:250) at room temperature for 30 min [30]. Membranes were washed three times with PBS. The bound anti-TTR antibodies were detected using ECL non-radioactive luminescence system (ECL, Gibco) by incubating with HRP-labeled anti-rabbit secondary antibody (1:1000) in PBS at room temperature for 1 h. The membranes were then washed and autoradiographed with Kodak Biomax MR film (Eastman Kodak, Rochester, NY) for 5–15 s.
2. 9. Northern blot of transferrin receptor (TfR) mRNA
Total RNA was extracted from immortal choroidal epithelial cells using RNA Zol™ B kit (Tel-Test) following manufacturer’s instructions. Total RNA (15 µg) was electrophoresed on 1.5% agarose gels, followed by soaking in 0.05 mM NaOH for 20 min and then 20× saline–sodium citrate (SSC) for 40 min. After the RNA was transferred to nylon filter in 20× SSC overnight, the filter was UV-irradiated, prehybridized in 0.5 M phosphate buffer (pH 7.2) containing 7% SDS and 1% BSA at 65 °C for 3 h, and then hybridized at 65 °C overnight in the same solution containing 2×106 cpm/ml of random-primed 32P-labeled transferrin receptor cDNA probe. The probe was a 507-base-pair fragment of 5′-end of a full length 3.4-kb rat transferrin receptor cDNA, subcloned to pTZ19U-RTR, which was a gift of Dr. Griswold at Washington State University. The filters were washed three times for 15 min each using 2× SSC with 0.1% SDS at 65 °C, followed by 0.2× SSC with 0.1% SDS at 65 °C for 15 min by three times. The filters were then autoradiographed with Kodak Biomax MR film in an intensifying screen at −70 °C for 3 days.
2. 10. Culture on a two-chamber system
Permeable membranes attached to the Transwell-COL culture wells (12 mm in diameter, 0.4 µm of pore size) were pretreated with laminin (14 µg/ml) for 10 min and allowed to air dry for at least 45 min prior to cell seeding. Aliquots (0.8 ml) of cell suspension were plated in 12-mm laminin-coated culture wells (2×105 cells per well). This was designated as the inner (apical) chamber. The inner chambers were then inserted into the outer (basal) chambers which contained 1.2 ml of culture medium. The cultures continued for 48 h and the medium was changed every 2 days thereafter.
Transepithelial electrical resistance was measured using an epithelial volt-ohmmeter (EVOM, World Precision Instruments, Sarasota, FL) after culture in the bicameral chambers for at least 2 days. The net value of electrical resistance was computed by subtracting the background, which was measured on laminin-coated, control cell-free chambers, from values of epithelial cells-seeded chambers. The formation of confluent cell monolayers was judged by three criteria: (1) the cells grew to a confluent monolayer without visible spaces between cell clusters under light microscope; (2) the height of the culture medium in the inner chamber had to be at least 2 mm higher than that in the outer chamber for at least 24 h; and (3) the electrical resistance across the cell layer had to fall into the range of 180–200 Ω-cm2.
To compare the paracellular leakage of the barrier between Z310 cells and primary culture, the cells grown on the inner chamber for 5 days were used in the study. [14C]sucrose was added into the outer chamber to a final concentration of 1 µM (0.4 µCi/ml) [15]. Aliquots (10 µl) of media in the inner chamber were removed at the designated times, mixed with liquid scintillation solution, and counted using a Packard Tri-Carb model 2100TR scintillation counter. The data collected during the first 5 h were analyzed by a linear regression fitting; the slopes of the curves were used to estimate the initial permeability rate constant (ki). The procedure to grow the primary epithelial cells on the Transwell system has been described in our previous publications [30,31].
3. Results
3. 1. Genotypic characterization of immortalized choroidal epithelial cells
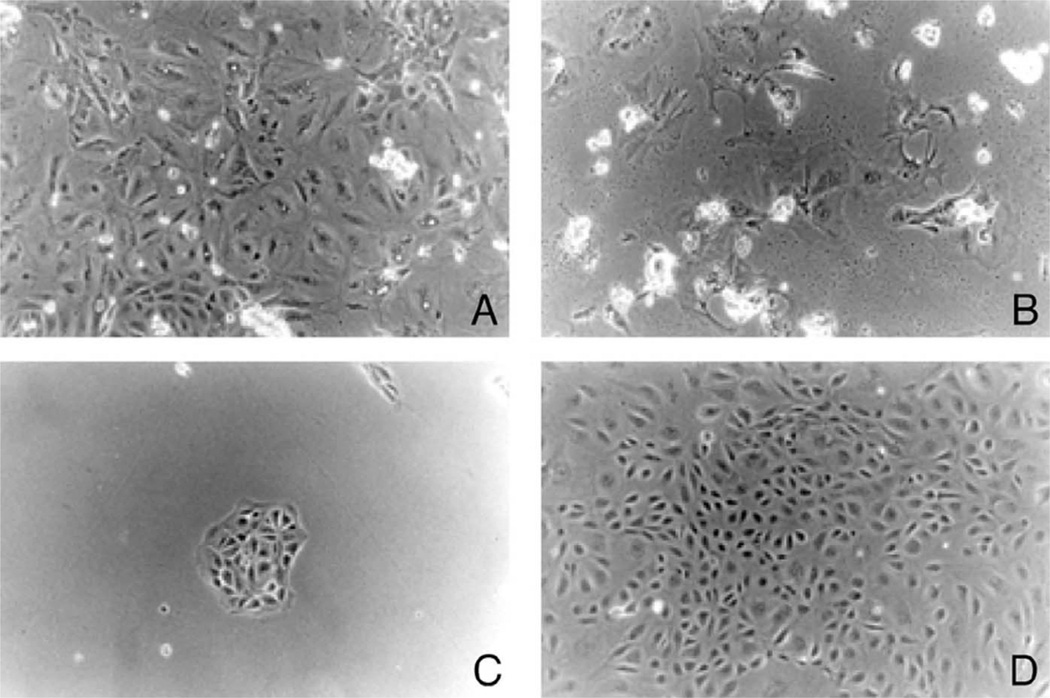
Transfection of the pSV3neo plasmid was performed on a 5-day-old primary culture of murine choroidal epithelial cells. Twenty-four hours after transfection (in the absence of G418), a heterogeneous population of cells attached to the culture plate (Fig. 1A). Selection of transfected cells began at day 3 by culturing the cells in a selection medium containing G418. After 2–3 weeks cultured in the selection medium, most of the cells died (Fig. 1B), while a few of resistant colonies were formed (Fig. 1C). The frequency of these G418-resistant transformants was usually two to four colonies per 3×5 10 cells transfected. Among those picked colonies which had an obvious epithelial phenotype (closely packed cuboidal each growing in discrete islands), the Batch 3 (Fig. 1D) maintained a typical choroidal epithelial characteristics before and after the critical ‘crisis’ period. To date, these cells, even after more than 110 passages, retain the ability to express TTR and possess stable cell growth dynamics.
Fig. 1.
Time course of pSV3neo-transfected murine choroidal epithelial cells. The primary choroidal epithelial cells were transfected with the plasmid pSV3neo and plated in the presence of cytotoxic agent G418. (A) Primary culture of choroidal epithelial cells. (B) Cell death of the majority of transfected cells 3 days following addition of G418 in culture medium. The cells floating on the surface (with a strong light reflection) were either dead or detached cells. (C) Appearance of the single colony of immortalized cells 2 weeks after G418 selection. (D) Expansion of the survived cells. The single colony was selected and expanded to yield independent choroidal epithelial cell line (Z310) at the 50th passage (×100). Note: the cells maintain the same polygonal cell type even at the 110th passage.
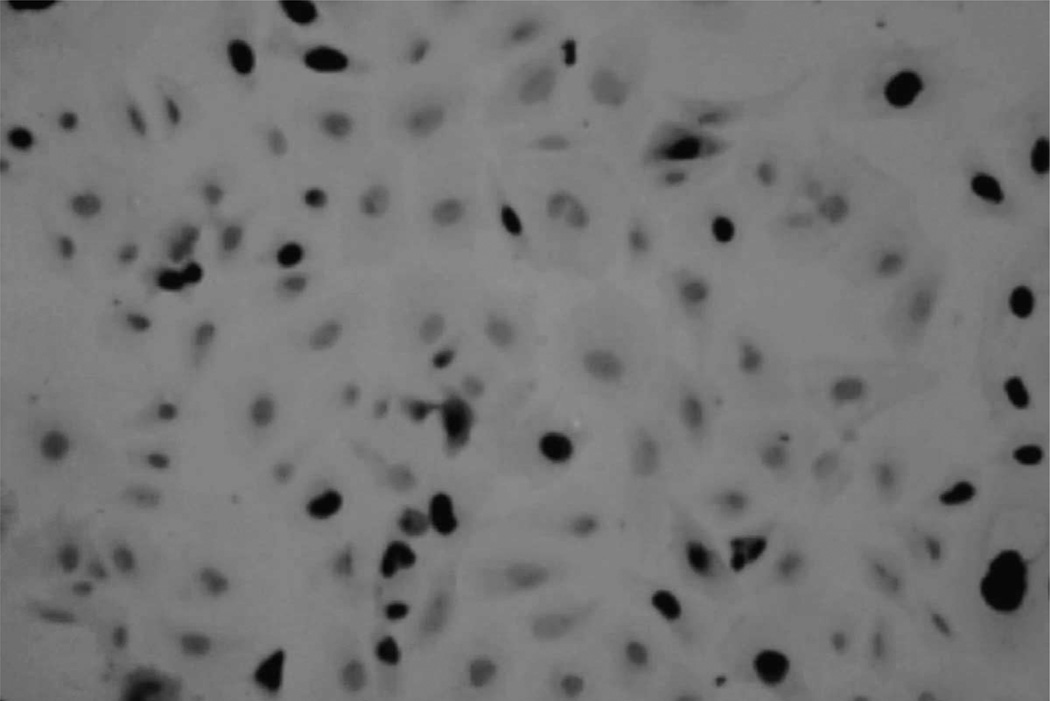
Immunocytochemical staining demonstrated predominantly nuclear localization of SV40 large T antigen immunoreactivity in transfected cells (Fig. 2). Throughout the entire culture period, the SV40 T-antigen was consistently expressed in these cells, indicating that the gene encoding the SV40 large T antigen had been integrated into the host genome. Thus, the transfected cells derived from the Batch 3 are now referred to as the Z310 immortal choroidal epithelial cell line. It is noticed that the expression of the positive staining was about 53% of the cell population. The low percentage of staining probably reflects a low level of expression of large T antigen due to the cell growth in the different stages, or the loss of staining due to experimental variations.
Fig. 2.
Immunocytochemical staining of SV40 large T antigen in cultured Z310 cells. The cells were cultured on a glass coverslip and incubated with monoclonal anti-SV40 large T antigen antibody, followed by reaction with biotinylated goat anti-mouse IgG secondary antibody. The positively stain of SV40 large T was seen in nuclei (×200) at the 52nd passage.
3. 2. Morphological characteristics and growth dynamics of immortalized choroidal epithelial cells
Z310 cells are clearly of choroidal epithelial origin. When studied under light microscope, the cells displayed uniform polygonal epithelial type (Fig. 1D) similar to the normal primary cells from which they were derived [31]. Unlike primary cells (Fig. 1A), there were no other cell types identifiable under light microscopy. These cells have a tendency to form closely packed islands. They are mitotically more active than primary cells.
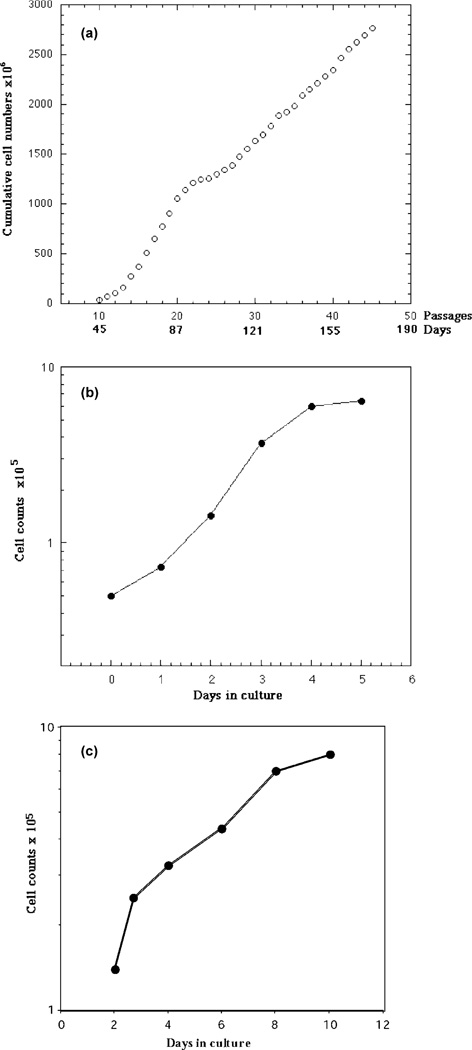
Initially, the selected transfected cells grew rapidly after about the 12th passage. Cell growth gradually declined after the 20th passage and ceased between the 23rd and 26th passages (the crisis period) (Fig. 3A). Thereafter, the cells recovered their growth and divided at a relatively steady rate. A growth dynamic study using cells in the 110th passage showed a population doubling time of 20–22 h in established cultures (Fig. 3B), in comparison to the doubling time of 3–4 days in the starting primary cells (Fig. 3C).
Fig. 3.
Growth patters of immortalized Z310 cells. (A) Cumulative growth curve following transfection with pSV3neo. The growth ‘crisis’ was seen between the 23rd and 26th passages. (B) Normal growth curves of Z310 cells at the 70th passage. (C) Normal growth curve of the primary choroidal cells.
3. 3. Phenotypic characterization of immortalized choroidal epithelial cells
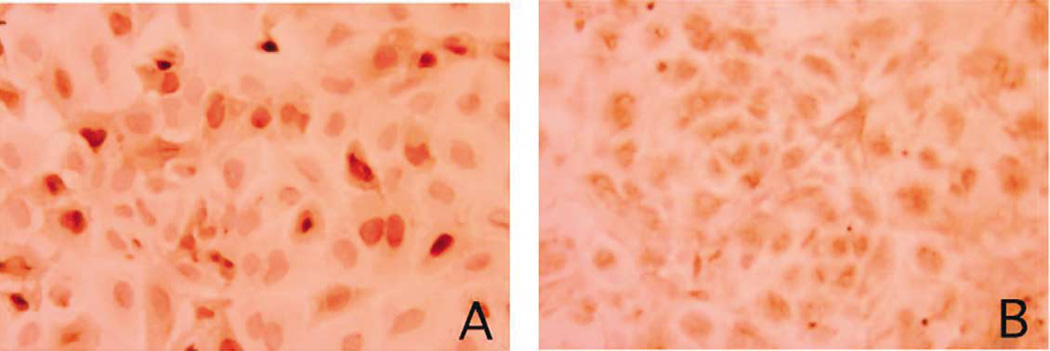
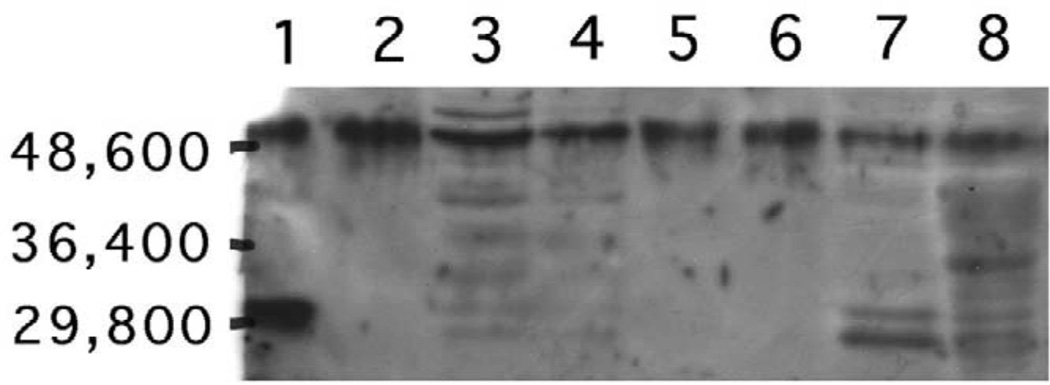
The existence of cytosolic TTR is a phenotypic hallmark of choroidal epithelial cells. The expression of a choroidal epithelium-specific marker, TTR, was investigated using immunohistochemical staining with anti-TTR antibody. Most of the immortalized cells had positively stained monocytosolic TTR (Fig. 4A), which was highly similar to those observed in primary culture of rat choroidal epithelial cells (Fig. 4B). Further identification of TTR proteins using Western blot analysis demonstrated the existence of TTR molecules in the immortalized cells, which was comparable to those observed in primary choroidal cells, plexus tissue extracts, as well as liver cytosolic fractions (Fig. 5). More interestingly, the media collected from the cultured immortal cells, which were free of serum, also displayed the band corresponding to TTR, indicating the secretion of TTR by the cultured cells (Fig. 5).
Fig. 4.
Immortalized choroidal Z310 cells possess cytosolic TTR by immunocytochemical staining. The cells were cultured on a glass coverslip and incubated with rabbit anti-rat TTR antiserum, followed by reaction with biotinylated secondary antibody. (A) Cultured Z310 cells show the positive stain for cytosolic TTR (×250). (B) Primary culture of rat choroidal epithelial cells was used as the positive control (×250).
Fig. 5.
Western blot analyses of TTR in immortalized choroidal epithelial cells. Cytosolic proteins from the cells of selected colonies, choroid plexus tissues, and liver homogenates, as well as proteins in culture media, were electrophoresized on 8.5% SDS–polyacrylamide gel, followed by transferred on to a PVDF membrane. Immunoblots resulting from the reaction with anti-rat TTR antibody were visualized by ECL luminescence method. TTR migrates with apparent MW of 55 KDa. Lanes: (1) rat TTR standard; (2) primary cultured plexus cells; (3): clone 3-2 (developed into Z310 cell line); (4): clone 1–2; (5) culture medium for clone 3-2; (6) culture medium for clone 1–2; (7) choroid plexus tissues; (8) liver cytosolic fractions.
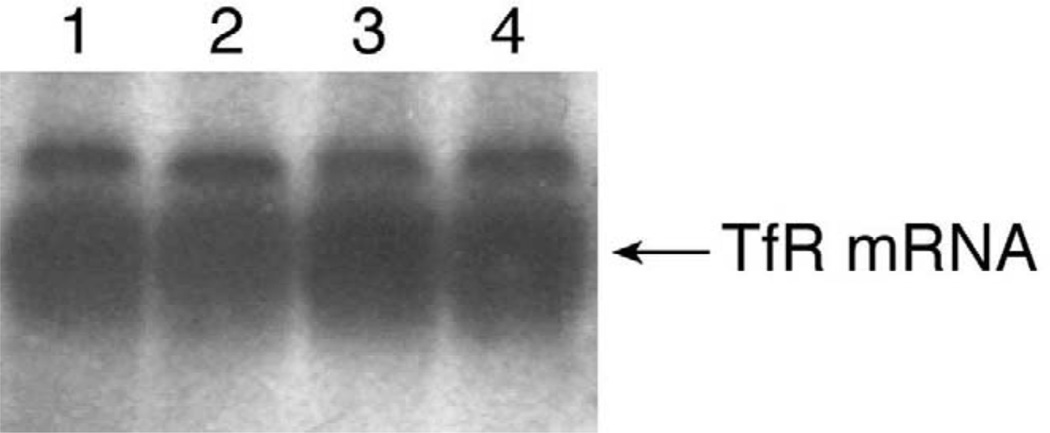
Choroidal epithelial cells in the primary culture express high levels of transferrin receptor mRNA [32]. Northern blot analysis of immortalized Z310 cells clearly demonstrated the presence of mRNAs encoding transferrin receptor in these cells (Fig. 6).
Fig. 6.
Expression of TfR mRNA in immortalized choroidal epithelial cells by Northern blot analyses. Total cellular RNA was extracted from the cells of selected colonies, electrophoresed on 1.5% agarose gels, and hybridized with random-primed 32P-labeled TfR cDNA probe. Lanes (1,2) Z310 cells; (3,4) primary choroidal cells.
When Z310 cells were seeded onto a semipermeable membrane in the inner chambers of a Transwell apparatus, the cells displayed similar morphology to those observed in the culture dishes. The formation of a confluent monolayer occurred within 48 h after seeding. A higher fluid level in the inner chamber than in the outer chamber was observed (differences >3 mm). This uneven fluid level was consistent throughout the duration of the culture. The transepithelial electrical resistance between the inner and outer chambers was approximately 150–200 Ω-cm2, and increased only when the monolayer of cells grew to the confluence.
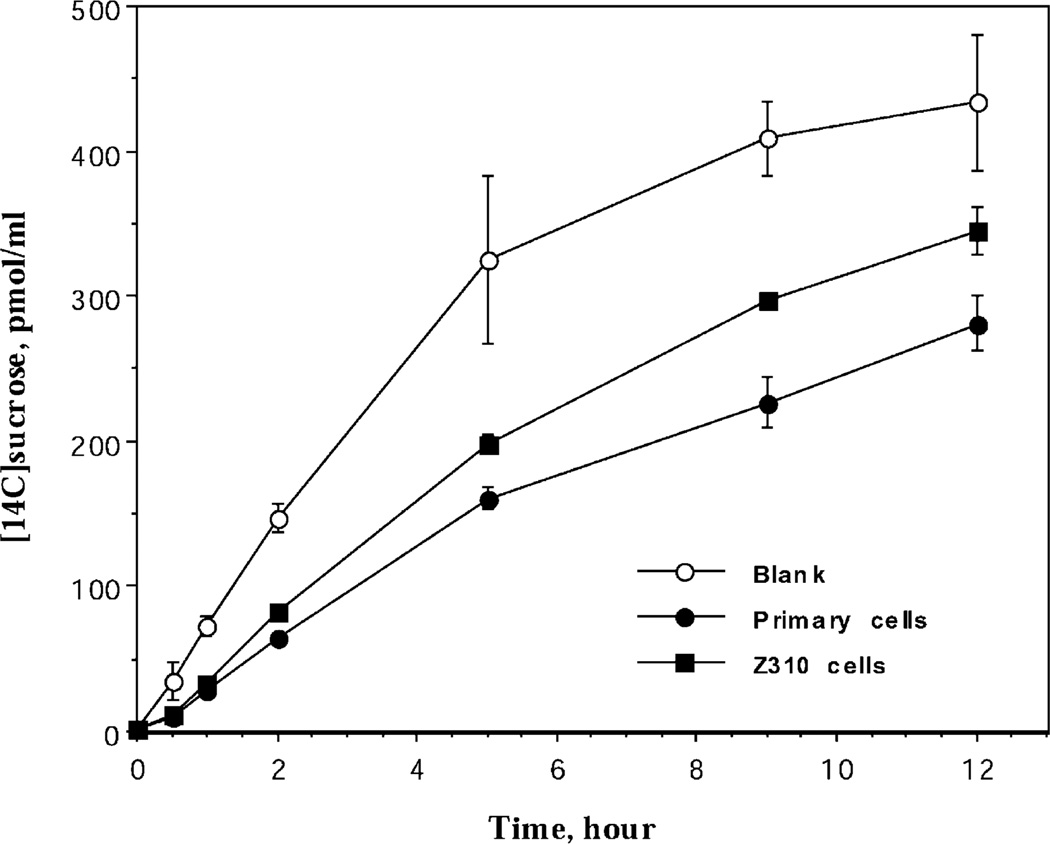
The paracellular permeability has also been investigated using [14C]sucrose as an extracellular marker in the Transwell model established by either Z310 cells or primary cells. The concentration of [14C]sucrose was increased over the time course of incubation in the inner chamber of both immortal and primary cells, suggesting the existence of a paracellular passage of [14C]sucrose between the monolayer of epithelial cells (Fig. 7). The initial permeability rate constant (ki) in the control group without cells was 862 fmol/min, whereas the ki values in the Transwell with the primary and Z310 cells were 432 and 538 fmol/min, respectively. Thus, the cell monolayer with Z310 cells appears to be slightly leakier than that with primary epithelial cells.
Fig. 7.
Paracellular passage of [14C]sucrose by immortalized Z310 cells or primary epithelial cells. Z310 cells or primary epithelial cells were cultured on Transwell-COL membranes in the inner chamber to confluence. [14C]sucrose was added into the outer chamber to a final concentration of 1 µM. Data represent means±S.D. (n = 3–5).
4. Discussion
This study demonstrates a successful immortalization of rat choroidal epithelial cells by stable transfection of SV40 large T into primary choroidal cells. The cultures possess a uniform epithelial cell type similar to the primary culture from which they are derived. Biochemical and functional characterization provides strong evidence that the immortal cells retain most of the differential properties and functions of primary choroidal cells. Thus, these cells represent a new cell line of choroidal epithelia. This cell line can be used to study the function of the choroid plexus in vitro.
Establishment of primary choroidal epithelial cell culture has been described in the literature [4,19,31]. While the primary culture serves as an ideal model for in vitro investigation of the blood–CSF barrier, it suffers from numerous disadvantages, such as the lack of cell abundance, difficulty in obtaining pure forms, reliance on large numbers of animals, as well as a brief life span in culture. These undesirable natures have limited the application of primary cultures to wider investigation. Thus, establishment of a continuing choroidal cell line has become a critical strategy for in vitro study of the blood–CSF barrier. Immortalization of specific cell types can be achieved by random integration of viral immortalizing genes to the host genome. Available approaches include the use of simian virus 40 large T antigen (SV40), papillomaviruses E6 and E7, adenovirus E1A, Epstein–Barr virus, human T-cell leukemia virus, herpesvirus saimiri, oncogenes, and mutant p53 [16]. We chose SV40 large T because most SV40 large T-immortalized cell lines retain the phenotype of the parent cells and preserve the contact inhibition while the transformed cells have a substantially increased growth rate [1,7,18]. In the current study, following gene transfection, Z310 cells displayed the genotypic characteristics of immortalized cells such as resistance to G418 selection and persistent expression of predominantly nuclear staining for SV40 large T antigen.* The cells that survived from the crisis exhibited an extended life span in vitro. By these criteria, the immortalization appeared to be successful in Z310 cells.
Phenotypically, Z310 cells share many similarities with primary choroidal cells. A uniform polygonal epithelial type was consistently observed throughout the life span of the culture. An important property from a functional point of view is the cells’ ability to produce TTR. TTR is a 55 000-Da protein consisting of four identical subunits in a tetrahedral symmetry [11]. Per unit of weight, the choroid plexus contains 10 times more TTR mRNA than liver, which produces TTR for serum [6]. Since the choroid plexus epithelium is the only cell type capable of synthesis and secretion of TTR in the mammalian brain, the presence of TTR in the culture should reflect exclusively the choroidal epithelial origin of the transfected cells. Current studies using different approaches have repeatedly demonstrated that Z310 cells possess the capability to produce TTR. These data clearly show that the immortalized Z310 cells carry the same phenotypic traits as the original primary choroidal epithelia.
One of the concerns in cell immortalization practice is the long-term genetic drift that may lead to the loss of phenotypic characteristics of immortalized cells. Our recent studies at the 110th passage showed that Z310 cells retain the original polygonal morphology. More importantly, the cells continue to express TTR and transferrin receptor.
Transferrin receptor has been shown to be expressed extensively in both blood–brain barrier and blood–CSF barrier [3,12,32]. By modulating the transport of transferrin-conjugated iron, both barriers contribute to the homeostasis of iron in the central milieu. Our previous results have revealed that among three cultured cell types, i.e., primary choroidal epithelia, dopaminergic PC12 cell line, and primary astrocytes, the choroidal epithelia appear to express transferrin receptor mRNA far more abundantly than astrocytes [28,29,32]. The current study provides evidence of the presence of transferrin receptor in Z310 cells, suggesting that Z310 cells may possess an iron-regulatory mechanism similar to that of the ascendant primary choroidal cells.
The primary choroidal epithelial cells can form an impermeable barrier when they are cultured on a porous membrane between inner and outer chambers of a Transwell apparatus [22,31]. The formation of such a ‘barrier’ type of feature is usually characterized by observations such as the formation of a confluent monolayer, the maintenance of an uneven fluid level between inner and outer chambers for the duration of the culture, and the establishment of electrical resistance of 99–200 Ω-cm2 which is increased once the monolayer of cells grows to confluence. In the current study, the net electrical resistance across Z310 cell layer ranged between 150 and 200 Ω-cm2, which approximated the values obtained from the primary choroidal cell cultures. On freshly isolated plexus tissues, the electrical resistance tends to be lower than those measured in cultured cells. For example, Zuethen and Wright [25] reports a value of 26 Ω-cm2 from fresh bullfrog choroid plexus, which is about 20 times higher than the value of peripheral endothelial capillaries (1–2 Ω-cm2). At present, it is still unclear what constitutes reasonable values of electrical resistance in cultured cells, which values are comparable to those in live plexus tissues land whether they are representative of the barrier structure. However, the values from early reports of 170 Ω-cm2 [20], to 99.4 Ω-cm2 [22], and most recently of 178 Ω-cm2 [23] appear to be commonly observed (150–200 Ω-cm2), although few studies using serum-depleted medium have reported an electrical resistance value as high as 1500 Ω-cm2 [10]. Cautions must be taken, however, in interpreting the electrical resistance as an indicator of tight junctions between the epithelial cells. The electrical resistance reflects only the tightness of the monolayer of confluent cells, but not the physical trademark of tight junctions. We have not yet examined the tight junctions via electron microscopy. In addition, the functional permeability of the cell layer to a variety of molecular markers such as creatinine, mannitol, blue dextran, etc., has not yet been tested.
During the course of preparation of this work, a report on the establishment of immortal plexus cells (TR-CSFB cells) from a transgenic animal model that carries an immortalizing gene has come to our notice [17]. By comparison, TR-CSFB cells display the phenotypical polygonal cell shape similar to the current Z310 cells. Both Z310 and TR-CSFB cell lines expressed functional marker TTR. Based on the availability of experimental settings, TR-CSFB cells were capable of taking up various amino acids from culture media, while Z310 cells transported thyroxine across the monolayer barrier (data not shown). It is, however, not clear as to the effect of long-term in vitro maintenance on the stability of TR-CSFB cell line with regards to its culture dynamics (e.g., doubling time, etc.), its generation parameters (e.g., crisis period, passage information, etc.), and its genotypic characters (e.g., the loss of expression of SV40 large T and/or TTR). The other differences between these two cell lines include a longer doubling time (35–40 h) and a lower electrical resistance (50 Ω-cm2) in TR-CSFB cells than in Z310 cells.
In summary, we have successfully inserted the pSV3neo plasmid into the host genome of rat choroidal epithelial cells by using gene transfection techniques. The transfected epithelial cells that survived cytotoxic selection display typical epithelial morphology. Moreover, these cells possess functions unique to the choroidal epithelia. This newly established cell line may not completely correspond to the primary cultures in many functional assessments, but may conceivably become an invaluable tool for studying biology, physiology, pharmacology, pathology, as well as the toxicology of the blood–CSF barrier.
Acknowledgements
We gratefully acknowledge Susan Lai and J. Richard Pilsner for their technical assistance and Mark Opler for his editing with this manuscript. This research was supported in part by National Institute of Environmental Health Grant ES08146, Calderone Foundation, and Wellcome Foundation.
Abbreviations
- CSF
cerebrospinal fluid
- BBB
blood–brain barrier
- BCB
blood–CSF barrier
- TTR
transthyretin
References
- 1.Burns JS, Lemoine L, Lemoine NR, Williams ED, Wynford-Thomas D. Thyroid epithelial cell transformation by a retroviral vector expressing SV40 large T. Br. J. Cancer. 1989;59:755–760. doi: 10.1038/bjc.1989.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc. Res. Tech. 2001;52:65–82. doi: 10.1002/1097-0029(20010101)52:1<65::AID-JEMT9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Connor JR, Benkovic SA. Iron regulation in the brain: histochemical, biochemical, and molecular considerations. Ann. Neurol. 1992;32(Suppl):S51–S61. doi: 10.1002/ana.410320710. [DOI] [PubMed] [Google Scholar]
- 4.Crook RB, Kasagami H, Prusiner SB. Culture and characterization of epithelial cells from bovine choroid plexus. J. Neurochem. 1981;37:845–854. doi: 10.1111/j.1471-4159.1981.tb04470.x. [DOI] [PubMed] [Google Scholar]
- 5.Davson H, Segal MB. Physiology of the CSF and Blood–Brain Barriers. New York: CRC Press; 1996. pp. 25–47. [Google Scholar]
- 6.Dickson PW, Howlett GJ, Schreiber G. Rat transthyretin (pre-albumin): molecular cloning, nucleotide sequence, and gene expression in liver and brain. J. Biol. Chem. 1985;260:8214–8219. [PubMed] [Google Scholar]
- 7.Eves EM, Tucker MS, Roback JD, Downen M, Rosner MR, Wainer BH. Immortal rat hippocampal cell lines exhibit neuronal and glial lineages and neurotrophin gene expression. Proc. Natl. Acad. Sci. USA. 1992;89:4373–4377. doi: 10.1073/pnas.89.10.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedheim E, Corvi C, Graziano J, Donnelli T, Breslin D. Choroid plexus as protective sink for heavy metals? Lancet. 1983;i(8331):981–982. doi: 10.1016/s0140-6736(83)92099-8. [DOI] [PubMed] [Google Scholar]
- 9.Graham FL, Van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 10.Hakvoort A, Haselbach M, Wegener J, Hoheisel D, Galla HJ. The polarity of choroid plexus epithelial cells in vitro is improved in serum-free medium. J. Neurochem. 1998;71:1141–1150. doi: 10.1046/j.1471-4159.1998.71031141.x. [DOI] [PubMed] [Google Scholar]
- 11.Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: Nutritional implications. Ann. Rev. Nutr. 1994;14:495–533. doi: 10.1146/annurev.nu.14.070194.002431. [DOI] [PubMed] [Google Scholar]
- 12.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 13.Jha KK, Banga S, Palejwala V, Ozer HL. SV40-Mediated immortalization. Exp. Cell Res. 1998;245:1–7. doi: 10.1006/excr.1998.4272. [DOI] [PubMed] [Google Scholar]
- 14.Johanson CE. Ventricles and cerebrospinal fluid. In: Conn PM, editor. Neuroscience in Medicine. Philadelphia, PA: Lippincott; 1995. pp. 171–196. [Google Scholar]
- 15.Johnson MD, Anderson BD. In vitro models of blood–brain barrier to polar permeants: comparison of transmonolayer flux measurements and cell uptake kinetics using cultured cerebral capillary endothelial cells. J. Pharm. Sci. 1999;88:620–625. doi: 10.1021/js9803149. [DOI] [PubMed] [Google Scholar]
- 16.Katakura Y, Alam S, Shirahata S. Immortalization by gene transfection. Methods Cell Biol. 1998;57:69–91. doi: 10.1016/s0091-679x(08)61573-3. [DOI] [PubMed] [Google Scholar]
- 17.Kitazawa T, Hosoya K, Watanabe M, Takashima T, Ohtsuki S, Takanaga H, Ueda M, Yanai N, Obinata M, Terasaki T. Characterization of the amino acid transport of new immortalized choroid plexus epithelial cell lines: a novel in vitro system for investigating transport functions at the blood–cerebrospinal fluid barrier. Pharm. Res. 2001;18:16–22. doi: 10.1023/a:1011014424212. [DOI] [PubMed] [Google Scholar]
- 18.Muruganandam A, Herx LM, Monette R, Durkin JP, Stanimirovic DB. Development of immortalized human cerebromicrovascular endothelial cell line as an in vitro model of the human blood–brain barrier. FASEB J. 1997;11:1187–1197. doi: 10.1096/fasebj.11.13.9367354. [DOI] [PubMed] [Google Scholar]
- 19.Peraldi-Roux S, Nguyen-Than Dao B, Hirn M, Gabrion J. Choroidal ependymocytes in culture: expression of markers of polarity and function. Int. J. Dev. Neurosci. 1990;8:575–588. doi: 10.1016/0736-5748(90)90050-c. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Wright EM. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J. Physiol. 1983;336:635–648. doi: 10.1113/jphysiol.1983.sp014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J. Mol. Appl. Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- 22.Southwell BR, Duan W, Alcorn D, Brack C, Richardson SJ, Kohrle J, Schreiber G. Thyroxine transport to the brain: role of protein synthesis by the choroid plexus. Endocrinology. 1993;133:2116–2126. doi: 10.1210/endo.133.5.8404661. [DOI] [PubMed] [Google Scholar]
- 23.Strazielle N, Ghersi-Egea JF. Demonstration of a coupled metabolism-efflux process at the choroid plexus as a mechanism of brain protection toward xenobiotics. J. Neurosci. 1999;19:6275–6289. doi: 10.1523/JNEUROSCI.19-15-06275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsumi M, Skinner MK, Sanders-Bush E. Transferrin gene expression and synthesis by cultured choroid plexus epithelial cells. J. Biol. Chem. 1989;264:9626–9631. [PubMed] [Google Scholar]
- 25.Zeuthen T, Wright EM. Epithelial potassium transport: tracer and electrophysiological studies in choroid plexus. J. Membr. Biol. 1981;60:105–128. doi: 10.1007/BF01870414. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W. Toxicology of choroid plexus: a special reference to metal-induced neurotoxicities. Microsc. Res. Tech. 2001;52:89–103. doi: 10.1002/1097-0029(20010101)52:1<89::AID-JEMT11>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng W. Neurotoxicology of brain barrier systems: new implications. J. Toxicol. Clin. Toxicol. 2001;39:711–719. doi: 10.1081/clt-100108512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W. Blood–brain barrier and blood–CSF barrier in metal-induced neurotoxicities. In: Massaro EJ, editor. Handbook of Neurotoxicology. Vol. 1. Totowa, NJ: Humana Press; 2002. pp. 161–193. [Google Scholar]
- 29.Zheng W, Zhao Q. Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–179. doi: 10.1016/s0006-8993(01)02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng W, Blaner WS, Zhao Q. Inhibition by Pb of production and secretion of transthyretin in the choroid plexus: Its relationship to thyroxine transport at the blood–CSF barrier. Toxicol. Appl. Pharmacol. 1999;155:24–31. doi: 10.1006/taap.1998.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng W, Zhao Q, Graziano JH. Primary culture of rat choroidal epithelial cells: a model for in vitro study of the blood–cerebrospinal fluid barrier. In Vitro Cell Biol. Dev. 1998;34:40–45. doi: 10.1007/s11626-998-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]