Abstract
Objective. To identify the risk factors for HCV infection within married couples in Egypt. Methods. In 2008 Egypt conducted its first nationally representative survey of HCV prevalence. 11126 of the 12780 individuals aged 15–59 year who were sampled agreed to participate and provided information via a questionnaire about demographic and behavioural characteristics and blood for HCV antibody and RNA analysis. We assessed the risk factors for HCV infection in a subsample of 5182 married individuals via multivariate logistic regression. Results. Overall HCV antibody prevalence in the married couples was 18.2% (95% CI, 16.8–19.6). HCV antibody prevalence was higher in the husbands (23.7%) than the wives (12.1%; P < 0.001). Having a spouse who was infected with HCV was an independent risk factor for HCV infection with odds ratios of 2.1 (95% CI, 1.6–2.9) and 2.2 (95% CI, 1.6–3.1) for women and men, respectively. Husbands whose wives had experienced female genital cutting (FGC) had a higher prevalence of HCV and this relationship was driven by a strong association in urban areas. Amongst the women there was no association between FGC and HCV overall but in urban areas only women who had experienced FGC were HCV infected. Conclusions. This study provides additional evidence of the importance of intrafamilial transmission of HCV in Egypt.
1. Introduction
With 14.7% of 15–59-year-olds testing anti-HCV positive, Egypt has the highest HCV prevalence in the world [1]. Although parenteral antischistosomiasis therapy (PAT) was important in the genesis of Egypt's HCV epidemic this was stopped over 25 years ago and HCV incidence remains high estimated between 150 000 and 500 000 new infections per year [2–4]. Infection from inadequate sterility of dental and medical devices has been shown to play a role in this regard [1, 2, 5–12]. Intrafamilial transmission is an alternative explanation [6]. Support for this theory comes from studies such as a longitudinal study of incidence in two villages in Egypt, which found that the strongest predictor of incident of HCV was having an anti-HCV positive family member [13]. Among those that did and did not have a family member infected with HCV, HCV incidence was 5.8 and 1.0/1000 person years, respectively. Parenteral exposure increased the risk of HCV but was not statistically significant.
This elevated risk of incident of HCV of family members could be due to sharing of implements such as razors or toothbrushes or due to sexual transmission between family members [14, 15]. Alternatively, the elevated risk may be due to shared risk factors (such as the family members all attending a particular health practitioner) rather than being caused by direct transmission between family members [13].
To disentangle these relationships it would be useful to know how HCV is patterned within families. If a husband, is infected is this associated with an increased risk of his wife being infected and vice versa? Is the risk higher for a spouse than nonspousal family members? Are these relationships affected by whether the affected individuals are HCV RNA as opposed to antibody positive?
In 2008 Egypt conducted its first nationally representative survey of HCV prevalence—the 2008 Egyptian Demographic and Health Survey (EDHS). A recently published analysis of this survey found that HCV prevalence increased steadily with age but more so in men than women, reaching, in the 50–59-year-age group, 46.3% in men and 30.8% in women [1]. HCV was also more prevalent in rural than urban areas and on multivariate analysis it was found to be associated with male sex, age, poverty, past history of PAT, and blood transfusion. In urban regions, those with a lack of education and females with genital cutting were more likely to be HCV infected.
This analysis did not however examine the extent to which HCV infection covaried within couples and families. The EDHS is the first HCV survey in the world that is both nationally representative and done in a way which enables researchers to link the HCV status of husbands and wives. In this paper we assess the correlates of HCV infection in 2591 married couples from the EDHS.
2. Materials and Methods
The EDHS entailed a three-stage probability sample that provided a nationally representative sample of 16527 ever-married women aged 15–49 who were interviewed about a range of key population indicators (KPI). In addition, in a subsample of 4953 households, 6702 women and 6078 men aged 15–59 were sampled for a special health topics (SHT) component (see Figure 1). The overall response rate for this latter section was 96.2% and 87.6% for the men and women, respectively. 11126 (87.1%) of these agreed to provide blood for HCV testing. This SHT component was selected so as to provide a sample which was representative for Egypt and the six major areas that the EDHS was stratified by: Urban and Frontier governorates and Upper and Lower Egypt (each of the latter two was divided into rural and urban areas). In order to link husbands and wives, we made use of the fact that 3877 women completed both questionnaires. These were the women who had ever been married, were 14–49 years old, and completed the KPI questionnaire. If the respondent was currently married, then the KPI questionnaire specified the husband's line number within their house. This provides a unique identifier for each husband. Via this mechanism, we established that, in the case of 2591 individuals, the husband of a respondent also completed the SHT component of the EDHS. In this paper we study the relationships between HCV and various risk factors in these 2591 husband-wife pairs. Apart from the 5182 individuals in this married subgroup, further 2338 persons aged 15–59 years, living in the same houses as the married couples, were included in the SHT survey. Although the outcome variable used in this study is the presence of anti-HCV antibodies in the 5182 members of the married subgroup, the relationship between the HCV serostatus of the married couples and that of the other household members is also of relevance. We therefore included the HCV antibody and RNA status of these 2338 individuals as exposure variables in our analyses. We also calculated the HCV prevalence for each of Egypt's 26 governorates. These were used as a measure of local or community HCV prevalence.
Figure 1.
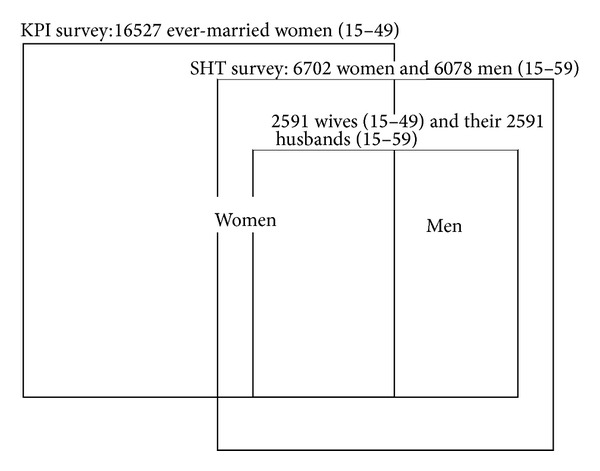
The structure of the Egyptian DHS 2008 and the derivation of the married couples subsample. 16527 ever-married women aged 15–49 were sampled in the key population indicators (KPI) survey. In a subsample of households surveyed in the KPI, 6702 women and 6078 men aged 15–49 were sampled in the special health topics (SHT) component. 2591 wives (aged 15–49) and their 2591 husbands (aged 15–59) could be linked to generate the married couples subsample.
Unless otherwise stated the terms “HCV prevalence” and “infection” refer to HCV antibody prevalence. The HCV antibody prevalence rates were calculated for a range of potential risk factors available in the special health topics questionnaire. Because of the strong association between age and HCV prevalence all the odds ratios and P values given are age-adjusted. Logistic regression was used to explore the strength of the association of each variable with HCV infection in the 5192 individuals in the married couples cohort.
Tests for interaction between variables were conducted. These tests revealed that the effect of several of the variables varied according to urban/rural location and men/women. As a result, separate models were constructed for men and women as well as urban and rural areas. All the urban women who had not undergone female genital cutting were HCV negative. To avoid the collinearity that this created in the analyses, for the analysis limited to urban women, we randomly selected one urban woman who had not undergone female genital cutting and changed her HCV status as positive. The final models were constructed by including all variables with P values <0.2 on univariate logistic regression. The education variable was not included due to significant collinearity with the income variable. The HCV status of the spouse and that of the other household members (both exposure variables) were represented by HCV RNA instead of HCV antibody positivity in the multivariate models due to exerting a stronger effect on the outcome variable (and considerable collinearity between the RNA and antibody HCV tests). All analyses were weighted to account for the sampling and survey design. Statistical analysis was conducted using STATA version 12.0 (StataCorp, College Station, TX).
The HCV prevalence rates for the husbands and wives were also stratified by the wives' excision status to explore how HCV prevalence in both husbands and wives varies according to the excision status of the woman. The terms excision and female genital cutting (FGC) are used synonymously in the paper. The FGC variable was defined as follows: both the women who had experienced FGC and the men whose wives had undergone FGC were coded as 1 and the women and men whose wives had not undergone FGC were coded as 0. To assess the impact of whether HCV prevalence in women was associated with who conducted the FGC, a second FGC variable, termed FGC-operator, was constructed as follows: women with no history of FGC coded 0, FGC performed by doctor and nondoctor coded as 1 and 2, respectively. The multivariate models for women were run separately with the FGC and FGC-operator variables.
A third generation enzyme-linked immunosorbent assay was used to detect HCV antibodies (Adaltis EIAgen HCV Ab, Casalecchio di Reno, Italy). Positive tests were confirmed by a chemiluminescent microplate immunoassay (CIA). Seropositive specimens were tested for HCV RNA using the RealTime_m2000 system (Abbott Laboratories, Abbott Park, IL, USA). Full details of the survey and sampling strategy have been previously published [1, 16].
3. Results
Overall HCV antibody prevalence in the married couples was 18.2% (95% CI, 16.8–19.6). HCV antibody prevalence was higher in the husbands (23.7%) than the wives (12.1%; P < 0.001; see Table 1). Restricting this analysis to the 15–49-year-olds reduced the difference in HCV between the husbands and wives (18.8% and 11.6% resp. P < 0.001). HCV prevalence was also higher in rural (20.4%) than urban (12.0%) regions (P < 0.001). HCV prevalence increased steadily with age reaching 30.2% (95% CI, 26.8–33.8) in men and 23.9% (95% CI, 20.4–27.7) in women in the 41–49-year-old category. Amongst women, there was a stepwise increase in HCV prevalence with increasing number of children: 6.9% if 0–2 children, 14.1% if 3–5 children, and 24.5% if more than 5 children. There was a lower HCV prevalence in those who had completed secondary level education (14.3%) compared to those with no education (23.5%; P = 0.001) and those in the top two income quintiles (12.1 and 12.9%) compared to those in the poorest quintile (22.8%; P < 0.001). HCV prevalence in persons who had received PAT (32.1%) was higher than in those who had not (16.5%; P < 0.001). Women with excision had a trend to higher HCV prevalence (12.5%) than those without (3.9%; P = 0.096; see Tables 1 and 2). Men whose wives had been excised had a higher HCV prevalence than those whose wives had not (23.7% versus 8.3%; P = 0.003). Women who had been excised by a doctor had a lower HCV prevalence than those excised by a nondoctor (5.6% versus 13.7%; P = 0.003). Respondents who had received a blood transfusion had nonsignificantly higher HCV prevalence rates than those who had not (26.9% versus 17.8%; P = 0.132). HCV prevalence increased with length of marriage, increasing from 8.2% to 17.6 and 29.6% in those married for ten years or less, 11–20 years, and over 20 years, respectively. Having received injections and dental treatment were not associated with HCV seropositivity.
Table 1.
HCV seroprevalence and age-adjusted odds ratios for selected characteristics (Egyptian DHS 2008).
| Risk factors | Number of exposed (%)a | Number of HCV antibody positive (%)b | Age-adjusted OR (95% CI) | P value (age-adjusted) |
|---|---|---|---|---|
| Place of residence | ||||
| Rural | 3234 (37.6) | 661 (20.4) | 2.4 (1.9–3.0) | <0.001 |
| Urban | 1948 (62.4) | 233 (12.0) | 1 | |
| Region | ||||
| Urban governorates | 610 (11.8) | 76 (12.6) | 1 | |
| Lower Egypt—urban | 554 (10.7) | 69 (12.3) | 1.0 (0.7–1.5) | 0.994 |
| Lower Egypt—rural | 1608 (31.0) | 364 (22.9) | 2.7 (1.9–3.8) | <0.001 |
| Upper Egypt—urban | 600 (11.6) | 79 (13.5) | 1.1 (0.7–1.7) | 0.778 |
| Upper Egypt—rural | 1524 (29.4) | 294 (20.1) | 2.1 (1.5–3.0) | <0.001 |
| Frontier governorates | 286 (5.5) | 12 (4.5) | 0.3 (0.2–0.6) | 0.001 |
| Gender | ||||
| Women | 2591 (50) | 300 (12.1) | 0.7 (0.6–0.9) | <0.001 |
| Men | 2591 (50) | 594 (23.7) | 1 | |
| Men's age (years) | ||||
| 15–20 | 9 (0.4) | 0 (0) | ||
| 21–30 | 524 (20.2) | 50 (9.5) | 1 | |
| 31–40 | 877 (33.9) | 117 (13.3) | 1.5 (1.0–2.1) | 0.034 |
| 41–49 | 827 (31.9) | 268 (32.4) | 4.5 (3.3–6.3) | 0.000 |
| 50–59 | 354 (13.7) | 159 (44.9) | 7.7 (5.4–11.1) | 0.000 |
| Women's age (years) | ||||
| 15–20 | 154 (5.9) | 5 (3.3) | 1 | |
| 21–30 | 1018 (39.3) | 60 (5.9) | 1.8 (0.7–4.7) | 0.188 |
| 31–40 | 860 (33.2) | 104 (12.1) | 4.1 (1.6–10.2) | 0.002 |
| 41–49 | 559 (21.6) | 131 (23.4) | 9.1 (3.6–22.7) | 0.000 |
| Educational attainment | ||||
| Secondary completed | 2424 (46.8) | 282 (14.3) | 0.7 (0.6–0.9) | 0.001 |
| Incomplete secondary or less | 1473 (28.4) | 282 (19.9) | 0.9 (0.7–1.1) | 0.219 |
| No education | 1285 (24.8) | 330 (23.5) | 1 | |
| Wealth index quintile | ||||
| Richest | 1010 (19.5) | 118 (12.1) | 0.4 (0.3–0.5) | <0.001 |
| Rich | 942 (18.2) | 112 (12.9) | 0.5 (0.4–0.7) | <0.001 |
| Middle | 1128 (21.7) | 225 (21.2) | 0.9 (0.7–1.2) | 0.413 |
| Poor | 1056 (20.4) | 220 (22.0) | 0.9 (0.7–1.2) | 0.600 |
| Poorest | 1046 (20.2) | 219 (22.8) | 1 | |
| Parenteral antischistosomiasis therapy | ||||
| No | 4582 (88.4) | 707 (16.5) | 1 | |
| Yes | 600 (11.6) | 187 (32.1) | 1.7 (1.4–2.2) | <0.001 |
| Women: reports FGCc | ||||
| No | 132 (5.1) | 4 (3.9) | 1 | |
| Yes | 2459 (94.9) | 296 (12.5) | 2.9 (0.8–10.1) | 0.096 |
| Men: his wife reports FGCd | ||||
| No | 132 (5.1) | 11 (8.3) | 1 | |
| Yes | 2459 (94.9) | 583 (23.7) | 3.1 (1.5–6.6) | 0.003 |
| FGC performed byg | ||||
| Doctor | 386 (15.7) | 21 (5.6) | 1 | |
| Nondoctor | 2073 (84.3) | 275 (13.7) | 1.4 (1.1–1.7) | 0.003 |
| Blood transfusion | ||||
| No | 4931 (95.3) | 832 (17.8) | 1 | |
| Yes | 244 (4.7) | 60 (26.9) | 1.3 (0.9–1.8) | 0.132 |
| Multiple injectionse | ||||
| No | 4301 (83.0) | 751 (18.4) | 1 | |
| Yes | 881 (17.0) | 143 (17.1) | 0.9 (0.7–1.1) | 0.312 |
| Dental treatmente | ||||
| No | 1829 (35.3) | 270 (15.3) | 1 | |
| Yes | 3353 (64.7) | 624 (19.6) | 1.1 (0.8–1.2) | 0.609 |
| Total number of childrenh | ||||
| 0–2 | 1098 (42.4) | 73 (6.9) | 1 | |
| 3–5 | 1162 (44.9) | 154 (14.1) | 1.2 (1.0–1.6) | 0.427 |
| ≥6 | 331 (12.8) | 73 (24.5) | 2.1 (1.0–2.5) | 0.035 |
| Partner is seropositive for HCV | ||||
| No | 4288 (82.8) | 608 (15.1) | 1 | |
| Yes | 894 (17.3) | 286 (32.6) | 2.1 (1.6–2.7) | <0.001 |
| Partner is HCV RNA positive | ||||
| No | 4569 (88.2) | 686 (15.9) | 1 | |
| Yes | 613 (11.8) | 208 (34.7) | 2.3 (1.7–2.9) | <0.001 |
| Wife is HCV RNA positive | ||||
| No | 2291 (88.4) | 451 (20.4) | 1 | |
| Yes | 300 (11.6) | 143 (47.4) | 2.6 (2.0–3.4) | <0.001 |
| Husband is HCV RNA positive | ||||
| No | 1997 (77.0) | 157 (8.3) | 1 | |
| Yes | 594 (22.9) | 143 (24.3) | 2.5 (1.9–3.3) | <0.001 |
| Another household member is seropositive for HCV | ||||
| No | 4760 (91.9) | 798 (17.7) | 1 | |
| Yes | 422 (8.1) | 96 (23.3) | 1.6 (1.2–2.1) | 0.004 |
| Another household member is HCV RNA positive | ||||
| No | 4896 (94.5) | 834 (17.9) | 1 | |
| Yes | 286 (5.5) | 60 (23.0) | 1.5 (1.1–2.1) | 0.015 |
| Length of marriage | ||||
| 0–10 years | 2430 (46.9) | 258 (8.2) | 1 | |
| 11–20 years | 1574 (30.4) | 339 (17.6) | 1.2 (1.0–1.5) | 0.051 |
| >20 years | 1178 (22.7) | 461 (29.8) | 1.4 (1.1–1.8) | 0.016 |
FGC: female genital cutting.
aUnweighted percentage.
bWeighted percentage.
cNumbers for this row are for women only.
dNumbers for this row are for men only.
eDefined as 2 or more injections reported in the preceding 6 months.
fEver received dental treatment of any sort.
gOf all women who report undergoing FGC
hThe total number of children that women report giving birth to.
Table 2.
Prevalence of HCV antibodies in 2591 husband-wife pairs, stratified by female genital cutting (FGC) status of the woman (Egyptian DHS 2008).
| Wife's HCV antibody status (%) | |||
|---|---|---|---|
| Negative | Positive | Totalb (%) | |
| Wife has undergone FGC | |||
| Husband HCV negative | 1721 | 155 | 1876 (76.3) |
| Husband HCV positive | 442 | 141 | 583 (23.7) |
| Totala (%) | 2163 (88.0) | 296 (12.0) | 2459 (100) |
| Wife has not undergone FGC | |||
| Husband HCV negative | 119 | 2 | 121 (91.7) |
| Husband HCV positive | 9 | 2 | 11 (8.3) |
| Totala (%) | 128 (97.0) | 4 (3.0) | 132 (100) |
aRow percentages.
bColumn percentages.
Persons with an HCV seropositive partner had a higher HCV prevalence than those who did not (32.6% versus 15.1%; P < 0.001). This effect was also evident if one's partner was RNA positive for HCV (34.7% versus 15.9%; P < 0.001). The effect was not as marked if it was another member of the household who was HCV antibody (23.3% versus 17.7%; P = 0.004) or RNA positive (23% versus 17.9%; P = 0.015).
In the multivariate logistic regression analyses, three variables were associated with HCV infection in all models; see Table 3. These were age, local HCV prevalence, and having a spouse who was infected with HCV. Having a nonspousal household member who was HCV infected was not independently associated with HCV. For both men and women HCV was less prevalent in the richer quintiles but in the case of men this effect was evident in the urban but not the rural areas.
Table 3.
Factors associated with 5182 husbands and wives testing seropositive for hepatitis C in the 2008 Egyptian Demographic and Health Survey: multivariate logistic regression model results (odds ratios, 95% confidence intervals, and P values).
| Women | Men | Urban women | Urban men | Rural women | Rural men | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| N | 2546 | 2546 | 953 | 953 | 1593 | 1593 | ||||||||||||
| Wife had FGC | 2.0 | 0.7–5.1 | 0.166 | 2.1 | 1.1–3.9 | 0.026 | 4.0 | 0.5–31 | 0.188 | 3.0 | 1.1–7.9 | 0.025 | 0.9 | 0.3–2.5 | 0.85 | 1.4 | 0.6–3.3 | 0.458 |
| Spouse is HCV RNA positive | 2.1 | 1.6–2.9 | 0.000 | 2.2 | 1.6–3.1 | 0.000 | 2.4 | 1.3–4.4 | 0.005 | 2.5 | 1.2–5.1 | 0.015 | 2.0 | 1.4–2.8 | 0.000 | 2.2 | 1.5–3.2 | 0.000 |
| Wealth index quintile | ||||||||||||||||||
| Poorest | Ref | |||||||||||||||||
| Poor | 1.2 | 0.9–1.8 | 0.260 | 0.9 | 0.7–1.3 | 0.698 | 1.9 | 0.5–7.6 | 0.379 | 0.2 | 0.1–0.6 | 0.002 | 1.2 | 0.8–1.8 | 0.333 | 1.1 | 0.8–1.5 | 0.527 |
| Middle | 1.0 | 0.6–1.5 | 0.922 | 1.2 | 0.9–1.6 | 0.248 | 0.9 | 0.2–3.5 | 0.855 | 0.4 | 0.2–0.9 | 0.035 | 1.1 | 0.7–1.7 | 0.670 | 1.4 | 1.0–1.9 | 0.063 |
| Rich | 0.7 | 0.4–1.1 | 0.097 | 0.7 | 0.5–0.9 | 0.017 | 0.8 | 0.2–3.1 | 0.796 | 0.2 | 0.1–0.5 | 0.000 | 0.8 | 0.4–1.4 | 0.378 | 0.9 | 0.6–1.5 | 0.727 |
| Richest | 0.5 | 0.3–0.8 | 0.003 | 0.7 | 0.5–1.0 | 0.087 | 0.6 | 0.2–2.5 | 0.508 | 0.3 | 0.1–0.6 | 0.001 | 0.5 | 0.2–1.1 | 0.082 | 1.3 | 0.7–2.2 | 0.381 |
| Ageb | 1.1 | 1.0-1.1 | 0.000 | 1.1 | 1.0-1.1 | 0.000 | 1.1 | 1.1-1.2 | 0.000 | 1.1 | 1.0-1.1 | 0.000 | 1.1 | 1.0-1.1 | 0.000 | 1.1 | 1.0-1.1 | 0.000 |
| Blood transfusion | 1.9 | 1.1–3.5 | 0.028 | 1.1 | 0.7–1.7 | 0.604 | 1.2 | 0.4–3.3 | 0.789 | 1.4 | 0.8–2.7 | 0.273 | 2.3 | 1.1–4.7 | 0.025 | 0.9 | 0.6–1.6 | 0.820 |
| Parenteral antischistosomiasis therapy | 1.7 | 1.1–2.8 | 0.016 | 1.3 | 1.0–1.7 | 0.025 | 1.9 | 0.6–5.8 | 0.254 | 2.3 | 1.4–3.8 | 0.002 | 1.7 | 1.1–2.9 | 0.028 | 1.1 | 0.9–1.5 | 0.412 |
| Another household member is HCV RNA positive | 0.8 | 0.5–1.4 | 0.455 | 1.2 | 0.8–1.9 | 0.361 | 1.1 | 0.3–4.1 | 0.915 | 1.5 | 0.5–4.6 | 0.518 | 0.7 | 0.4–1.4 | 0.319 | 1.2 | 0.7–1.9 | 0.496 |
| Length of marriage | ||||||||||||||||||
| 0–10 years | Ref | |||||||||||||||||
| 11–20 years | 1.0 | 0.7–1.6 | 0.887 | 1.3 | 1.0–1.8 | 0.044 | 0.6 | 0.2–1.5 | 0.285 | 1.4 | 0.8–2.3 | 0.243 | 1.2 | 0.7–2.1 | 0.416 | 1.4 | 1.0-2.0 | 0.056 |
| >20 years | 1.0 | 0.5–1.9 | 0.941 | 1.6 | 1.1–2.4 | 0.019 | 0.6 | 0.2–1.9 | 0.355 | 1.5 | 0.7–3.0 | 0.264 | 1.2 | 0.6–2.6 | 0.601 | 1.7 | 1.0–2.8 | 0.036 |
| Local HCV prevalencec | 1.1 | 1.1-1.1 | 0.000 | 1.1 | 1.0-1.1 | 0.000 | 1.1 | 1.0–1.2 | 0.001 | 1.0 | 1.0-1.1 | 0.033 | 1.1 | 1.1-1.1 | 0.000 | 1.1 | 1.0-1.1 | 0.000 |
| Number of childrend | 1.0 | 0.9–1.1 | 0.674 | NA | 1.0 | 0.8–1.3 | 0.695 | NA | 1.0 | 0.9-1.0 | 0.347 | NA | ||||||
NA: not applicable.
aNot entered into model due to collinearity; all nonexcised women were HCV negative (see text).
bAge is defined continuously in years.
cLocal HCV prevalence is defined as the HCV prevalence in the surrounding governorate.
dThe total number of children that women report giving birth to, here defined continuously.
HCV was associated with a blood transfusion in women but this association only applied to the rural areas. PAT was associated with HCV in all the models except in the men in the rural and the women in the urban areas. Being married for longer than 20 years was associated with HCV, but only for men. FGC was associated with HCV infection in the men but not the women overall. This relationship in men was driven by a relatively strong association in urban areas. Amongst the women, there was no association between FGC and HCV overall but in urban areas none of the women who were not excised were HCV infected. In the second set of women's models substitution of the FGC variable with the FGC-operator variable had little effect. FGC had no effect in rural areas and a strong effect in urban areas regardless of whether it was conducted by a doctor or nondoctor (data not shown).
The EDHS reveals that, of the women who had undergone FGC, 99.9% had done so by the age of 18. Of the women aged 15–18 surveyed in the EDHS, the HCV prevalence was significantly higher in those who had been excised (39/723; 5.4%) than those who had not (0/164; 0%, P = 0.028).
There was no evidence of interaction between the wife's FGC status and HCV status of the partner variables.
4. Discussion
Linking husbands and wives allowed us to test the association of HCV infection between husbands and wives. This represents the first time that this has been done in a nationally representative HCV survey. The sampling strategy used to describe the epidemiology of HCV in the USA, although nationally representative, does not include sexual partners in a linked way that would allow a similar analysis [17]. We found an association between the HCV status of the respondent and their partner. This is true for analyses limited to rural and urban areas and for subanalyses of men and women within these areas. The association remains after controlling for other members of the household being HCV infected. The relationship is slightly stronger when the HCV in the partner is measured with an RNA-based as opposed to an antibody-based test.
If not due to confounding, this association may be due to nonsexual intrafamilial transmission (such as shared utensils, toothbrushes, and razors), sexual transmission, or shared risk exposures (such as attending to the same health care practitioner). If the former was predominant then we should expect an association between HCV infection in nonspousal family members and in respondents. There was no evidence of such an association in any of the multivariate models. In our models we controlled for a large number of plausible, shared risk exposure types (such as blood transfusions, multiple injections, and PAT), but these did not affect the strength of the relationship between respondent and partner HCV status. The stronger association between the respondent's HCV status and that of their wife/husband as opposed to that of other family members may be mediated by the greater length of time they spent together. The fact that there is a relationship between length of marriage and HCV infection (for men) could be interpreted as supporting evidence for this idea. It does not however explain why this relationship only applies to men. An alternative explanation, and one that is also supported by the relationship between HCV infection and length of marriage, is that sexual transmission between partners is responsible for the relationship of HCV infection in married couples.
We cannot however exclude the possibility that the reason why the association between the wife and the husband's HCV status remains strongly positive after controlling for the HCV status of the other household members is due to the partner's HCV status being a better measure of general (nonsexual) infection pressure than the HCV status of the other household members. In the models we do control for the HCV in the surrounding community, but this is defined at the level of the governorate. This may not be a local enough measure of community HCV prevalence.
The relationship between HCV and FGC is complex. There is a strong relationship between HCV infection and FGC in the urban areas but none in the rural areas. There was little sex-based difference. For the men in the urban areas there is an association between HCV infection and having a wife who was excised (OR 3; 95% CI, 1.1–7.9). In the case of women, none of the nonexcised women had HCV infection.
How do we explain the discrepancy between the rural and urban areas? One possibility is that circumcision in urban areas is more likely to transmit HCV. Though this is possible, it should be noted that it is circumcision by nondoctors that is most strongly correlated with HCV infection [18] and in rural areas the proportion of FGC performed by nondoctors is higher (84.2%) than in urban areas (73.0%; P < 0.001) [18]. Another possibility is that FGC is so prevalent in the rural areas (97.2%) that there are too few nonexcised women to be able to demonstrate an effect of FGC on HCV prevalence. For example, in two of the other studies, to consider the impact of FGC on HCV in Egypt, no effect was found, but this may have been due to the extremely low numbers of persons not excised. In the first study there was only one person (out of 1989 individuals in the survey over the age of 20) who was not excised [5]. In the second study, only 4 women out of 1051 (0.4%) over the age of 30 were not excised. This study found a nonsignificant increase in the risk of HCV infection in those women who had been excised by an informal health care provider as opposed to those nonexcised combined with those excised by a formal health care provider (OR 1.6; 95% CI, 0.7–3.8). In a separate analysis of the EDHS a strong ecological association was found between the prevalence of FGC and HCV at the governorate level [18].
FGC has been associated with range of infections [19]. A population-based, cross-sectional study from the Gambia, for example, found a strong association between prevalent FGC and herpes simplex virus-2 infection (OR 4.7, 95% CI, 3.7–6.4) and a weaker association between FGC and bacterial vaginosis [20]. A case-control study of primary infertility in Sudan found more extensive forms of FGC to be more prevalent in the cases [21]. There were too few cases and controls without FGC in Sudan study to allow any analysis of those with versus those without FGC. The evidence from Egypt is mixed. A case control study of the determinants of infertility found that cases were more likely to have been excised by a traditional practitioner and more likely to have had more extensive forms of FGC [22]. A later study found no association between FGC and infertility [23].
What could be the possible mechanisms for FGC to result in increased rates of HCV for both men and women? Inadequate sterilization of implements used to perform FGC could be a factor. The higher HCV prevalence in excised versus nonexcised 15–18-year-olds in the EDHS could be interpreted as evidence supporting this nonsterility hypothesis. In addition, HCV transmission at the time of FGC could have been greater in the past when a considerably greater proportion of FGC procedures were performed by nondoctors [18]. Two studies from Egypt have found an association between male circumcision performed by informal health care providers and prevalent HCV infection [5, 24].
The anatomical changes produced by FGC, particularly the more extensive forms of FGC, could also promote subsequent female to male and male to female HCV transmission. It is biologically plausible that FGC could both enhance women's susceptibility to the sexual transmission of HCV and increase the chances that HCV is transmitted to their partner [23]. We cannot however exclude the possibility that the relationship between FGC and HCV is due to an unmeasured confounding variable.
There is considerable controversy in the literature about the extent to which HCV can be transmitted by sexual contact and cohabitation. In general most studies and two systematic reviews have found that HCV can be transmitted sexually but that the risk of infection is low [14, 25]. The most recent systematic review tried to make sense of the conflicting results by dividing the studies into those from high (Japanese) and low (non-Japanese) prevalence regions [14]. They found that pooling the results of studies along these lines provided strong evidence of increased HCV prevalence in offspring of affected persons in endemic areas but no such effect in nonendemic areas. In contrast they found evidence of an increased HCV prevalence amongst the spouses of persons who were HCV seropositive in nonendemic areas but no evidence for this effect in endemic areas. One interpretation of these apparently discordant findings is that HCV prevalence in spouses of HCV seropositive persons was not higher than controls in endemic areas as the prevalence in the controls was so high [14]. In endemic settings, transmission rates may be so high that close to all susceptible persons are infected by the time they are married. This may mask any effect that domestic and sexual transmission may play. An analogous effect was observed with hepatitis B virus. Sexual transmission was shown to occur in low prevalence areas such as USA but not in high prevalence areas such as East Asia [26, 27]. More recent studies have found evidence of spousal transmission of HCV in endemic areas [5, 6, 11, 13, 28–30]. Genotypic studies provide further evidence of the spousal transmission of HCV [8, 15, 30, 31].
In Egypt there is an increasing amount of evidence that intrafamilial transmission is an important source of new infections [5, 13, 29]. Two prospective studies investigating the correlates of incident of HCV in Egypt have found evidence of intrafamilial transmission [13, 32]. One of these was a study that followed up a cohort of 6734 HCV antibody negative persons from 2 rural villages over a median of 1.6 years [13]. In this time there were 33 new HCV infections, 27 of which occurred in families with an anti-HCV positive member. Parenteral factors were not associated with an elevated HCV incidence and in 21 of the cases there was no history of any parenteral exposure. HCV incidence per 1000 person years was higher in spouses of HCV antibody positive as opposed to antibody negative persons (13.1 versus 1.9; P = 0.08). Men and women with anti-HCV positive spouses were 7 and 2 times as likely to seroconvert as those with seronegative spouses. HCV incidence in children increased in a stepwise manner if they had one of two parents who is HCV antibody positive. A number of other studies have found marriage to be a risk factor for HCV infection but not all of these are controlled for age, which is likely a significant confounder [5, 6, 29]. One study found that parenteral factors only play a part in explaining prevalent cases in those over the age of 20 in Egypt [6].
One way of tying together the seemingly discordant findings about the extent of intrafamilial HCV transmission from different studies around the world is to apply the insight from hepatitis B virus epidemiology that the predominant mode of transmission may vary considerably between different regions of the world. Hepatitis B transmission in East Asia is predominantly perinatal, in USA it is largely sexual and intravenous drug use [33, 34], and in sub-Saharan Africa an important cause is horizontal transmission between children through poorly defined mechanisms [35–37].
In USA, iatrogenic and intravenous drug usage have been shown to be the dominant modes of HCV transmission [17]. There is mounting evidence that sexual transmission is important in HCV outbreaks of men who have sex with men [38]. The best quality evidence however suggests that sexual transmission has not played a large role in HCV transmission among heterosexuals in the USA [17, 39].
The composite evidence from Egypt reveals a somewhat different epidemiology for HCV. PAT was clearly important in the initial amplification of HCV in Egypt [3]. What perpetuated the spread of HCV thereafter? Perinatal transmission can take place. However, most individuals infected by this route clear the virus spontaneously [6, 40]. Unsterile procedures have clearly played an important role [1, 2, 6–8, 11]. A large proportion of cases are however not explained by these factors [1, 6, 9, 13]. Our study backs up the evidence from elsewhere of the likelihood of horizontal spread between family/household members [5, 9]. Some of this may be sexual but much is likely to be via other, as yet unclearly defined, mechanisms [13]. The findings presented here also build on the evidence from elsewhere [1] that FGC may have played a role in the spread of HCV—both at the time of the procedure and via enhancing the sexual transmission of HCV.
This analysis has a number of serious limitations. The EDHS was a cross-sectional survey and thus the direction of any implied causation cannot be established. Only 5182 individuals (out of 11126 individuals surveyed in the special health topics sample) could be linked together to provide the wife-husband dyad sample used for this analysis. Furthermore the limitations imposed by the linking process meant that the ages of the husbands were from a wider age-band (15–59 years old) than that of the wives (15–49). Because of these limitations, the sample we used cannot be assumed to be representative of whole Egypt.
The uni- and multivariate analyses of the married couple subsample are, however, remarkably similar to those found in analyses of the entire sample of 11126 respondents (presented in Guerra et al. [1]). This suggests that our subsample is not significantly biased.
Given the ongoing high incidence of HCV in Egypt [2], further research is needed to better define the mechanisms for intrafamilial spread so as to guide new prevention strategies. In particular further research is needed to ascertain if FGC is an effect-modifier in the sexual transmission of HCV.
Acknowledgment
The authors would like to thank Measure DHS (http://www.measuredhs.com) for making these data available.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. Journal of Viral Hepatitis. 2012;19(8):560–567. doi: 10.1111/j.1365-2893.2011.01576.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller FD, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14757–14762. doi: 10.1073/pnas.1008877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. The Lancet. 2000;355(9207):887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 4.Breban R, Doss W, Esmat G, et al. Towards realistic estimates of HCV incidence in Egypt. Journal of Viral Hepatitis. 2013;20:294–296. doi: 10.1111/j.1365-2893.2012.01650.x. [DOI] [PubMed] [Google Scholar]
- 5.Habib M, Mohamed MK, Abdel-Aziz F, et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33(1):248–253. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 6.Arafa N, El Hoseiny M, Rekacewicz C, et al. Changing pattern of hepatitis C virus spread in rural areas of Egypt. Journal of Hepatology. 2005;43(3):418–424. doi: 10.1016/j.jhep.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Talaat M, Kandeel A, Rasslan O, et al. Evolution of infection control in Egypt: achievements and challenges. American Journal of Infection Control. 2006;34(4):193–200. doi: 10.1016/j.ajic.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Paez Jimenez A, Sharaf Eldin N, Rimlinger F, et al. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59(11):1554–1560. doi: 10.1136/gut.2009.194266. [DOI] [PubMed] [Google Scholar]
- 9.Saleh DA, Shebl FM, El-Kamary SS, et al. Incidence and risk factors for community-acquired hepatitis C infection from birth to 5 years of age in rural Egyptian children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010;104(5):357–363. doi: 10.1016/j.trstmh.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleh DA, Shebl F, Abdel-Hamid M, et al. Incidence and risk factors for hepatitis C infection in a cohort of women in rural Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(9):921–928. doi: 10.1016/j.trstmh.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez AP, Mohamed MK, Eldin NS, et al. Injection drug use is a risk factor for HCV infection in urban Egypt. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0007193.e7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmat G, Hashem M, El-Raziky M, et al. Risk factors for hepatitis C virus acquisition and predictors of persistence among Egyptian children. Liver International. 2012;32(3):449–456. doi: 10.1111/j.1478-3231.2011.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed MK, Abdel-Hamid M, Mikhail NN, et al. Intrafamilial transmission of hepatitis C in Egypt. Hepatology. 2005;42(3):683–687. doi: 10.1002/hep.20811. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman Z, Ackerman E, Paltiel O. Intrafamilial transmission of hepatitis C virus: a systematic review. Journal of Viral Hepatitis. 2000;7(2):93–103. doi: 10.1046/j.1365-2893.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 15.de Cavalheiro NP, de la Rosa A, Elagin S, Tengan FM, Barone AA. Hepatitis C virus: molecular and epidemiological evidence of male-to-female transmission. Brazilian Journal of Infectious Diseases. 2010;14(5):427–432. doi: 10.1590/s1413-86702010000500001. [DOI] [PubMed] [Google Scholar]
- 16.Fatma EZ, Way A. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health; 2009. [Google Scholar]
- 17.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kenyon C, Buyze J, Apers L, Colebunders R. female genital cutting and hepatitis C spread in Egypt. ISRN Hepatology. 2013;2013:3 pages. doi: 10.1155/2013/617480.617480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iavazzo C, Sardi TA, Gkegkes ID. Female genital mutilation and infections: a systematic review of the clinical evidence. Archives of Gynecology and Obstetrics. 2013;287(6):1137–1149. doi: 10.1007/s00404-012-2708-5. [DOI] [PubMed] [Google Scholar]
- 20.Morison L, Scherf C, Ekpo G, et al. The long-term reproductive health consequences of female genital cutting in rural Gambia: a community-based survey. Tropical Medicine and International Health. 2001;6(8):643–653. doi: 10.1046/j.1365-3156.2001.00749.x. [DOI] [PubMed] [Google Scholar]
- 21.Almroth L, Elmusharaf S, El Hadi N, et al. Primary infertility after genital mutilation in girlhood in Sudan: a case-control study. The Lancet. 2005;366(9483):385–391. doi: 10.1016/S0140-6736(05)67023-7. [DOI] [PubMed] [Google Scholar]
- 22.Inhorn MC, Buss KA. Infertility, infection, and iatrogenesis in Egypt: the anthropological epidemiology of blocked tubes. Medical anthropology. 1993;15(3):217–244. doi: 10.1080/01459740.1993.9966092. [DOI] [PubMed] [Google Scholar]
- 23.Yount KM, Carrera JS. Female genital cutting and reproductive experience in Minya, Egypt. Medical Anthropology Quarterly. 2006;20(2):182–211. doi: 10.1525/maq.2006.20.2.182. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed MK, Magder LS, Abdel-Hamid M, et al. Transmission of hepatitis C virus between parents and children. American Journal of Tropical Medicine and Hygiene. 2006;75(1):16–20. [PubMed] [Google Scholar]
- 25.Rooney G, Gilson RJC. Sexual transmission of hepatitis C virus infection. Sexually Transmitted Infections. 1998;74(6):399–404. doi: 10.1136/sti.74.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lok AS, Lai CL, Wu PC, Wong VC, Yeoh EK, Lin HJ. Hepatitis B virus infection in Chinese families in Hong Kong. American Journal of Epidemiology. 1987;126:492–499. doi: 10.1093/oxfordjournals.aje.a114681. [DOI] [PubMed] [Google Scholar]
- 27.Szmuness W, Much MI, Prince AM. On the role of sexual behavior in the spread of hepatitis B infection. Annals of Internal Medicine. 1975;83(4):489–495. doi: 10.7326/0003-4819-83-4-489. [DOI] [PubMed] [Google Scholar]
- 28.Akahane Y, Kojima M, Sugai Y, et al. Hepatitis C virus infection in spouses of patients with type C chronic liver disease. Annals of Internal Medicine. 1994;120(9):748–752. doi: 10.7326/0003-4819-120-9-199405010-00005. [DOI] [PubMed] [Google Scholar]
- 29.Magder LS, Fix AD, Mikhail NNH, et al. Estimation of the risk of transmission of hepatitis C between spouses in Egypt based on seroprevalence data. International Journal of Epidemiology. 2005;34(1):160–165. doi: 10.1093/ije/dyh370. [DOI] [PubMed] [Google Scholar]
- 30.Kao J-H, Chen P-J, Yang P-M, et al. Intrafamilial transmission of hepatitis C virus. The important role of infections between spouses. Journal of Infectious Diseases. 1992;166(4):900–903. doi: 10.1093/infdis/166.4.900. [DOI] [PubMed] [Google Scholar]
- 31.Healey CJ, Smith DB, Walker JL, et al. Acute hepatitis C infection after sexual exposure. Gut. 1995;36(1):148–150. doi: 10.1136/gut.36.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mostafa A, Taylor SM, El-Daly M, et al. Is the hepatitis C virus epidemic over in Egypt? Incidence and risk factors of new hepatitis C virus infections. Liver International. 2010;30(4):560–566. doi: 10.1111/j.1478-3231.2009.02204.x. [DOI] [PubMed] [Google Scholar]
- 33.Alter MJ, Hadler SC, Margolis HS, et al. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. Journal of the American Medical Association. 1990;263(9):1218–1222. [PubMed] [Google Scholar]
- 34.Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis—United States, 2006. MMWR Surveillance Summaries: Morbidity and Mortality Weekly Report Surveillance Summaries/CDC. 2008;57(2):1–24. [PubMed] [Google Scholar]
- 35.Martinson FEA, Weigle KA, Royce RA, Weber DJ, Suchindran CM, Lemon SM. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. American Journal of Epidemiology. 1998;147(5):478–487. doi: 10.1093/oxfordjournals.aje.a009474. [DOI] [PubMed] [Google Scholar]
- 36.Whittle H, Inskip H, Bradley AK, et al. The pattern of childhood hepatitis B infection in two Gambian villages. Journal of Infectious Diseases. 1990;161(6):1112–1115. doi: 10.1093/infdis/161.6.1112. [DOI] [PubMed] [Google Scholar]
- 37.Karim SSA, Thejpal R, Coovadia HM. Household clustering and intra-household transmission patterns of hepatitis B virus infection in South Africa. International Journal of Epidemiology. 1991;20(2):495–503. doi: 10.1093/ije/20.2.495. [DOI] [PubMed] [Google Scholar]
- 38.Urbanus AT, Van De Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23(12):F1–F7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- 39.Vandelli C, Renzo F, Romanò L, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. American Journal of Gastroenterology. 2004;99(5):855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 40.Shebl FM, El-Kamary SS, Saleh DA, et al. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. Journal of Medical Virology. 2009;81(6):1024–1031. doi: 10.1002/jmv.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]


