Abstract
Lymphocytic esophagitis (LE) is a newly described entity characterized histopathologically by peripapillary lymphocytosis (PL) without significant granulocytes (neutrophils and eosinophils). In an initial study, a significant portion of patients with LE had Crohn’s disease (CD). A subsequent study revealed LE in one quarter of children with CD. The aim of this study was to test the hypothesis that LE is associated with adult inflammatory bowel disease (IBD) and assess the disease variables that link LE and IBD. Random esophageal biopsies from consecutive adults with CD, ulcerative colitis (UC), or indeterminate colitis (IC) were evaluated. The numbers of lymphocytes, eosinophils, and neutrophils were counted from 3 high-power fields (HPF) in each specimen. Four of 47 patients (8.5%; 3/30 CD, 1/15 UC, and 0/2 IC) had PL (esophageal biopsies with ≥50 lymphocytes/HPF; mean, 100.5±31.1/HPF). A significant number of granulocytes were seen in biopsies from 3 of the 4 patients with PL, leaving 1 who met criteria for LE (PL without significant granulocytes). PL was associated with a higher erythrocyte sedimentation rate (90.3±17.6 mm/hr vs 24.5±3.6 mm/hr; P<.001) and C-reactive protein level (5.5±2.2 mg/dL vs 1.0±0.2 mg/dL; P<.001), with risk ratios of 2.06 (95% confidence interval [CI], 1.45-2.93; P=.031) and 3.56 (95% CI, 2.04-6.19; P=.033), respectively, for elevated values. All patients with PL had a relapsing CD course. The mean Harvey-Bradshaw index (HBI) was higher in these patients (8.5±0.6 vs 4.3±0.7; P=.026), with a risk ratio of 4.78 for moderate-to-severe disease (95% CI, 2.67-8.54; P=.004). We found a less frequent association between IBD and LE than was previously reported. This may be due to differences between pediatric and adult IBD. Alternatively, it may be methodologic because, unlike in previous reports, we evaluated consecutive patients with IBD. PL was associated with elevated inflammatory markers and HBI. These observations suggest that PL may be a marker of disease activity in IBD.
Keywords: Crohn’s disease, esophagitis, lymphocytic esophagitis, lymphocytes, inflammatory bowel disease, ulcerative colitis
Lymphocytic esophagitis (LE) is a recently recognized entity characterized by the occurrence of peripapillary lymphocytosis (PL) with high numbers of intraepithelial lymphocytes (IELs), but only occasional or absent granulocytes (neutrophils and eosinophils) on esophageal biopsy.1 The histologic features of LE were first described in baboons after striking lymphocyte infiltration of the esophagus was noticed while reviewing histologic features of the stomach.2
The most common cause of esophageal inflammation or injury is gastroesophageal reflux disease (GERD). The characteristic response of esophageal epithelia to GERD is epithelial cell proliferation that produces thickening of the basal layer and elongation of the epithelial papillae.3,4 Nonspecific histopathologic features of reflux esophagitis include pale, distended squamous cells called balloon cells, dilated vascular channels in papillae of the lamina propria, and scattered intraepithelial eosinophils and/or neutrophils. Investigators also have identified a lymphocytic intraepithelial infiltrate associated with reflux esophagitis.5,9 Esposito and colleagues7 and Mangano and colleagues8 described the presence of IELs with irregular nuclear contours, also called squiggle cells because these cells appear tightly intermingled with epithelial cells9 in the esophageal epithelium of children and adults with suspected GERD. Wang and colleagues reported a numerical correlation of T lymphocytes with eosinophils but not with neutrophils.9 While more lymphocytes were seen in specimens with histologic evidence of esophagitis, their numbers were not statistically different from those of controls.
LE differs from other types of esophagitis (eg, reflux, postradiation, and Candida albicans esophagitis) in that it is characterized by many IELs and few or no intraepithelial granulocytes. Other forms of esophagitis are characterized by the presence of intraepithelial granulocytes. LE also appears to be localized to the esophagus. In a study by Rubio and colleagues that documented 20 patients with LE, none of the biopsy specimens obtained from sites other than the esophagus showed IELs.1 Similarly, in a separate study, duodenal mucosal biopsies from patients with esophagitis and/or gastritis revealed normal or slightly increased numbers of IELs.10 Few of the patients with LE had symptoms and/or endoscopic findings consistent with GERD, implying the presence of a disparate disease that is not triggered by GERD. These data were corroborated by Haque and Genta who reviewed biopsy specimens from 129,252 unique patients and found that the complaint of GERD was less frequent (18%) in patients with LE than in those with normal esophageal biopsies (37%).11 In an evaluation of the histologic importance of lymphocytes in eosinophilic esophagitis and reflux esophagitis, we saw a similar number of patients with GERD and PL (8/39; 21%).12 These findings potentially blur the line between reflux and LE and have led some doctors to postulate that perhaps LE is an extreme pathology in the spectrum of GERD.13
The incidence of LE varies among studies and may be secondary to differences in study sizes and patient populations. We detected LE in 2 (5%) of 39 patients with GERD, whereas Haque and Genta reported a diagnosis of LE in 119 (~0.1%) of 129,252 patients.11 In contrast, Ronkainen and colleagues could not identify LE in 1,000 randomly selected community subjects.14
Perhaps the most intriguing finding by Rubio and colleagues1 was an association between LE and Crohn’s disease (CD). Forty percent of patients with LE had CD. Endoscopic findings in this subset of patients included esophagitis (n=2), gastroduodenitis (n=2), and aphthoid ulcerations (n=l). The findings led the authors to ask whether LE is a manifestation of CD or whether the association found was “haphazard.”
A subsequent investigation reported a histologic diagnosis of LE in 17 (28%) of 60 pediatric patients with CD, suggesting that LE may be a manifestation of upper gastrointestinal CD.15 Of note, 11 (55%) of the 20 patients with LE in the study by Rubio and colleagues were age 17 years or younger.1 Another retrospective analysis of 42 LE cases from a combined pediatric and adult population concluded that patients with LE were not statistically more likely than controls to have CD.16 In the most recently published investigation of LE, none of the 119 patients with LE had concurrent pathology consistent with CD.11
Given the possible association with inflammatory bowel disease (IBD) and our own clinical experiences with this novel entity, we sought to determine the incidence of PL and LE in esophageal biopsies from adults with IBD and to identify predictive disease variables.
Methods
Subjects
Consecutive patients with IBD (CD, ulcerative colitis, and indeterminate colitis) who underwent upper endoscopy between September 2009 and April 2010 at our IBD center were included in the study. Esophageal biopsy specimens were collected from all patients. It is the routine of the investigators to obtain biopsies from both the mid- and distal esophagus. Previously, all subjects had either conclusively diagnosed or suspected IBD. IBD was diagnosed from biopsies obtained at the same endoscopy as the aforementioned esophageal biopsies. Exclusion criteria included age less than 18 years, history of eosinophilic esophagitis, collagen vascular disease, radiation therapy involving the chest, metastatic cancer, Behcet disease, graft-versus-host-disease, sarcoidosis, chronic granulomatous disease, certain medications (including montelukast, loratadine, nitrogen-containing bisphosphonates, antibiotics, nonsteroidal anti-inflammatory drugs, potassium supplementation, quinidine, and chemotherapy), and an uncertain diagnosis of IBD. The exclusion criteria were selected based on assessment of variables that potentially may lead to confounding results. The Institutional Review Board approved this study.
Chart Review
Each subjects electronic medical record was evaluated to assess the following variables: age, gender, type of IBD, IBD duration, CD behavior (inflammatory, stricturing, or penetrating), history of relapsing disease, clinical activity (remission or relapsed/active), Harvey-Bradshaw index (HBI),17 history and type (if applicable) of surgery, tobacco and alcohol use, history of GERD, endoscopic findings, and laboratory data (white blood cell [WBC] count, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP] level, and fecal calprotectin). The endoscopic finding of esophagitis was based on the impression of the endoscopist. An endoscopic classification scheme for esophagitis was not applied. Endoscopic features described included—but were not limited to—mucosal breaks, erythema, and superficial ulcerations. Endoscopic findings from other locations in the gastrointestinal tract were also documented (if available).
Histologic Analysis
After hematoxylin and eosin staining, the numbers of lymphocytes, eosinophils, and neutrophils were counted in 3 separate high-power fields (HPF; 400 x 0.22 mm2). Three HPFs were counted in hopes of obtaining a better global assessment of the inflammatory process, as previous work demonstrated variations in the density of cellular infiltrates within a single specimen.18 The most inflamed fields were chosen in the setting of patchy inflammation, and random fields were evaluated in the background of diffuse inflammation. The most complete fields were chosen in each respective setting. Two expert gastrointestinal pathologists read all of the pathology specimens and agreed on the reported result.
Statistical Analysis
Student t test was used to assess for significant differences between mean values. Statistical relationships between proportions and percentages were evaluated by chi-square analysis. P-values of .05 or less were assumed to be statistically significant. Odds ratio (OR) (or risk ratio [RR] if OR was infinite) with 95% confidence interval (CI) was also assessed for a number of parameters. Standard deviation (SD) was given for age, and the standard error of the mean was calculated for all other continuous variables.
Results
Primary Characteristics of the Subjects
Forty-seven patients with IBD were evaluated (Table 1). The average age was 39.3±14.6 (SD) years (range, 18-74 years). Thirty (64%) patients had CD, 15 (32%) patients had ulcerative colitis, and 2 (4%) patients had indeterminate colitis. Comorbidities included GERD symptoms (n=l4; 30%), asthma (n=3; 6%), and diabetes mellitus (n=3; 6%). There were no patients with celiac disease. One patient was an active smoker, and 7 patients were previous smokers. Active alcohol consumption was documented in 5 patients and previous use in 6 patients. The mean WBC count was 7.8±0.4 x 1,000/µL, the mean ESR was 29.9±4.4 mm/hr, and the mean CRP level was 1.4±0.3 mg/dL.
Table 1.
Primary Characteristics of the Subjects
| Age (years) | 39.3±14.6 |
| Male (%) | 51 |
| GERD (%) | 30 |
| Asthma (%) | 6 |
| Diabetes mellitus (%) | 6 |
| Celiac disease (%) | 0 |
| Active smoker (%) | 2 |
| Former smoker (%) | 15 |
| Active alcohol use (%) | 11 |
| Previous alcohol use (%) | 13 |
| WBC count (×l,000/µL) | 7.8±0.4 |
| ESR (mm/hr) | 29.9±4.4 |
| CRP level (mg/dL) | 1.4±0.3 |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GERD, gastroesophageal reflux disease; WBC, white blood cell.
Characteristics of Inflammatory Bowel Disease
The mean disease duration was 12.5±9.9 (SD) years (Table 2). Forty-three patients (91%) had a relapsing disease course, and 30 patients (64%) had active disease, with a mean HBI of 4.6±0.6. Inflammatory, stricturing, and penetrating disease were documented in 21 patients, 7 patients, and 9 patients, respectively. Gastrointestinal surgery related to IBD had been performed in 24 patients (51%).
Table 2.
Characteristics of Inflammatory Bowel Disease
| Crohn’s disease (%) | 64 |
| Ulcerative colitis (%) | 32 |
| Indeterminate colitis (%) | 4 |
| Disease duration (years) | 12.5±9.9 |
| Relapsing course (%) | 89 |
| Active disease (%) | 64 |
| HBI | 4.6±0.6 |
| Phenotype (n) | |
| Inflammatory | 21 |
| Penetrating | 9 |
| Stricturing | 7 |
| Surgery (%) | 51 |
| Ileal/IC resection | 21 |
| Colectomy | 15 |
| Proctocolectomy | 9 |
| Other SB resection | 6 |
HBI, Harvey-Bradshaw index; IC, ileocolonic; SB, small bowel.
Peripapillary Lymphocytosis
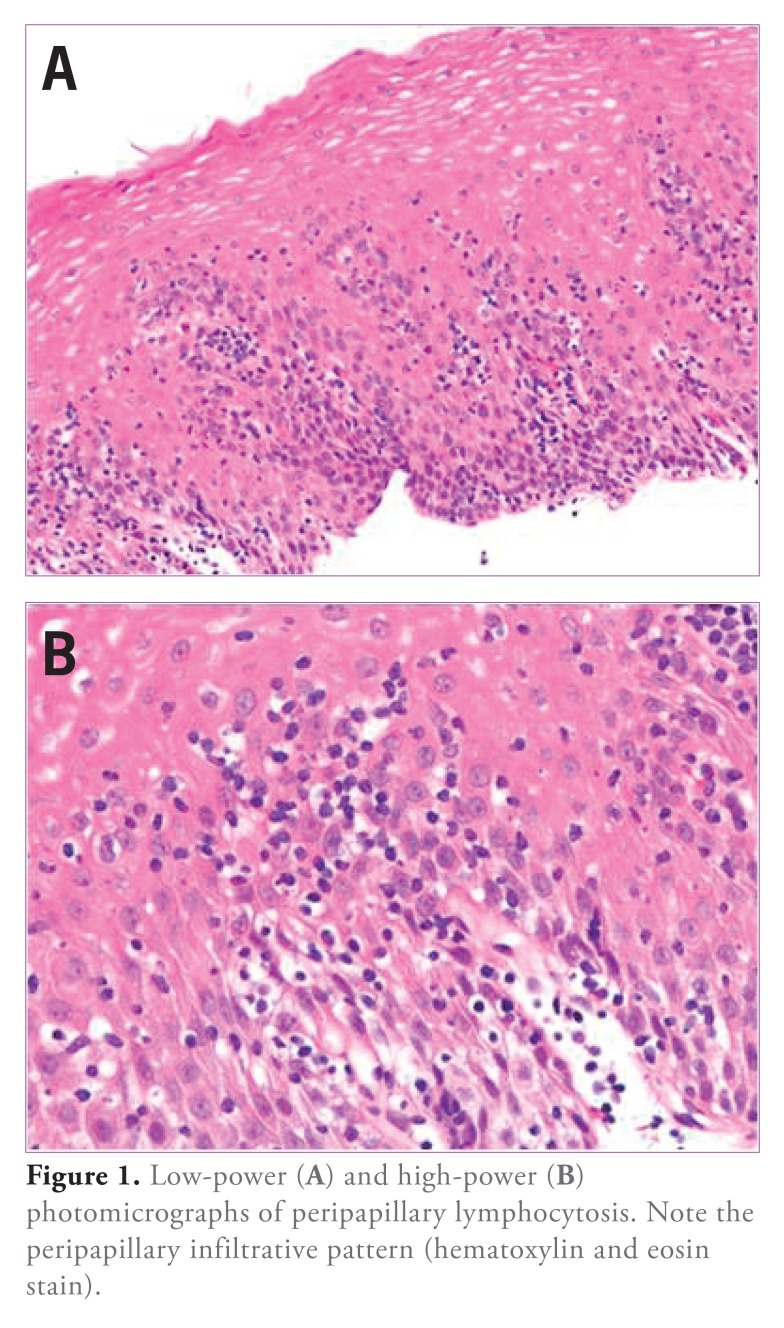
The overall mean lymphocytes/HPF was 20.6±4.5. Neutrophils (2.0±0.9/HPF) and eosinophils (0.6±0.2/HPF) were less common. Esophageal biopsies from 4 patients (8.5%; 3 CD and 1 UC) demonstrated PL (Figure 1).
Figure 1.
Low-power (A) and high-power (B) photomicrographs of peripapillary lymphocytosis. Note the peripapillary infiltrative pattern (hematoxylin and eosin stain).
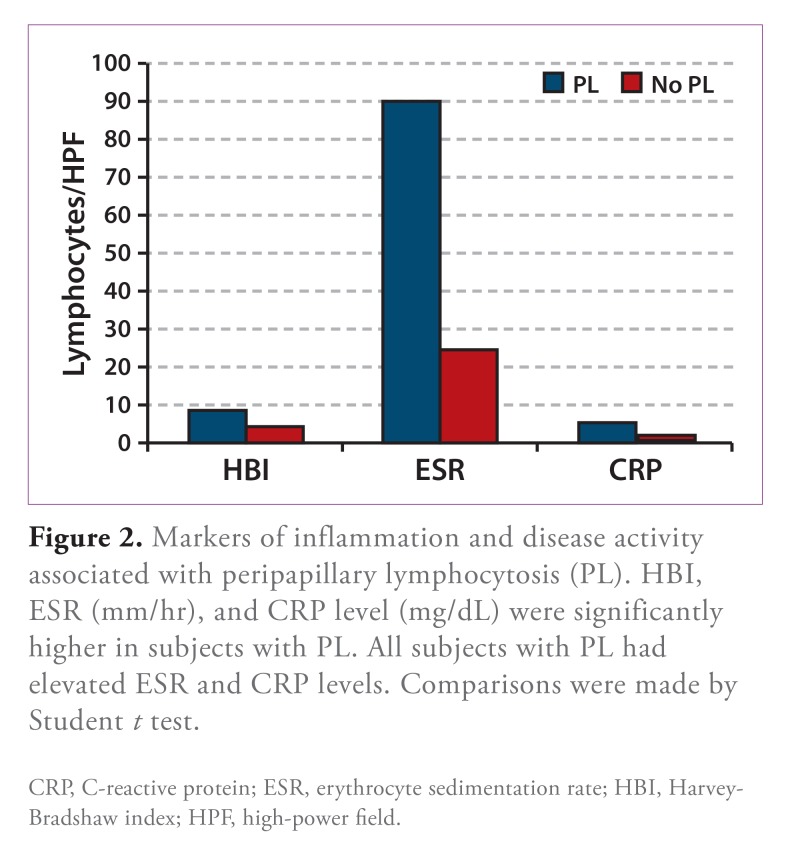
In addition to markedly greater lymphocyte density, PL was associated with greater numbers of neutrophils/ HPF (Table 3). GERD symptoms were present in three fourths of patients (75% vs 28% without PL; OR, 7.75; 95% CI, 0.73-82.02; P=.089), while other symptoms included abdominal pain (n=3), odynophagia (n=l), and diarrhea (n=l). All of the patients with PL had a relapsing IBD disease course (3 with active disease), and the mean HBI was almost twice that of patients without PL (Table 4 and Figure 2), with an RR of 4.78 for an HBI that was indicative of moderate or severe disease (95% CI, 2.67-8.54; P=.004). Endoscopic and histologic findings included white plaques (n=2) and friable mucosa (n=l) in the esophagus, gastric erythema (n=2), active duodenitis (n=2), duodenal lymphocytosis (n=l), and active colitis (n=2). There were no striking associations between the location of extraesophageal disease activity and PL. ESR and CRP level were significantly higher in patients with PL (Table 4 and Figure 2), with RRs of 2.06 (95% CI, 1.45-2.93; P=.031) and 3.56 (95% CI, 2.04-6.19; P=.033), respectively for elevated values.
Table 3.
Cell Type Distributions in Patients with and without Peripapillary Lymphocytosis (PL)
| Cell type | PL | NO PL | P-value |
|---|---|---|---|
| Mean±SE | Mean±SE | ||
| Lymphocytes | 100.5±31.1 | 13.2±1.5 | <.001 |
| Neutrophils | 14.5±4.9 | 0.9±0.6 | <.001 |
| Eosinophils | 0.9±0.6 | 0.6±0.2 | .723 |
SE, standard error of the mean. Data compared by Student t test.
Table 4.
Markers of Inflammation and Disease Activity Associated with Peripapillary Lymphocytosis (PL)
| Marker | PL | NO PL | P-value |
|---|---|---|---|
| Mean±SE | Mean±SE | ||
| HBI | 8.5±0.6 | 4.3±0.7 | .026 |
| ESR (mm/hr) | 90.3±17.6 | 24.5±3.6 | <.001 |
| CRP level (mg/dL) | 5.5±2.2 | 1±0.2 | <.001 |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HBI, Harvey-Bradshaw index; SE, standard error of the mean. Data compared by Student t test.
Figure 2.
Markers of inflammation and disease activity associated with peripapillary lymphocytosis (PL). HBI, ESR (mm/hr), and CRP level (mg/dL) were significantly higher in subjects with PL. All subjects with PL had elevated ESR and CRP levels. Comparisons were made by Student t test.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HBI, Harvey-Bradshaw index; HPF, high-power field.
Lymphocytic Esophagitis
One patient (2%) had PL without significant granulocytes, which is consistent with a diagnosis of LE. This patient was a man, age 20 years, in whom CD had been diagnosed 1 year prior. His disease was active at the time, with a HBI of 10. He did not have any of the evaluated comorbidities, and he did not use alcohol or tobacco. An endoscopy showed several erosions in the distal esophagus consistent with esophagitis as well as duodenal nodularity, superficial ulceration, and scalloping of the mucosa from the bulb to the third portion. Granulomas were noted on histologic examination of biopsies from both the esophagus and duodenum.
Threshold for Peripapillary Lymphocytosis
Because LE is a relatively recently described process, which is not well described in the literature, we sought to evaluate our data using various proposed thresholds for the number of peripapillary lymphocytes required to diagnose LE. The total and additional (in parenthesis) numbers of patients in whom PL was diagnosed using thresholds of 40 or greater, 30, 20, and 10 IELs/HPF were 5 (1), 6 (2), 12 (8), and 24 (20), respectively. The numbers of additional patients who met histopathologic criteria for LE at these thresholds were 0, 1, 6, and 12, respectively. Disease duration and prevalence of GERD symptoms were similar at all thresholds; however, there was a trend toward a decrease in the values of WBC, ESR, and CRP level and a decreased likelihood of active disease at lower thresholds.
Discussion
PL was observed in 4 (8.5%) of 47 patients with IBD, whereas the histopathologic criteria for LE (ie, PL without significant granulocytes) was present in only 1 (2%) of the 47 patients in our adult IBD population. Our findings support data that did not show an association between LE and IBD in a large pathology database11 and in a mixed IBD population of children and adults.16 Our data differ from those previously reported in a pediatric IBD population.12
Our study population had relatively aggressive IBD, as indicated by the presence of a relapsing disease course in approximately 90% of patients, active disease in approximately two thirds of patients, and a history of gastrointestinal surgery related to IBD in over half of the patients. This is not surprising given that subjects were evaluated at a large IBD referral center. Under the presumption that LE may be a manifestation of more advanced IBD, the rarity of this entity in our patient population further argues against an association between adult IBD and LE.
Although LE was present in only a single subject, the role of lymphocytes may still be underappreciated in esophageal inflammation. We previously reported the presence of PL in 20% of patients with GERD and saw an inverse correlation between lymphocytes and proton pump inhibitor therapy in eosinophilic esophagitis.12 Eosinophilic esophagitis was suspected in 33% of patients with LE in Haque and Genta’s study.11 Increased numbers of IELs and active chronic inflammation have been described in suspected esophageal involvement with CD.19,20 Approximately 9% of our study population demonstrated PL on esophageal biopsies. Three of these 4 patients had a history of reflux (2 with GERD symptoms and 1 with esophagitis; P=.050), supporting previous reports of increased IELs seen with GERD.12,21,22 However, other studies have not shown a strong association between PL and GERD; in our aforementioned study, PL was present in 20% of patients with GERD,12 and studies from Haque and Genta reported a decreased prevalence of GERD symptoms in patients with LE compared with those with normal esophageal biopsy results.11
PL also was associated with elevated ESR, CRP level, HBI, and neutrophil density in the esophagus. These associations with both inflammation and clinical activity indicate that PL may be a marker of more aggressive or advanced IBD. This hypothesis is supported by the presence of active duodenitis and/or colitis in most of our patients with PL and by the presence of granulomas in both the esophagus and duodenum of our patient with histologic LE. While supporting this hypothesis, the finding of granulomas in esophageal biopsies raises the possibility that our patient actually had severe, active upper-gut CD. Nonetheless, the criteria for LE were met in this case. The downward trend in inflammatory markers (ie, WBC, ESR, and CRP level) with fewer IELs/HPF and the increased likelihood of active disease with higher numbers of IELs/HPF also suggest an association between PL and IBD severity.
Lowering the threshold number of peripapillary lymphocytes needed to diagnose LE is accompanied by a concomitant decrease in the number of granulocytes/HPF and a decrease in overall inflammation. The presence of fewer granulocytes might make it easier to meet the criteria for LE. For this reason, maintaining a high threshold number of peripapillary lymphocytes to diagnose LE, as is the case currently, is probably warranted.
There are a few key reasons that our results might differ from those reported by Rubio and colleagues1 and Ebach and colleagues.15 Rubio and colleagues evaluated a pathology database in search of patients with LE.1 This report does not document how many patients were in the database and how many of them had IBD. The pool of patients with IBD evaluated to obtain the 8 with LE was not specified, so we cannot postulate the prevalence of LE in IBD based on this study. Ebach and colleagues conducted a blinded retrospective analysis in which 28% of evaluated pediatric patients with IBD had LE.15 The fundamental difference between this study and our study is the pediatric patient population. Additionally, 11 (55%) of the 20 patients with LE in the study by Rubio and colleagues were age 17 years or younger,1 although, as with patients with IBD, we do not know how many pediatric patients were in the study database. The disparity between our adult population and the pediatric population from the study by Ebach and colleagues15 suggests that LE may be an age-related phenomenon in IBD.
Esophageal involvement in IBD is traditionally believed to be a rare phenomenon. More recent studies, however, have noted esophageal changes in 13—54% of children with IBD undergoing upper endoscopy.23-26 Ulcers are commonly seen on endoscopy, and concomitant gastric and/or duodenal involvement is present in the majority of cases,25 although granulomas occur in less than 25% of cases.27 Strictures and fistulae are uncommon. A higher pediatric CD activity index in patients with esophageal involvement supports an association with more severe IBD.26 Although there is a greater detection of esophageal abnormalities in pediatric IBD, the reported adult prevalence remains low (0.2—13% in patients with coexisting ileocolonic disease).28
As differences in study design (ie, pediatric vs adult study population) might help explain differences we observed, such differences may also help explain why our study size could be smaller than others reported in the literature. Whereas some of these studies examined large databases in search of patients with LE and IBD,1,11 a definitive history of IBD was an inclusion criterion in our study. The exclusion of patients without IBD significantly reduced our study size. The mean lymphocyte count (20.6/HPF) in patients with IBD in our study was higher than that (3.9/HPF) reported in a selection of 20 patients with CD in one of these larger studies.11 The severity of CD was not reported in that study, but it is possible that the difference in lymphocyte count may be associated with the likely more aggressive IBD in our study population. Despite this difference, the detection of LE was similar (2.1% vs 0%) in the 2 studies.
Conclusion
We found a less frequent association between IBD and LE than previously reported. According to histopathologic criteria, 2% of adult patients with IBD had LE. Differences between pediatric and adult IBD, such as a higher prevalence of esophageal involvement of CD reported in the pediatric group, may help explain these findings. Alternatively, the disparate findings may be methodologic because, unlike previous studies, we evaluated consecutive patients in whom IBD was diagnosed. PL was seen in 8.5% of patients with CD and was associated with elevated inflammatory markers and HBI. These observations suggest that PL may be a marker of disease activity in IBD. Further evaluation is required to assess the relationships among LE, GERD, and IBD.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Rubio CA, Sjodahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125(3):432–437. [PubMed] [Google Scholar]
- 2.Rubio CA, Hubbard GB. Hyperplastic foveolar gastropathy and hyperplastic foveolar gastritis in baboons. In Vivo. 1996;10(5):507–510. [PubMed] [Google Scholar]
- 3.Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58(2):163–174. [PubMed] [Google Scholar]
- 4.Black DD, Haggitt RC, Orenstein SR, Whitington PF. Esophagitis in infants: morphometric histological diagnosis and correlation with measures of gastroesophageal reflux. Gastroenterology. 1990;98(6):l408–l4l4. [PubMed] [Google Scholar]
- 5.Ismail-Beigi F, Pope CE., 2nd Distribution of the histological changes of gastroesophageal reflux in the distal esophagus of man. Gastroenterology. 1974;66(6):1109–1113. [PubMed] [Google Scholar]
- 6.Cucchiara S, D’Armiento F, Alfieri E, et al. Intraepithelial cells with irregular nuclear contours as a marker of esophagitis in children with gastroesophageal reflux disease. Dig Dis Sci. 1995;40(11):2305–2311. doi: 10.1007/BF02063229. [DOI] [PubMed] [Google Scholar]
- 7.Esposito S, Valente G, Zavallone A, Guidali P, Rapa A, Oderda G. Histological score for cells with irregular nuclear contours for the diagnosis of reflux esophagitis in children. Hum Pathol. 2004;35(1):96–101. doi: 10.1016/j.humpath.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Mangano MM, Antonioli DA, Schnitt SJ, Wang HH. Nature and significance of cells with irregular nuclear contours in esophageal mucosal biopsies. Mod Pathol. 1992;5(2):191–196. [PubMed] [Google Scholar]
- 9.Wang HH, Mangano MM, Antonioli DA. Evaluation of T-lymphocytes in esophageal mucosal biopsies. Mod Pathol. 1994;7(1):55–58. [PubMed] [Google Scholar]
- 10.Yousef MM, Yantiss RK, Baker SP. Banner BF Duodenal intraepithelial lymphocytes in inflammatory disorders of the esophagus and stomach. Clin Gastroenterol Hepatol. 2006;4(5):631–634. doi: 10.1016/j.cgh.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61(8):1108–1114. doi: 10.1136/gutjnl-2011-301014. [DOI] [PubMed] [Google Scholar]
- 12.Basseri B, Levy M, Wang HL, et al. Redefining the role of lymphocytes in gastroesophageal reflux disease and eosinophilic esophagitis. Dis Esophagus. 2010;23(5):368–376. doi: 10.1111/j.1442-2050.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 13.Ronkainen J, Walker MM, Aro P, et al. Lymphocytic oesophagitis, a condition in search of a disease? Gut. 2012;61(12):1776. doi: 10.1136/gutjnl-2012-302329. [DOI] [PubMed] [Google Scholar]
- 14.Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol. 2005;40(3):275–285. doi: 10.1080/00365520510011579. [DOI] [PubMed] [Google Scholar]
- 15.Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn’s disease. Inflamm Bowel Dis. 2010;17(1):45–49. doi: 10.1002/ibd.21347. [DOI] [PubMed] [Google Scholar]
- 16.Purdy JK, Appelman HD, Golembeski CP, McKenna BJ. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact. dermatitis. Am J Clin Pathol. 2008;130(4):508–513. doi: 10.1309/D3PCF6D6YYMQRX9A. [DOI] [PubMed] [Google Scholar]
- 17.Harvey RF, Bradshaw JM. A simple index of Crohn’s disease activity. Lancet. 1980;1(8167):514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 18.Furuta GT, Straumann A. Review article: the pathogenesis and management of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2006;24(2):173–182. doi: 10.1111/j.1365-2036.2006.02984.x. [DOI] [PubMed] [Google Scholar]
- 19.Decker GAG, Loftus EV, Pasha TM, Tremaine WJ, Sandborn WJ. Crohn’s disease of the esophagus: clinical features and outcomes. Inflamm Bowel Dis. 2001;7(2):113–119. doi: 10.1097/00054725-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Remes-Troche JM, Martinez-Benitez B, Valdovinos-Diaz MA. Crohn’s disease of the esophagus. Gastroenterology. 2006;130(4):1029–1376. doi: 10.1053/j.gastro.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 21.Resnick MB, Finkelstein Y, Weissler A, Levy J, Yakirevich E. Assessment and diagnostic utility of the cytotoxic T-lymphocyte phenotype using the specific markers granzyme-B and TIA-1 in esophageal mucosal biopsies. Hum Pathol. 1999;30(4):397–402. doi: 10.1016/s0046-8177(99)90114-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang HH, Mangano MM, Antonioli DA. Evaluation of T-lymphocytes in esophageal mucosal biopsies. Mod Pathol. 1994;7(1):55–58. [PubMed] [Google Scholar]
- 23.Pytrus T, Mowszet K, Krzesiek E, Rzeszutko M, Iwariczak B. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel diseases. Pol Merkur Lekarski. 2008;25(150):460–464. [PubMed] [Google Scholar]
- 24.Castellaneta SP, Afzal NA, Greenberg M, et al. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39(3):257–261. doi: 10.1097/00005176-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ammoury RF, Pfefferkorn MD. Significance of esophageal Crohn disease in children. J Pediatr Gastroenterol Nutr. 2011;52(3):291–294. doi: 10.1097/MPG.0b013e3181ec21b5. [DOI] [PubMed] [Google Scholar]
- 26.Ramaswamy K, Jacobson K, Jevon G, Israel D. Esophageal Crohn disease in children: a clinical spectrum. J Pediatr Gastroenterol Nutr. 2003;36(4):454–458. doi: 10.1097/00005176-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds HL, Jr, Stellato TA. Crohn’s disease of the foregut. Surg Clin N Am. 2001;81(1):117–135. doi: 10.1016/s0039-6109(05)70276-0. [DOI] [PubMed] [Google Scholar]
- 28.Naranjo-Rodriguez A, Solorzano-Peck G, Lopez-Rubio F, et al. Isolated oesophageal involvement of Crohn’s disease. Eur J Gastroenterol Hepatol. 2003;15(10):1123–1126. doi: 10.1097/00042737-200310000-00010. [DOI] [PubMed] [Google Scholar]