Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) is a therapeutic procedure used to treat problems associated with biliary and pancreatic diseases. The benefits of ERCP over surgical treatment are well documented; however, complications including infection, pancreatitis, hemorrhage, and perforation can occur even in expert hands. Several factors, such as patient selection, skill of the operator, and the complexity of the procedure, can add to the intrinsic risks of ERCP This review outlines the current knowledge regarding ERCP complications and solutions for improved outcomes.
Keywords: ERCP, post-ERCP pancreatitis, post-ERCP hemorrhage, post-ERCP perforation, post-ERCP infection
Endoscopic retrograde cholangiopancreatography (ERCP) is a procedure that is frequently used in the management of a variety of pancreatobiliary disorders. ERCP is generally considered to be effective and safe. Post-ERCP complication rates vary widely depending on the complexity of the intervention and the individual patient. In a prospective, 2-year study of 2,347 patients from 17 institutions, 9.8% had post-ERCP complications, with pancreatitis (5.4%) and hemorrhage (2%) being the most common.1 The overall complication rate in a Chinese study of 3,178 patients who underwent ERCP was 7.9%.2 The rate reported in a British study of 4,561 patients was 5%.3 Retrospective studies show similar post-ERCP complication rates. In a study of 16,855 patients undergoing ERCP from 1977-2006, the post-ERCP complication rate was 6.85%.4 In this study, the majority (5.17%) of complications were mild. High-risk patients and/or patients who undergo more complex interventions reportedly have higher complication rates.5-7
Infectious Complications
One of the most serious post-ERCP complications is cholangitis leading to subsequent septicemia. Enteric bacteria enter the biliary tree by the hematogenous route or following endoscopic or radiologic manipulation. Improperly disinfected endoscopes and accessories may also introduce infection into the biliary tree.8 The most common organisms responsible for infection after ERCP are the Enterobacteriaceae (especially Escherichia coli and Klebsiella species), alpha hemolytic streptococci, Pseudomonas aeruginosa, Enterococcus, and Staphylococcus epidermidis.9,10 In most patients with acute cholangitis, a single organism is isolated from blood cultures.11
Risk factors for post-ERCP infection include the use of combined percutaneous and endoscopic procedures, stent placement in malignant strictures, the presence of jaundice, low case volume, and incomplete or failed biliary drainage.12 Patients who are immunocompromised are more likely to experience an infectious complication.11 Although transient bacteremia has been reported in up to 27% of therapeutic procedures, cholangitis has been reported in 1 % or fewer procedures.1,10,13 In a large retrospective study of 16,855 patients undergoing ERCP, infection was reported in only 1.4%; however, the mortality rate attributed to infections was 7.85%.4 The importance of complete drainage of the biliary tree cannot be overstated. In a prospective study of 242 patients, acute cholangitis occurred in 75% of patients who had retained stones and had failed biliary drainage, and it occurred in only 3% of patients who had successful drainage.14 Unrelieved hilar obstruction from cholangiocarcinoma also carries a risk of sepsis, especially when complete drainage of intrahepatic segments cannot be achieved. Contrast injection without the ability to drain the biliary tree should be avoided.
Similar to all pancreatitis, post-ERCP pancreatitis (PEP) also carries a risk of infectious complications. The risk of pancreatic parenchymal infection is related to the extent of pancreatic necrosis and usually does not manifest until 1—2 weeks after the onset of pancreatitis. Infected necrosis develops in approximately 30% of patients with pancreatic necrosis and is caused by translocation of bacteria across a disrupted intestinal barrier into nonviable pancreatic tissue.15,16 The diagnosis of infected pancreatic necrosis is suspected in patients with clinical deterioration, persistent organ failure, or signs of sepsis.17 Although fluid collections are common sequelae of pancreatitis, when persisting after 4 weeks, they are termed pseudocysts, which also carry a small risk of infection. All that is required when faced with clinical signs of a pseudocyst in an asymptomatic patient is close monitoring.
Strategies to Prevent, Reduce, and Manage Infectious Complications
Preprocedural use of magnetic resonance cholangiopancreatography and limiting contrast injection to segments previously accessed with a guidewire appear to reduce the risk of cholangitis. In several studies, air cholangiography was used to minimize the need for contrast injection in patients with obstruction.18,19 The debate regarding the need for unilateral or bilateral biliary drainage in patients with malignant hilar obstruction continues. In a randomized, controlled study, a lower rate of cholangitis with equal relief of jaundice was noted in the unilateral drainage group.20 A retrospective review by Chang and colleagues that included 141 patients with bifurcation tumors showed that the best survival rate was seen in patients in whom both liver lobes were drained.21 The worst survival rate was seen in patients in whom both liver lobes were opacified and only 1 was drained. In a recent study from Japan, a more durable response in terms of cumulative stent patency was seen with bilateral stent placement; there were no significant differences in the success of biliary drainage or the complication rate.22
Prevention and/or reduction of the risk of post-ERCP infectious complications can be achieved by judicious use of preprocedural antibiotics and intraprocedural steps, such as minimizing or avoiding contrast injection in patients with known biliary obstruction or cholangitis, endoscopic decompression, including the placement of a biliary stent or nasobiliary drain when complete drainage cannot be achieved, and prompt percutaneous drainage if endoscopic drainage is not possible or incomplete. Prophylactic preprocedural antibiotics should be given to patients with jaundice and suspected mechanical obstruction. In addition, patients with sclerosing cholangitis, pancreatic pseudocysts, and those who are immunocompromised should also receive preprocedural antibiotics.23
A recent Cochrane review concluded that routine prophylactic antibiotics reduce bacteremia and appear to prevent cholangitis; however, in a subgroup of patients with uncomplicated ERCP, the effect of antibiotics was less evident, and, therefore, preprocedural antibiotics may not be needed.24 In general, it is not necessary to give antibiotics post-ERCP if the endoscopist believes that the biliary tree has been completely drained. Exceptions include patients with sclerosing cholangitis and those with post-transplantation biliary strictures.25 It is a common practice to continue such patients on oral antibiotics for 3—5 days postprocedure. The antibiotic regimen should cover enteric gram-negative bacteria. Antibiotic agents that appear to reduce infection after ERCP include cephalosporins, aminoglycosides, and fluoroquinolones.26,27 Although the ideal antibiotic for biliary sepsis has not been found, ciprofloxacin and related fluoroquinolones are effective against most common organisms and are easy to administer with minimal adverse effects.28,29 In a study of 77 patients undergoing therapeutic ERCP for an obstructed biliary system, no patient treated with ciprofloxacin experienced cholangitis postprocedure.30
Management of pancreatic necrosis has shifted from early surgical debridement to initial less-invasive techniques such as percutaneous or laparoscopic necrosectomy. In the recent PANTER (Minimally Invasive Step Up Approach versus Maximal Necrosectomy in Patients with Acute Necrotising Pancreatitis) trial, a step-up approach where conservative management with percutaneous management preceded open necrosectomy was associated with reduced rates of major complications and death.31
Hemorrhage
Postsphincterotomy bleeding has been reported in up to 2% of ERCP cases.1,12 Immediate bleeding is seen in up to 30% of patients. Delayed bleeding can occur up to 2 weeks after the procedure.32 Several studies have addressed the risk factors for bleeding after endoscopic sphincterotomy.32,33 In one study, multivariable analysis suggested that definite risk factors were coagulopathy, anticoagulation within 3 days of endoscopic sphincterotomy, cholangitis before ERCP, bleeding during initial endoscopic sphincterotomy, and a lower case volume.33
Factors such as aspirin and nonsteroidal antiinflammatory drug (NSAID) use, ampullary tumors, long sphincterotomy, and extension of prior sphincterotomy were not associated with a greater risk of postsphincterotomy bleeding, whereas liver cirrhosis, dilated common bile ducts, periampullary diverticulum, precut sphincterotomy, and common bile duct stones appear to increase the risk of postsphincterotomy bleeding.33 Fortunately, most bleeding episodes are not clinically significant. The presence of melena, hematochezia, or hematemesis associated with a hemoglobin level decrease of at least 2 g/dL and/or the need for a blood transfusion signals a major bleeding episode and requires intervention.1
Severe bleeding following ERCP has been reported in 0.1-0.5% of cases.1,12 To reduce the risk of bleeding, special attention should be paid to the anatomy of arterial supply to the major papilla when performing a sphincterotomy. The 11—1 o’clock arc above the duodenal papilla reportedly has the least concentration of arteries, which validates the current practice of performing biliary sphincterotomy by incising the papilla in this axis to minimize the risk of bleeding.34 This risk of bleeding can be further decreased by identifying risk factors such as coagulopathy (international normalized ratio >1.5) or a low platelet count (<50,000/cu mm). Accurate positioning, proper electrocautery technique, and prevention of long, erratic cuts during sphincterotomy (referred to as “zippers”) are also important.35
The choice of electrosurgical current for biliary sphincterotomy is a source of controversy. The “pure cut” setting in electrocautery, which uses a low-voltage sawtooth waveform to cut the tissue without major heating, theoretically should reduce the risk of postsphincterotomy pancreatitis. A “blended” current, which is a mixture of cutting and coagulating (sinusoidal waveform) current, causes more coagulation, and heating is some- what mitigated by a brief “cooling off” interval between pulses. A meta-analysis has shown that pure current used for sphincterotomy is associated with more episodes of mild, transient, intraprocedural bleeding.36 Sequential use of pure cutting current then blended current does not appear to reduce the rate of postsphincterotomy bleeding.37,38 The use of microprocessor-controlled sphincterotomy (ENDO CUT mode on an ERBE generator) has been associated with a significant decrease in endoscopically observed bleeding but no change in clinically significant bleeding.39 The use of a partially covered (coated) sphincterotome wire, thought to provide more controlled cutting, does not decrease the rate of immediate or delayed bleeding.40
Treatment of Postsphincterotomy Bleeding
Treatment options for postsphincterotomy bleeding include balloon tamponade, injection of dilute epinephrine solution through a sclerotherapy needle, heater probe or bipolar coagulation, and/or the placement of endoscopic clips. Most episodes of bleeding cease spontaneously; thus, treatment should be reserved for patients who have clinically significant bleeding. Injection of epinephrine solution around the bleeding site or at the apex of the sphincterotomy incision is the most common method of hemostasis and is quite effective.36
Epinephrine injection was used with 100% success in a case series of 61 patients with immediate postsphincterotomy bleeding.41 Although injection therapy is reportedly very effective in stopping immediate postsphincterotomy bleeding—with rates as high as 96%— not all bleeding can be controlled by this technique.42,43 Delayed bleeding after injection therapy can occur in 4—16% of cases, and systemic absorption of epinephrine has been reported to cause cardiac arrhythmias in patients with coronary artery disease.42-44
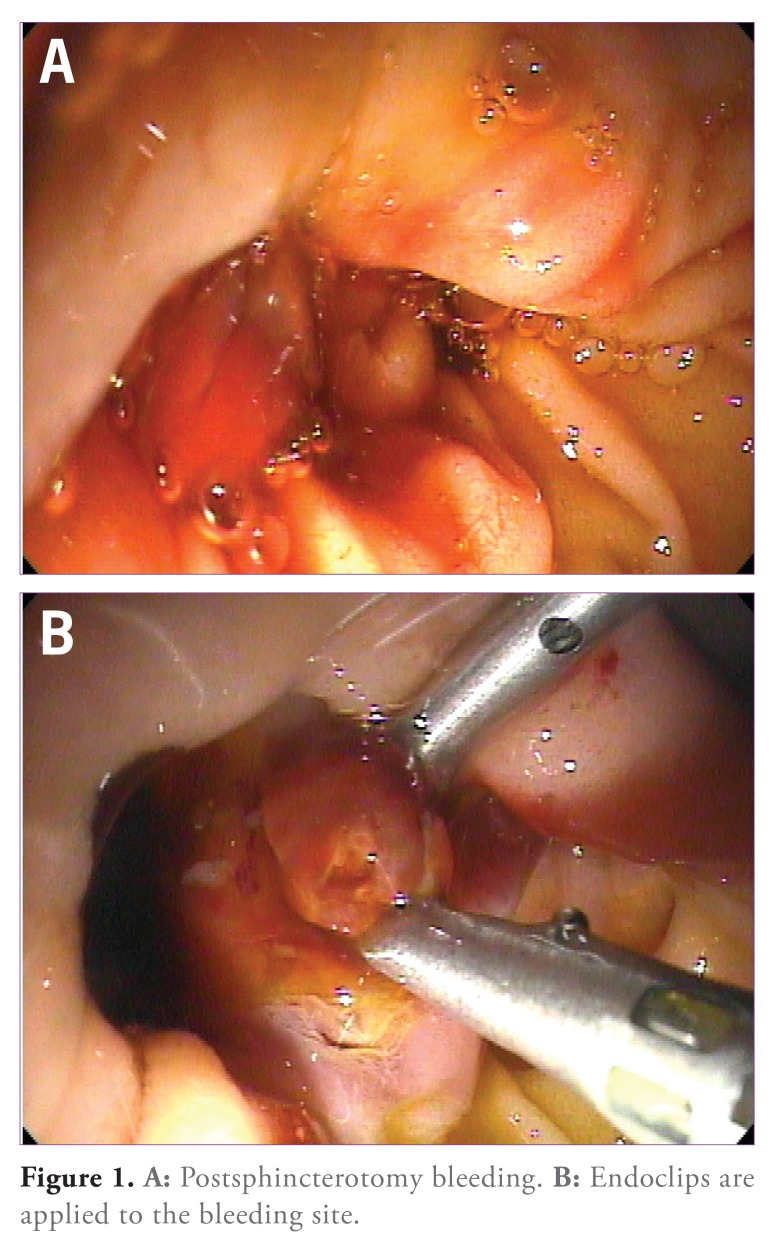
When injection fails to stop the bleeding, or when there are contraindications to its use, monopolar electrocautery can be applied to the bleeding site. In a prospective study of 11 patients with postsphincterotomy bleeding that was unresponsive to spray irrigation or injection of 1:10,000 epinephrine solution, monopolar coagulation was successful in all of the cases.40 When applying heat in the vicinity of the duodenal papilla, the pancreatic duct opening, located at the 5 o’clock position, should be avoided. The application of endoscopic clips (Figure 1) can achieve hemostasis. Although success has been reported in the literature, data from randomized, controlled trials are lacking.40,45,46 Applying endoscopic clips is technically difficult when using a duodenoscope, as the plastic sheath may bend and kink passing over the elevator at the bottom of the instrument channel, preventing clip deployment.
Figure 1.
A: Postsphincterotomy bleeding. B: Endoclips are applied to the bleeding site.
A recently developed treatment for postsphincterotomy bleeding is the insertion of fully covered self-expandable metal stents (SEMS) into the bile duct. This technique has been reported to stop bleeding that is unresponsive to more traditional methods.47,48 However, the cost of inserting a coated metal mesh stent is a considerable disadvantage when comparing this approach with other modalities. When endoscopic treatment fails and hemodynamically significant bleeding persists, the patient may need to undergo selective mesenteric angiography with embolization or even open surgery.32
Perforation
Perforation of the bile duct, pancreatic duct, or duodenum is reported in less than 1 % of patients undergoing ERCP.49-51 Bile duct perforation can be a result of guidewire or sphincterotome manipulation and, if significant, leads to development of an encapsulated collection of bile (a biloma). Free air following ERCP can be observed in 13-29% of asymptomatic patients; therefore, it should not be the sole reason for medical or surgical intervention.52,53 Duodenal perforations during ERCP can be retroperitoneal (usually from sphincterotomy or guidewire manipulation) or free/ intraperitoneal (typically resulting from endoscopic trauma or stent impaction). In a recent retrospective review of 12,427 patients undergoing ERCP, 75 (0.6%) had postprocedural perforation.51 The most common presumed causes were guidewire manipulation (32%), sphincterotomy (15%), endoscope manipulation (11%), cannulation (11%), stent placement (9%), or stricture dilation (7%).51 In 15% of patients, the exact cause for perforation was unknown. The majority (94%) of patients were undergoing therapeutic ERCP Retroperitoneal perforation rarely requires surgery; however, free duodenal perforations usually require open surgical toilet and repair.49
In one study, suspected sphincter of Oddi dysfunction (SOD), dilated bile ducts, and performance of sphincterotomy carried an increased risk of perforation.49 Post—Billroth II gastrectomy anatomy and biliary stricture dilation also have been noted to be risk factors.12,51 ERCP in patients with post—Billroth II anatomy was associated with an increased risk of jejunal loop perforation when using a side-viewing endoscope compared with a forward-viewing endoscope.54
Management of Perforation
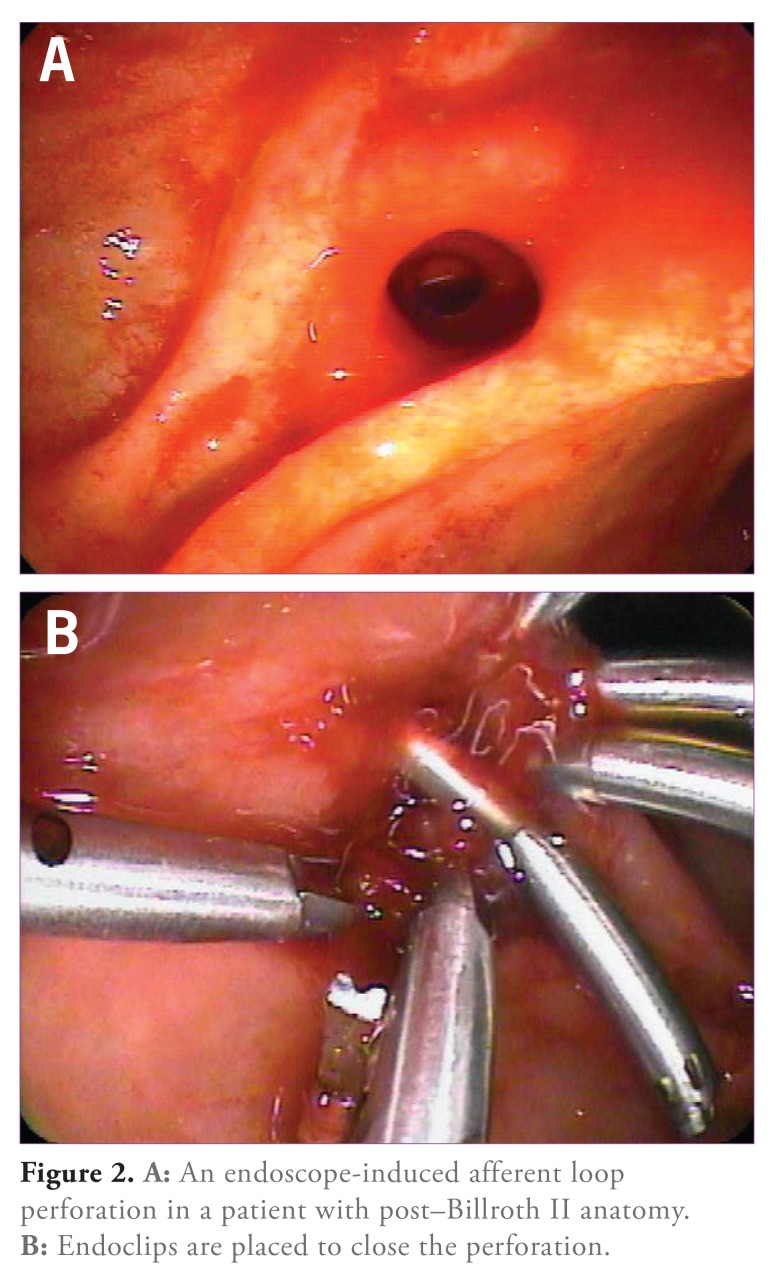
Management is usually guided by clinical symptoms and computed tomography (CT) findings. Suspected luminal perforation seen during the procedure should be immediately closed, if possible, using endoscopic clips (Figure 2), followed by a postprocedural CT scan with oral contrast to look for any evidence of leakage. Several case reports have demonstrated the efficacy of this approach.51,53 Bile duct or pancreatic duct perforation/leaks are usually managed with stent placement and further observation. If a biloma develops, antibiotics and percutaneous drainage in addition to endobiliary stent placement are key in management. Most small retroperitoneal perforations (with minimal or no evidence of contrast leakage on CT) can be managed medically. “Free” intraperitoneal perforation usually requires operative intervention. Clinical evaluation, abdominal CT scanning (with oral contrast to look for extravasation), and early surgical consultation are essential elements in the successful management of post-ERCP duodenal perforations.
Figure 2.
A: An endoscope-induced afferent loop perforation in a patient with post—Billroth II anatomy. B: Endoclips are placed to close the perforation.
Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis
Although asymptomatic elevation of serum amylase is a common occurrence after ERCP, the incidence of clinically significant PEP ranges from 1-15.7%.12,33,55-58 PEP is defined as new-onset or worsening abdominal pain with the elevation of serum amylase of 3 or more times the upper limit of normal at 24 hours postprocedure and the need for more than 2 days of pancreatitis-related hospitalization.55 Several studies and reviews have identified several risk factors. High PEP rates have been reported after interventional ERCP, especially those involving extensive biliary or pancreatic manipulation, after which PEP rates can approach 30%.5-7
Patient selection is a critical step in preventing PEP. Female sex, young age, clinical suspicion of SOD, a history of prior PEP and the absence of chronic pancreatitis were noted to be risk factors in multivariate analyses.33,59 Multiple risk factors can synergistically increase the chances of PER56 Women, in particular, have an increased risk compared with men.60 Critical review of the indication for ERCP and the use of less-invasive imaging modalities, such as magnetic resonance cholangiopancreatography and endoscopic ultrasound, should render diagnostic ERCP unnecessary in almost all cases and, thereby, limit manipulation of the ampulla/pancreas in high-risk patients.
Whereas careful patient selection and the availability of an experienced endoscopist reduce the risk of PEP, certain technical variables increase the risk. These variables include more than 1 injection of contrast into the pancreatic duct, difficult or failed cannulation, pancreatic sphincterotomy, balloon dilation of an intact biliary sphincter, failed clearance of bile duct stones, and precut sphincterotomy.56 The type of current used (pure vs blended) does not appear to have an impact on the risk of pancreatitis.36
What constitutes a difficult or prolonged cannulation is hard to define, as the time to successful cannulation varies widely. Many authors have used periods of 10-30 minutes before abandoning the procedure.61-63 Variables including operator experience, type of accessories used, and patient anatomy are important in determining the difficulty of ERCP. In a large, prospective, multicenter US study, the risk of PEP after a difficult cannulation increased from 4.3-11.3%.56 In another study, the risk of PEP increased from 3.3-14% when difficulty was encountered using standard cannulation methods.50 Mechanical trauma to the pancreatic sphincter with subsequent edema is thought to decrease drainage of pancreatic secretions and increase pressure within the duct, leading to pancreatitis.
Multiple contrast injections into the pancreatic duct have been cited as a major risk factor for PER2,50 Incre of the pancreatic parenchyma.59 In a multivariate analysis, the extent of pancreatic duct opacification was an independent predictor of PER59 In another study, however, pancreatic duct injection did not appear to play such a role.64
The osmolarity of injected contrastased frequency of PEP has been reported with increasing number of injections into the pancreatic duct and after acinarization media does not appear to impact the incidence of PEP. Low-osmolarity contrast media had been hypothesized to be safer because the media osmotically draw less fluid into the pancreatic duct, thereby keeping the pancreatic ductal pressure low. However, a meta-analysis that examined the role of the osmolarity of contrast media in the development of PEP did not show any difference between low- and highosmolarity contrast media.65
Several studies have reported that wire-guided biliary cannulation is associated with a reduced incidence of PEP.61,66,67 However, a recent study did not demonstrate such a benefit.68 Ten or more failed cannulation attempts, main pancreatic duct cannulation or opacification, suspected SOD, and precut papillotomy were significant risk factors for PEP. Overall, wire-guided biliary cannulation may be advisable before contrast injection as away to reduce trauma to the ampulla from repeated catheter probing.
Placement of a pancreatic duct stent allows the free flow of pancreatic exocrine secretions, preventing ductal hypertension and reducing the risk of pancreatitis. Multiple randomized, controlled trials and 2 meta-analyses have demonstrated that pancreatic duct stenting reduces the incidence of PEP in high-risk patients.4,69 A recent meta-analysis of 8 randomized, controlled trials and 10 nonrandomized studies concluded that placement of prophylactic pancreatic stents decreased the odds of PEP in high- and low-risk patients (odds ratio, 0.22; 95% confidence interval, 0.12-0.38; P<.01).70 When using a pancreatic stent, the risk of PEP was reduced from 19% to 6%.70 In the same analysis, it was estimated that 8 prophylactic stents need to be placed to prevent 1 episode of PEP.
Prophylactic pancreatic stents also appear to reduce the severity of PER71 The ideal stent is easy to place and migrates out of the pancreatic duct within 3—7 days (allowing sufficient time for papillary edema to resolve) while creating minimal risk of pancreatic duct injury. In most studies, 5 French (Fr) and 4Fr gauge plastic pancreatic stents have been used.6,72 3Fr gauge plastic stents also can be used; however, their insertion requires the use of a guidewire with an 0.021-inch diameter, which is difficult to manipulate, making the technique unpopular. Pahk and colleagues found that short 5Fr gauge stents were associated with less spontaneous migration, necessitating endoscopic removal in up to 40% of patients.6 In other studies, unflanged, short 5Fr stents had a much better spontaneous migration rate.73,74
Although temporary pancreatic stent placement will reduce the frequency and severity of PEP, the act of placing the stent itself can lead to potential complications. Pancreatic stent placement can be challenging. Various reports suggest a failure rate of 4-10%.71,75-77 Trauma to the duodenal papilla during failed pancreatic duct cannulation efforts likely increases the risk of pancreatitis. In a prospective study by Freeman, pancreatitis developed in 66.7% of patients after multiple failed pancreatic duct cannulation attempts.71 Other potential complications of stent placement include perforation of the pancreatic duct with a guidewire or stent, bleeding, and pain.78 Late complications include the development of pancreatic ductal changes, stent occlusion, and too-rapid stent migration following the procedure. Pancreatic duct stricturing and segmental dilation resembling chronic pancreatitis have been noted to occur in patients with previously normal pancreatic ducts after stenting.79 In a study by Kozarek, pancreatic ductal changes attributed to stent placement/occlusion developed in 72% of patients with previously normal pancreatograms.80
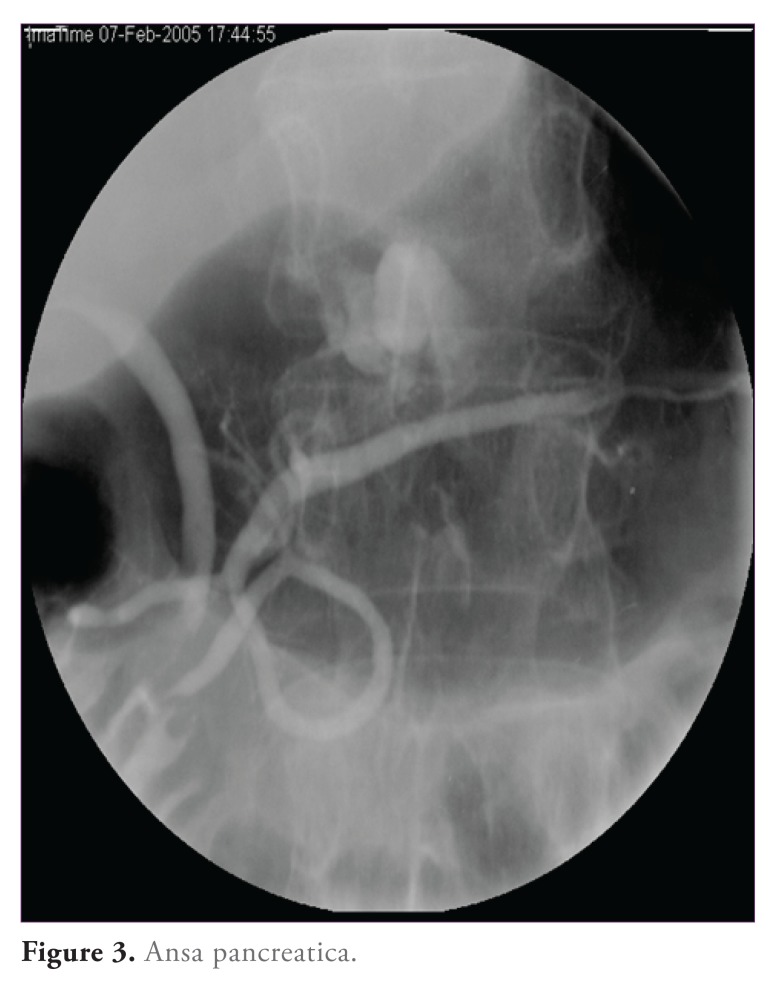
Long pancreatic stents may have less of a tendency to migrate early. In patients with aberrant pancreatic duct anatomy, such as ansa pancreatica (Figure 3), and others whose anatomy prevents deep insertion of a guidewire, very short stents (2—3 cm) are effective in reducing PER71
Figure 3.
Ansa pancreatica.
Pharmacotherapy for Post—Endoscopic Retrograde Cholangiopancreatography Pancreatitis
Pharmacotherapy for the prevention of PEP has been disappointing. Many drugs have been tried with variable success. Multiple pathways of pancreatic inflammation have been identified; compounds known to interfere with those biochemical processes have been tried as PEP prophylaxis. Somatostatin and its synthetic analog, octreotide, inhibit pancreatic exocrine secretion, nitroglycerin is a nitrogen oxide donor that relaxes SOD, and NSAIDs are inhibitors of phospholipase A2, cyclooxygenase, and neutrophil-endothelial interactions.81 A partial list of drugs that have been tried with variable success includes allopurinol, ceftazidime, diclofenac, gabexate, glyceryl trinitrate, hydrocortisone and other corticosteroids, indomethacin, interleukin-10, nafamostat mesylate, somatostatin/octreotide, and ulinastatin.82 A meta-analysis by Elmunzer and colleagues suggested that NSAIDs may be effective in reducing the risk of PER83 In a recent prospective, randomized clinical trial of rectally administered indomethacin (100 mg) in patients at high risk for PEP, the incidence of PEP was reduced from 16.9% to 9.2% in the treatment group; however, most patients also had prophylactic pancreatic stents.5
The use of prophylactic pancreatic duct stents is highly recommended in patients at predicted high risk for PEP. However, the benefits of prophylactic pancreatic duct stenting in patients not at high risk for PEP are uncertain because the stent placement carries some risk of triggering an attack. In light of recent data suggesting that postprocedural rectal insertion of two 50-mg indomethacin suppositories (ie, a 100-mg dose) reduces PEP in high-risk patients, this relatively benign intervention is being adopted as routine in many centers.
Postsphincterotomy Strictures
Historically seen in up to 8% of patients, postsphincterotomy biliary stricturing is now an uncommon complication.84 Fibrosis at the sphincterotomy site causes mechanical obstruction to bile flow, and patients can present with cholangitis and/or obstructive jaundice months to years after the index ERCP84-86 If stenosis is right at the duodenal wall (type 1 stenosis), then simply extending the prior sphincterotomy will relieve the narrowing. However, if the stenosis extends deep into the bile duct (type 2 stenosis), then sphincterotomy may not be definitive treatment, and stricture dilation is needed. In a 6-year retrospective study of 49 patients, endoscopic therapy of postsphincterotomy biliary stenosis resulted in sustained relief of symptoms in 83% of type 1 cases and 65% of type 2 cases.87 In another study, sequential insertion of an increasing number of biliary stents was successful in 90% of patients followed for up to 15 months.88 In yet another study, patients with postsphincterotomy strictures were successfully treated with sphincterotome strictureplasty in which electrocautery through the cutting wire of a sphincterotome (papillotome) was used to incise the biliary stricture.85 This technique differs from standard endoscopic sphincterotomy, during which one half to two thirds of the length of cutting wire remains outside the papilla. More data are needed to confirm the safety and efficacy of this technique. It cannot be recommended for routine management of biliary strictures because it is a blind cutting procedure, which may carry an increased risk of perforation.
Pancreatic duct orifice stenosis following pancreatic sphincterotomy also can occur, leading to recurrent pancreatitis and abdominal pain. A series of 7 patients with both bile duct and pancreatic duct orifice stenosis and 3 patients with accessory duct orifice stenosis were treated with stent placement and either one or both of the following: repeat sphincterotomy and dilation of the stricture.89 After a median of 140 days, strictures resolved in 4 of 7 patients with both bile duct and pancreatic duct orifice stenosis and none with accessory pancreatic duct orifice stenosis. At a median of 720 days after stent removal, 57% of patients with both bile duct and pancreatic duct orifice stenosis had improved symptoms, whereas only 33% of patients in the accessory pancreatic duct orifice stenosis group reported improvement. However, this is a small study, and further investigations need to be performed to evaluate the efficacy of such an approach.
Conclusions
ERCP will continue to play an important role in the management of patients with a variety of pancreatic and biliary disorders. Complications of ERCP can and do occur even when a skilled endoscopist is involved and all relevant guidelines are adhered to. Endoscopists need to be aware of the potential for complications of ERCP and be proactive in diagnosing and managing them. An experienced surgical colleague should be consulted early in the decision-making process since surgery may be required to manage a complication. Endoscopy units should have quality assurance programs to track negative outcomes prospectively so that issues that need intervention can be identified.
Footnotes
Dr. Szary has no conflicts of interest to disclose. Dr. Al-Kawas is a consultant for Glaxo and Boston Scientific.
References
- 1.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335(13):909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, Li ZS, Liu F, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104(1):31–40. doi: 10.1038/ajg.2008.5. [DOI] [PubMed] [Google Scholar]
- 3.Williams EJ, Taylor S, Fairclough P, et al. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy. 2007;39(9):793–801. doi: 10.1055/s-2007-966723. [DOI] [PubMed] [Google Scholar]
- 4.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102(8):178I–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 5.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366(15):1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pahk A, Rigaux J, Poreddy V, et al. Prophylactic pancreatic stents: does size matter? A comparison of 4-Fr and 5-Fr stents in reference to post-ERCP pancreatitis and migration rate. Dig Dis Sci. 2011;56(10):3058–3064. doi: 10.1007/s10620-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101(1):139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 8.Classen DC, Jacobson JA, Burke JP, et al. Serious Pseudomonas infections associated with endoscopic retrograde cholangiopancreatography. Am J Med. 1988;84(3 pt 2):590–596. doi: 10.1016/0002-9343(88)90141-6. [DOI] [PubMed] [Google Scholar]
- 9.Parker HW, Greenen JE, Bjork JT, et al. A prospective analysis of fever and bacteraemia following ERCP. Gastrointest Endosc. 1989;25(3):102–103. doi: 10.1016/s0016-5107(79)73385-2. [DOI] [PubMed] [Google Scholar]
- 10.Kullman E, Borch K, Lindström E, et al. Bacteremia following diagnostic and therapeutic ERCP. Gastrointest Endosc. 1992;38(4):444–449. doi: 10.1016/s0016-5107(92)70474-x. [DOI] [PubMed] [Google Scholar]
- 11.Subhani JM, Kibbler C, Dooley JS. Review article: antibiotic prophylaxis for endoscopic retrograde cholangiopancreatography (ERCP) Aliment Pharmacol Ther. 1999;13(2):103–116. doi: 10.1046/j.1365-2036.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 12.Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48(1):1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 13.Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96(2):4l7–423. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 14.Boender J, Nix GA, de Ridder MA, et al. Endoscopic sphincterotomy and biliary drainage in patients with cholangitis due to common bile duct stones. Am J Gastroenterol. 1995;90(2):233–238. [PubMed] [Google Scholar]
- 15.Beger HG, Rau B, Mayer J, et al. Natural course of acute pancreatitis. World J Surg. 1997;21(2):130–135. doi: 10.1007/s002689900204. [DOI] [PubMed] [Google Scholar]
- 16.Buchler MW, Gloor B, Muller CA, et al. Acute necrotising pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232(5):619–626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhl W, Warshaw A, Imrie C, et al. International Association of Pancreatology. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2(6):565–573. doi: 10.1159/000071269. [DOI] [PubMed] [Google Scholar]
- 18.Sud R, Puri R, Hussain S, et al. Air cholangiogram: a new technique for biliary imaging during ERCP. Gastrointest Endosc. 2010;72(1):204–208. doi: 10.1016/j.gie.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 19.Singh V, Singh G, Gupta V, et al. Contrast-free air cholangiography-assisted unilateral plastic stenting in malignant hilar biliary obstruction. Hepatobiliary Pancreat Dis Int. 2010;9(1):88–92. [PubMed] [Google Scholar]
- 20.De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized and controlled study. Gastrointest Endosc. 2001;53(6):547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 21.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47(5):354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 22.Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24(4):552–557. doi: 10.1111/j.1440-1746.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S, Shen B, Nelson DB, et al. ASGE Standards of Practice Committee. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791–798. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 24.Brand M, Bizos D, O’Farrell P., Jr Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database Syst Rev. 2010;6:CD007345. doi: 10.1002/14651858.CD007345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson MA, Fisher L, Jain R, et al. ASGE Standards of Practice Committee. Complications of ERCP. Gastrointest Endosc. 2012;75(3):467–473. doi: 10.1016/j.gie.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Byl B, Deviere J, Struelens MJ, et al. Antibiotic prophylaxis for infectious complications after therapeutic retrograde cholangiopancreatography: a randomized, double-blind, placebo-controlled study. Clin Infect Dis. 1995;20(5):1236–1240. doi: 10.1093/clinids/20.5.1236. [DOI] [PubMed] [Google Scholar]
- 27.Sauter G, Grabein B, Huber G, et al. Antibiotic prophylaxis of infectious complications with endoscopic retrograde cholangiopancreatography. A randomized controlled study. Endoscopy. 1990;22(4):164–167. doi: 10.1055/s-2007-1012830. [DOI] [PubMed] [Google Scholar]
- 28.Alveyn CG, Robertson DA, Wright R, Lowes JA, Tillotson G. Prevention of sepsis following endoscopic retrograde cholangiopancreatography. J Hosp Infect. 1991;19(suppl C):65–70. doi: 10.1016/0195-6701(91)90169-9. [DOI] [PubMed] [Google Scholar]
- 29.Wajahat Z, Culshaw KD, Tillotson GS, Chapman RW. Antibiotic prophylaxis for ERCP: a randomized clinical trial comparing ciprofloxacin and cefuroxime in 200 patients at high risk of cholangitis. Eur J Gastroenterol Hepatol. 1995;7(9):84l–845. [PubMed] [Google Scholar]
- 30.Davis AJ, Kolios G, Alveyn CG, Robertson DA. Antibiotic prophylaxis for ERCP: a comparison of oral ciprofloxacin with intravenous cephazolin in the prophylaxis of high-risk patients. Aliment Pharmacol Ther. 1998;12(3):207–211. doi: 10.1046/j.1365-2036.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 31.van Santvoort HC, Besselink MG, Bakker OJ, et al. Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira LE, Baron TH. Post-sphincterotomy bleeding: who, what, when, and how. Am J Gastroenterol. 2007;102(12):2850–2858. doi: 10.1111/j.1572-0241.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 33.Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56(suppl):S273–S382. doi: 10.1067/mge.2002.129028. [DOI] [PubMed] [Google Scholar]
- 34.Mirjalili SA, Stringer MD. The arterial supply of the major duodenal papilla and its relevance to endoscopic sphincterotomy. Endoscopy. 2011;43(4):307–311. doi: 10.1055/s-0030-1256229. [DOI] [PubMed] [Google Scholar]
- 35.Ratani RS, Mills TN, Ainley CC, Swain CP. Electrophysical factors influencing endoscopic sphincterotomy. Gastrointest Endosc. 1999;49(1):43–52. doi: 10.1016/s0016-5107(99)70444-x. [DOI] [PubMed] [Google Scholar]
- 36.Verma D, Kapadia A, Adler DG. Pure versus mixed electrosurgical current for endoscopic biliary sphincterotomy: a meta-analysis of adverse outcomes. Gastrointest Endosc. 2007;66(2):283–290. doi: 10.1016/j.gie.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Gorelick A, Cannon M, Barnett J, Chey W, Scheiman J, Elta G. First cut, then blend: an electrocautery technique affecting bleeding at sphincterotomy. Endoscopy. 2001;33(11):976–980. doi: 10.1055/s-2001-17918. [DOI] [PubMed] [Google Scholar]
- 38.Stefanidis G, Karamanolis G, Viazis N, et al. A comparative study of postendoscopic sphincterotomy complications with various types of electrosurgical current in patients with choledocholithiasis. Gastrointest Endosc. 2003;57(2):192–197. doi: 10.1067/mge.2003.61. [DOI] [PubMed] [Google Scholar]
- 39.Perini RF, Sadurski R, Cotton PB, et al. Post-sphincterotomy bleeding after the introduction of microprocessor-controlled electrosurgery: does the new technology make the difference? Gastrointest Endosc. 2005;61(1):53–57. doi: 10.1016/s0016-5107(04)02454-x. [DOI] [PubMed] [Google Scholar]
- 40.Katsinelos P, Paroutoglou G, Kountouras J, et al. Partially covered vs uncovered sphincterotome and post-endoscopic sphincterotomy bleeding. World J Gastroenterol. 2010;16:5077–5083. doi: 10.3748/wjg.v16.i40.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung JW, Chan FK, Sung JJ, Chung S. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factors and the role of epinephrine injection. Gastrointest Endosc. 1995;42(6):550–554. [PubMed] [Google Scholar]
- 42.Wilcox CM, Canakis J, Monkemuller KE, et al. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Am J Gastroenterol. 2004;99(40):244–248. doi: 10.1111/j.1572-0241.2004.04058.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsou YK, Lin CH, Liu NJ, et al. Treating delayed endoscopic sphincterotomy-induced bleeding: epinephrine injection with or without thermotherapy. World J Gastroenterol. 2009;15(38):4823–4828. doi: 10.3748/wjg.15.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Delius S, Thies P, Umgelter A, Prinz C, Schmid RM, Huber W. Hemodynamics after endoscopic submucosal injection of epinephrine in patients with nonvariceal upper gastrointestinal bleeding: a matter of concern. Endoscopy. 2006;38(12):1284–1288. doi: 10.1055/s-2006-944959. [DOI] [PubMed] [Google Scholar]
- 45.Guo SB, Gong AX, Leng J, Ma J, Ge LM. Application of endoscopic hemoclips for nonvariceal bleeding in the upper gastrointestinal tract. World] Gastroenterol. 2009;15(34):4322–4326. doi: 10.3748/wjg.15.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baron TH, Norton ID, Herman L. Endoscopic hemoclip placement for postsphincterotomy bleeding. Gastrointest Endosc. 2000;52(5):662. doi: 10.1067/mge.2000.108621. [DOI] [PubMed] [Google Scholar]
- 47.Shah JN, Marson F, Binmoeller KF. Temporary self-expandable metal stent placement for treatment of post-sphincterotomy bleeding. Gastrointest Endosc. 2010;72(6):1274–1278. doi: 10.1016/j.gie.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Di Pisa M, Tarantino I, Barresi L, et al. Placement of covered self-expandable metal biliary stent for the treatment of severe postsphincterotomy bleeding: outcomes of two cases. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/138748. 2010:138748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enns R, Eloubeidi MA, Mergener K, et al. ERCP-related perforations: risk factors and management. Endoscopy. 2002;34(4):293–298. doi: 10.1055/s-2002-23650. [DOI] [PubMed] [Google Scholar]
- 50.Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56(5):652–656. doi: 10.1067/mge.2002.129086. [DOI] [PubMed] [Google Scholar]
- 51.Fatima J, Baron TH, Topazian MD, et al. Pancreaticobiliary and duodenal perforations after periampullary endoscopic procedures: diagnosis and management. Arch Surg. 2007;l42(5):448–455. doi: 10.1001/archsurg.142.5.448. [DOI] [PubMed] [Google Scholar]
- 52.de Vries JH, Duijm LE, Dekker W, Guit GL, Ferwerda J, Scholten ET. CT before and after ERCP: detection of pancreatic pseudotumor, asymptomatic retroperitoneal perforation, and duodenal diverticulum. Gastrointest Endosc. 1997;45(3):231–235. doi: 10.1016/s0016-5107(97)70264-5. [DOI] [PubMed] [Google Scholar]
- 53.Wu HM, Dixon E, May GR, Sutherland FR. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): a population-based review. HPB (Oxford). 2006;8(5):393–399. doi: 10.1080/13651820600700617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim MH, Lee SK, Lee MH, et al. Endoscopic retrograde cholangiopancreatography and needle-knife sphincterotomy in patients with Billroth II gastrectomy: a comparative study of the forward-viewing endoscope and the side-viewing duodenoscope. Endoscopy. 1997;29(2):82–85. doi: 10.1055/s-2007-1004080. [DOI] [PubMed] [Google Scholar]
- 55.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 56.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54(4):425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 57.Cotton PB, Garrow DA, Gallagher J, et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70(1):80–88. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 58.Barthet M, Lesavre N, Desjeux A, et al. Complications of endoscopic sphincterotomy: results from a single tertiary referral center. Endoscopy. 2002;34(12):991–997. doi: 10.1055/s-2002-35834. [DOI] [PubMed] [Google Scholar]
- 59.Cheong YK, Bum K, Watkins JL, et al. Frequency and severity of post-ERCP pancreatitis correlated with the extent of pancreatic ductal opacification. Gastrointest Endosc. 2007;65(3):385–398. doi: 10.1016/j.gie.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 60.Trap R, Adamsen S, Hart-Hanses O, et al. Severe and fatal complications after diagnostic and theraputic ERCP: a prospective series of claims to insurance covering public hospitals. Endoscopy. 1999;31(2):125–130. doi: 10.1055/s-1999-13659. [DOI] [PubMed] [Google Scholar]
- 61.Lee TH, Park do H, Park JY, et al. Can wire-guided cannulation prevent post-ERCP pancreatitis? A prospective randomized trial. Gastrointest Endosc. 2009;69(3 pt 1):444–449. doi: 10.1016/j.gie.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 62.Maeda S, Hayashi H, Hosokawa O, et al. Prospective randomized pilot trial of selective biliary cannulation using pancreatic guide-wire placement. Endoscopy. 2003;35(9):721–724. doi: 10.1055/s-2003-41576. [DOI] [PubMed] [Google Scholar]
- 63.Kaffes AJ, Sriram PV, Rao GV, Santosh D, Reddy DN. Early institution of pre-cutting for difficult biliary cannulation: a prospective study comparing conventional vs. a modified technique. Gastrointest Endosc. 2005;62(5):669–674. doi: 10.1016/j.gie.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Halttunen J, Keranen I, Udd M, Kylänpää L. Pancreatic sphincterotomy versus needle knife precut in difficult biliary cannulation. Surg Endosc. 2009;23(4):745–749. doi: 10.1007/s00464-008-0056-0. [DOI] [PubMed] [Google Scholar]
- 65.George S, Kulkarni AA, Stevens G, et al. Role of osmolality of contrast media in the development of post-ERCP pancreatitis: a meta-analysis. Dig Dis Sci. 2004;49(3):503–508. doi: 10.1023/b:ddas.0000020511.98230.20. [DOI] [PubMed] [Google Scholar]
- 66.Artifon EL, Sakai P, Cunha JE, Halwan B, Ishioka S, Kumar A. Guidewire cannulation reduces risk of post-ERCP pancreatitis and facilitates bile duct cannulation. Am J Gastroenterol. 2007;102(10):2147–2153. doi: 10.1111/j.1572-0241.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 67.Hisa T, Matsumoto R, Takamatsu M, Furutake M. Impact of changing our cannulation method on the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis after pancreatic guidewire placement. World J Gastroenterol. 2011;17(48):5289–5294. doi: 10.3748/wjg.v17.i48.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mariani A, Giussani A, Di Leo M, Testoni S, Testoni PA. Guidewire biliary cannulation does not reduce post-ERCP pancreatitis compared with the contrast injection technique in low-risk and high-risk patients. Gastrointest Endosc. 2012;75(2):339–346. doi: 10.1016/j.gie.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60(4):544–550. doi: 10.1016/s0016-5107(04)02013-9. [DOI] [PubMed] [Google Scholar]
- 70.Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc. 2011;73(2):275–282. doi: 10.1016/j.gie.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 71.Freeman ML. Pancreatic stents for prevention of post-endoscopic ret rograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5(11):1354–1365. doi: 10.1016/j.cgh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2010;42(10):842–852. doi: 10.1055/s-0030-1255781. [DOI] [PubMed] [Google Scholar]
- 73.Chahal P, Baron TH, Petersen BT, Topazian MD, Gostout CJ, Levy MJ. Pancreatic stent prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: spontaneous migration rates and clinical outcomes. Minerva Gastroenterol Dietol. 2007;53(3):225–230. [PubMed] [Google Scholar]
- 74.Kawaguchi Y, Ogawa M, Omata F, et al. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18(14):1635–1641. doi: 10.3748/wjg.v18.i14.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fazel A, Quadri A, Catalano MF, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57(3):291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 76.Smithline A, Silverman W, Rogers D, et al. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy—induced pancreatitis in high-risk patients. Gastrointest Endosc. 1993;39(5):652–657. doi: 10.1016/s0016-5107(93)70217-5. [DOI] [PubMed] [Google Scholar]
- 77.Aizawa T, Ueno N. Stent placement in the pancreatic duct prevents pancreatitis after endoscopic sphincter dilation for removal of bile duct stones. Gastrointest Endosc. 2001;54(2):209–213. doi: 10.1067/mge.2001.115730. [DOI] [PubMed] [Google Scholar]
- 78.Smits ME, Badiga SM, Rauws EA, Tytgat GN, Huibregtse K. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc. 1995;42(5):461–467. doi: 10.1016/s0016-5107(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 79.Bakman YG, Safdar K, Freeman ML. Significant clinical implications of prophylactic pancreatic stent placement in previously normal pancreatic ducts. Endoscopy. 2009;41(12):1095–1098. doi: 10.1055/s-0029-1215317. [DOI] [PubMed] [Google Scholar]
- 80.Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointest Endosc. 1990;36(2):93–95. doi: 10.1016/s0016-5107(90)70958-3. [DOI] [PubMed] [Google Scholar]
- 81.Bang UC, Semb S, Nojgaard C, Bendtsen F. Pharmacological approach to acute pancreatitis. World] Gastroenterol. 2008;14(19):2968–2976. doi: 10.3748/wjg.14.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woods KE, Willingham FF. Endoscopic retrograde cholangiopancreatography asso ciated pancreatitis: a 15-year review. World J Gastrointest Endosc. 2010;16(5):165–178. doi: 10.4253/wjge.v2.i5.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elmunzer BJ, Waljee AK, Elta GH, Taylor JR, Fehmi SM, Higgins PD. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut. 2008;57(9):1262–1267. doi: 10.1136/gut.2007.140756. [DOI] [PubMed] [Google Scholar]
- 84.Bourke MJ, Elfant AB, Alhalel R, Scheider D, Kortan P, Haber GB. Sphincterotomy-associated biliary strictures: features and endoscopic management. Gastrointest Endosc. 2000;52(4):494–499. doi: 10.1067/mge.2000.108970. [DOI] [PubMed] [Google Scholar]
- 85.Tang SJ, Singh S, Singh S. Sphincterotome stricturoplasty for long ampullary stenoses and benign biliary strictures. Surg Endosc. 2011;25(4):1313–1318. doi: 10.1007/s00464-010-1340-3. [DOI] [PubMed] [Google Scholar]
- 86.Escourrou J, Cordova JA, Lazorthes F, Frexinos J, Ribet A. Early and late complications after endoscopic sphincterotomy for biliary lithiasis with and without the gallbladder “in situ.”. Gut. 1984;25(6):598–602. doi: 10.1136/gut.25.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veldkamp MC, Rauws EA, Dijkgraaf MG, Fockens P, Bruno MJ. Iatrogenic ampullary stenosis: history, endoscopic management, and outcome in a series of 49 patients. Gastrointest Endosc. 2007;66(4):708–716. doi: 10.1016/j.gie.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 88.Pozsár J, Sahin P, László F, Topa L. Endoscopic treatment of sphincterotomyassociated distal common bile duct strictures by using sequential insertion of multiple plastic stents. Gastrointest Endosc. 2005;62(1):85–91. doi: 10.1016/s0016-5107(05)00547-x. [DOI] [PubMed] [Google Scholar]
- 89.Khandekar S, DiSario JA. Endoscopic therapy for stenosis of the biliary and pancreatic orifices. Gastrointest Endosc. 2000;52(4):500–505. doi: 10.1067/mge.2000.108715. [DOI] [PubMed] [Google Scholar]