Abstract
Disruptions in emotional, cognitive, and social behavior are common in neurodegenerative disease and many forms of psychopathology. Because neurodegenerative diseases have much clearer patterns of brain atrophy, they may provide a window into the neural bases of these common symptoms. We discuss five common symptoms that occur in both neurodegenerative disease and psychopathology (i.e., anxiety, dysphoric mood, apathy, disinhibition, and euphoric mood) and their associated neural circuitry. We focus on two neurodegenerative diseases (i.e., Alzheimer’s disease and frontotemporal dementia) that are common and well-characterized in terms of emotion, cognition, and social behavior and in patterns of associated neuropathology. Neurodegenerative diseases provide a powerful model system for studying the neural correlates of psychopathological symptoms; this is supported by evidence indicating convergence with psychiatric syndromes (e.g., symptoms of disinhibition associated with dysfunction in orbitofrontal cortex and inferior frontal gyrus in both frontotemporal dementia and bipolar disorder). We conclude that neurodegenerative diseases can play an important role in future approaches to the assessment, prevention, and treatment of mental illness.
Keywords: Neurodegeneration, psychopathology, emotion, neural circuits, Alzheimer’s disease, frontotemporal dementia
Introduction
The major premise of this chapter is that neuropathology provides a royal road for understanding psychopathology. Historically, neurological injuries have largely been seen as causing deficits in cognitive domains such as memory. Although certainly relevant to some forms of mental illness (e.g., amnesias in dissociative disorders), these kinds of deficits do not really capture the dramatic emotional and social changes that form the bedrock symptoms of psychopathology. Moreover, although prevailing diathesis-stress models of mental illness (Monroe & Simons 1991) surely could accommodate both biological and psychological vulnerabilities and exacerbations, mid-20th century research on the etiology of severe psychopathologies put greatest emphasis on the importance of psychological factors rather than biological ones. During this period, the spotlight shined most brightly on psychological etiologies such as communication patterns (e.g., double bind communications and schizophrenia; Bateson et al 1956), parental styles (e.g., refrigerator mothers and autism; Kanner 1943), and the familial emotional climate (e.g., expressed emotion and relapse; Brown 1959).
Today, the spotlight in psychopathology research has clearly shifted toward a more biological, brain-centric focus. This transition arguably began with the introduction of pharmacological and biological treatments for severe mood disorders and psychosis (e.g., the introduction of chlorpromazine and electroconvulsive therapy into psychiatric practice in the 1940s and 1950s) and the accumulating evidence of the heritability of psychiatric syndromes arising from twin and family studies (e.g., Gottesman 1991). In more recent times, the dramatic methodological advances in brain imaging opened the floodgates for a deluge of studies reporting that the brains of the mentally ill are different in structure, function, resting activity, and connectivity from those of the mentally healthy. Contemporary biological models of mental illness often emphasize the importance of a holy trinity consisting of genes, molecules, and circuits. Most recently, the place of the brain at the top of this rarified hierarchy was underscored with the emergence of the Research Domain Criteria initiative (RDoC; Insel et al 2010) at the National Institute of Mental Health (NIMH). In RDoC, psychopathology is not viewed in terms of traditional Diagnostic and Statistical Manual (DSM, A.P.A. 1994) syndromes (e.g., schizophrenia, major depressive disorder), but rather in terms of dimensions of neurobiology and observable behavior (e.g., sustained responsiveness to reward, perception and understanding of self and others, Cuthbert & Insel 2013). For a “construct” (i.e., a behavior that spans a dimension of normal to abnormal functioning) to be included in RDoC, there must be a plausibly associated brain circuit. Genes and molecules still play an important role in RDoC, representing specifications of the constructs at different levels of analysis, but it is the existence of an associated brain circuit that is necessary for a behavior to be included.
Psychopathology research has clearly embraced imaging studies of psychiatric patients. These studies take advantage of significant advances in scanner sensitivity; ways to study brain networks and assess their intrinsic connectivity; and methods for pipelining, processing, and analyzing imaging data. Against this backdrop, we will be presenting a somewhat contrarian position, namely that patients with neurodegenerative disease may provide an ideal model system for understanding the neural circuitry associated with key symptoms of mental illness. In addition to differences in the types of patients, the neurodegenerative disease approach differs in its greater use of structural relative to functional magnetic resonance imaging (fMRI). Because neurodegenerative diseases can produce widespread damage in multiple brain regions in ways that differ among individual patients, structural imaging is critical for precisely characterizing areas that are injured and those that are spared. These differences notwithstanding, we believe studies of neurodegenerative disease can be extremely useful in understanding the neural circuitry that underlies some of the most important symptoms of mental illness.
Neurological patients versus functional imaging
At their core, psychopathologies are manifest as dysfunctions in emotional, cognitive, and social functioning. Historically, studies of neurological patients have contributed immensely to fundamental discoveries in psychology related to the neural circuitry that underlies these processes. As we previously noted:
“Studies of neurological patients have been critical to advancing our understanding of the human brain. In some instances, fundamental insights were gained and doors opened to entire new areas of inquiry based on findings from a single patient such as Phineas Gage and the frontal lobes (Harlow 1848) and Patient H.M. and the organization of memory (Scoville & Milner 1957). In other instances, findings from a small group of patients were seminal, such as epileptics treated with cerebral commissurotomy and hemispheric specialization (Gazzaniga & Sperry 1967). In addition to these spectacular advances, there has been a steady and continuing parade of findings derived from the deceptively simple strategy of identifying individuals with loss in particular brain areas of interest, determining how their abilities and functioning differ from the norm, and studying the ways that they change over time (Levenson 2007, p. 158).
Ironically, despite this illustrious history, studies with neurological patients seem much less prevalent in contemporary clinical science than studies using functional imaging. However, it is important to recognize that functional imaging methodologies come with their own set of constraints and limits, including imposing an experimental environment in which participants must lie down in a cramped tube, stay still, and endure a symphony of banging sounds. Fortunately for psychology, there are many cognitive processes that can be studied in this environment without great compromise (e.g., learning, memorizing, deciding, recognizing, calculating, choosing). But this is not an ideal environment for studying the kinds of complex emotional and social behaviors that are impacted by psychopathology; such full-blown emotional and social processes tend to involve action, movement, other people, and are highly vulnerable to environmental distractions.
Even if it were possible to induce powerful emotions in the scanner, the attendant muscular activity in the body and face and vocalizations would produce huge signal artifacts. As a result, a great deal of emotional and social research conducted in the scanner falls into the realms of social and emotional cognition where the focus is on how we think about and make judgments about social and emotional processes rather than the actual processes as they unfold in real time. An example in the realm of emotion may be illustrative. Most scanner studies of fear are more likely to assess brain activity when people are recognizing fear (e.g., in a photograph) rather than when they are actually experiencing fear. Or if they are experiencing fear, it is likely to be of the mildest and most non-motoric form. Similar constraints exist when studying social behaviors, with scanner studies more likely to involve assessing brain activity when individuals make social judgments rather than when they engage in actual social interactions (although some simple, non-active social behaviors such as hand-holding have been successfully brought into the scanner; Coan et al 2006). These constraints in studying social and emotional behaviors become all the more critical when applied to the study of psychopathology. Although problems with emotional and social cognition are important in some psychopathologies (e.g., distorted views of self and others in narcissistic personality disorder and in schizophrenia, inability to recognize emotions in others in antisocial personality disorder and autism), dysfunctions in these domains often involve high levels of activation and high levels of interpersonal complexity.
In studies of patients with brain damage, the scanner can play an important, but very different role, obtaining a “snapshot” that documents the morphology of the injury. Behaviors are not constrained by the need to be assessed in the scanner. Instead, the behaviors of interest can be observed under controlled conditions in the laboratory (Levenson 2007) or clinic or in more naturalistic conditions in the patient’s world, including at home and at work. Importantly, given the impact that mental illness has on social functioning, patient behavior can be observed in a full range of social contexts including actual interactions with friends, family, and co-workers. As we have previously noted:
“The great power and advantage of patient studies is that the behavioral, cognitive, and emotional “dependent measures” need not be constrained. Patient studies allow use of the entire armamentarium of methods and techniques available for studying the full range of basic behavioral and social processes under both controlled and naturalistic conditions.” (Levenson 2007, pp. 158–159)
Neurodegenerative disease: An opportunity to study neural circuits
Brain lesions can result from a number of processes including trauma, inflammation, tumor, stroke, and neurodegenerative disease. With H.M. and the split brain (i.e., cerebral commissurotomy) patients described earlier, the lesions were produced surgically (to control epilepsy) and thus could be specified quite precisely. As a result, they provided a remarkable opportunity to examine the behavioral deficits that accompanied injury to particular brain regions (e.g., hippocampus and medial temporal lobe structures in H.M., corpus callosum in the split brain patients). Studying the function of particular brain structures and relating them to psychopathology remains an active area of inquiry (e.g., the specialization of the fusiform gyrus for face recognition and its role in autism; Kanwisher et al 1997, Weigelt et al 2012). Increasingly, however, the focus in contemporary neuroscience has turned to consideration of how multiple structures participate in more distributed circuits. This influence can be clearly seen in RDoC with its explicit emphasis on neural circuits.
When Phineas Gage had his unfortunate encounter with the errant tamping iron, he suffered quite extensive damage throughout his left frontal lobe (Harlow 1848). Debate about the exact anatomy of this injury (Damasio et al 1994, Ratiu et al 2004) and the nature of the associated behavioral change continues to this day (Macmillan 2000). The uncertainty around the injury to Gage’s brain and the associated changes in behavior underscore the importance of having precise ways to characterize both the anatomy of brain injuries and the associated behavioral changes.
Neurodegenerative disease: From cons to pros
Neurodegenerative diseases (e.g., Alzheimer’s disease [AD], Parkinson’s disease, frontotemporal dementia [FTD]) present challenges for studying brain-behavior when compared to studies of focal lesions. Historically, neurodegenerative diseases have been seen as presenting problems in: (a) anatomy (the damage is diffuse, far-flung, and sometimes seemingly random), (b) stability (the damage is progressive and constantly expanding), and (c) measurement (the damage is difficult to characterize precisely in living patients, often requiring autopsy to specify the underlying neuropathology). Moreover, neurodegenerative diseases have been thought to affect “cognitive” processes primarily (e.g., memory, visuospatial abilities, language, executive control, computation), and thus do not capture the emotional and social processes that are so important in psychopathology. As often is the case, there are elements of truth in each of these arguments. However, increasingly the tide seems to be turning in the other direction, with neurodegenerative diseases emerging as highly promising model systems for studying brain-behavior relationships in general and for studying the neural circuitry associated with psychopathology in particular. The causes of these changes are instructive.
In terms of anatomy, increasingly it appears that neurodegenerative diseases are not diffuse and random but rather target and spread along neural networks that are structurally connected and have functional significance (Buckner et al 2005, Seeley et al 2009). Put in simple terms, major neural networks in normal brains serve as highways along which neurodegeneration progresses. For example, AD targets the “default mode” network (medial temporal, posterior cingulate/precuneus, lateral temporo-parietal) involved in episodic memory (i.e., memory for specific events and experiences), whereas behavioral variant FTD targets a very different network (anterior insula, temporal pole, pregenual anterior cingulate cortex, ventral striatum, hypothalamus, periaqueductal gray, amygdala) linked to emotional salience processing (Seeley et al 2009). This affinity for networks means that neurodegenerative diseases can provide an ideal model for understanding the functions these networks serve in normal brains and for documenting the behavioral changes associated with network damage.
In terms of stability, patients with focal lesions are often studied long after the brain injury occurred. As a result, compensatory mechanisms (in both brain and behavior) may have developed that obscure the original relationships between brain injuries and behavioral deficits. With neurodegenerative diseases, behavioral changes can be studied quite close to the time of injury. One particularly promising and still largely untapped approach is to use repeated assessments and longitudinal data analyses to link changes in brain circuits with changes in behavior. This kind of approach can help control for the confounding effects of premorbid individual differences in the circuits and behaviors of interest.
In terms of measurement, remarkable advances have been made in ways to characterize the location and extent of brain injury in living patients with neurodegenerative diseases. These include: (a) measuring the extent and location of volume loss using voxel-based morphometry (Ashburner & Friston 2000) and semi-automated methods such as FreeSurfer (Dale et al 1999); (b) assessing the integrity of white-matter tracts using diffusion tensor imaging (Basser et al 1994); and (c) assessing the accumulation of particular neurotoxic substances using marking compounds and positron-emission tomography (e.g., Pittsburgh compound B to detect beta-amyloid accumulation, Klunk et al 2004).
The high prevalence rates of neurodegenerative diseases constitute an enormous public health problem. For example, estimates are that there are over 5 million Americans currently living with AD. Sizeable patient populations also create remarkable opportunities to study brain-behavior relationships using much larger samples than has been the case in typical studies of focal lesion patients. These large samples beget significant scientific benefits including greater statistical power, ability to aggregate anatomically similar cases to increase signal-to-noise ratios, improved control for premorbid behavioral differences, availability of replication samples, and ability to study exemplars of particular degeneration patterns of interest.
Using neurodegenerative patients to study the neural circuitry of psychopathology
Neurodegenerative diseases that target brain circuits involved in social and emotional processes create symptoms that mimic those seen in psychiatric disorders. This mimicry can be quite close, so close in fact that they confound the diagnostic efforts of even highly experienced clinicians (including psychiatrists, neurologists, family physicians, and clinical psychologists). Many of the patients we see in our research who will go on to receive confirmed diagnosis of FTD at autopsy have histories of prior diagnosis with psychiatric disorders (e.g., mood and psychotic disorders, Woolley et al 2011, Woolley et al 2007). Moreover, many received psychotropic medications (e.g., anti-depressants, mood stabilizers, anti-psychotics) prior to being diagnosed with dementia. As neurodegenerative diseases with prominent behavioral symptoms (such as FTD) become more familiar outside of university and medical research centers, diagnosticians are more likely to consider neurodegenerative diseases in their differential diagnoses. Nonetheless, similarities in presenting behavioral symptoms between neurodegenerative and psychiatric diseases can make differential diagnosis quite difficult.
The argument for using neurological diseases to shed light on the likely neural circuitry underlying psychiatric symptoms is based on large part on the similarity of the behavioral phenotypes. Thus, if a group of neurological patients with known brain atrophy (e.g., FTD patients with damage to the circuit involving the anterior cingulate cortex and temporal pole documented on structural images via voxel-based morphometry) present with symptoms (e.g., blunted affect) that are essentially identical to those seen in psychiatric patients (e.g., those with schizophrenia or autism), then the same circuitry is a prime candidate for being involved in producing the symptoms in the psychiatric patients.
Clearly, this proof-by-analogy requires several significant assumptions and is not without controversy. Importantly, it is premised on an assumed one-to-one relationship between particular well-defined behavioral symptoms and particular neural circuits. The alternative hypothesis, that particular symptoms are associated with different underlying neural circuits in neurological and psychiatric patients is certainly possible, but seems unlikely and unparsimonious. Moreover, a small number of studies of psychiatric patients using sensitive assessments of brain structure and function to link symptoms with underlying neural structures show striking overlap with the links derived from the study of neurodegenerative patients (see below).
We believe that in the new RDoC era, the use of neurodegenerative patients can assume a place of prominence among the various approaches for identifying neural circuitry (e.g., animal models, functional imaging studies with patients and patient analogs, transcranial magnetic stimulation, focal lesion patients). Each approach has its strengths, weaknesses, and inherent biases; thus, there is great value in seeking areas of convergence in brain-behavior links established using different methods and different patient populations.
Neurodegenerative diseases
Neurodegenerative diseases typically have an onset in mid-to late-life and are characterized by insidious onset and gradual decline. In the early stages of progression, each neurodegenerative disease is characterized by degeneration of a specific large-scale brain network that is associated with the clinical manifestations of the disease in terms of motor, cognitive, behavioral, and emotional symptoms (Seeley et al 2009). As the diseases progress, the pathological process can become more diffuse and spread to other networks in patterns constrained by network-based organization (Zhou et al 2012). Although post-mortem pathological studies are the gold standard for determining diagnosis and disease etiology, clinical evaluations that include neurological, neuropsychological, and neuroimaging studies can distinguish between these diseases during life with increasing reliability (Miller 2009).
In this chapter, we focus on AD and FTD, two neurodegenerative diseases that target different neural networks (Zhou et al 2010) and present with different socioemotional phenotypes (for a review see Teng et al 2009). Both of these diseases present major public health problems. AD is the most common form of dementia with prevalence rates of about 11% among individuals age 65 or older and at least 32% among individuals age 85 and older (Thies & Bleiler 2013). FTD is much less prevalent than AD among individuals over the age of 65, but is at least as common (Ratnavalli et al 2002) or even more prevalent (Knopman et al 2004) in those under the age of 65. Clinical and neuroanatomical profiles for both diseases are summarized in Table 1.
Table 1.
Alzheimer’s disease (AD) and frontotemporal dementia (FTD) have distinct neuroanatomical and clinical profiles.
| Neurodegenerative Disease |
Typical Brain Atrophy |
Primary Cognitive Symptoms |
Primary Emotional and Behavioral Symptoms |
|---|---|---|---|
| AD | Medial temporal lobe (entorhinal cortex and hippocampus) Parietal (precuneus) Posterior cingulate |
Episodic memory Language Visuospatial functioning |
Anxiety Low mood Apathy |
| FTD | Frontal lobe (anterior insula, anterior cingulate cortex, orbitofrontal cortex) Anterior temporal lobe and amygdala |
Executive control | Apathy Disinhibition Loss of Empathy Elation |
Although other neurodegenerative diseases (e.g., corticobasal syndrome, progressive supranuclear palsy, amyotrophic lateral sclerosis, dementia with Lewy bodies) may also affect cognition, emotion, and behavior, they have been the focus of much less relevant research and thus are not included in this review.
Alzheimer’s disease
In AD, one of the earliest sites of pathology is in the medial temporal lobe (for a review of AD's molecular pathology, see Jagust 2013). Progressive accumulations of β-amyloid plaques and tau tangles in the brain (Jack et al 2013) are thought to begin in the entorhinal cortex and hippocampus and gradually progress to other limbic and then cortical structures (Braak & Braak 1995). The hippocampus, with connections to precuneus, posterior cingulate cortex, lateral prefrontal cortex, and lateral temporoparietal cortex, is a key hub in the default mode network (Raichle et al 2001), a network that supports episodic memory processes (Buckner et al 2008). In AD, deterioration of default mode network connectivity is accompanied by the emergence of cognitive symptoms including episodic memory impairment (Buckner et al 2005) as well as decline in visuospatial processing, language, and executive functioning (Mendez 2012).
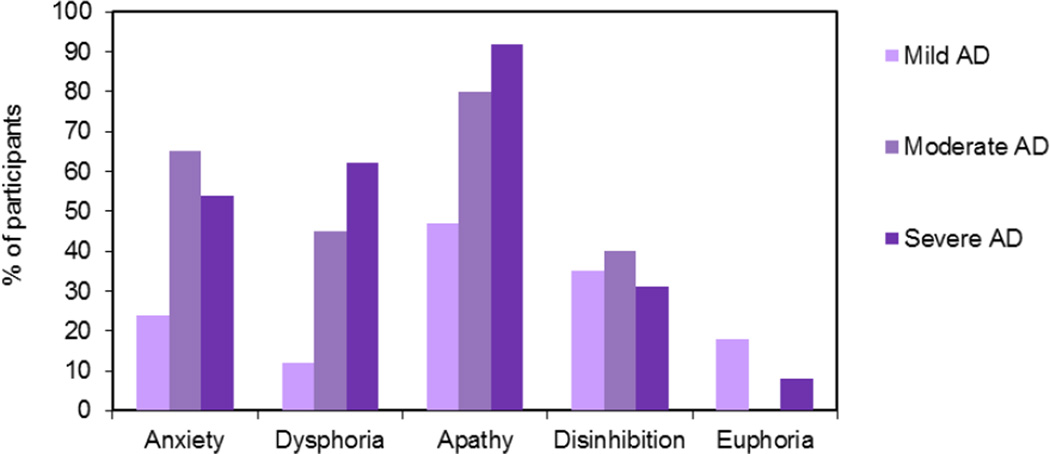
Social behavior is often preserved in AD, and patients may retain their social graces and interpersonal relationships even late into the disease (Sturm et al 2011). This preservation may result from a disjunction between network changes. As intrinsic connectivity declines in the default mode network in AD, connectivity may actually increase in the emotional salience network (anterior insula, anterior cingulate cortex, and thalamus, among others), which is devoted to affective stimulus detection and visceromotor emotion generation (Seeley et al 2007, Zhou et al 2010). These changes can result in a large number of behavioral changes, some of which can be considered as positive (e.g., preservation of emotional reactivity) and others that are quite troublesome (e.g., irritability, aberrant motor behavior, agitation, and aggression; Balthazar et al 2013). In Figure 1, prevalence rates for common symptoms of psychopathology in AD are depicted.
Figure 1.
Prevalence estimates for symptoms of psychopathology in Alzheimer’s disease (AD) reported by caregivers using the Neuropsychiatric Inventory (Cummings 1997). Data from Mega and colleagues (1996). Mini Mental State Examination scores for Mild AD: 21–30; Moderate AD: 11–20; Severe AD: 0–10
Frontotemporal dementia
FTD is a clinically heterogeneous disease characterized by gradual neurodegeneration of the frontal and anterior temporal lobes. Pathologically, FTD is most often caused by abnormal aggregation of tau or TDP-43 proteins (Dickson et al 2011, Josephs et al 2011). Although most cases are sporadic, occurring without known cause, some cases are associated with known genetic mutations (Cohn-Hokke et al 2012).
FTD is an umbrella term that includes three clinical subtypes: (a) behavioral variant FTD, (b) semantic variant primary progressive aphasia, and (c) non-fluent variant primary progression aphasia (Gorno-Tempini et al 2011, Rascovsky et al 2007). In behavioral variant FTD, impairments in social behavior and emotion are common early features. Patients have dramatic declines in their interpersonal functioning and exhibit empathy deficits, disinhibition, compulsive behavior, apathy, aberrant motor behavior, and executive dysfunction (Neary et al 1998, Rascovsky et al 2007). These early behavioral changes are often accompanied by neurodegeneration of salience network structures that are essential for social behavior and emotion including right anterior insula, pregenual anterior cingulate cortex, and amygdala (Seeley et al 2008). Deterioration of this salience network may hinder patients’ ability to detect relevant affective cues, monitor their own emotional behavior, respond empathically, and interact appropriately with others (Banks & Weintraub 2008, Eslinger et al 2005, Rankin et al 2005, Snowden et al 2003).
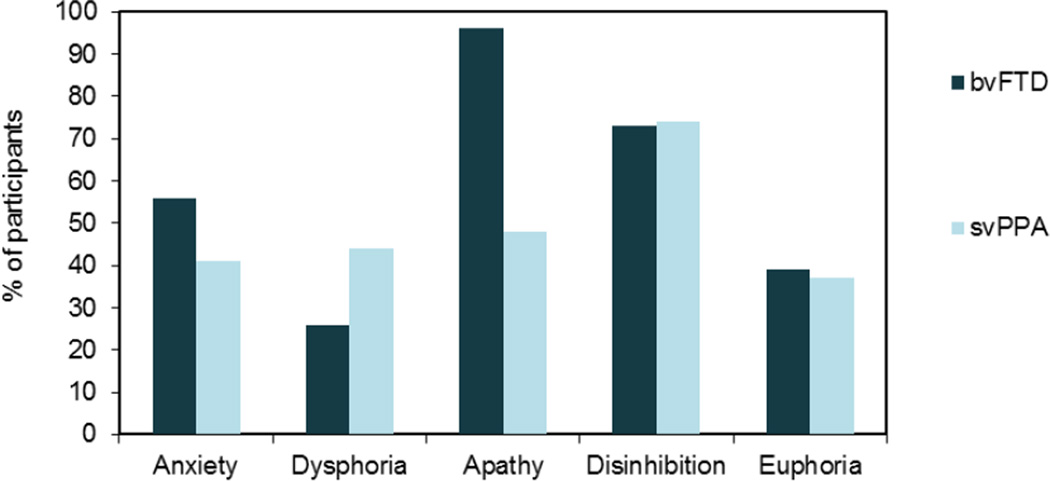
In semantic variant primary progressive aphasia, predominantly left-hemisphere atrophy in the temporal pole leads to decline in single-word object knowledge. In non-fluent variant primary progressive aphasia, predominantly left-hemisphere atrophy in the inferior frontal gyrus and anterior insula disrupts motor-speech output and syntax (Gorno-Tempini et al 2011, Grossman et al 2012). Each of the clinical subtypes of FTD is initially characterized by a somewhat unique constellation of symptoms and anatomy. However, as the disease progresses regions involved in socioemotional regulation become affected and behavioral changes become increasingly common (Rosen et al 2002, Seeley et al 2005). In Figure 2, prevalence rates for common symptoms of psychopathology in FTD are depicted.
Figure 2.
Prevalence estimates for symptoms of psychopathology in frontotemporalobar dementia (FTD) reported by caregivers using the Neuropsychiatric Inventory (Cummings 1997). Data on behavioral variant FTD (bvFTD) and semantic variant primary progressive aphasia (svPPA) from Liu and colleagues (2004).
Neuropathology and five major symptoms of psychopathology
Traditional DSM diagnoses (e.g., schizophrenia, major depressive disorder) are notorious for heterogeneity of symptoms, both within and across syndromes. As an example of within-syndrome heterogeneity, some patients diagnosed with schizophrenia have primarily negative symptoms (e.g., social withdrawal, emotional blunting) while others have primarily positive symptoms (e.g., hallucinations, delusions). That such widely disparate symptoms would have a common underlying neural circuitry seems highly unlikely. As an example of across-syndrome heterogeneity, the same symptom (e.g., anxiety, sleep disturbance) can appear in multiple disorders (e.g., Harvey et al 2004). Together, these two kinds of heterogeneities powerfully argue against DSM diagnoses serving as the appropriate level of analysis for linking psychopathology with underlying neural circuitry.
For this review, we adopt a different approach, emphasizing specific behaviors rather than DSM diagnoses. The five behaviors associated with psychopathology that we have chosen (anxiety, dysphoric mood, apathy, disinhibition, and euphoric mood) have some overlap with constructs in the current version of RDoC (Cuthbert & Insel 2013), but are not identical. RDoC was designed to be a dynamic system, with the behavioral constructs evolving over time to reflect new knowledge (Insel et al 2010). We believe that these five behaviors are ones for which we can make a strong case for associated neural circuitry based on brain-behavior links using data from patients with neurodegenerative diseases. Only time will tell whether the constructs in the current RDoC, the ones we will be describing, or others yet to be proposed will be most useful for understanding the neural substrates of psychopathology.
Anxiety
Anxiety reflects uncontrolled feelings of apprehension and persistent worry or concern (Brown & Barlow 2009). It is a prime exemplar of a symptom that appears in multiple DSM disorders. For example, focal forms of anxiety can arise in response to specific triggers including social situations (social phobia), animals or objects (specific phobia), traumatic memories (post-traumatic stress disorder), or fear of contamination (obsessive-compulsive disorder). There is also a more diffuse, pervasive, and non-specific variant that appears in generalized anxiety disorder. Despite the great variability in the specific triggers that can cause anxiety and in the duration and intensity of the anxiety response itself (i.e., a sudden panic attack versus chronic unease), an overlapping characteristic that unites all anxiety disorders is dysregulation of fear (Ohman & Mineka 2001). When operating in a functional manner, fear is an extremely useful emotion that arises in response to threatening stimuli, mobilizes needed physiological resources (e.g., somatic, autonomic), and helps protect us from danger (Levenson 2003). When fear is out of control or occurs in response to non-threatening cues, however, it can be debilitating, creating unneeded physiological activation and dramatically interfering with normal functioning.
Anxiety is a common symptom in multiple neurodegenerative diseases, with prevalence estimates ranging from 5% to 21% for anxiety disorders and from 8% to 71% for anxiety symptoms (see review in Seignourel et al 2008). In mild cognitive impairment (MCI, cognitive impairment that exceeds expected age-related changes but is less profound than that seen in dementia) and AD, the majority of patients report anxiety symptoms (Apostolova & Cummings 2008, Spalletta et al 2010). Common manifestations of anxiety in patients with AD include worried appearance, fearfulness (e.g., fear of being left alone), tension, restlessness, and fidgeting (Ferretti et al 2001). Although there is some debate as to whether these symptoms reflect a psychological reaction to cognitive and functional decline or a biological change in emotion-relevant neural circuits, there is strong evidence for the latter. This includes findings of heightened intrinsic connectivity in the salience network in AD (Balthazar et al 2013) and hypo-metabolism in the medial temporal lobe, superior temporal gyrus, and insula (Hashimoto et al 2006), both associated with attendant increases in anxiety. In FTD, anxiety is more variable, with some studies suggesting lower prevalence rates than AD (de Vugt et al 2006, Liu et al 2004) and others reporting higher rates (Porter et al 2003). The anatomical correlates of anxiety in FTD have not been as well established. Although one study found that anxiety is more common in FTD patients with predominantly frontal (compared to temporal) damage (Liu et al 2004), another suggested that anxiety was associated with temporal hypo-metabolism (Mendez et al 2006b). One challenge with FTD is determining whether behavioral symptoms that are often associated with anxiety in other patient groups (e.g., aberrant motor behavior) are actually associated with feelings of anxiety when manifest by FTD patients.
Previous studies conducted in both animals and humans have delineated a neural circuit that supports fear. These neuroanatomical models emphasize the amygdala (central nucleus in particular) as a key structure in fear reactivity (LeDoux 2000). This framework has served as the foundation for more elaborate models in humans that propose that anxiety may be due to a combination of hyperactivity in emotional generation systems and hypoactivity in emotion regulation systems that help to modulate the fear response. Consistent with this hypothesis, multiple fMRI studies have found that individuals with anxiety disorders have higher activity in the amygdala, insula, anterior cingulate cortex, and ventromedial prefrontal cortex in response to negative emotional cues (Etkin & Wager 2007, Freitas-Ferrari et al 2010). Analyses of intrinsic connectivity in individuals with anxiety have also found heightened intrinsic connectivity between the amygdala and anterior cingulate cortex and other emotion generating structures (Pannekoek et al 2013) and decreased coupling between emotion generators (e.g., amygdala) and regions that promote emotional control including orbitofrontal and dorsolateral prefrontal cortex (Etkin et al 2009, Hahn et al 2011). Structural neuroimaging analyses, which measure regional brain volumes, have found mixed results as to whether individuals with anxiety have smaller or larger brain volumes in emotion-relevant structures. For example, smaller volume in left middle and superior temporal gyri and pregenual anterior cingulate cortex has been reported in anxiety disorders broadly (van Tol et al 2010), and larger volume in the central nucleus of the amygdala has been found in individuals with generalized anxiety disorder (Etkin et al 2009). Similarly, smaller volume in the temporal lobes and bilateral amygdala (but normal hippocampal volume) has been reported in individuals with panic disorder (Massana et al 2003, Vythilingam et al 2000). Both types of findings lend themselves to plausible explanations. For example, findings of larger volumes in emotion generators can be interpreted as indicating greater neural support for mounting fear responses. On the other hand, findings of smaller volumes in these structures can be interpreted as resulting from the accumulated wear and tear associated with chronic activation of the fear system over time. The influences of symptom severity and duration, medication and psychological treatment, and other biological and lifestyle factors likely also contribute to these differing results.
Dysphoric mood
In recent years, our understanding of the mood changes that accompany DSM depressive disorders has been evolving. Whereas at one time depression was thought to largely consist of high levels of sad affect (perhaps accompanied by other negative emotions such as anger), the emerging picture is more complex. Of course, sad mood is often seen in major depressive disorder, but laboratory studies have found a profile of affective responding that is “context insensitive.” In these studies, depressed patients have been shown to have blunted emotional reactivity to both positive and negative stimuli (Bylsma et al 2008). Moreover, when they become emotionally activated, they may have trouble recovering from negative emotional events (Salomon et al 2009), suggesting problems with downregulating negative emotion. As with so many DSM syndromes, we expect that the depressive disorders encompass multiple dysfunctional processes that are associated with different neural circuitry. In this regard, symptoms of overwhelming sadness seem likely to be dissociable from symptoms of blunted reactivity, apathy, and social withdrawal.
In neurodegenerative disease, symptoms of depression are common. Although the depressive symptoms experienced by individuals with dementia are quite similar to those seen in depressed older adults who are cognitively normal (Chemerinski et al., 2001), there is some indication that certain symptoms (e.g., irritability and social isolation) may be more common than others (e.g., diminished interest in activities) in depressed individuals with dementia (Olin et al., 2002a, 2002b). Some studies of depression in dementia do not specify the particular symptoms that are present, but those that do indicate that sadness, crying spells, and hopelessness commonly occur in depressed MCI patients (Apostolova & Cummings 2008, Gabryelewicz et al 2004, Lopez et al 2005) and that feelings of sadness are also common in depressed AD patients (Engedal et al 2011, Spalletta et al 2010). The picture in FTD is quite different. Depression is often reported in FTD (de Vugt et al 2006), but it is not as common as in AD (Levy et al 1996). Importantly, when depression is reported in FTD, it is more likely to be associated with apathy and social withdrawal (Blass & Rabins 2009) and less likely to be associated with sadness (Suzuki et al 1999). In our work with FTD patients, we have been struck by how often they are described by clinicians as being relatively “happy,” which may reflect an absence of sadness (and anxiety).
Influential models of the neurobiology of depression have proposed that altered connections between emotion generating and emotion regulating systems underlie mood symptoms. Consistent with this, individuals with depression have been shown to have heightened intrinsic connectivity in visceromotor emotion generators including subgenual anterior cingulate cortex and thalamus (Greicius et al 2007) and reduced dorsolateral prefrontal cortex metabolic activity (Bench et al 1992, Rogers et al 2004) at rest and in response to emotional stimuli (Davidson et al 2003). They also exhibit greater (Davidson et al 2003) and sustained (Siegle et al 2002) activity in the amygdala to emotional cues, a pattern that abates with successful treatment (Mayberg 2009, Sheline et al 2001). Some authors have concluded that depression involves an inability to modulate amygdala reactivity to changing emotional cues (Anand et al 2005, Sheline et al 2001), a hypothesis that is consistent with findings of context-insensitive emotional responding. Structural neuroimaging studies of depressed patients have found volume reductions in regions important for both emotion generation and regulation including the pregenual anterior cingulate cortex, dorsomedial prefrontal cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, hippocampus, and thalamus (Bora et al 2012, Du et al 2012). Although there is some evidence for a relationship between disease duration and smaller volume in these structures (Bora et al 2012, Sheline et al 1996, van Tol et al 2010), intrinsic connectivity analyses have found that longer mood episodes are associated with greater connectivity in subgenual anterior cingulate cortex (Greicius et al 2007). Of course, it is difficult to know the direction of causality in these relationships; anatomical differences may reflect pre-existent vulnerabilities or may result from disease processes, medications, and other factors.
Apathy
Apathy is a multi-faceted construct that refers to a constellation of cognitive, behavioral, and affective symptoms including emotional blunting, lack of initiative and motivation, and lack of interest in pursuing goal-directed activities (Marin 1991, Robert et al 2002). Because many patients do not endorse feelings of apathy, most studies of apathy rely on informant reports (e.g., Eslinger et al 2012, Robert et al 2002). Apathy has also been reported in studies of schizophrenia (Bonilha et al 2008). In addition, there are a number of related symptoms that have been reported in major depressive disorder (including psychomotor retardation, diminished interest or pleasure, and diminished ability to think or concentrate) and in schizophrenia (flat affect, alogia, anhedonia, and avolition). Apathy and this constellation of related symptoms span the domains of cognition, behavior, and affect and are related to problems in motivation, emotional responsiveness, social and intellectual interest, and self-initiated activity.
Apathy is one of the most common behavioral symptoms in neurodegenerative disease and is one of the most distressing symptoms for family members (de Vugt et al 2006, Massimo et al 2009). Apathy is quite common in AD, with prevalence rates ranging from 55–80% (Mega et al 1996, Spalletta et al 2010, van Reekum et al 2005). Apathy may be even more ubiquitous in FTD (Liu et al 2004, Massimo et al 2009) and is one of the core diagnostic criteria of behavioral variant FTD (Rascovsky et al 2007). Most studies evaluate apathy in dementia quite broadly, using instruments that are not designed to tease apart its various components. Nonetheless there is emerging evidence that different dementias are characterized by different forms of apathy. For example, apathy in AD is more likely to be associated with dysphoric mood than in FTD (Chow et al 2009). Conversely, apathy in FTD is more likely to be characterized by diminished motor activity than in AD (Shinagawa et al 2006).
Given the substantial heterogeneity in the definition and measurement of apathy across studies, it is not surprising that there is also significant variability in the neuroanatomical regions that have been associated with it. Apathy, broadly defined, has been associated with lesions in a neural system that includes anterior cingulate cortex, ventral striatum (nucleus accumbens and globus pallidus), and thalamus (Bonelli & Cummings 2007). In FTD, atrophy in many of these regions (i.e., anterior cingulate cortex caudate and ventral striatum) in addition to ventromedial prefrontal cortex and temporoparietal junction, among others, has been associated with greater apathy (Eslinger et al 2012, Levy & Dubois 2006, Rosen et al 2005). In AD, there is a similar pattern of results with apathy being related to hypoperfusion in anterior cingulate cortex, inferior frontal gyrus, and orbitofrontal cortex (Benoit et al 2002, Migneco et al 2001). In terms of more specific apathy symptoms, lack of initiative and interest has been associated with hypoperfusion in anterior cingulate cortex in AD (Robert et al 2006).
Taken together, the emotional component of apathy in dementia appears to be associated with deficient activation of medial frontal systems that support reward responsivity and positive emotional reactivity. A similar conclusion can be drawn from functional imaging studies of patients with schizophrenia that focused on specific symptoms. Thus, in schizophrenia, negative symptoms such as flat affect, avolition, anhedonia, and social withdrawal have been associated with medial frontal hypoactivity (Mazza et al 2013) and less activation in the ventral striatum during reward anticipation (Simon et al 2010).
Disinhibition
Disinhibition refers to the presence of unwanted actions, thoughts, and feelings. As control systems that are involved in self-monitoring, impulse control, and emotion regulation fail, behavior becomes dysregulated, inappropriate, and error-prone. In social contexts, these deficits lead to poor maintenance of social boundaries and violations of social norms. These kinds of problems are often seen in patients with bipolar disorder, who tend to be impulsive and have problems with decision-making regardless of whether they are in the euthymic or manic phase of their illness (Henry et al 2013, Mazzola-Pomietto et al 2009, Swann et al 2003). They are also seen in Axis II disorders such as borderline personality disorder, where difficulties with interpersonal boundaries are common (Linehan & Kehrer 1993), as well as in narcissistic and histrionic personality disorders.
Disinhibition is more common in FTD than AD (Hirono et al 1999, Liu et al 2004) and is one of the core diagnostic criteria of behavioral variant FTD (Rascovsky et al 2007). Clinical reports suggest that disinhibited FTD patients often approach others without concern for negative consequences, commit social faux pas without feelings of embarrassment, and are overly familiar with strangers. This pattern of behavior may reflect underlying deficits in the ability to generate embarrassment and other self-conscious emotions (e.g., shame, guilt), which normally signal violations of social norms and motivate corrective behaviors (Sturm et al 2006, Sturm et al 2008).
Behavioral, cognitive, and emotional control all rely on a network that has hubs in ventrolateral prefrontal cortex and orbitofrontal cortex and connections with dorsal anterior insula, anterior mid-cingulate cortex, dorsolateral prefrontal cortex, medial frontal cortex, frontal pole, and lateral parietal cortex (Aron 2007, Dosenbach et al 2007). In patients with dementia, disinhibition is associated with orbitofrontal cortex damage, which may alter connections with basal ganglia and thalamus (Bonelli & Cummings 2007). Consistent with this, disinhibition in FTD relates to dysfunction in orbitofrontal cortex and inferior frontal gyrus (Hornberger et al 2010, Massimo et al 2009, Peters et al 2006, Possin et al 2012), regions that are less vulnerable in AD (Liu et al 2004, Whitwell et al 2008). Studies with psychiatric patients that focus on particular symptoms also converge on this circuitry. For example, in patients with bipolar disorder, hypoactivation in these regions has been linked to higher error rates on response inhibition tasks (Mazzola-Pomietto et al 2009).
Euphoric mood
We close this section by discussing a fifth important symptom of psychopathology, euphoric mood. However, in contrast to the symptoms discussed earlier, evidence about the associated neural circuitry is largely derived from studies of psychiatric rather than neurological patients. Feelings of euphoria and elation reflecting an elevated positive emotional state are the hallmark symptom of bipolar disorder. Among DSM disorders, bipolar disorder may be least vulnerable to criticisms of heterogeneity. The hallmark symptoms of intermittent elation and euphoria found in bipolar disorder are not found in many other diagnoses. Moreover, bipolar disorder is one of the few DSM disorders that has a fairly unique pharmacologic treatment (i.e., Lithium and other mood stabilizers). For these reasons, neuroimaging studies of bipolar patients may be much more useful in detailing the associated neural circuitry of euphoric mood than is the case with more heterogeneous DSM disorders. This is fortunate because there are not many studies of euphoric mood in neurodegenerative disease to draw upon.
A diagnosis of bipolar disorder requires at least one episode of mania, a period of elevated or irritable mood that is accompanied by symptoms including decreased need for sleep, increased activity, racing thoughts, extreme self-confidence, and pursuit of rewards with disregard of associated risks (Johnson et al 2012). Although depression may also occur intermittently in bipolar disorder, it has been proposed that persistent elevations in positive emotion (even between manic or depressive episodes) are central to this illness (Gruber 2011). At one time, positive emotion was not seen as highly differentiated as negative emotion (which includes sadness, fear, anger, disgust, contempt, etc.). However, increasingly positive emotion is being viewed as a family of emotions that includes amusement, attachment love, awe, contentment, enthusiasm, and nurturant love (Griskevicius et al 2010). These positive emotions serve important interpersonal functions, facilitating approach behavior, fostering the development of new relationships, and supporting creativity and expansiveness (Fredrickson 2004, Shiota et al 2011). Moreover, they serve valuable functions in quelling physiological arousal produced by negative emotions (Fredrickson & Levenson 1998). Arguably, enthusiasm is the hallmark positive emotion in bipolar disorder, but this has not been established empirically. Individuals with bipolar disorder place high value on reward (with disregard for negative consequences), have elevated levels of positive emotional experience, and have high levels of vagal tone and reactivity (a measure of parasympathetic nervous system activity that has been linked with with positive emotion, Gruber et al 2011, Gruber et al 2008, Johnson et al 2012). Together, these findings suggest that there may be elevated positive emotional experience in bipolar disorder that promotes reward-seeking and approach behaviors and puts people at risk for manic episodes.
In most neurodegenerative diseases, euphoric mood is relatively rare (Teng et al 2009). However, euphoric mood is relatively common in FTD (Hirono et al 1999) with prevalence rates of up to 40% (Liu et al 2004). FTD patients often exhibit accentuated positive affect (e.g., greater smiling and laughing, overfamiliarity, jocularity, and silliness) and increased reward-seeking behaviors (e.g., overeating, risk-taking, gambling, and excessive use of drugs and alcohol, Mendez et al 2006a, Woolley et al 2007). Given the substantial symptom overlap with bipolar disorder, it not surprising that FTD patients who display high levels of positive affect are often diagnosed as having bipolar disorder (Woolley et al 2011, Woolley et al 2007).
Functional neuroimaging studies of individuals with bipolar disorder have found hypermetabolism in structures important for appraisal and emotion-generation (e.g., pregenual and subgenual anterior cingulate cortex, parahippocampal gyrus, gyrus rectus, and medial and inferior temporal lobe). In addition, hypometabolism has been found in emotion-regulating systems (e.g., dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, dorsal anterior cingulate cortex, superior temporal gyrus, and precuneus) during mania (Brooks et al 2010). Other studies have found smaller volumes in emotion regulatory structures (e.g., orbitofrontal cortex) to be associated with greater amygdala reactivity to emotional stimuli (Foland-Ross et al 2010, Foland et al 2008). Volumetric analyses have found atrophy in emotion-relevant structures including dorsal anterior insula, ventrolateral prefrontal cortex, and pregenual anterior cingulate cortex that is somewhat attenuated with pharmacotherapy (Bora et al 2010, Ellison-Wright & Bullmore 2010). Importantly, these are some of the same regions that are affected in FTD (Seeley et al 2008), which may account for the overlapping symptomatology and the frequent diagnostic confusion.
Discussion
There is striking overlap in the cognitive, emotional, and behavioral symptoms produced by psychiatric disorders and neurodegenerative disease. In this review, we examined five core symptoms of psychopathology (anxiety, dysphoric mood, apathy, disinhibition, euphoric mood) that also appear in two common neurodegenerative diseases, AD and FTD. Our goal was to explore the neural circuitry associated with these symptoms in neurodegenerative diseases and, when possible, compare this to available evidence about the neural circuitry associated with these symptoms in psychiatric diseases. AD and FTD are particularly useful in this regard because they are common, well-characterized behaviorally, and have quite different clinical presentations. Importantly, they also have different anatomical features, including their patterns of atrophy, breakdowns in intrinsic neural connectivity, and neuropathology. AD is a disease that targets more posterior neuroanatomical systems important for episodic memory and other cognitive functions; in contrast, FTD is a disease that targets more anterior systems important for social and emotional behavior. Between them they present opportunities to study some of the most important symptoms of psychopathology in the realms of cognition, emotion, and social behavior and their associated neural circuitry.
Our analysis takes advantage of neurodegenerative diseases having much more clearly discernible patterns of neural atrophy than those typically associated with psychiatric symptoms. New understanding about the patterns of atrophy in neurodegenerative diseases suggests that they are not random, but rather target major structural and functional networks in the brain (Seeley et al 2009). This emphasis on specific behaviors and symptoms is consistent with RDoC’s basic tenet that greater progress in the treatment and prevention of mental illness will come from a symptom-centric approach than a more traditional syndrome-centric approach (Cuthbert & Insel 2013). Our review is also consistent with the RDoC emphasis on underlying neural circuitry. In our approach, when a neurodegenerative disease with a well-characterized pattern of damage to neural circuits creates a symptom that closely resembles a psychiatric symptom, we consider the circuit associated with the neurodegenerative disease likely to be critically involved in producing the psychiatric symptom. While fully cognizant of the logical leaps involved and the viability of alternative hypotheses, when appropriate data are available from both neurodegenerative and psychiatric populations, the overlap has been impressive (see, for example, our earlier discussion of neural circuits related to symptoms of disinhibition in FTD and bipolar disorder). Arguably this can be considered to provide a preliminary proof-of-concept for this approach. Nonetheless, there is clearly much more research that will need to be done before the final verdict is known.
Sharpening the behavioral phenotypes
The exploration of brain-behavior relationships lies at the very heart of psychology, psychiatry, neuroscience, and neurology. In the last decade, our ability to characterize the brain side of these relationships has improved dramatically, with the advent of magnetic resonance imaging representing the breakthrough technology that opened the floodgates. Since then, there have been continuing and dramatic improvements in the methodologies available for quantifying imaging information in ways that enable characterization of many important aspects of brain structure and function. Ironically, our ability to characterize the behavioral side of these relationships has lagged behind. In recent years, as funding at NIMH has shifted away from basic behavioral science toward more translational and disease-centric science (NIMH 1999), human and non-human animal research that could have provided deeper understanding of basic cognitive, social, and emotional processes and better ways to measure these functions has suffered. Simply stated, when 21st century brain science is coupled with 20th (and 19th) century behavioral science, the result is destined to sink to the lowest common denominator. Moreover, these mismatches are destined to hinder progress in other areas of psychopathology research involving bio-behavioral relationships (e.g., linking genes with behavior, linking proteins and molecules with behavior).
RDoC clearly throws down the gauntlet in favor of much finer-grained behavioral analysis. Attempting to match specific circuits, molecules, and genes with broad, heterogeneous DSM syndromes (e.g., schizophrenia, depression, autism) is rejected in favor of building links with more specific behaviors. But how are these behaviors to be measured and quantified? Focusing on clinical symptoms rather than syndromes is clearly a step toward greater behavioral precision. However, such symptoms are still quite complex, likely involving a number of different emotional, cognitive, and motor processes, each mediated by different neural mechanisms. The symptom of apathy explored earlier in this review illustrates this problem. Apathy is a highly heterogeneous behavioral construct that can arise from disruption of cognitive, emotional, or motor systems (Marin 1991, Robert et al 2002). Studies of apathy describe its behavioral features using a panoply of descriptors including remoteness, disinterest, passivity, mental sluggishness, boredom, social withdrawal, social avoidance, lessened drive, lessened motivation, less caring, less concern, self-centeredness, loss of awareness, and aspontaneity (Merrilees et al 2013). This raises the question of whether some or all of these terms actually refer to the same underlying process and thus share a common underlying neural circuitry or refer to different processes with different associated neural circuitry.
In our literature review, we encountered the related problem of how to determine whether a given symptom said to occur in both a neurodegenerative disease and a psychiatric disorder was really the same symptom? Thus, for example, how similar is the disinhibition shown by an FTD patient to the disinhibition shown by a bipolar disorder patient? Or if skilled observers blind to diagnosis were shown thin slices of apathy behavior in a group of schizophrenic and FTD patients, would they be able to match the behavior samples with the patient diagnosis? The frequent misdiagnosis of FTD and bipolar disease by experienced clinicians indicates that this would not be an easy task, suggesting that the symptoms are actually quite similar in the two diseases. However, this is only anecdotal evidence, underscoring the need for serious research on symptom equivalency.
Addressing issues of symptom equivalency would also greatly benefit from detailed descriptions and reliable, well-validated measures of symptoms in neurological and psychiatric patients. There was a time when richly detailed observations of psychiatric symptoms by skilled clinicians were relatively common (e.g., Shapiro 1965); however, these promising descriptive forays did not lead to the development of measures with sound psychometric properties. As a result, the literature is replete with data on symptom intensities and frequencies but less well endowed with the kinds of information that enables determination of symptom equivalency.
A related problem is found when symptom-related terminology is used by different investigators in very different ways. A good example is “emotion regulation,” a critical locus of dysfunction in a number of neuropathologies and psychopathologies. In many studies, emotion regulation is assessed using self-reports and other-reports, which ultimately reflect respondents’ beliefs about how, how often, and how well they or others regulate their emotions. However, in other studies, emotion regulation is assessed by measuring performance (e.g., placing the person in a situation that elicits emotion and measuring how quickly they can restore emotional equilibrium, e.g., Bloch et al in press). Furthermore, within performance measures, some studies assess spontaneous performance (regulation that occurs without explicit instructions, e.g., Bloch et al in press) while others assess instructed performance (telling participants how and when to regulate, e.g., Shiota & Levenson 2009). And finally, the actual kind of regulation assessed (e.g., up-regulation versus down-regulation; suppression versus numerous kinds of reappraisal) often differs between studies (for a more extensive discussion of these issues, see Levenson et al in press). As noted earlier, heterogeneity of symptoms within broad DSM diagnoses has long worked against establishing reliable bio-behavioral links. Heterogeneity of behaviors (and RDoC behavioral constructs) can create similar kinds of problems.
Finally, it is important to note that, when it comes to emotion, neurological and psychiatric diseases may not affect all kinds of emotions equally. Thus, for example, phobias may be associated with hyper-reactivity in emotions such as fear and disgust in response to the phobic object, but not have much effect on sadness. Depression may be more associated with elevations in sadness and anger and less in fear and disgust. Similarly, neurological diseases can target particular emotions (e.g., deficits in disgust generation and recognition in Huntington’s disease, Sprengelmeyer et al 1997) and emotion-related behaviors (e.g., laughing and crying in pseudobulbar affect in amyotrophic lateral sclerosis, Olney et al 2011). Although there has been a lively debate in the affective science literature about the existence of separable neural circuitry for different emotions in the mammalian brain (Barrett et al 2007, Izard 2007, Panksepp 2007), a great deal of the discussion rightfully centers on the quality of existing empirical evidence. For purposes of linking emotional behaviors with neural circuits, it seems prudent to make sure that studies that are being aggregated are well-matched in terms of the particular emotions (e.g., fear, anger), emotional dimensions (e.g., valence, arousal), and/or emotion families (positive, negative, self-conscious) that have been studied.
To summarize, in studying bio-behavior links related to psychopathology and neuropathology, there is a pressing need for greater precision and care in specifying the behaviors, constructs, and symptoms that are candidates for linkage with neural circuits (and genes and molecules). Equivalencies among measures, procedures, and paradigms that purport to assess particular behaviors, constructs, and symptoms (including, but not limited to, those reviewed in the present chapter) cannot be assumed, but rather must be established empirically.
Implications for etiology, diagnosis, and treatment
Identifying neural circuits associated with core symptoms of psychopathology will surely contribute greatly to our fundamental understanding of the brain, of functional and dysfunctional behaviors, and of brain-behavior relationships. However, in public health-relevant initiatives such as RDoC, there is a clear expectation that studying these relationships will have significant applied and practical benefits as well, leading to palpable advances in diagnosis, prevention, and treatment that will help relieve the enormous burden and suffering associated with mental illness. Envisioning how the latter might work requires a bit of extrapolation and conjecture.
In the realm of diagnosis, it is highly likely that future assessments (now largely based on structured interviews, questionnaires and rating scales, and behavioral observations) will be expanded to include more detailed assessment of the status of key neural circuits in the brain. This may include measures of brain structure (e.g., evidence of neurodegeneration and tract integrity obtained from highly sensitive structural scans) as well as measures of brain function (e.g., assessments of intrinsic connectivity and of activation in response to standardized behavioral probes). These kinds of assessments may be particularly helpful for diagnosing symptoms of mental illness, for identifying pre-clinical vulnerabilities, and for suggesting targets for early preventative interventions.
The ultimate utility of improved assessment methods will dramatically increase when there are more specific treatments available that target different disorders. When thinking about biological treatments for mental illness, pharmacologic approaches loom prominently. Currently available psychiatric drugs may target particular neurotransmitters and specific neurotransmitter mechanisms but are still relatively broad in action. When administered using current delivery systems, these drugs affect large areas of the brain and many different neural circuits (some producing the desired beneficial effects and some creating unwanted side-effects). A great deal of research is being devoted to improved drug delivery systems. As these delivery systems improve, pharmacological treatments may become much more effective in targeting particular circuits in particular brain regions.
Biological treatments that directly target particular brain circuits are likely to include both electrical and surgical approaches. As with pharmacological treatments, there is a pressing need for greater precision in these approaches. Methods with long histories, such as electroconvulsive therapy and psychosurgery (e.g., lobotomy), although much improved in precision in recent incarnations, are still very broad in their action. Newer approaches that are more focused in action are already starting to be utilized in the treatment of mental illness. These include deep brain stimulation of particular neural tracts (Holtzheimer et al 2012), transcranial magnetic stimulation (e.g., Mosimann et al 2000), and biofeedback (providing subjects with information about the level of activation in particular brain regions).
In thinking about the implications of an increased focus on neural circuits in the assessment and treatment of psychopathology, it is important to note that this approach can be quite agnostic as to the original cause of circuit dysfunction and the type of treatment that will be most effective. Thus, for example, a problem that is thought to involve hyper-reactivity in the emotional salience network might have been caused by some combination of biological and psychosocial factors including disease (e.g., inflammation, infection), injury (e.g., early or recent brain trauma), genes (e.g., inherited vulnerabilities), environments (e.g., poverty, toxins, childhood adversity), life stress, and faulty learning. Similarly, treatment aimed at improving function of the affected circuitry might include some combination of biological, pharmacologic, psychosocial (e.g., psychotherapy targeting quite specific dysfunctional behaviors), and environmental interventions.
Conclusion
Neurodegenerative diseases that produce symptoms similar to those found in psychiatric disorders can provide powerful tools for exploring the neural circuitry associated with a number of the major symptoms of psychopathology. This approach takes advantage of the more clear-cut and more readily quantifiable patterns of neural loss and changes in intrinsic connectivity that accompany neurodegenerative diseases and the proclivity of these diseases to target major structural/functional networks in the brain. For this approach to be most useful in characterizing brain-behavior relationships, behavioral symptoms need to be characterized with a level of care and precision comparable to that typically applied to structural and functional brain measures. This approach is consistent with new approaches to the study of mental illness that emphasize the importance of specific behaviors and neural circuits (e.g., RDoC). Studying patients with neurodegenerative disease holds great promise for helping identify neural structures that will be important for understanding the etiology of psychopathology as well as for playing a critical role in future approaches to the diagnosis, prevention, and treatment of mental illness.
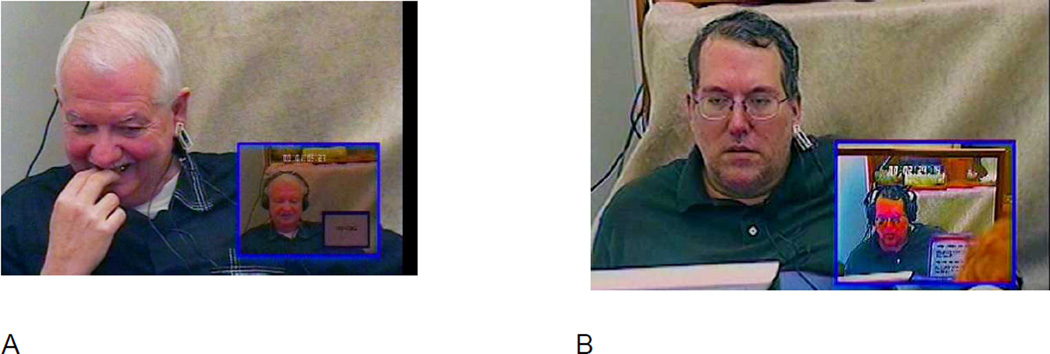
Figure 3.
Karaoke singing task elicits self-conscious emotion in healthy participants but not in patients with FTD.
Note. The large pictures show the participants while watching themselves singing Karaoke style (the small picture insets show the video recordings of them singing “My Girl,” which were recorded earlier in the testing session). Still images were taken from videotaped recordings of the laboratory testing session. The participants provided consent for their videotape being used for scientific publication. (A) An embarrassment response in a healthy control participant. The participant exhibits an embarrassment response, as indexed by smiling, smile suppression, gaze aversion, and face touching. (B) A patient with behavioral variant FTD watching himself singing Karaoke style. The patient exhibited no embarrassment response during the task.
Sidebar 1: Methodological Challenges in the Measurement of Symptoms.
Most of the existing research on symptoms of psychopathology in neurodegenerative disease is based on caregiver reports (Teng et al 2009). These studies use scales that measure emotional and behavioral symptoms such as depression, agitation, apathy, hallucinations, mood lability, and wandering (e.g., Cohen–Mansfield Agitation Inventory, Cornell Scale for Depression in Dementia, Neuropsychiatric Inventory, Apathy Inventory, Behavioral Pathology in Alzheimer’s Disease Rating Scale, and Behavioral Rating Scale for Dementia). While caregivers have unique insights into the everyday functioning of patients, their assessment of symptoms can be negatively biased, especially when their own well-being is compromised (Schulz et al 2013). For example, prevalence rates of certain symptoms (e.g., apathy) in AD vary from 29% using reports from trained personnel such as psychometricians and nurses (Lyketsos et al 2000) to 72% using caregiver-reports (Mega et al 1996). Studies that rely on caregiver reports typically find higher prevalence rates of symptoms of psychopathology than studies that rely on reports by trained personnel.
Sidebar 2: Laboratory Approaches to the Study of Emotion in Neurodegenerative Disease.
Laboratory-based approaches for assessing emotional functioning that were developed in basic affective science are an effective way to identify areas of functional loss and preservation in neurodegenerative disease (Levenson 2007). These approaches often distinguish between different emotion processes (i.e., emotion reactivity, regulation, understanding), different emotions and emotion families (e.g., positive, negative, self-conscious), and different emotion response systems (e.g., peripheral and central physiology, facial expression, body posture, voice tone, emotional language, and subjective experience). One example paradigm that we have used in the laboratory is the Karaoke task. Participants sing a song (“My Girl”) over instrumental background music with lyrics presented on a monitor. Afterwards, without warning, participants are shown a recording of their just-completed singing performance and their emotional response is assessed. For healthy participants, the Karaoke task is a highly effective elicitor of self-conscious emotional (i.e., embarrassment). FTD patients, in contrast, show deficits in physiological responding and self-conscious emotional behavior while watching themselves singing. Impairment in self-conscious emotion likely contribute to key behavioral symptoms in FTD such as disinhibition and social inappropriateness. See Figure 3.
Acknowledgements
Preparation of this manuscript was supported by NIH grants AG17766 and AG19724 to Robert W. Levenson and by NIH grant 1K23AG040127 and support from the Larry L. Hillblom Foundation to Virginia E. Sturm.
Acronyms and Definitions
- Dementia
A decline in cognitive ability that impacts daily functioning.
- Neurodegeneration
Progressive deterioration of the structure and function of neurons.
- Neurodegenerative disease
A progressive neurological disease that targets specific, distributed brain networks critical for cognitive, emotional, behavioral, and motor functioning.
- Alzheimer’s disease (AD)
A neurodegenerative disease characterized by neurodegeneration in the medial temporal and parietal lobes that typically causes prominent deficits in episodic memory and may also affect visuospatial processing, language, and executive functioning.
- Frontotemporal dementia (FTD)
A neurodegenerative disease characterized by neurodegeneration in the frontal and anterior temporal lobes that causes pronounced deficits in emotion, social behavior, and empathy.
- Clinical subtypes of FTD
FTD is an umbrella term that includes three clinical subtypes: (1) behavioral variant FTD, (2) semantic variant primary progressive aphasia, and (3) non-fluent variant primary progressive aphasia.
- Emotions
Emotions are short-lived, psychological-physiological phenomena that are efficient modes of adaptation to changing environmental demands.
- Laboratory studies
Laboratory studies of emotion examine emotion processes (reactivity, regulation, and empathy), types (positive, negative, and self-conscious), and response systems (experience, behavior, physiology, and language).
- Mild cognitive impairment (MCI)
A clinical condition in which cognitive decline exceeds normal age-related changes but does not severely disrupt daily functioning. MCI often precedes Alzheimer’s disease.
- Symptom-based approach
An approach to understanding psychopathology (e.g., Research Domain Criteria; RDoC) that conceptualizes mental illness as involving particular behaviors that can become dysfunctional. In RDoC, for these behaviors to be included they must be associated with a plausible neural circuit.
- Syndrome-based approach
The traditional approach to classifying psychopathological disorders (e.g., Diagnostic and Statistical Manual of Mental Disorders), which conceptualizes mental illness as being categorical entities.
Contributor Information
Robert W. Levenson, Email: boblev@berkeley.edu.
Virginia E. Sturm, Email: virginia.sturm@ucsf.edu.
Claudia M. Haase, Email: claudia.haase@northwestern.edu.
References
- A.P.A. Diagnostic and statistical manual of mental disorders. Fourth edition (DSM-IV) Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, et al. Neuropsychiatric symptoms in Alzheimer's disease are related to functional connectivity alterations in the salience network. Human Brain Mapping. 2013 doi: 10.1002/hbm.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S, Weintraub S. Self-awareness and self-monitoring of cognitive and behavioral deficits in behavioral variant frontotemporal dementia, primary progressive aphasia and probable Alzheimer’s disease. Brain and Cognition. 2008;67:58–68. doi: 10.1016/j.bandc.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Lindquist KA, Bliss-Moreau E, Duncan S, Gendron M, et al. Of mice and men: Natural kinds of emotions in the mammalian brain? A response to Panksepp and Izard. Perspectives on Psychological Science. 2007;2:297–311. doi: 10.1111/j.1745-6916.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson G, Jackson DD, Haley J, Weakland J. Toward a Theory of Schizophrenia. Behavioral Science. 1956;1:251–264. [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia--focal abnormalities of cerebral blood flow in major depression. Psychological Medicine. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Benoit M, Koulibaly PM, Migneco O, Darcourt J, Pringuey DJ, Robert PH. Brain perfusion in Alzheimer's disease with and without apathy: a SPECT study with statistical parametric mapping analysis. Psychiatry Research. 2002;114:103–111. doi: 10.1016/s0925-4927(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Blass DM, Rabins PV. Depression in frontotemporal dementia. Psychosomatics. 2009;50:239–247. doi: 10.1176/appi.psy.50.3.239. [DOI] [PubMed] [Google Scholar]
- Bloch L, Haase CM, Levenson RW. Emotion regulation predicts marital satisfaction: More than a wives' tale. Emotion. doi: 10.1037/a0034272. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Molnar C, Horner MD, Anderson B, Forster L, et al. Neurocognitive deficits and prefrontal cortical atrophy in patients with schizophrenia. Schizophrenia Research. 2008;101:142–151. doi: 10.1016/j.schres.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorders. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biological Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary change. Neurobiology of Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Brooks JO, 3rd, Hoblyn JC, Ketter TA. Metabolic evidence of corticolimbic dysregulation in bipolar mania. Psychiatry Research. 2010;181:136–140. doi: 10.1016/j.pscychresns.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Brown GW. Experiences of Discharged Chronic Schizophrenic Patients in Various Types of Living Group. Milbank Memorial Fund Quarterly. 1959;37:105–131. [PubMed] [Google Scholar]
- Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: implications for assessment and treatment. Psychol Assess. 2009;21:256–271. doi: 10.1037/a0016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chow TW, Binns MA, Cummings JL, Lam I, Black SE, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Archives of Neurology. 2009;66:888–893. doi: 10.1001/archneurol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cohn-Hokke PE, Elting MW, Pijnenburg YA, van Swieten JC. Genetics of dementia: update and guidelines for the clinician. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:628–643. doi: 10.1002/ajmg.b.32080. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. Biomed Central Medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer's disease and frontotemporal dementia. Dement Geriatr Cogn. 2006;22:35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) Journal of Molecular Neuroscience. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MY, Wu QZ, Yue Q, Li J, Liao Y, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;36:11–16. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Engedal K, Barca ML, Laks J, Selbaek G. Depression in Alzheimer's disease: specificity of depressive symptoms using three different clinical criteria. International Journal of Geriatric Psychiatry. 2011;26:944–951. doi: 10.1002/gps.2631. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol. 2012;25:127–136. doi: 10.3233/BEN-2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti L, McCurry SM, Logsdon R, Gibbons L, Teri L. Anxiety and Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology. 2001;14:52–58. doi: 10.1177/089198870101400111. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Altshuler LL, Bookheimer SY, Lieberman MD, Townsend J, et al. Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness. Journal of Neuroscience. 2010;30:16673–16678. doi: 10.1523/JNEUROSCI.4578-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Research. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The broaden-and-build theory of positive emotions. Philosophical Transactions of the Royal Society of London Series B Biological Series. 2004;359:1367–1378. doi: 10.1098/rstb.2004.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition & Emotion. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, Filho AS, Machado-de-Sousa JP, et al. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, et al. Prevalence of major and minor depression in elderly persons with mild cognitive impairment--MADRS factor analysis. International Journal of Geriatric Psychiatry. 2004;19:1168–1172. doi: 10.1002/gps.1235. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Sperry RW. Language after section of the cerebral commissures. Brain: A Journal of Neurology. 1967;90(1):131–148. doi: 10.1093/brain/90.1.131. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia genesis: The origins of madness. US: W H Freeman/Times Books/ Henry Holt & Co; 1991. [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griskevicius V, Shiota MN, Neufeld SL. Influence of different positive emotions on persuasion processing: A functional evolutionary approach. Emotion. 2010;10:190–206. doi: 10.1037/a0018421. [DOI] [PubMed] [Google Scholar]
- Grossman M, Powers J, Ash S, McMillan C, Burkholder L, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain and Language. 2012 doi: 10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clin Psychol Psychother. 2011;18:356–365. doi: 10.1002/cpp.776. [DOI] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Purcell A. What goes up can come down? A preliminary investigation of emotion reactivity and emotion recovery in bipolar disorder. Journal of Affective Disorders. 2011;133:457–466. doi: 10.1016/j.jad.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Johnson SL, Oveis C, Keltner D. Risk for mania and positive emotional responding: too much of a good thing? Emotion. 2008;8:23–33. doi: 10.1037/1528-3542.8.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Passage of an iron rod through the head. Boston Medical and Surgical Journal. 1848;39:389–393. [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. New York: Oxford University Press; 2004. [Google Scholar]
- Hashimoto H, Monserratt L, Nguyen P, Feil D, Harwood D, et al. Anxiety and regional cortical glucose metabolism in patients with Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:521–528. doi: 10.1176/jnp.2006.18.4.521. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, et al. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. Journal of Affective Disorders. 2013 doi: 10.1016/j.jad.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono N, Mori E, Tanimukai S, Kazui H, Hashimoto M, et al. Distinctive neurobehavioral features among neurodegenerative dementias. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:498–503. doi: 10.1176/jnp.11.4.498. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Archives of General Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, Hodges JR. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer's disease. Dement Geriatr Cogn Disord. 2010;30:547–552. doi: 10.1159/000321670. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Izard CE. Emotion feelings stem from evolution and neurobiological development, not from conceptual acts: Corrections for Barrett et al. 2007. Perspectives on Psychological Science. 2007;2:404–405. doi: 10.1111/j.1745-6916.2007.00053.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, et al. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77:219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annu Rev Clin Psychol. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathologica. 2011;122:137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology. 2004;62:506–508. doi: 10.1212/01.wnl.0000106827.39764.7e. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Blood, sweat, and fears: The autonomic architecture of emotion. Annals of the New York Academy of Sciences. 2003;1000:348–366. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Emotion elicitation with neurological patients. In: Coan JA, Allen JJB, editors. The handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007. pp. 158–168. [Google Scholar]
- Levenson RW, Haase CM, Bloch L, Holley SR, Seider BH. Emotion regulation in couples. In: gross JJ, editor. Handbook of emotion regulation (2nd edition) New York, NY: Guilford Press; in press. [Google Scholar]
- Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions. Archives of Neurology. 1996;53:687–690. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Kehrer CA. Borderline personality disorder. US: Guilford Press; 1993. [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA. Non-cognitive symptoms in mild cognitive impairment subjects. Neurocase. 2005;11:65–71. doi: 10.1080/13554790490896893. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. American Journal of Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- Macmillan M. An odd kind of fame: stories of Phineas Gage. Cambridge, Mass: MIT Press; 2000. [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gasto C, Junque C, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. NeuroImage. 2003;19:80–90. doi: 10.1016/s1053-8119(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. Journal of Clinical Investigation. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Catalucci A, Pino MC, Giusti L, Nigri A, et al. Dysfunctional neural networks associated with impaired social interactions in early psychosis: an ICA analysis. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9223-6. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. Journal of Psychiatric Research. 2009;43:432–441. doi: 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer's disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- Mendez MF. Early-onset Alzheimer's disease: nonamnestic subtypes and type 2 AD. Archives of Medical Research. 2012;43:677–685. doi: 10.1016/j.arcmed.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Lu PH, Miller BL. Acquired extroversion associated with bitemporal variant of frontotemporal dementia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006a;18:100–107. doi: 10.1176/jnp.18.1.100. [DOI] [PubMed] [Google Scholar]
- Mendez MF, McMurtray AM, Chen AK, Shapira JS, Mishkin F, Miller BL. Functional neuroimaging and presenting psychiatric features of frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2006b;77:4–7. doi: 10.1136/jnnp.2005.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees J, Dowling GA, Hubbard E, Mastick J, Ketelle R, Miller BL. Characterization of apathy in persons with frontotemporal dementia and the impact on family caregivers. Alzheimer Disease and Associated Disorders. 2013;27:62–67. doi: 10.1097/WAD.0b013e3182471c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migneco O, Benoit M, Koulibaly PM, Dygai I, Bertogliati C, et al. Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: a study in Alzheimer's disease and nondemented patients. NeuroImage. 2001;13:896–902. doi: 10.1006/nimg.2000.0741. [DOI] [PubMed] [Google Scholar]
- Miller BL. Basic clinical approaches to diagnosis. In: Miller BL, Boeve BF, editors. The behavioral neurology of dementia. Cambridge: Cambridge University Press; 2009. pp. 1–6. [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Rihs TA, Engeler J, Fisch H-U, Schlaepfer TE. Mood effects if repetitive transcranial magnetic stimulation of left prefrontal cortex in healthy volunteers. Psychiatry Research. 2000;94:251–256. doi: 10.1016/s0165-1781(00)00146-3. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- NIMH. Washington, D.C.: National Institute of Mental Health; 1999. Report of the National Advisory Mental Health Council's Behavioral Science Workgroup: Translating behavioral science into actionNIH 00-4699. [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olney NT, Goodkind MS, Lomen-Hoerth C, Whalen PK, Williamson CA, et al. Behaviour, physiology and experience of pathological laughing and crying in amyotrophic lateral sclerosis. Brain. 2011;134:3458–3469. doi: 10.1093/brain/awr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Neurologizing the psychology of affects: How appraisal-based constructivism and basic emotion theory can coexist. Perspectives on Psychological Science. 2007;2:281–295. doi: 10.1111/j.1745-6916.2007.00045.x. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, et al. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. European Neuropsychopharmacology. 2013;23:186–195. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21:373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Porter VR, Buxton WG, Fairbanks LA, Strickland T, O'Connor SM, et al. Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:180–186. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- Possin KL, Chester SK, Laluz V, Bostrom A, Rosen HJ, et al. The frontal-anatomic specificity of design fluency repetitions and their diagnostic relevance for behavioral variant frontotemporal dementia. Journal of the International Neuropsychological Society. 2012;18:834–844. doi: 10.1017/S1355617712000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer Disease and Associated Disorders. 2007;21:S14–S18. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Ratiu P, Talos IF, Haker S, Lieberman D, Everett P. The tale of Phineas Gage, digitally remastered. Journal of neurotrauma. 2004;21:637–643. doi: 10.1089/089771504774129964. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Robert PH, Clairet S, Benoit M, Koutaich J, Bertogliati C, et al. The apathy inventory: assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. International Journal of Geriatric Psychiatry. 2002;17:1099–1105. doi: 10.1002/gps.755. [DOI] [PubMed] [Google Scholar]
- Robert PH, Darcourt G, Koulibaly MP, Clairet S, Benoit M, et al. Lack of initiative and interest in Alzheimer's disease: a single photon emission computed tomography study. European Journal of Neurology. 2006;13:729–735. doi: 10.1111/j.1468-1331.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno–Tempini ML, Goldman WP, Perry RJ, Schuff N, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Salomon K, Clift A, Karlsdottir M, Rottenberg J. Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychology. 2009;28:157–165. doi: 10.1037/a0013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Cook TB, Beach SR, Lingler JH, Martire LM, et al. Magnitude and causes of bias among family caregivers rating Alzheimer disease patients. American Journal of Geriatric Psychiatry. 2013;21:14–25. doi: 10.1016/j.jagp.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery & Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seignourel PJ, Kunik ME, Snow L, Wilson N, Stanley M. Anxiety in dementia: A critical review. Clinical Psychology Review. 2008;28:1071–1082. doi: 10.1016/j.cpr.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. Neurotic styles. xii. New York: Basic Books; 1965. p. 207. [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa S, Ikeda M, Fukuhara R, Tanabe H. Initial symptoms in frontotemporal dementia and semantic dementia compared with Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:74–80. doi: 10.1159/000090139. [DOI] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, Perea EF. Feeling good: autonomic nervous system responding in five positive emotions. Emotion. 2011;11:1368–1378. doi: 10.1037/a0024278. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, et al. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophrenia Research. 2010;118:154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Gibbons ZC, Blackshaw A, Doubleday E, Thompson J, et al. Social cognition in frontotemporal dementia and Huntington's disease. Neuropsychologia. 2003;41:688–701. doi: 10.1016/s0028-3932(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Musicco M, Padovani A, Rozzini L, Perri R, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. American Journal of Geriatric Psychiatry. 2010;18:1026–1035. doi: 10.1097/JGP.0b013e3181d6b68d. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Sprenglemeyer A, Calder AJ, Rowland D, et al. Recognition of facial expressions: Selective impairment of specific emotions in Huntington's disease. Cognitive Neuropsychology. 1997;14:839–879. [Google Scholar]
- Sturm VE, Allison SC, Rosen HJ, Miller BL, Levenson RW. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129:2508–2516. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8:861–869. doi: 10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, McCarthy ME, Yun I, Madan A, Yuan JW, et al. Mutual gaze in Alzheimer's disease, frontotemporal and semantic dementia couples. Social Cognitive and Affective Neuroscience. 2011;6:359–367. doi: 10.1093/scan/nsq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kuroda S, Ishizu H, Fujisawa Y, Sasaki K. Depression in the early stages of Pick's disease. Acta Medica Okayama. 1999;53:253–257. doi: 10.18926/AMO/31619. [DOI] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. Journal of Affective Disorders. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Teng E, Marshall GA, Cummings JL. Neuropsychiatric features of dementia. In: Miller BL, Boeve BF, editors. The behavioral neurology of dementia. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- Thies W, Bleiler L. 2013 Alzheimer's disease facts and figures. Alzheimer's and Dementia. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- van Reekum R, Stuss DT, Ostrander L. Apathy: why care? Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:7–19. doi: 10.1176/jnp.17.1.7. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, et al. Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson ER, Goddard A, Woods SW, Staib LH, et al. Temporal lobe volume in panic disorder--a quantitative magnetic resonance imaging study. Psychiatry Research. 2000;99:75–82. doi: 10.1016/s0925-4927(00)00055-x. [DOI] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience and Biobehavioral Reviews. 2012;36:1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. Journal of Clinical Psychiatry. 2011;72:126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Wilson MR, Hung E, Gorno-Tempini ML, Miller BL, Shim J. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164:1811–1816. doi: 10.1176/appi.ajp.2007.07061001. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]